Abstract
Introduction
Melioidosis is caused by Burkholderia pseudomallei and primarily affects non-vascular organ systems. We present a case of a melioidotic penetrating aortic ulcer (PAU) with unusual clinical features of vascular infection. The patient was successfully treated with a single-stage neoaortoiliac system procedure, highlighting the challenges in managing melioidotic aortic infections.
Presentation of case
We present a case of melioidotic PAU that was successfully treated using a single-stage neoaortoiliac system procedure. A 70-year-old male with type 2 diabetes and ischemic heart disease presented with acute abdominal and back pain, later found to have an infrarenal PAU without aneurysmal changes. Following an emergency endovascular aortic repair (EVAR), blood cultures revealed Burkholderia pseudomallei bacteraemia, leading to the diagnosis of melioidosis-associated aortitis. The patient underwent a complex surgical procedure to remove the infected aorta and reconstruct it using a neoaortoiliac system, followed by a challenging recovery that included wound infection, prolonged antibiotic therapy, and subsequent hospitalization for sepsis. Despite complications, the patient remains alive and functionally independent 15 months post-surgery.
Discussion
Burkholderia pseudomallei, found in contaminated soil and water, can lead to severe infections, including mycotic aneurysms, with a high mortality rate despite treatment. Management of vascular involvement is complex, often requiring emergency interventions like EVAR to enable survival for definitive treatment.
Conclusion
This case emphasizes the importance of recognizing melioidosis as a potential cause of infectious aortitis, particularly in patients with a travel history to endemic regions.
Keywords: Melioidosis, Burkholderia pseudomallei, Infective aortitis, Penetrating aortic ulcer, Case report
Highlights
-
•
First report of melioidotic-penetrating aortic ulcer causing infectious aortitis.
-
•
Successful management involved emergent EVAR followed by NAIS reconstruction.
-
•
Early recognition of Burkholderia pseudomallei is crucial to prevent high mortality.
-
•
Multidisciplinary approach is needed for optimal outcomes in complex melioidosis.
-
•
Awareness of vascular melioidosis is vital, especially in endemic regions.
1. Introduction
Vascular manifestations of infection with Burkholderia pseudomallei are uncommon. However, given the high mortality rate, prompt recognition and treatment are crucial to optimize patient outcomes. We are reporting an aortic infection caused by a melioidotic penetrating aortic ulcer (PAU) to enhance awareness of its presentation and outline key management aspects. The patient provided written informed consent for the report of case details, imaging studies, and clinical photography.
2. Methods
This case has been reported in line with SCARE criteria [1].
3. Case report
A 70-year-old male patient presented to the emergency department with sharp, acute-onset, sharp abdominal and lower back pain. The patient had no bladder or bowel symptoms or clinical features of infection. The medical history included noninsulin-dependent type 2 diabetes mellitus and ischaemic heart disease. The patient was of Asian descent but lived in an Australian city, south of the tropics of Capricorn and had visited the Indian subcontinent and the Australian Kimberley region 3 and 2 months prior, respectively.
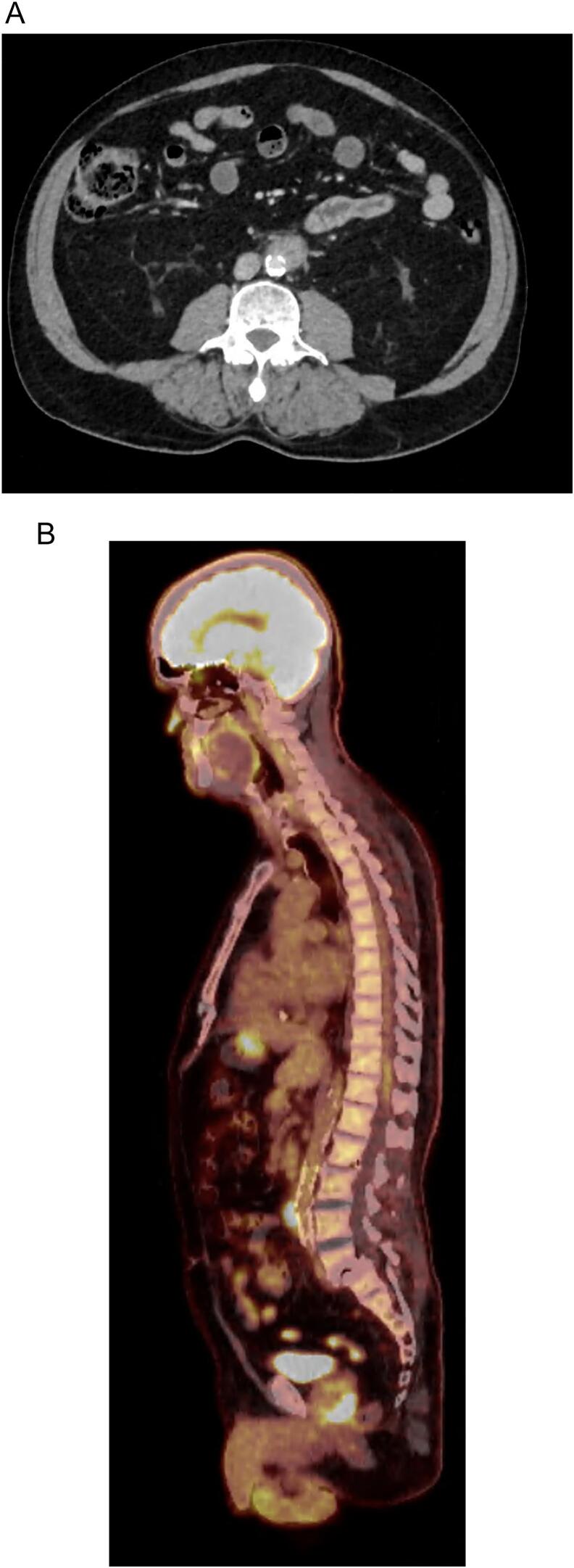
The patient was afebrile and comfortable and had mild lower abdominal tenderness without peritonism. Haematology and biochemistry test results were unremarkable, except for a white cell count of 11.11 × 10 [9]/L, a C-reactive protein level of 44 mg/L, and mildly elevated liver function results. Computed tomography (CT) and subsequent CT angiography revealed an infrarenal PAU with peri-ulcerative inflammation and no aneurysmal changes (Fig. 1).
Fig. 1.
Computed tomography angiography demonstrating an infrarenal penetrating aortic ulcer with inflammatory mass (A) and positron emission tomography-computed tomography demonstrating endovascular aortic repair stent-graft in situ and increased fluorodeoxyglucose uptake around infrarenal aorta (B).
Given the low clinical suspicion of infection and the risk of aortic rupture, the patient underwent emergent endovascular aortic repair (EVAR) using a reverse-deployed 16 × 20 × 82 mm Endurant Stent Graft System (Medtronic, Minneapolis, MN, USA) iliac limb as a single-tube endograft, as the distal aortic diameter was larger than the proximal diameter. The sterile graft was externally deployed, reversed, collapsed manually into the delivery system, and reintroduced by reverse-winding the delivery handle. Care was taken to avoid kinking the delivery system. Standard graft introduction and deployment procedures were then followed.
Two days later, the preoperative blood culture results confirmed Burkholderia pseudomallei bacteraemia. The patient developed acute testicular pain, dysuria, and fever, with a negative screening result for sexually transmitted infection. Ultrasonography confirmed orchitis. Positron emission tomography (PET) additionally demonstrated increased fluorodeoxyglucose (FDG) uptake in the left ureter and prostate. Following positive blood cultures, intravenous meropenem (2 g, 8-hourly and oral trimethoprim/sulfamethoxazole 1600/320 mg 12-hourly were commenced. Trimethoprim/sulfamethoxazole was later substituted with oral doxycycline 100 mg 12-hourly due to hyperkalaemia.
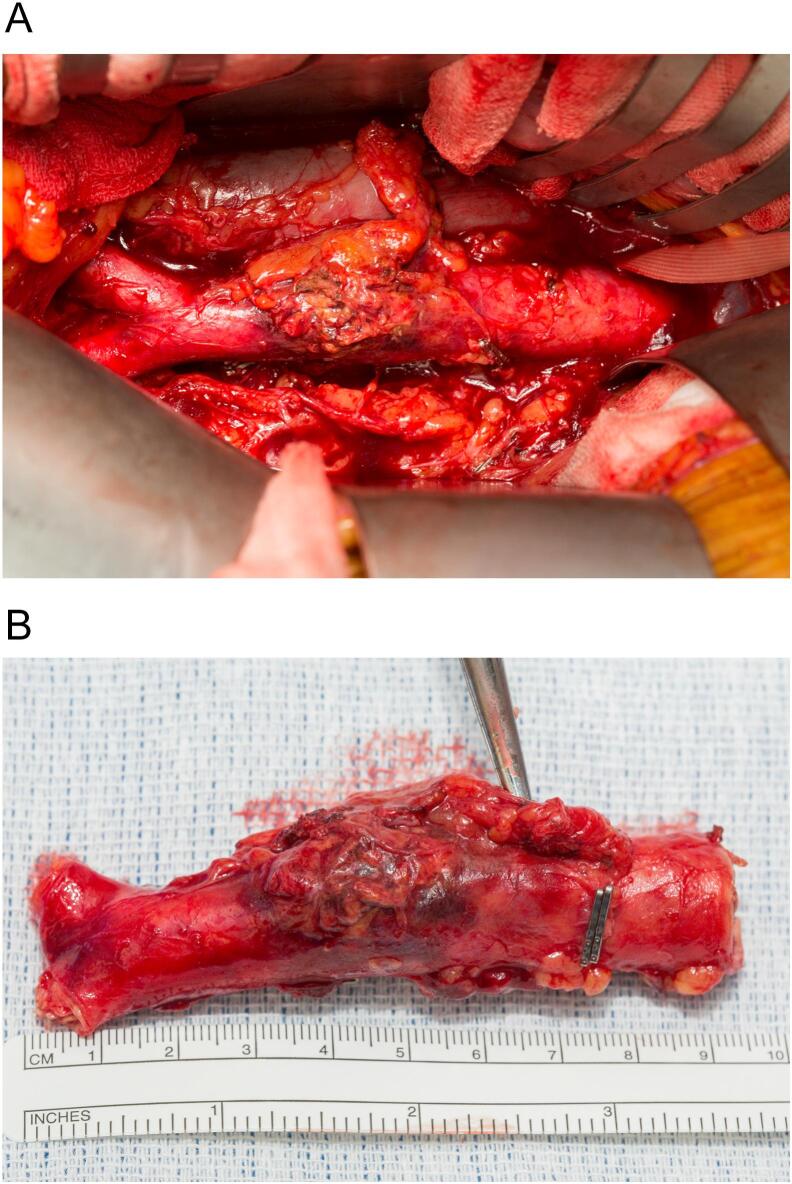
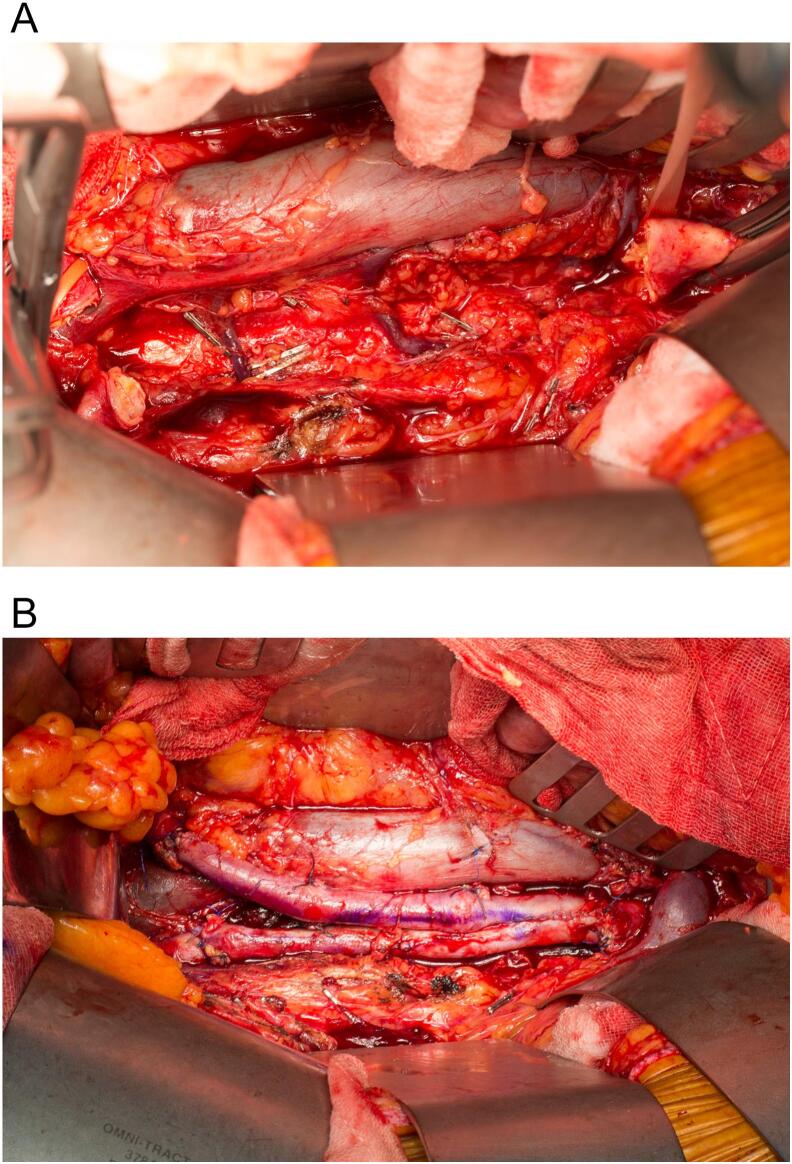
Three weeks post-EVAR and after the multidisciplinary intervention, the patient underwent explantation of the EVAR stent graft and a neoaortoiliac system (NAIS) single-stage procedure for the reconstruction of the infrarenal abdominal aorta using the bilateral femoral veins anastomosed in a pantalogue. Open repair is considered the only curative option for these patients, and a bifurcated graft was planned to facilitate resection of the infected aorta with adequate margins. The delay in the procedure was due to investigations for confirmation of the diagnosis and preoperative planning. Preoperative lower limb vein mapping confirmed an adequate femoral vein diameter (5.3–6.5 mm bilaterally) with a patent and competent venous system. Bilateral ureteric stents were placed under the same general anaesthesia, and one surgical team harvested the bilateral femoral veins, preserving the profunda and popliteal veins and fashioning the aortic vein graft in tandem. The femoral veins were anastomosed in a pantalogue configuration using 5/0 Prolene sutures to create an aortic graft. The second surgical team initiated a transperitoneal approach with evisceration and protection of the small bowel, which facilitated the visualisation of the suprarenal aorta to the level of the bilateral common iliac arteries. Inspection of the aorta (Fig. 2A) revealed an isolated infrarenal inflammatory/infected mass (Fig. 2B) without involvement of adjacent structures, such as the inferior vena cava or ureter. The inferior mesenteric and lumbar arteries were ligated before infrarenal aortic and common iliac clamping and excision of the infected tissues (Fig. 3A). The aortic graft was anastomosed with 4/0 Prolene (Fig. 3B), checked for leaks, and omentalised prior to abdominal closure.
Fig. 2.
Inflammatory/infective infrarenal abdominal aortic mass in situ (A) and after excision (B).
Fig. 3.
Exposure of retroperitoneum after infrarenal aortic excision (A) and after anastomosis of pantalooned femoral vein graft (B).
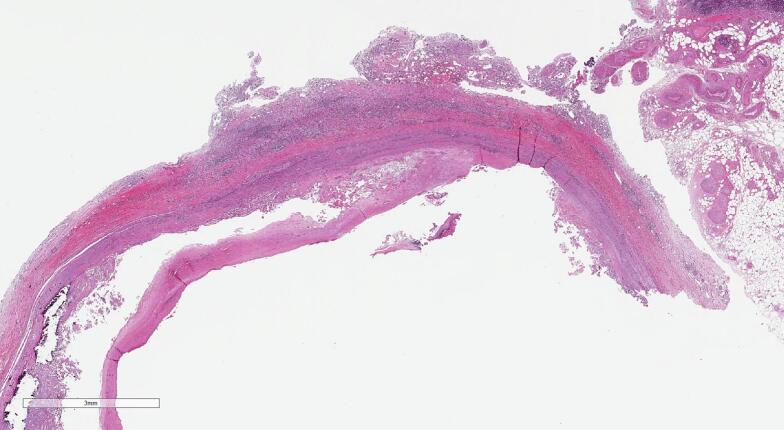
Intraoperative tissue samples returned negative microbiological results, which was expected because Burkholderia pseudomallei is not readily isolated from clinical specimens [2,3]. Sections of the aorta showed non-specific plasma cell-rich peri-aortitis with atherosclerotic changes, a pattern observed in both infectious and non-infectious aortitis/peri-aortitis (Fig. 4) [4,5]. The surrounding tunica media and adventitia demonstrated chronic inflammatory cell infiltration, comprising plasma cells, lymphocytes, and histiocytes. This finding was consistent with our diagnosis of melioidosis and aortitis.
Fig. 4.
Sections from the midpoint of the exudative area seen macroscopically demonstrated atheroma formation within the intima associated with cholesterol clefts, calcification and haemorrhage (A) (hematoxylin and eosin, original magnification x1).
The patient remained in the intensive care unit for the management of vasoplegic syndrome before being transferred to the ward. The patient's recovery was initially complicated by infective dehiscence of the laparotomy wound, which required debridement. The patient remained in the hospital for 2 and 18 days, respectively. Intravenous meropenem was continued for 6 weeks postoperatively, with a plan to continue lifelong doxycycline suppression, given the potential consequences of relapse. Three months later, the patient was readmitted for sepsis secondary to ischaemic colitis in the inferior mesenteric artery region with sigmoid perforation, requiring Hartmann's procedure and colostomy. The patient survived until discharge and returned home functionally independent after 2 months of rehabilitation. Fifteen months after his index procedure, he remains alive, functionally independent and is considering whether he wants to undergo further surgery for reversal of his colostomy.
4. Discussion
Melioidosis is a rare but significant cause of infectious aortitis. Burkholderia pseudomallei is a facultative, saprophytic, intracellular, gram-negative bacillus found in contaminated soil and groundwater [3]. It is ubiquitous and endemic across the tropical regions [6,7]. It infects 165,000 individuals annually and causes 89,000 deaths [8]. In Northern Australia, the incidence of infection is up to 20 times higher than that in Southeast Asia, and it disproportionately affects Indigenous Australians [9,10].
Infection occurs through ingestion, inhalation, or direct exposure of broken skin to contaminated water or soil [11]. Vertical, sexual, and zoonotic transmissions are rare [[12], [13], [14]]. Immunosuppressed individuals are at a considerable risk of infection and adverse outcomes [9]. The major risk factors include diabetes mellitus, hazardous alcohol use, and chronic lung or renal disease [9]. Infections can be acute, chronic, or latent with reactivation [9]. Typical incubation periods range from 1 day to 3 weeks, although the latency can last decades [15]. Mortality is as high as 50 % but lowers to 10 % with effective treatment [8,9].
Melioidosis presents as pneumonia in half of the cases, frequently accompanied by sepsis and commonly bacteremia [9]. Uncommon presentations include intra-abdominal abscesses, urinary tract infections, and infections of the central nervous system, musculoskeletal system, and soft tissue [7]. Acceptable antibiotic therapies include meropenem, ceftazidime, trimethoprim/sulfamethoxazole, and doxycycline, as Burkholderia pseudomallei demonstrates intrinsic resistance to penicillin and first and second-generation cephalosporins [16]. The United States Centers for Disease Control and Prevention classifies melioidosis as a Category B selected agent, although no vaccination is currently available [17].
Melioidosis rarely presents vascular involvement. No clear epidemiological data exist that describe the vascular patterns of melioidotic infectious diseases. Published case reports and series indicate that it presents as a mycotic aneurysm, a pseudoaneurysm of the aorta or its major branches, or as an infected endograft [[18], [19], [20], [21], [22], [23], [24]]. Atherosclerotic intimal lesions may predispose individuals to infection, likely due to the high prevalence of PAU in men aged >70 years [25,26]. Management strategies for melioidotic mycotic aneurysms include antibiotic suppression, in situ repair, and extra-anatomical repair. One case series found that 8 of 159 patients with melioidosis presented with vascular involvement, all with mycotic aneurysms, and had a 25 % mortality rate despite source control and optimal antibiotic therapy [18].
Burkholderia pseudomallei contains several virulence factors that aid in cell invasion, evasion of immune-mediated host defences, and promotion of intracellular survival [3,27]. It infects a range of cell types, although protease-activated receptor 1, which is expressed on several cell types, including endothelial cells, appears to promote Burkholderia pseudomallei invasion and persistence [3,27]. Although the initial inflammatory response is bactericidal, excessive inflammation is associated with mortality [28].
The initial emergent EVAR performed for symptomatic penetrating atherosclerotic ulcer (PAU) increased the complexity of the case. Although EVAR allowed the patient to receive definitive treatment, its timing may have been premature, considering the subsequent need for multistage intervention.
5. Conclusion
This case contributes to the current understanding of the spectrum of melioidosis and infectious aortitis presentations. This study outlined several key aspects of clinical management. Melioidosis is associated with high mortality rates. Therefore, awareness of this uncommon infection is important for clinicians when treating patients visiting endemic areas.
Author contribution
James Dodd: Concept, information collection, analysis, interpretation, writing
Bibombe Mwipatayi: Concept, analysis, interpretation, writing
Amber Louw: Analysis, interpretation, writing
Simon Joseph: Information collection, analysis, interpretation, writing
Fernando Picazo-Pineda: Concept, information collection, analysis, interpretation, writing
Guarantor
James Dodd
Bibombe Patrice Mwipatayi
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Case reports are exempt from requiring ethical approval in our institution as per Royal Perth Hospital Human Research Ethics Committee (Research Hub, East Metropolitan Health Service, Perth, Australia), although require written consent by the patient.
Declaration of Generative AI and AI-assisted technologies in the writing process
No generative AI or AI-assisted technologies were used in the writing process.
Funding
No funding was required.
Declaration of competing interest
The authors declare they have no competing interests.
Acknowledgements
I would like to express my appreciation to the entire team of theatre staff and ward nurses who have been diligently involved in addressing the demands of this particularly challenging case.
References
- 1.Sohrabi C., Mathew G., Maria N., et al. The SCARE 2023 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2023;109(5):1136–1140. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau S.K., Sridhar S., Ho C.C., et al. Laboratory diagnosis of melioidosis: past, present and future. Exp. Biol. Med. (Maywood) 2015;240(6):742–751. doi: 10.1177/1535370215583801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga W.J., Virk H.S., Torres A.G., et al. Melioidosis. Nat Rev Dis Primers. 2018;4:17107. doi: 10.1038/nrdp.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halushka M.K., Angelini A., Bartoloni G., et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the association for European cardiovascular pathology: II. Noninflammatory degenerative diseases - nomenclature and diagnostic criteria. Cardiovasc. Pathol. 2016;25(3):247–257. doi: 10.1016/j.carpath.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Aneurysm Mycotic. In: Diagnostic Pathology: Cardiovascular (Second Edition) Miller D.V., Revelo M.P., editors. Elsevier; Philadelphia: 2018. pp. 314–317. [Google Scholar]
- 6.Birnie E., Biemond J.J., Wiersinga W.J. Drivers of melioidosis endemicity: epidemiological transition, zoonosis, and climate change. Curr. Opin. Infect. Dis. 2022;35(3):196–204. doi: 10.1097/QCO.0000000000000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birnie E., Virk H.S., Savelkoel J., et al. Global burden of melioidosis in 2015: a systematic review and data synthesis. Lancet Infect. Dis. 2019;19(8):892–902. doi: 10.1016/S1473-3099(19)30157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Limmathurotsakul D., Golding N., Dance D.A., et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016;1(1) doi: 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- 9.Currie B.J., Mayo M., Ward L.M., et al. The Darwin prospective Melioidosis study: a 30-year prospective, observational investigation. Lancet Infect. Dis. 2021;21(12):1737–1746. doi: 10.1016/S1473-3099(21)00022-0. [DOI] [PubMed] [Google Scholar]
- 10.Pang L., Harris P.N.A., Seiler R.L., et al. Melioidosis, Singapore, 2003-2014. Emerg. Infect. Dis. 2018;24(1):140–143. doi: 10.3201/eid2401.161449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Limmathurotsakul D., Kanoksil M., Wuthiekanun V., et al. Activities of daily living associated with acquisition of melioidosis in Northeast Thailand: a matched case-control study. PLoS Negl. Trop. Dis. 2013;7(2) doi: 10.1371/journal.pntd.0002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ralph A., McBride J., Currie B.J. Transmission of Burkholderia pseudomallei via breast milk in northern Australia. Pediatr. Infect. Dis. J. 2004;23(12):1169–1171. [PubMed] [Google Scholar]
- 13.Webling D.D. Genito-urinary infections with pseudomonas pseudomallei in Australian aboriginals. Trans. R. Soc. Trop. Med. Hyg. 1980;74(1):138–139. doi: 10.1016/0035-9203(80)90036-x. [DOI] [PubMed] [Google Scholar]
- 14.Choy J.L., Mayo M., Janmaat A., et al. Animal melioidosis in Australia. Acta Trop. 2000;74(2–3):153–158. doi: 10.1016/s0001-706x(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 15.Chodimella U, Hoppes WL, Whalen S, et al. Septicemia and suppuration in a Vietnam veteran. Hosp. Pract. (1995) 1997; 32(5):219–21. [DOI] [PubMed]
- 16.Crowe A., McMahon N., Currie B.J., et al. Current antimicrobial susceptibility of first-episode melioidosis Burkholderia pseudomallei isolates from the Northern Territory. Australia. Int J Antimicrob Agents. 2014;44(2):160–162. doi: 10.1016/j.ijantimicag.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Peacock S.J., Limmathurotsakul D., Lubell Y., et al. Melioidosis vaccines: a systematic review and appraisal of the potential to exploit biodefense vaccines for public health purposes. PLoS Negl. Trop. Dis. 2012;6(1) doi: 10.1371/journal.pntd.0001488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu H., Wang X., Zhou X., et al. Mycotic aneurysm secondary to melioidosis in China: a series of eight cases and a review of literature. PLoS Negl. Trop. Dis. 2020;14(8) doi: 10.1371/journal.pntd.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong TK, Shan G, Sibangun FJ, et al. Melioidosis-related mycotic aneurysm: Three cases. IDCases 2021; 26:e01295. [DOI] [PMC free article] [PubMed]
- 20.Padmaja K., Lakshmi V., Sudhaharan S., et al. Unusual presentation of Melioidosis in a case of Pseudoaneurysm of descending thoracic aorta: review of two case reports. Research in cardiovascular medicine. 2015;4(2) doi: 10.5812/cardiovascmed.4(2)2015.27205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barry M., Dada H., Barry M., et al. Unusual presentation of melioidosis in a returning traveler. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auvens C., Neuwirth C., Piroth L., et al. Infected aneurysm after returning from Southeast Asia: think Burkholderia pseudomallei! BMJ Case Rep. 2019;12(5) doi: 10.1136/bcr-2018-228856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harison S., Bhat P., Rai R. Melioidosis: an unusual presentation as mycotic pseudoaneurysm of left superficial femoral artery. Journal of Microbiology and Infectious Diseases. 2018;08:162–164. [Google Scholar]
- 24.Cheok S., Gan L.S.C., Chung S.J., et al. Aortic endograft infection secondary to Burkholderia pseudomallei: a case report and review of the literature. J. Vasc. Surg. Cases Innov. Tech. 2021;7(3):421–424. doi: 10.1016/j.jvscit.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriyama Y., Toda R., Iwamura H., et al. Infected atherosclerotic ulcer of the abdominal aorta as a cause of mycotic aneurysm treated by in-situ prosthetic graft reconstruction: report of a case. Surg. Today. 1998;28(3):325–327. doi: 10.1007/s005950050132. [DOI] [PubMed] [Google Scholar]
- 26.DeMartino R.R., Sen I., Huang Y., et al. Population-based assessment of the incidence of aortic dissection, intramural hematoma, and penetrating ulcer, and its associated mortality from 1995 to 2015. Circ. Cardiovasc. Qual. Outcomes. 2018;11(8) doi: 10.1161/CIRCOUTCOMES.118.004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun G.W., Lu J., Pervaiz S., et al. Caspase-1 dependent macrophage death induced by Burkholderia pseudomallei. Cell. Microbiol. 2005;7(10):1447–1458. doi: 10.1111/j.1462-5822.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- 28.Gan Y.H. Interaction between Burkholderia pseudomallei and the host immune response: sleeping with the enemy? J Infect Dis. 2005;192(10):1845–1850. doi: 10.1086/497382. [DOI] [PubMed] [Google Scholar]