Abstract
Background
Immune checkpoint inhibitors (ICIs) have improved survival outcomes in melanoma. Studies exploring the correlations between body mass index (BMI), type 2 diabetes (T2DM) and the outcomes of ICI treatment have yielded inconsistent results. In this study, we aim to investigate the effects of BMI and T2DM on survival outcomes of patients with melanoma receiving ICIs.
Methods
A retrospective multicenter cohort of patients with melanoma treated with ICIs was analyzed. Overall survival was evaluated with Kaplan-Meier survival analysis, univariate Cox and multivariate Cox proportional hazards model. Propensity-score matching (1:1) analysis between overweight and non-overweight groups was done and survival analyses and Cox analyses were performed again. Subgroup analyses and secondary analyses stratifying patients with different weights and T2DM statuses were also performed.
Results
A total of 2,078 patients were included, of whom 1,412 were overweight (BMI≥25 kg/m2) and 666 were non-overweight (BMI<25 kg/m2). Overweight patients had better overall survival compared with non-overweight (median 71.7 vs 36.7 months, p<0.001). Patients with T2DM had worse overall survival compared with patients without T2DM (median 28.5 vs 67.3 months, p<0.001). After propensity-score matching (666 overweight were matched to 666 non-overweight), overweight patients remained to have better overall survival compared with non-overweight (median 67.7 vs 36.7 months, p<0.001). Patients with T2DM had worse survival in univariate Cox (HR 1.71, (95% CI: 1.20 to 2.43)) and multivariate Cox (HR 1.58, (95% CI: 1.08 to 2.31)) analyses. Overweight patients without T2DM had the best survival outcomes compared with other weight and T2DM combinations.
Conclusion
In patients with melanoma treated with ICIs, being overweight had better survival outcomes compared with non-overweight. Having T2DM was associated with worse survival compared with those without T2DM. Further studies are needed to investigate the underlying mechanisms of these associations.
Keywords: Immune Checkpoint Inhibitor, Melanoma, Diabetes
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
In this large propensity score-matched cohort study evaluating patients with melanoma treated with immune checkpoint inhibitors, being overweight had better survival outcomes compared with non-overweight. Having T2DM was associated with worse survival compared with those without T2DM. Being overweight and without T2DM was associated with the best overall survival among all subgroups.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Metabolic factors such as body mass index and T2DM may be used to predict the outcomes of patients with melanoma receiving immune checkpoint inhibitors. Further studies are needed to elucidate the complex relationships between body composition, T2DM, and the responses to immune checkpoint inhibitors.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment in recent years and have significantly improved survival outcomes in multiple malignancies, including melanoma.1,10 However, the treatment response of ICIs has remained heterogeneous among different patient cohorts, highlighting the importance of discovering predictive biomarkers.11 Metabolic factors such as body mass index (BMI, defined as weight divided by the square of height12 and type 2 diabetes mellitus (T2DM) have been investigated for their association with treatment outcomes for patients with cancer who received ICIs.13,30 In melanoma, several studies have investigated the association between being overweight or obese (defined as BMI≥25 kg/m2 and BMI≥30 kg/m2, respectively,12 and treatment outcomes of ICIs.13,21 While most studies have shown possible positive survival outcomes in overweight/obese patients treated with ICIs (compared with patients with BMI<25 kg/m2),13,20 a recent meta-analysis study showed no significant survival benefit in overweight/obese patient groups.21
On the other hand, the relationship between T2DM and ICI treatment outcomes remains complicated. Recent reports have shown that patients with cancer with T2DM might have a negative survival outcome when treated with ICIs compared with patients without T2DM.29 30 However, when investigating the effect of glucose-lowering medications, especially metformin, on ICI survival outcomes, there are mixed reports with both positive and negative associations.29,32 In melanoma specifically, additional studies are needed to investigate the relationship between T2DM and ICI treatment outcomes.
In recent years, a new phenotype of obesity has been proposed, termed “metabolically healthy obesity (MHO)”, which describes a subgroup of obese individuals that lack cardiometabolic comorbidities.33,37 Studies have shown that MHO patients might have more favorable mortality outcomes compared with patients with metabolically unhealthy obesity (MUO), including cancer mortalities.33 38 This phenomenon should also be considered when elucidating the heterogeneous effect of different metabolic derangements on ICI treatment outcomes.
In this study, we aimed to investigate the impact of different metabolic factors such as overweight/obesity and T2DM on the survival outcomes of patients with melanoma treated with ICIs with a large real-world multicenter cohort.
Methods
Study design, setting, and population
This was a multicenter, retrospective cohort study with propensity-score matching analysis.39 The database was constructed using a protocol approved by institutional review boards at the Mass General Brigham (MGB), detailing electronic medical records access in patients with diagnosis of metastatic or non-metastatic melanoma who have received ICIs including anti-programmed cell death protein 1 (anti-PD-1, eg, pembrolizumab, nivolumab), anti-programmed cell death-ligand 1 (anti-PD-L1, eg, atezolizumab), anti-cytotoxic T lymphocyte antigen-4 (anti-CTLA-4, eg, ipilimumab) and anti-lymphocyte-activation gene-3 (eg, relatlimab) therapies between January 2014 and December 2022 within the hospitals in the MGB network. The index date was determined as the date of the first ICI administration after the diagnosis of melanoma. Patients who had unknown data for the ICI therapy, multiple cancer diagnoses and incomplete records were excluded from the study. Patients were classified into overweight (BMI≥25 kg/m2) or non-overweight (BMI<25 kg/m2) based on the WHO definitions.12
Covariates and outcomes
We extracted clinical characteristics including patient demographics, baseline glucose level, underlying comorbidities (defined by International Classification of Diseases (ICD) codes), and medication usage at the time of starting ICI treatment from the MGB Research Patient Data Registry (RPDR) and Severe Immunotherapy Complications (SIC) registry. Oncology-specific data including the specific type of ICI, date of ICI initiation, and the presence of metastatic disease were also collected from the RPDR and SIC registry. The primary endpoint was all-cause mortality in overweight and non-overweight patients.
Statistical analysis
Patient characteristics were compared between overweight and non-overweight groups with appropriate statistical tests: t-test for continuous variables, and χ2 for discrete variables. We performed Kaplan-Meier analysis to evaluate time of survival between the BMI groups (overweight or non-overweight) and T2DM status (with T2DM and without T2DM). Time of survival was calculated from the index date to the date of death or date of last follow-up, which censoring would occur. Univariate and multivariate Cox proportional hazards model was used to evaluate the association between baseline characteristic variables and overall survival. The proportional HR assumption was assessed using the Schoenfeld residuals. In the multivariate Cox proportional hazards analysis, we incorporated variables including age, sex, metastatic disease, different ICI types and underlying comorbidities to further adjust for baseline differences.
We later performed a 1:1 propensity-score matching39 to minimize the baseline differences between the overweight and non-overweight cohorts. The propensity score was constructed using the following predetermined variables: age, sex, metastatic disease, ICI type, underlying comorbidities including hypertension, T2DM, hyperlipidemia, ischemic heart disease, chronic obstructive pulmonary disease, and statin use. We selected these variables because they predict the likelihood of the exposure (ie, overweight or non-overweight), are potential confounders, or are related to the primary outcome. The selection of such variables has been shown to provide optimal propensity-score matching models.40 We used a nearest neighbor matching approach, with a caliper set at 0.2 of the SD of the logit of the propensity score. After matching, we compared the differences in clinical characteristics between patients who were overweight and non-overweight using standardized mean differences (SMD). A covariate with less than 10% SMD is considered adequately matched.
After propensity-score matching, Kaplan-Meier analysis was used to evaluate the time of survival between the BMI groups (overweight or non-overweight) of the new cohort. We subsequently analyzed the new cohort using the univariate and multivariate Cox proportional hazards model as described previously. Similarly, we incorporated variables including age, sex, metastatic disease, different ICI types, and underlying comorbidities to adjust for baseline differences in the multivariate model. In subgroup analyses, we assessed the effects of BMI on overall survival in prespecified subgroups including age, sex, metastatic disease, different ICI types, and underlying comorbidities. We also performed Kaplan-Meier analyses to evaluate the effect of BMI in both male and female subgroups.
In secondary analyses, we compared the survival of patients across four different combination groups: BMI≥25 kg/m2 with or without diabetes mellitus and BMI<25 kg/m2 with or without diabetes mellitus. We also compared the survival of patients across five different BMI (kg/m2) groups: underweight (BMI<18.5), healthy weight (18.5≤BMI<25), overweight (25≤BMI<30), obese (30≤BMI<35), and severely obese (BMI≥35). In addition, Kaplan-Meier analyses comparing patients with or without baseline hyperglycemia (glucose level ≥126 mg/dL) and their BMI groups and DM status were also performed.
A p value<0.05 for a two-sided test was used to indicate statistical significance. The analyses were conducted using Stata V.16.0 (StataCorp LLC, College Station, Texas, USA) and R statistical software V.4.3 (http://www.r-project.org/).
Results
Patient demographics
We identified 2,254 patients with melanoma who were treated with ICIs between January 2014 and December 2022 cross referencing the MGB RPDR database and SIC registry. After excluding patients with unknown data for the ICI therapy, multiple cancer diagnosis and incomplete records, 2,078 patients remained eligible for analysis (online supplemental figure 1). Baseline characteristics were shown in table 1. Among the 2,078 patients, 1,412 patients were overweight and 666 patients were non-overweight. Among all patients, 43% received pembrolizumab, 31% received ipilimumab and nivolumab combination, 15% received nivolumab, 9.4% received ipilimumab, 1% received nivolumab and relatlimab combination and 1% received atezolizumab. Patients who were overweight had a higher proportion of underlying comorbidities including hypertension, T2DM and hyperlipidemia. Overweight patients were also more commonly prescribed cardiovascular and metabolic medications than non-overweight patients.
Table 1. Patient baseline characteristics.
| Total | BMI≥25(overweight) | BMI<25(non-overweight) | P value | |
| N=2,078 | N=1,412 | N=666 | ||
| Age | 67 (57–75) | 66 (57–75) | 67 (56–76) | 0.66 |
| Male | 1283 (62%) | 949 (67%) | 334 (50%) | <0.001 |
| Metastatic disease | 1180 (57%) | 785 (56%) | 395 (59%) | 0.11 |
| Glucose on ICI initiation, median | 106 (95–124) | 108 (97–127) | 103 (91–118) | <0.001 |
| ICI type | ||||
| Atezolizumab | 18 (1%) | 13 (1%) | 5 (1%) | 0.22 |
| Ipilimumab | 195 (9%) | 121 (9%) | 74 (11%) | |
| Ipilimumab and nivolumab | 635 (31%) | 422 (30%) | 213 (32%) | |
| Nivolumab | 305 (15%) | 220 (16%) | 85 (13%) | |
| Nivolumab and relatlimab | 30 (1%) | 19 (1%) | 11 (2%) | |
| Pembrolizumab | 895 (43%) | 617 (44%) | 278 (42%) | |
| Targeted therapy | ||||
| MEK inhibitors | 23 (1%) | 13 (1%) | 10 (2%) | 0.24 |
| BRAF inhibitors | 29 (1%) | 16 (1%) | 13 (2%) | 0.14 |
| Underlying comorbidities | ||||
| T2DM | 215 (10%) | 184 (13%) | 31 (5%) | <0.001 |
| Hypertension | 875 (42%) | 666 (47%) | 209 (31%) | <0.001 |
| Hyperlipidemia | 734 (35%) | 558 (40%) | 176 (26%) | <0.001 |
| Heart failure | 99 (5%) | 76 (5%) | 23 (3%) | 0.054 |
| Ischemic heart disease | 11 (1%) | 9 (1%) | 2 (0%) | 0.32 |
| Chronic kidney disease | 124 (6%) | 91 (6%) | 33 (5%) | 0.18 |
| COPD | 103 (5%) | 75 (5%) | 28 (4%) | 0.28 |
| Cardiovascular and metabolic medications | ||||
| Beta-blocker | 461 (22%) | 342 (24%) | 119 (18%) | 0.001 |
| Diuretics | 191 (9%) | 136 (10%) | 55 (8%) | 0.31 |
| Calcium channel blocker | 261 (13%) | 192 (14%) | 69 (10%) | 0.038 |
| Aspirin | 249 (12%) | 177 (13%) | 72 (11%) | 0.26 |
| Renin-angiotensin system inhibitor | 384 (18%) | 300 (21%) | 84 (13%) | <0.001 |
| Metformin | 121 (6%) | 102 (7%) | 19 (3%) | <0.001 |
| Insulin | 87 (4%) | 76 (5%) | 11 (2%) | <0.001 |
| Statin | 452 (22%) | 343 (24%) | 109 (16%) | <0.001 |
BMI unit: kg/m2.
Glucose unit: mg/dL.
BMIbody mass indexCOPDchronic obstructive pulmonary diseaseICIimmune checkpoint inhibitorT2DMtype 2 diabetes mellitus
Analysis for the overall cohort
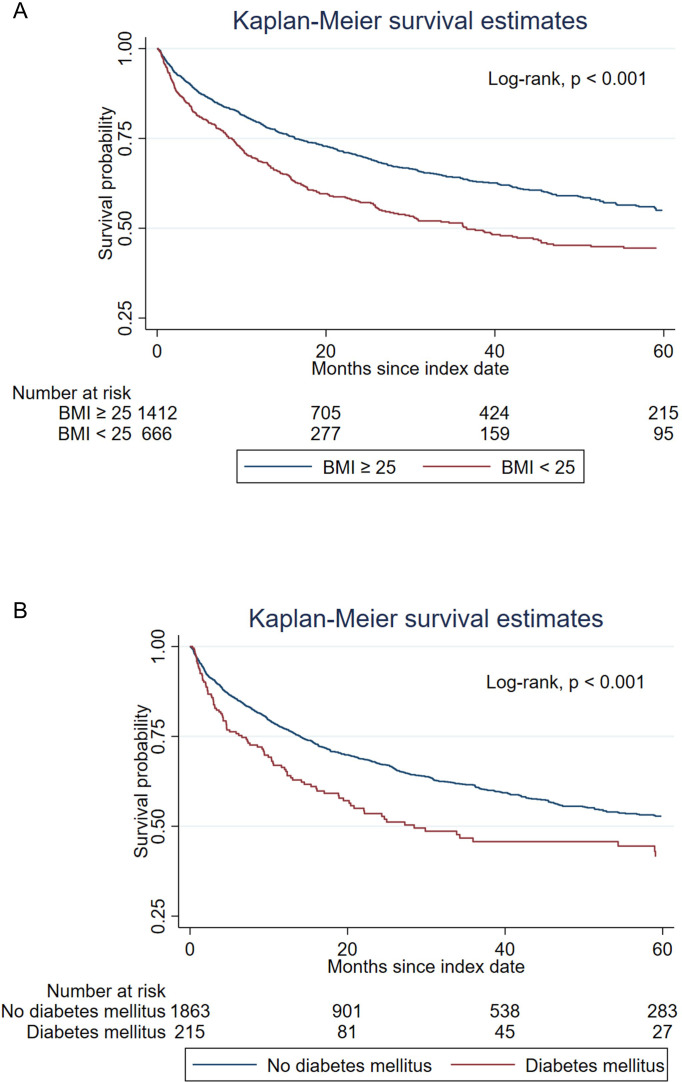
Kaplan-Meier analysis showed that overweight patients (BMI≥25 kg/m2) had a longer overall survival compared with non-overweight patients (BMI<25 kg/m2) (log-rank, p<0.001; median 71.7 months vs 36.7 months) (figure 1A). When comparing patients with T2DM versus without T2DM, Kaplan-Meier analysis showed patients with T2DM had worse survival (log-rank, p<0.001; median 28.5 months vs 67.3 months) (figure 1B). Univariate and multivariate Cox regression analyses of overall survival were performed and shown in online supplemental table 1. In the univariate Cox proportional hazard model, BMI≥25 kg/m2 was associated with an approximately 30% reduction in the risk of mortality (HR, 0.68 (95% CI: 0.60 to 0.79), p<0.001). In the multivariate Cox proportional hazard model, BMI≥25 kg/m2 remained associated with a reduction in the risk of mortality after adjusting for the variables including age, sex, metastatic disease, different ICI type and underlying comorbidities (HR, 0.69 (95% CI: 0.60 to 0.80), p<0.001). In univariate Cox analysis, T2DM was associated with a 46% increased risk of mortality (HR, 1.46 (95% CI: 1.19 to 1.80), p<0.001) and similarly so after adjusting for the variables in multivariate Cox analysis (HR, 1.44 (95% CI: 1.14 to 1.81), p=0.002).
Figure 1. Kaplan-Meier survival analysis comparing overall survival between patients with (A) BMI≥25 kg/m2 and BMI<25 kg/m2 (median survival 71.7 (IQR: 16.3 to not reached) months vs 36.7 (IQR: 8.8 to not reached) months), and (B) with and without type 2 diabetes mellitus (median survival 28.5 (IQR: 6.6 to not reached) months vs 67.3 (IQR: 14.0 to not reached) months). BMI, body mass index.
In addition, in univariate Cox analysis, other comorbidities such as hypertension, heart failure and chronic kidney disease showed statistically significant association with increased risk of mortality. However, only heart failure remained with a statistically significant association in the multivariate Cox analysis (HR, 1.39 (95% CI: 1.02 to 1.90), p=0.036). Furthermore, hyperlipidemia did not show a significant association with mortality in univariate Cox analysis, but it showed a statistically significant association with decreased risk of mortality in the multivariate Cox model (HR, 0.83 (95% CI: 0.70 to 0.99), p=0.035).
Propensity-score matching analysis
A total of 666 overweight patients were matched to 666 non-overweight patients. After propensity-score matching, all covariates including underlying comorbidities were well balanced between the two groups (table 2 and online supplemental figure 2).
Table 2. Patient baseline characteristics after propensity-score matching.
| Total | BMI≥25(overweight) | BMI<25(non-overweight) | P value | |
| N=1,332 | N=666 | N=666 | ||
| Age | 67 (57–76) | 67 (57–75) | 67 (56–76) | 0.75 |
| Male | 669 (50%) | 335 (50%) | 334 (50%) | 0.96 |
| Metastatic disease | 780 (59%) | 385 (58%) | 395 (59%) | 0.58 |
| Glucose on ICI initiation, median | 104 (93–121) | 105 (94–123) | 103 (91–118) | <0.001 |
| ICI type | ||||
| Atezolizumab | 10 (1%) | 5 (1%) | 5 (1%) | 0.3 |
| Ipilimumab | 137 (10%) | 63 (9%) | 74 (11%) | |
| Ipilimumab and nivolumab | 409 (31%) | 196 (29%) | 213 (32%) | |
| Nivolumab | 198 (15%) | 113 (17%) | 85 (13%) | |
| Nivolumab and relatlimab | 19 (1%) | 8 (1%) | 11 (2%) | |
| Pembrolizumab | 559 (42%) | 281 (42%) | 278 (42%) | |
| Targeted therapy | ||||
| MEK inhibitors | 18 (1%) | 8 (1%) | 10 (2%) | 0.64 |
| BRAF inhibitors | 19 (1%) | 6 (1%) | 13 (2%) | 0.11 |
| Underlying comorbidities | ||||
| T2DM | 62 (5%) | 31 (5%) | 31 (5%) | 1 |
| Hypertension | 412 (31%) | 203 (30%) | 209 (31%) | 0.72 |
| Hyperlipidemia | 352 (26%) | 176 (26%) | 176 (26%) | 1 |
| Heart failure | 51 (4%) | 28 (4%) | 23 (3%) | 0.48 |
| Ischemic heart disease | 5 (0%) | 3 (0%) | 2 (0%) | 0.65 |
| Chronic kidney disease | 56 (4%) | 23 (3%) | 33 (5%) | 0.17 |
| COPD | 57 (4%) | 29 (4%) | 28 (4%) | 0.89 |
| Cardiovascular and metabolic medications | ||||
| Beta-blocker | 234 (18%) | 115 (17%) | 119 (18%) | 0.77 |
| Diuretics | 109 (8%) | 54 (8%) | 55 (8%) | 0.92 |
| Calcium channel blocker | 129 (10%) | 60 (9%) | 69 (10%) | 0.4 |
| Aspirin | 136 (10%) | 64 (10%) | 72 (11%) | 0.47 |
| Renin-angiotensin system inhibitor | 185 (14%) | 101 (15%) | 84 (13%) | 0.18 |
| Metformin | 46 (3%) | 27 (4%) | 19 (3%) | 0.23 |
| Insulin | 22 (2%) | 11 (2%) | 11 (2%) | 1.00 |
| Statin | 208 (16%) | 99 (15%) | 109 (16%) | 0.45 |
BMI unit: kg/m2.
Glucose unit: mg/dL.
BMIbody mass indexCOPD, chronic obstructive pulmonary disease; ICI, immune checkpoint inhibitorT2DM, type 2 diabetes mellitus
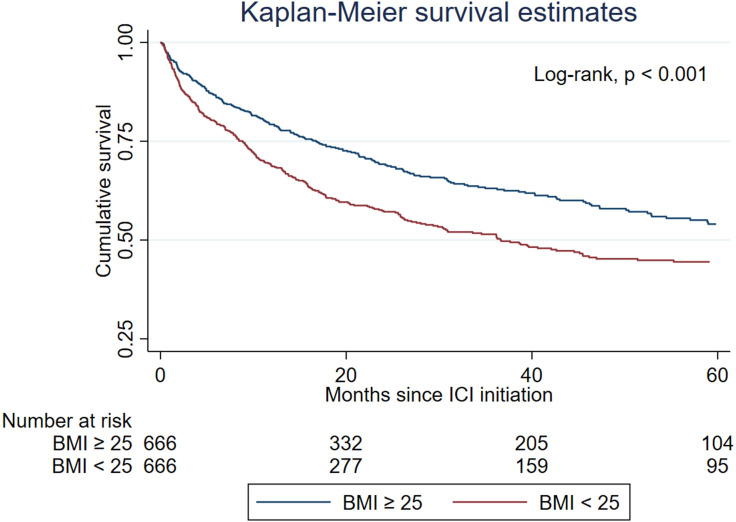
During a median follow-up period of 17.3 (IQR: 6.6–43.7) months, 235 (35%) and 303 (45%) patients died in the overweight and non-overweight groups, respectively. Kaplan-Meier analysis of the propensity-score matched cohort showed that overweight patients (BMI≥25 kg/m2) had higher overall survival compared with non-overweight patients (BMI<25 kg/m2) (log-rank, p<0.001; median 67.7 months vs 36.7 months) (figure 2). Univariate and multivariate Cox analyses of the overall survival of the propensity-score matched cohort were shown in table 3. In univariate Cox analysis, BMI≥25 kg/m2 was significantly associated with a 29% reduction in the risk of mortality (HR, 0.71 (95% CI: 0.60 to 0.84), p<0.001). In multivariate Cox analysis, BMI≥25 kg/m2 remained significantly associated with a reduction in the risk of mortality after adjusting for the variables listed (HR, 0.71 (95% CI: 0.60 to 0.84), p<0.001). In the univariate Cox proportional hazard model, T2DM was associated with a 71% increased risk of mortality (HR, 1.71 (95% CI: 1.20 to 2.43), p=0.003) and remained associated with an increased risk after adjusting for the variables in multivariate analysis (HR, 1.58 (95% CI: 1.08 to 2.31), p=0.019).
Figure 2. Kaplan-Meier survival analysis comparing overall survival between BMI≥25 kg/m2 and BMI<25 kg/m2 after propensity-score matching. Median survival 67.7 (IQR: 16.7 to not reached) months versus 36.7 (IQR: 8.8 to not reached) months. BMI, body mass index.
Table 3. Cox regression analysis comparing all-cause mortality after-propensity-score matching.
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95% CI) | P value | HR (95% CHR) | P value | |
| Age | 1.02 (1.01 to 1.03) | <0.001 | 1.02 (1.01 to 1.03) | <0.001 |
| Sex | 1.00 (0.85 to 1.19) | 0.96 | 0.87 (0.73 to 1.04) | 0.125 |
| Metastatic disease | 1.24 (1.04 to 1.48) | 0.017 | 1.24 (1.04 to 1.48) | 0.018 |
| ICI type | 0.99 (0.95 to 1.02) | 0.49 | 0.96 (0.93 to 1.00) | 0.045 |
| BMI≥25 | 0.71 (0.60 to 0.84) | <0.001 | 0.71 (0.60 to 0.84) | <0.001 |
| T2DM | 1.71 (1.20 to 2.43) | 0.003 | 1.58 (1.08 to 2.31) | 0.019 |
| Hypertension | 1.26 (1.05 to 1.51) | 0.011 | 0.91 (0.73 to 1.14) | 0.42 |
| Heart failure | 2.10 (1.45 to 3.04) | <0.001 | 1.30 (0.85 to 1.98) | 0.22 |
| Hyperlipidemia | 1.13 (0.93 to 1.37) | 0.21 | 0.85 (0.68 to 1.07) | 0.162 |
| Chronic kidney disease | 2.00 (1.41 to 2.85) | <0.001 | 1.35 (0.91 to 1.99) | 0.135 |
| COPD | 1.27 (0.85 to 1.90) | 0.24 | 1.05 (0.69 to 1.60) | 0.81 |
| Ischemic heart disease | 0.76 (0.19 to 3.06) | 0.70 | 0.52 (0.12 to 2.20) | 0.37 |
BMI unit: kg/m2.
COPD, chronic obstructive pulmonary disease; ICI, immune checkpoint inhibitorT2DM, type 2 diabetes mellitus
Other comorbidities such as hypertension, heart failure and chronic kidney disease still showed statistically significant association with increased risk of all-cause mortality in univariate Cox analysis, however, none of these comorbidities showed statistically significant association in the multivariate Cox analysis. On the other hand, hyperlipidemia also did not show a significant association with all-cause mortality in both univariate and multivariate Cox analysis.
Subgroup analysis
In our subgroup analysis of the propensity-score matched cohort, overweight (BMI≥25 kg/m2) was mostly associated with a reduction or a trend towards a lower risk of all-cause mortality across different subgroups including age, sex, presence of metastatic disease and underlying comorbidities (online supplemental figure 3). In the analysis of different ICI regimens, overweight appeared to be associated with a statistically significant reduction in mortality in patients receiving anti-PD-1/PD-L1 therapy (HR, 0.64 (95% CI: 0.51 to 0.80)); however, overweight did not appear to have statistical significance in reduction in mortality in patients receiving anti-CTLA-4 (HR, 0.70 (95% CI: 0.43 to 1.14)) or anti-PD-1/anti-CTLA-4 combination (HR, 0.87 (95% CI: 0.63 to 1.19)).
Additional Kaplan-Meier analysis comparing overweight and non-overweight in male patients (online supplemental figure 4A). Log-rank, (p=0.001; median 67.7 months vs 36.6 months) and in female patients (online supplemental figure 4B). Log-rank, (p=0.012; median survival 67.7 months vs 36.3 months) were also performed, which showed consistent favorable survival outcomes of overweight among both male and female patients. We also
Secondary analyses
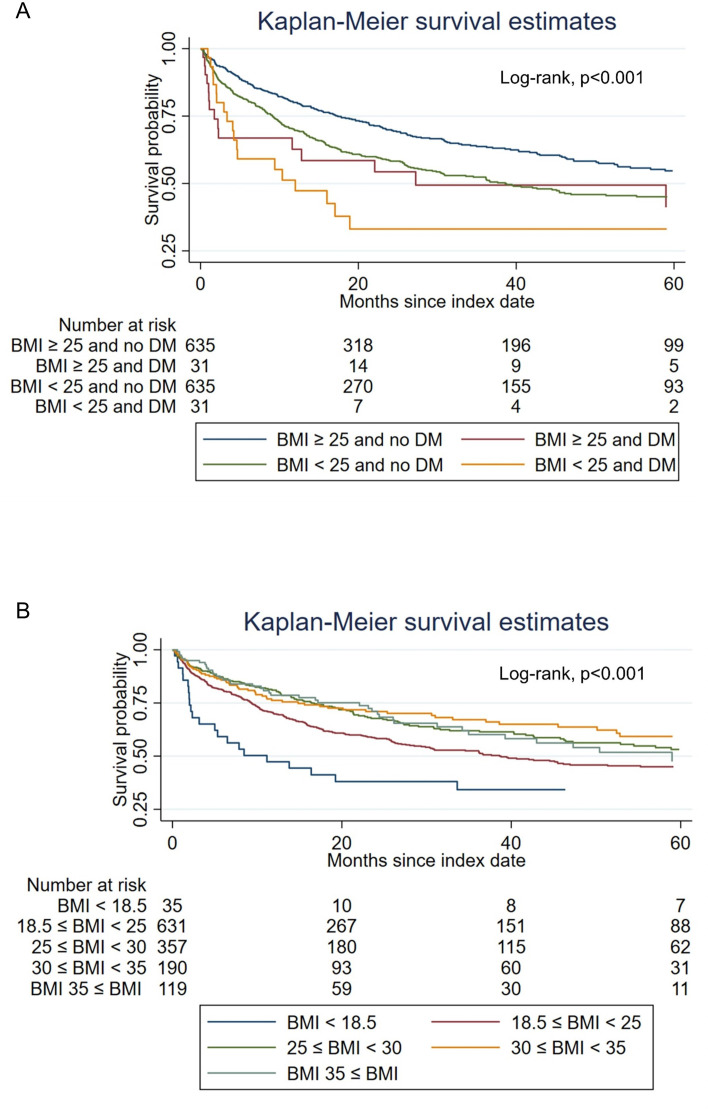
Furthermore, we compared the survival of patients in the following four subgroups: Patients with BMI≥25 kg/m2 with or without T2DM, and patients with BMI<25 kg/m2 with or without T2DM. Kaplan-Meier analysis showed that patients with BMI≥25 kg/m2 and without T2DM had the highest median overall survival (median 67.7 months) among the four subgroups, followed by patients who had BMI≥25 kg/m2 with T2DM (median 38.6 months), and followed by BMI<25 kg/m2 without T2DM (median 27.3 months). Patients who had a BMI<25 kg/m2 and with T2DM had the worse overall survival (median 12.0 months). Log-rank p value<0.001 (figure 3A).
Figure 3. Kaplan-Meier survival analysis comparing overall survival (A) between BMI≥25 kg/m2 with T2DM (median 38.6 (IQR: 9.1 to not reached) months)and without T2DM (median 67.7 (IQR: 17.4 to not reached) months), and BMI<25 kg/m2 with T2DM (median 12.0 (IQR: 3.4 to not reached) months) and without T2DM (median 27.3 (IQR: 1.7 to not reached) months), and (B) between five different BMI groups (underweight: median 11.2 (IQR: 2.0 to 64.6) months; healthy weight: median 38.6 (IQR: 9.3 to not reached) months; overweight: median 67.7 (IQR: 16.4 to 103.4) months; obese: median not reached (IQR: 14.8 to not reached) months; severely obese; median 59.0 (IQR: 22.1 to not reached) months) (BMI unit kg/m2). BMI, body mass index; T2DM, type 2 diabetes mellitus.
We also performed Kaplan-Meier analysis in five different BMI groups (underweight (BMI<18.5 kg/m2), healthy weight (18.5 kg/m2≤BMI<25 kg/m2), overweight (25 kg/m2≤BMI<30 kg/m2), obese (30 kg/m2≤BMI<35 kg/m2), and severely obese (BMI≥35 kg/m2)). Results showed that patients who were overweight (median 67.7 months), obese (median not reached months), or severely obese (median 59.0 months) had higher overall survival compared with patients who were underweight (median 11.2 months) or healthy weight (median 38.6 months). Log-rank p value<0.001 (figure 3B).
In addition, Kaplan-Meier analyses comparing patients with or without baseline hyperglycemia (glucose level ≥126 mg/dL) and their BMI groups (online supplemental figure 5) as well as their DM status (online supplemental figure 6) were performed. Patients with baseline hyperglycemia experienced worse overall survival in the respective BMI groups and DM groups.
Discussion
In this large retrospective cohort study with propensity-score matching analysis, we found that being overweight (BMI≥25 kg/m2) was associated with improved overall survival among patients with melanoma undergoing ICI therapy, while patients with T2DM were associated with worse overall survival. Furthermore, patients with BMI≥25 kg/m2 without T2DM had the highest median overall survival compared with other groups of BMI/T2DM combinations. Among all patients with BMI≥25 kg/m2, there were no significant differences between those who were overweight, obese, and severely obese. To the best of our knowledge, this was the largest retrospective cohort study to date to investigate the association between BMI, T2DM, and survival outcomes in patients with melanoma undergoing ICI therapy.
There have been several studies investigating the association between BMI and survival among patients with melanoma receiving ICI therapy, but the results have been inconsistent.21 In a multicenter cohort study conducted by McQuade et al, obesity (BMI≥30 kg/m2) was associated with improved progression-free survival and overall survival among male patients receiving anti-CTLA-4 or anti-PD-1/PD-L1 inhibitors.14 Similarly, in another cohort study performed by Naik et al, overweight to obese (25 kg/m2≤BMI<35 kg/m2) male patients receiving anti-PD-1 monotherapy or combination ICI therapy also experienced improved survival compared with normal-weight patients.15 Nevertheless, many studies did not find an association between BMI and survival outcomes.16,18 For example, in a multicenter cohort study performed by Di Filippo et al, a higher BMI was not associated with an improved progression-free survival or overall survival among patients receiving an anti-PD-1 monotherapy or combination ICI therapy.18 In our study, we found an improved overall survival among overweight patients compared with those who were non-overweight. The results remained consistent in propensity-score matched analysis. Furthermore, these survival benefits were observed consistently across the overweight, obese, and severely obese categories. In our subgroup analysis, the survival benefit of overweight remained consistent in both male and female patients. Interestingly, when looking into treatment types, although patients with BMI≥25 kg/m2 had favorable survival outcomes when receiving anti-PD-1/PD-L1 monotherapy, anti-CTLA-4 monotherapy and anti-CTLA-4 with anti-PD-1 combination therapies, only anti-PD-1/PD-L1 group had statistical significance in HR. It was unclear why there had been such a discordance among the published literature. However, factors such as differences in study population including cohort size, underlying comorbidities, cancer stages, ICI regimen, and differences in analysis such as adjustments for potential confounders, could all have contributed to the discrepancies in the reported results.
The mechanism underlying this observation, coined the “obesity paradox”, has not been fully elucidated. However, preclinical studies have shown that adipose tissues, which are increased in patients with higher BMIs, may exert immunomodulatory effects via the production of various cytokines and hormones that can activate antitumor immune responses.41 Furthermore, patients with higher BMIs have increased tumor-infiltrating cytotoxic T-lymphocytes in the tumor microenvironment and hence may be primmed to provide a more efficacious response to ICIs.42
Previously, there were few studies investigating pre-existing T2DM affecting patients with cancer undergoing ICI treatment.29 30 Cortellini et al previously reported an association between diabetes, use of glucose-lowering medications, and poorer outcomes in patients treated with ICIs in a mixed cancer cohort of 1,395 patients, among which 345 were patients with melanoma.30 To our knowledge, no dedicated melanoma study has established this association. Our study found that patients with melanoma with T2DM at baseline had worse overall survival compared with patients without T2DM when receiving ICI therapies. Furthermore, we found that overweight patients without T2DM experienced the best overall survival compared with overweight patients with T2DM and non-overweight patients with or without T2DM. Not surprisingly, non-overweight patients with T2DM experienced the worst overall survival. We also found that patients with baseline hyperglycemia experienced worse overall survival in the respective BMI groups and DM groups. Furthermore, patients with baseline hyperglycemia and no DM experienced similar survival outcomes compared with patients with DM. Our findings could be a possible validation of potential health benefits in the recently proposed concept of “metabolically healthy obesity (MHO)” status.33,37 Previously, Calori et al38 had shown that obese subjects with insulin resistance (ie, MUO) had higher cancer mortality compared with non-obese insulin-sensitive subjects. No significant mortality difference was found comparing obese insulin-sensitive (ie, MHO) subjects with non-obese insulin-sensitive subjects. Our results support the hypothesis that patients with MHO cancer will have potentially favorable outcomes when treated with ICIs. Further studies are needed to further elucidate this complex interaction.
We also had additional observations that patients with heart failure at the time of starting ICI therapy had a higher risk of all-cause mortality in multivariate Cox analyses in the overall cohort but not in the propensity-score matched cohort. Previous studies have shown that patients with pre-existing cardiac conditions such as heart failure may be prone to developing ICI-mediated cardiotoxicity and related immune-related adverse events when treated with ICIs.43,47 Heart failure was also a risk factor for developing major adverse cardiovascular events for patients with cancer treated with ICIs.48 These phenomena could potentially explain our observations of less favorable outcomes in the overall patient cohort. However, this association might be due to a confounding effect as we did not have the same observation in the propensity-score-matched cohort. Our study was also limited by a lack of cause of death annotation, therefore we were not able to distinguish cardiovascular-related mortality including ICI-toxicity from other causes of death. Additional research exploring the effect of pre-existing heart failure on patients with melanoma treated with ICIs is needed.
Another observation that we made was that patients with hyperlipidemia showed a statistically significant association with decreased risk of all-cause mortality in the multivariate Cox model in the overall patient cohort but not in the propensity-score matched cohort. There were recent studies showing that higher blood cholesterol level was associated with better outcomes in patients with cancer treated with ICIs.49,52 While our observation in the overall patient cohort is consistent with previous studies, we did not see the same association in the propensity-score-matched cohort. This could also be due to the possible confounding effect between high BMI and hyperlipidemia. By performing propensity-score matching, we removed this effect and showed that hyperlipidemia alone was not associated with a reduced risk of mortality. Further clinical and mechanistic studies are needed to understand the complicated interaction between obesity, hyperlipidemia and its effect on ICI treatment.
One major strength of our study was that we used propensity-score matching and subsequently multivariate analyses to minimize the baseline differences and potential confounders between patients who were overweight and non-overweight. The large sample size allowed us to explore possible effect modifiers in different subgroups of patients. Nevertheless, there were several limitations that need to be acknowledged. This was an observational study and despite the use of propensity-score matching and multivariate analyses, there were likely residual confounders that were not included in the models. We did not have data on the causes of death, ICI toxicity, and tumor-based endpoints such as relapse-free or progression-free survival and response to treatment. Therefore, we could not be entirely certain that the survival benefits associated with a higher BMI were attributed to an improved response to ICI therapy or other non-oncologic mechanisms. The different lines of melanoma therapy might affect our survival analysis as well. In both cohorts, about 1–2% of patients had received BRAF/MEK inhibitors prior to ICI. Also, although we used the first ICI encounter in the system as the patients’ ICI start date, we could not exclude a small number of patients who might have received ICIs in different institutions prior to the encounter. Although this was a multicenter study, the hospitals are in the same healthcare system in the New England area which may limit its generalizability. The identification of most comorbidities and conditions relied on physician diagnoses and ICD coding, which could potentially introduce some degree of inaccuracy or incompleteness of the data. Lastly, we only had the data on BMI, which may not be a representative surrogate for body composition as it does not differentiate between skeletal muscle and adipose tissue.53
Conclusion
In this large retrospective cohort study with propensity-score matching analysis, overweight patients with melanoma had better overall survival compared with non-overweight patients when treated with ICIs, particularly those undergoing anti-PD-1 therapy. Patients with T2DM were associated with worse overall survival compared with patients without T2DM. Overweight patients without T2DM experienced the best overall survival compared with other groups. Further prospective randomized trials and molecular mechanistic studies are needed to further investigate the relationships between BMI, T2DM and patients with melanoma treated with ICIs.
supplementary material
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability free text: Data of the study are available upon reasonable request.
Ethics approval: This study was approved by Mass General Brigham Institutional Review Board (Protocol #: 2023P000244) as an exempt study of medical record review.
Contributor Information
Yu Jen Alexander Jan, Email: yujenalex.jan@gmail.com.
Cho-Han Chiang, Email: chiangchohan1129@gmail.com.
Soravis Osataphan, Email: sosataph@bidmc.harvard.edu.
Aleigha R Lawless, Email: alawless@mgb.org.
Kerry L Reynolds, Email: kreynolds7@mgb.org.
Ryan J Sullivan, Email: rsullivan7@mgh.harvard.edu.
Data availability statement
Data are available upon reasonable request.
References
- 1.Shiravand Y, Khodadadi F, Kashani SMA, et al. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol. 2022;29:3044–60. doi: 10.3390/curroncol29050247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381:1535–46. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Carlino MS, McNeil C, et al. Seven-Year Follow-Up of the Phase III KEYNOTE-006 Study: Pembrolizumab Versus Ipilimumab in Advanced Melanoma. J Clin Oncol. 2023;41:3998–4003. doi: 10.1200/JCO.22.01599. [DOI] [PubMed] [Google Scholar]
- 4.Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390:1853–62. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 5.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 8.(NCCN®) NCCN . National Comprehensive Cancer Network, Inc; 2023. NCCN clinical practice guidelines in oncology (nccn guidelines®) for melanoma: cutaneous version 2.2023 — march 10, 2023. [Google Scholar]
- 9.O’Rourke K. Relatlimab plus nivolumab beneficial for previously untreated metastatic or unresectable melanoma. Cancer. 2022;128:1887. doi: 10.1002/cncr.34227. [DOI] [PubMed] [Google Scholar]
- 10.Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37–45. doi: 10.1016/j.ejca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–50. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. [PubMed] [Google Scholar]
- 13.Richtig G, Hoeller C, Wolf M, et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: An observational multi-centre study. PLoS ONE. 2018;13:e0204729. doi: 10.1371/journal.pone.0204729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310–22. doi: 10.1016/S1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naik GS, Waikar SS, Johnson AEW, et al. Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J Immunother Cancer. 2019;7:89. doi: 10.1186/s40425-019-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnelly D, Bajaj S, Yu J, et al. The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J Immunother Cancer. 2019;7:222. doi: 10.1186/s40425-019-0699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutkowski P, Indini A, De Luca M, et al. Body mass index (BMI) and outcome of metastatic melanoma patients receiving targeted therapy and immunotherapy: a multicenter international retrospective study. J Immunother Cancer. 2020;8:e001117. doi: 10.1136/jitc-2020-001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Filippo Y, Dalle S, Mortier L, et al. Relevance of body mass index as a predictor of systemic therapy outcomes in metastatic melanoma: analysis of the MelBase French cohort data☆. Ann Oncol. 2021;32:542–51. doi: 10.1016/j.annonc.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Young AC, Quach HT, Song H, et al. Impact of body composition on outcomes from anti-PD1 +/- anti-CTLA-4 treatment in melanoma. J Immunother Cancer. 2020;8:e000821. doi: 10.1136/jitc-2020-000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JH, Hyung S, Lee J, et al. Visceral adiposity and systemic inflammation in the obesity paradox in patients with unresectable or metastatic melanoma undergoing immune checkpoint inhibitor therapy: a retrospective cohort study. J Immunother Cancer. 2022;10:e005226. doi: 10.1136/jitc-2022-005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roccuzzo G, Moirano G, Fava P, et al. Obesity and immune-checkpoint inhibitors in advanced melanoma: A meta-analysis of survival outcomes from clinical studies. Semin Cancer Biol. 2023;91:27–34. doi: 10.1016/j.semcancer.2023.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Cortellini A, Bersanelli M, Buti S, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7:57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martini DJ, Kline MR, Liu Y, et al. Adiposity may predict survival in patients with advanced stage cancer treated with immunotherapy in phase 1 clinical trials. Cancer. 2020;126:575–82. doi: 10.1002/cncr.32576. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed M, von Itzstein MS, Sheffield T, et al. Association between body mass index, dosing strategy, and efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2021;9:e002349. doi: 10.1136/jitc-2021-002349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ringel AE, Drijvers JM, Baker GJ, et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell. 2020;183:1848–66. doi: 10.1016/j.cell.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25:141–51. doi: 10.1038/s41591-018-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kichenadasse G, Miners JO, Mangoni AA, et al. Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2020;6:512–8. doi: 10.1001/jamaoncol.2019.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat Rev Immunol. 2021;21:785–97. doi: 10.1038/s41577-021-00541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobi O, Landman Y, Reinhorn D, et al. The Relationship of Diabetes Mellitus to Efficacy of Immune Checkpoint Inhibitors in Patients with Advanced Non-Small Cell Lung Cancer. Oncology (Williston Park, NY) 2021;99:555–61. doi: 10.1159/000516671. [DOI] [PubMed] [Google Scholar]
- 30.Cortellini A, D’Alessio A, Cleary S, et al. Type 2 Diabetes Mellitus and Efficacy Outcomes from Immune Checkpoint Blockade in Patients with Cancer. Clin Cancer Res. 2023;29:2714–24. doi: 10.1158/1078-0432.CCR-22-3116. [DOI] [PubMed] [Google Scholar]
- 31.Finisguerra V, Dvorakova T, Formenti M, et al. Metformin improves cancer immunotherapy by directly rescuing tumor-infiltrating CD8 T lymphocytes from hypoxia-induced immunosuppression. J Immunother Cancer. 2023;11:e005719. doi: 10.1136/jitc-2022-005719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiang C-H, Chen Y-J, Chiang C-H, et al. Effect of metformin on outcomes of patients treated with immune checkpoint inhibitors: a retrospective cohort study. Cancer Immunol Immunother. 2023;72:1951–6. doi: 10.1007/s00262-022-03363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanriover C, Copur S, Gaipov A, et al. Metabolically healthy obesity: Misleading phrase or healthy phenotype? Eur J Intern Med. 2023;111:5–20. doi: 10.1016/j.ejim.2023.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Blüher M. Metabolically Healthy Obesity. Endocr Rev. 2020;41:bnaa004. doi: 10.1210/endrev/bnaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duque AP, Rodrigues Junior LF, Mediano MFF, et al. Emerging concepts in metabolically healthy obesity. Am J Cardiovasc Dis. 2020;10:48–61. [PMC free article] [PubMed] [Google Scholar]
- 36.Tsatsoulis A, Paschou SA. Metabolically Healthy Obesity: Criteria, Epidemiology, Controversies, and Consequences. Curr Obes Rep. 2020;9:109–20. doi: 10.1007/s13679-020-00375-0. [DOI] [PubMed] [Google Scholar]
- 37.Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019;129:3978–89. doi: 10.1172/JCI129186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calori G, Lattuada G, Piemonti L, et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care. 2011;34:210–5. doi: 10.2337/dc10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–56. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.AlZaim I, Hammoud SH, Al-Koussa H, et al. Adipose Tissue Immunomodulation: A Novel Therapeutic Approach in Cardiovascular and Metabolic Diseases. Front Cardiovasc Med. 2020;7:602088. doi: 10.3389/fcvm.2020.602088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rausch ME, Weisberg S, Vardhana P, et al. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–63. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 43.Chennamadhavuni A, Abushahin L, Jin N, et al. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front Immunol. 2022;13:779691. doi: 10.3389/fimmu.2022.779691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pirozzi F, Poto R, Aran L, et al. Cardiovascular Toxicity of Immune Checkpoint Inhibitors: Clinical Risk Factors. Curr Oncol Rep. 2021;23:13. doi: 10.1007/s11912-020-01002-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyon AR, Yousaf N, Battisti NML, et al. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–58. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 46.Mocan-Hognogi DL, Trancǎ S, Farcaş AD, et al. Immune Checkpoint Inhibitors and the Heart. Front Cardiovasc Med. 2021;8:726426. doi: 10.3389/fcvm.2021.726426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thuny F, Naidoo J, Neilan TG. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur Heart J. 2022;43:4458–68. doi: 10.1093/eurheartj/ehac456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laenens D, Yu Y, Santens B, et al. Incidence of Cardiovascular Events in Patients Treated With Immune Checkpoint Inhibitors. J Clin Oncol. 2022;40:3430–8. doi: 10.1200/JCO.21.01808. [DOI] [PubMed] [Google Scholar]
- 49.Tong J, III, Mao Y, Yang Z, et al. Baseline Serum Cholesterol Levels Predict the Response of Patients with Advanced Non-Small Cell Lung Cancer to Immune Checkpoint Inhibitor-Based Treatment. CMAR. 2021;Volume 13:4041–53. doi: 10.2147/CMAR.S304022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karayama M, Inui N, Inoue Y, et al. Increased serum cholesterol and long-chain fatty acid levels are associated with the efficacy of nivolumab in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2022;71:203–17. doi: 10.1007/s00262-021-02979-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pecci F, Cantini L, Cognigni V, et al. Prognostic Impact of Blood Lipid Profile in Patients With Advanced Solid Tumors Treated With Immune Checkpoint Inhibitors: A Multicenter Cohort Study. Oncologist. 2023 doi: 10.1093/oncolo/oyad273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perrone F, Minari R, Bersanelli M, et al. The Prognostic Role of High Blood Cholesterol in Advanced Cancer Patients Treated With Immune Checkpoint Inhibitors. J Immunother. 2020;43:196–203. doi: 10.1097/CJI.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 53.Shuster A, Patlas M, Pinthus JH, et al. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.