SUMMARY
Insufficient telomerase activity, stemming from low telomerase reverse transcriptase (TERT) gene transcription, contributes to telomere dysfunction and aging pathologies. Besides its traditional function in telomere synthesis, TERT acts as a transcriptional co-regulator of genes pivotal in aging and age-associated diseases. Here, we report the identification of a TERT activator compound (TAC) that upregulates TERT transcription via the MEK/ERK/AP-1 cascade. In primary human cells and naturally aged mice, TAC-induced elevation of TERT levels promotes telomere synthesis, blunts tissue aging hallmarks with reduced cellular senescence and inflammatory cytokines, and silences p16INK4a expression via upregulation of DNMT3B-mediated promoter hypermethylation. In the brain, TAC alleviates neuroinflammation, increases neurotrophic factors, stimulates adult neurogenesis and preserves cognitive function without evident toxicity, including cancer risk. Together, these findings underscore TERT’s critical role in aging processes and provide preclinical proof-of-concept for physiological TERT activation as a strategy to mitigate multiple aging hallmarks and associated pathologies.
Keywords: telomerase, telomere, senescence, inflammation, p16INK4a, epigenetics, adult neurogenesis, cognition
‘In Brief’ statement
This study identifies a small molecule (TAC) which restores physiological levels of TERT throughout aged tissues, resulting in rejuvenation of multiple tissues. Specifically, TAC administration in very aged mice alleviates multiple aging hallmarks such as cellular senescence and systemic inflammation, promotes new neuron formation with improved cognitive ability, enhances neuromuscular function, and was well tolerated with no evidence of toxicity.
Graphical Abstract

INTRODUCTION
Aging is characterized by time-dependent loss of physiological integrity and fitness in living organisms,1,2 driven by multiple converging mechanisms leading to the accumulation of cellular damage and eventual decline in organ function and tissue homeostasis. Genetic and epigenetic alterations are the major contributors to its functional and physiological decline with aging. Human and model organism studies have documented global changes in DNA damage and mutation, genomic instability, DNA methylation patterns, histone modifications, and chromatin remodeling with advancing age. These dynamic regulatory changes affect gene expression and protein functions governing diverse cellular pathways central to aging and age-related diseases.3 In particular, age-associated epigenomic alterations can induce significant transcriptional drift and initiate cellular aging processes, implicating altered epigenetic signatures in aging pathophysiology and aging-related diseases.4,5 Notably, modulating chromatin modifier activity has shown promise in mitigating multiple aging risk factors and associated disease phenotypes on the organismal level.6
Telomere dysfunction is a primary hallmark of aging, causing molecular and cellular damage and serving as a driver or amplifier of the molecular circuitries driving the aging process and associated diseases.1,2,7 Telomeres, specialized chromatin structures that preserve chromosomal integrity, undergo progressive attrition during aging-associated tissue renewal, leading to loss of their capping function. Telomere dysfunction is itself an aging hallmark and can also contribute to other hallmarks such as genome instability, stem cell exhaustion, mitochondrial dysfunction and cellular senescence.2,7 Telomere dysfunction has been causally implicated as a rate-limiting pathogenetic step in age-related diseases including premature aging (progeroid) syndromes, cardiovascular diseases, inflammatory bowel disease, pulmonary fibrosis, metabolic diseases and neurological diseases.2,7,8
Telomerase, a ribonucleoprotein complex responsible for extending telomeres, maintains telomere length and genome integrity. While essential for cell viability in normal healthy tissues, telomerase activity is tightly regulated in most adult somatic cells, primarily due to transcriptional repression of the core catalytic subunit of telomerase, TERT. Indeed, defects in telomerase activity due to germ-line mutations in core telomerase components are linked to the premature loss of tissue renewal and premature death, such as dyskeratosis congenita,9 aplastic anemia10 and idiopathic pulmonary fibrosis.11 Conversely, enforced expression of TERT stabilizes endogenous telomeres, reduces senescence-associated markers and restores the proliferative lifespan in mammals.12–14 Moreover, in genetically engineered mice, TERT reactivation studies revealed the dual role of TERT in the reversal of aging via telomere synthesis in proliferative tissues as well as via gene expression modulation in post-mitotic tissues in a telomere-independent manner. Indeed, in post-mitotic neurons, restoration of physiological TERT levels improve capacity for neural stem cell regeneration, enhance synaptic plasticity and preserve cognitive function in two models of Alzheimer’s Disease.15,16 Accordingly, natural or synthetic compounds that induce TERT expression have been shown to promote cellular survival and metabolic fitness.17–19 While promising, the development of these compounds has been hampered by a lack of understanding of their downstream mechanisms of action. In addition, while numerous pathways regulate TERT gene transcription, the importance of each pathway and their associated promoter binding elements in regulating the transcription of TERT in human adult somatic tissues remains unclear.
In this study, we conducted a high-throughput screen using mouse cells transgenic for the human TERT locus harboring a luciferase reporter element, screening a library of approximately 653,000 compounds. We identified and characterized a small molecule TERT activating compound (TAC) that enhances TERT transcription in human and mouse adult somatic cells. We elucidated the mechanism of TAC-mediated TERT up-regulation and explored its impact on regulatory factors governing aging including p16INK4a methylation. TAC promoted tissue rejuvenation, including new neuron formation, and alleviated multiple aging hallmarks in aged mice, revealing the regenerative potential of adult tissues through physiological TERT activation.
RESULTS
Identification of a small molecule activator of TERT expression.
Given the established benefits of genetic TERT activation on diseases of aging,2,14,16 we sought to identify small molecules that could induce transient expression of the human and mouse TERT gene in somatic cells. To this end, we developed a cell-based high-throughput screening (HTS) assay to enable large-scale screening of small molecules that modulate in vivo transcriptional activity of the human TERT transgene in adult mouse ear fibroblasts (Figures 1A, 1B, and S1A). These cells were derived from mice transgenic for a 160-kb bacterial artificial chromosome (BAC) which contains the hTERT gene, in which an Renilla luciferase (Rluc) reporter cassette was inserted into the hTERT initiation codon, and its neighboring loci; this human hTERT reporter transgene is capable of mirroring and recapitulating the key transcriptional and functional aspects of the regulation of TERT gene expression in normal mouse somatic tissues, thus providing a useful tool for preclinical drug screening.20 Following an initial screen of 653,000 compounds from the California Institute for Biomedical Research (Calibr)’s library, approximately 100 hits were further characterized by measuring reporter bioluminescence, yielding several TACs capable of modestly inducing the transcription activity of TERT gene (Figure S1B). Immunoblot analysis demonstrated that the screening hit TAC induced the highest level of protein expression and therefore it was chosen for further analyses (Figure S1C). TAC treatment resulted in a dose-dependent induction of TERT mRNA in primary human fibroblasts MRC-5 (Figures 1C and 1D). Consistent with TAC-induced TERT gene expression, TAC treatment led to the accumulation of the active enhancer/promoter mark H3K27ac and loss of repressive mark H3K9me3 upstream of the transcriptional start site of the TERT gene (Figure 1E), indicating that TAC can override the repressive chromatin state of the human TERT locus. Moreover, TAC (intraperitoneal (i.p.) injection of 6 mg/kg) increased human TERT gene expression across multiple tissues of hTERT-Rluc transgenic mice including brain, heart and skeletal muscle (Figure 1F). Further analysis of cell-type-specific effects in primary cells revealed that TAC (0.5 μM) can trigger expression of endogenous Tert gene in both mouse proliferating and post-mitotic cells within the physiological range (Figures S1D, S1E and S1F). We also assessed TAC activity in primary Werner syndrome (WS) fibroblasts which normally undergo rapid senescence that can be reversed by enforced hTERT expression.21 We found that TAC (0.5 μM) was able to induce TERT expression in WS fibroblasts (Figures 1G and S1G). In this WS model, both quantitative PCR and fluorescent in situ hybridization (FISH) analyses showed that long-term TAC treatment led to an increase in endogenous telomere length relative to the control group (Figures 1H and 1I). TAC-treated WS fibroblasts also exhibited a marked reduction in telomere dysfunction-induced DNA damage foci (TIFs) and an enhancement in proliferative potential relative to the vehicle control group (Figure S1H and S1I), suggesting that DNA damage at short telomeres is repaired efficiently by TAC/TERT-mediated telomere addition and that human primary fibroblasts retain the proliferative capacity. Further, our comprehensive survey demonstrates that TAC up-regulates human TERT transcription independently of cell- or tissue-types.
Figure 1. Identification of a small molecule activator of TERT.
(A) The workflow of high-throughput and confirmation screening strategy used to identify novel small molecule TERT activators. (B) Plate-based Z scores of hTERT-RLUC luminance measurements of all test compound screen in primary adult mouse fibroblasts of hTERT-Rluc transgenic mouse. (C) Molecular structure of TAC. (D) TERT mRNA levels in MRC-5 fibroblasts treated with the indicated concentration of TAC for 4 h. (E) The chromatin occupancy of active enhancer/promoter mark H3K27ac and repressive histone mark H3K9me3 in the TERT gene of vehicle- or TAC-treated MRC-5 fibroblasts. (F) hTERT promoter activity in the indicated tissues of hTERT-Rluc reporter transgenic mice at the indicated time points (hr) postinjection (i.p.) of 6 mg/kg TAC (n = 3~4 per group, two-way ANOVA with Tukey’s multiple comparisons test). (G) Immunoblots for the indicated endogenous proteins in vehicle- or TAC-treated primary WS fibroblasts. A tubulin was used as a loading control. (H) Relative telomere length of primary WS fibroblasts treated with vehicle or TAC. Relative telomere length was determined as the ratio of telomere repeat copy numbers to single copy gene 36B4 copy number measured by quantitative PCR (n = 4 per group, two-tailed unpaired t-test). (I) Left, representative FISH images for telomeres (red) in interphase nuclei of vehicle- or TAC-treated WS fibroblasts. Right, quantification of telomere FISH. Each value represents average intensity of each nuclei (n = 50 nuclei per group, two-tailed unpaired t-test) Scale bar, 10 μm. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. See also Figure S1.
TAC activates the MEK/ERK/AP-1 cascade to directly upregulate TERT gene transcription.
To gain insight into the downstream signaling cascades and TERT promoter elements linked to TAC-induced TERT expression, we first analyzed the phosphorylation profiles of kinases and their substrates using phospho-kinase arrays. We found that phosphorylation of extracellular signal-regulated kinase (ERK) and its downstream effector S6 kinase was consistently increased by TAC treatment in primary human MRC-5 cells (Figures 2A and 2B). Accordingly, inhibition of ERK with the MEK inhibitor trametinib abolished TAC-induced ERK phosphorylation and TERT upregulation in MRC-5 cells (Figures 2C and S2A).
Figure 2. TAC activates the MEK/ERK/AP-1 cascade to upregulate TERT transcription.
(A) Alteration of cellular signaling in TAC-treated MRC-5 cells. MRC-5 cells were treated with vehicle or TAC for 0.5 h and the cell lysates were subjected to the human phosphokinase array. (B) Quantification of p-ERK1/2 and p-S6K from the phosphokinase array in A (n = 2 per group, two-tailed unpaired t-test). (C) TERT and ERK levels in MRC-5 cells treated with TAC and/or trametinib, a selective MEK inhibitor. A tubulin was used as a loading control. (D) Venn diagrams showing overlaps of significantly upregulated genes upon TAC treatment in human fibroblasts and neurons (≥ 2-fold cutoff; P < 0.05). (E) Scatterplot comparing the statistical significance (p-values) of DEGs in human fibroblasts and neurons. Red dots indicate the genes significantly upregulated in both cells (n = 3 per group). (F) RNA-seq heatmap of genes up-regulated upon TAC treatment in human MRC-5 fibroblasts and human iPSC-derived neurons (n = 3). (G) Quantification of the expression of FOS genes in human MRC-5 fibroblasts and human iPSC-derived neurons treated with vehicle or TAC (n = 3 per group, two-tailed unpaired t-test). (H) Sequence comparison of a putative FOS binding site in human and mouse TERT 5’-UTR region. (I) Schematic representation and transcriptional activity of human -4 kb TERT promoter-Luc reporter constructs and deletion mutants in MRC-5 cells (n = 4 per group, two-way ANOVA with Tukey’s multiple comparisons test). Putative AP-1 binding sites are boxed. (J) c-FOS ChIP-qPCR enrichment at the endogenous promoter region of TERT in MRC-5 cells after TAC treatment (n = 4 per group, two-way ANOVA with Sidak’s multiple comparisons test). (K) TERT levels in MRC-5 cells treated with TAC and/or T-5224, a selective c-FOS/AP-1 inhibitor. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant. See also Figure S2.
Next, we investigated transcriptomic changes across different somatic cell types to elucidate potential transcription factors and their binding elements linking ERK activation to TERT transcriptional control. Through transcriptome profiling of human normal fibroblasts and induced pluripotent stem cell (iPSC)-derived neurons, we observed that acute treatment with TAC (0.5 μM for 1 hr) resulted in the significant induction of only a limited number of common genes in both human cell types (Figure 2D). Strikingly, only two genes, FOS and SOGA3, exhibited significant upregulation in both cell types following treatment (≥ 2-fold cutoff; P < 0.05), with FOS, encoding a major constituent of the activator protein 1 (AP-1) transcriptional complex, identified as the gene most significantly regulated by TAC in both cells (Figures 2E–2G). In addition, TAC treatment did not elicit any significant change in the global translation rate (Figure S2B), providing evidence that TAC treatment specifically induces TERT expression and its downstream transcriptional effects without affecting general mRNA translation.
The FOS gene encodes a critical component of AP-1 transcription factor complex, which binds to specific cis-acting elements on gene promoters stimulating the expression of these target genes.22 We identified two AP-1 binding sites residing within the 4-kb upstream regulatory region of the human TERT gene, with one of these binding motifs well conserved between human and mouse (Figures 2H and 2I). To explore whether AP-1 motifs are required for TERT promoter activation, we constructed a human TERT promoter-luciferase reporter containing 4 kb upstream sequences from the transcription start site of the TERT gene. Following transient transfection of MRC-5 cells with these luciferase reporter constructs, we stimulated the cells with TAC. Consistent with our observation of endogenous TERT levels, TAC induced TERT reporter activity in human fibroblasts (Figure 2I). Of note, single and double deletions including the AP-1 binding motif common in the promoters of both mouse and human significantly abolished TAC-induced TERT promoter activity (Figure 2I), highlighting that the conserved AP-1 cis-element has a more prominent function in inducing TERT transcription compared to the other. We next assessed whether the AP-1 complex is specifically recruited to the TERT promoter in response to TAC treatment in their endogenous chromatin context. Chromatin immunoprecipitation followed by real-time quantitative PCR (ChIP-qPCR) analysis showed that TAC treatment led to recruitment of endogenous FOS, a subunit of AP-1 complex, to two AP-1 binding motifs in the endogenous TERT promoter (Figure 2J). A selective AP-1 inhibitor T-5224 that specifically blocks FOS/AP-1 binding to DNA without affecting their expression23 impaired TAC-induced expression of TERT (Figures 2K and S2C). Together, these data indicate that TAC specifically activates the transcriptional activation of TERT via the MEK/ERK/AP-1 pathway.
TAC attenuates multiple aging hallmarks in vivo.
The capacity of genetic TERT reactivation to rejuvenate prematurely aged mice with telomere dysfunction15,24 prompted us to evaluate whether TAC-mediated TERT induction could similarly attenuate organismal aging in naturally aged mice with intact telomeres. Intraperitoneal administration of TAC, followed by the time-dependent quantification of TAC levels by mass spectrometry-based pharmacokinetics, demonstrated favorable plasma exposure for TAC (T1/2: 0.568 h, AUC: 285 h·ng/ml) (Figures S3A and S3B). Notably, TAC exhibited central nervous system exposure, with approximately two-fold partitioning of the compound in the central nervous system (CNS) relative to plasma. This CNS exposure corresponded to an increase in the signaling cascade upstream of TERT transcription within 0.5 to 3 hr post-administration, mirroring the rapid induction of hTERT promoter activity observed in brain tissues of TAC-treated transgenic mice (Figure 1F). TAC is cleared from the plasma by 3 hr with plasma levels tracking those in the brain (Figures S3C and S3D).
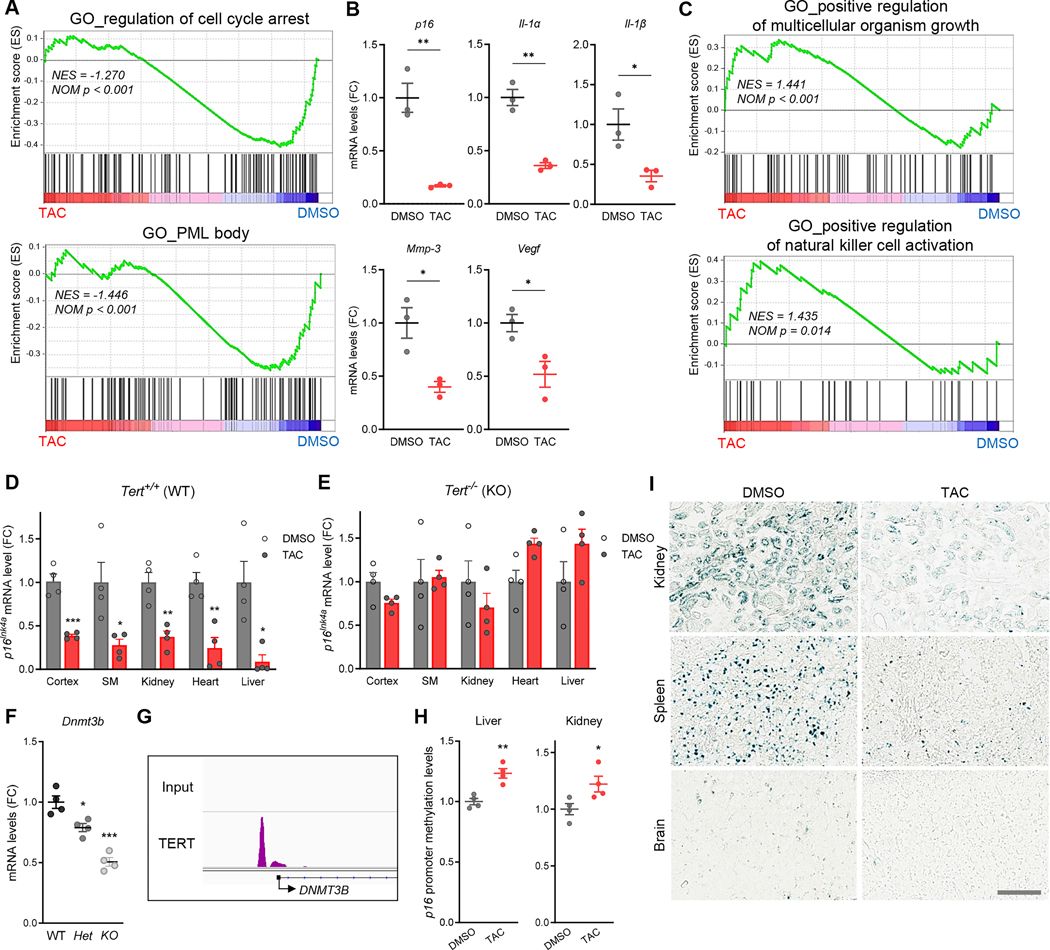
To further investigate transcriptomic changes following TAC treatment, we performed RNA-seq of peripheral blood mononuclear cells (PBMCs) from 12-month-old mice. Consistent with previous in vivo experiments, TERT protein levels were significantly increased in PBMCs from middle-aged mice treated with TAC (Figure S4A). Following a one-week treatment course (daily i.p. injection of 6 mg/kg/day), transcriptome analysis revealed a reduction in aging gene signatures including cell cycle arrest and PML body, which are established key cellular changes associated with aging PBMCs of middle-aged C57BL/6 mice (Figure 3A). Specifically, relative to vehicle-treated controls, TAC-treated PBMCs exhibited repression of p16Ink4a, a key driver and biomarker of in vivo senescence and aging, as well as other senescence-associated secretory phenotype (SASP) components – Il-1α, Il-1β, Mmp-3 and Vegf (Figure 3B). Conversely, TAC treatment concurrently induced signatures of organism growth and natural killer cell activation (Figure 3C), which are known to decline with age.25 Across a wide range of tissues, a one-week course of TAC treatment decreased expression of the classical senescence marker p16Ink4a, but not p21Cip1, in the brain, skeletal muscle, kidney, heart and liver of middle-aged (10~12-month-old) mice (Figures 3D, S4B–S4D). TAC-mediated repression of p16Ink4a was abolished in age- and sex-matched Tert-null mice (Figure 3E), indicating the requirement of TERT for TAC-induced silencing of p16Ink4a.
Figure 3. TAC attenuates diverse aging hallmarks in vivo.
(A) GSEA plots showing downregulated GO pathways in the PBMC of TAC-treated C57BL/6 mice relative to vehicle-treated controls (n = 4 per group; 10~12-month-old). (B) mRNA levels of senescence-related genes downregulated in TAC-treated PBMCs compared to control (n = 3 per group, two-tailed unpaired t-test). (C) GSEA plots showing upregulated GO pathways in the PBMC of TAC-treated mice relative to vehicle-treated controls. (D, E) p16Ink4a mRNA levels in the multiple tissues of TAC-treated wildtype (Tert+/+) (D) or Tert-KO (Tert−/−, first-generation [G1]) (E) mice relative to each control group (n = 4 per group; 10~12-month-old, two-tailed unpaired t-test). (F) mRNA levels of Dnmt3b gene in wild-type (Tert+/+), Tert heterozygous (Tert+/−) and Tert homozygous knockout (Tert−/−, G1) mouse brains (n = 4 per group, two-way ANOVA with Tukey’s multiple comparisons test). (G) TERT occupancy in the DNMT3B gene of human iPSC-derived neurons. (H) p16Ink4a promoter methylation levels in the liver and kidney tissues of vehicle- or TAC-treated C57BL/6 mice (n = 4 mice per group; 10~12-month-old, two-tailed unpaired t-test). (I) Representative images of SA-β-gal staining in the tissues of vehicle- or TAC-treated aged C57BL/6 mice (n = 4, 26~27-month-old). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001. See also Figure S3 and S4.
To determine the molecular mechanism of TAC-mediated p16Ink4a repression, we examined known regulators governing p16Ink4a transcription. Transcriptomic analysis of the brains of adult Tert+/+ and first generation (G1) Tert−/− mice (with intact telomeres)16 showed significant and specific reduction in the expression of Dnmt3b (Figures S4E and S4F), a DNA methyltransferase responsible for the hypermethylation of p16Ink4a promoter.26 Quantitative RT-PCR analysis of Tert+/+, Tert+/− and Tert−/− mouse brains confirmed marked down-regulation of Dnmt3b levels in Tert+/− mice which was further reduced in Tert−/− mice (Figure 3F), indicating a positive correlation between TERT levels and Dnmt3b expression in vivo. Correspondingly, chromatin IP followed by sequencing (ChIP-Seq) analysis demonstrated that TERT bound to the DNMT3b promoter in human iPSC-derived neurons (Figure 3G). These results are consistent with previous findings that TERT can act as a transcriptional modulator not only in highly proliferating cells but also in terminally differentiated cells.16,27
Given that de novo DNA methylation mediated by DNMT3b is associated with repression of p16INK4a transcription26, we examined whether TAC treatment could induce hypermethylation of the p16Ink4a promoter in vivo. Real-time methylation-specific PCR (MSR) was used to detect the methylation of CpG islands flanking the translation start site of murine p16Ink4a 28,29, revealing a significant increase in methylated CpG sites at the promoter region of p16Ink4a in middle-aged mouse tissues after TAC treatment compared with vehicle controls (Figure 3H). Furthermore, chronic administration of TAC for 6 months reduced senescence cell burden, as well as the production of pro-inflammatory IL-1β and IL-6 SASP factors in multiple tissues of naturally aged (26~27-month-old) mice (Figures 3I, S4G–S4J). Thus, TAC-driven TERT upregulation reduces age-dependent tissue senescence and regulates major genetic drivers of cellular senescence, including p16Ink4a and SASP components.
Chronic TAC administration ameliorates brain aging.
Genetic re-activation of TERT can restore the neurogenic and cognitive capacities in rodent models of premature aging and Alzheimer’s Disease and can increase levels of mature brain-derived neurotrophic factor (BDNF), a key molecule promoting the growth, survival and differentiation of newborn neurons in the normal mouse brain.14–16 Consistent with these reports, immunoblotting and mature BDNF (mBDNF)-specific enzyme-linked immunosorbent assay (ELISA) demonstrated a marked increase in the levels of mature BDNF as well as TERT in adult hippocampus following one-week administration of TAC (Figures S5A, S5B and S5C).
To gain further insight into the impact of TAC on gene expression programs, we performed RNA-seq analysis of microdissected hippocampal tissues from middle-aged (12~14-month-old) C57BL/6 mice treated with vehicle or TAC for 3 weeks (daily i.p. injection of 6 mg/kg/day). Remarkably, TAC treatment induced a significant increase in the expression of genes positively associated with adult hippocampus neurogenesis and brain function,30–35 including Eomes, a driver of immune cell development & neurogenesis, Dlk1, a mediator of adult hippocampal neurogenesis and cognition, Gdnf, a neurotrophic factor, Dcx, a marker for new-born neurons, Sox1/2, critical determinants of adult neurogenesis, Gdf11, a rejuvenation factor, and Fos, an in vivo marker of neural activity (Figure 4A). Moreover, pathway enrichment analysis revealed that TAC treatment resulted in the activation of pathways associated with synaptic potential, axon guidance, hippocampus/stem cell development, telomere maintenance, neuroblast proliferation, dopaminergic neurogenesis/CNS neuron differentiation, and MAPK family signaling cascades in the hippocampus of middle-aged mice (Figure 4B). Conversely, cytokines and inflammatory response, whose over-activation or dysregulation is known to contribute to age-related diseases and aging itself,36 were downregulated with TAC administration (Figure 4B). Notably, immunohistochemical and immunofluorescent analyses demonstrated that TAC treatment for 4 weeks markedly increased the number of doublecortin (DCX)-expressing newborn neurons in the hippocampal dentate gyrus (DG) (Figures 4C and 4D), indicating that TAC can indeed enhance the regenerative capacity of the aged brain. Further confirmation of TAC-induced adult neurogenesis was obtained with the immature neuron marker PSA-NCAM, revealing a higher number of DCX+ PSA-NCAM+ immature neurons in TAC-treated mice relative to controls (Figures 4E and S5D). No change in body weight was detected between the two groups throughout the study (Figure S5E). Thus, pharmacological activation of TERT induces rejuvenation-associated gene signatures and stimulates adult neurogenesis in the hippocampus of adult mice.
Figure 4. Chronic TAC administration ameliorates brain aging.
(A) A ranked list of genes in the observed transcriptional data from TAC-treated mouse hippocampi relative to control (n = 3 mice per group). (B) Pathways enriched (red) or depleted (blue) in TAC-treated hippocampi. Resource categories: #, Gene Ontology; ##, WikiPathways; ###, Reactome. (C, D) Representative images of DCX immunoreactivity by immunohistochemistry (C) or immunofluorescence (D) in the dentate gyrus of middle-aged (10~12-month-old) mice that were treated with vehicle and TAC for 1 month. Scale bars, 100 μm and 25 μm, respectively. (E) Quantifications of DCX+ and DCX+ PSA-NCAM+ cells in both groups (n = 4 per group, two-tailed unpaired t-test). (F) Representative images of IBA1 labelled microglia (left) and quantifications of microglial density (middle) and cell soma size (right) in the hippocampus of middle-aged (10~12-month-old) mice that were treated with vehicle and TAC for 1 month (n = 4 (IBA1+ cell numbers) and 80 (soma size), respectively, per group, two-tailed unpaired t-test). Scale bar, 100 μm. (G) mRNA levels of pro-inflammatory cytokines Il-1β, Il-6 and Tnf-α in the hippocampus of middle-aged mice (10~12month-old) treated with vehicle or TAC for 1 month (n = 4 per group, two-way ANOVA with Sidak’s multiple comparisons test). (H) Escape latency in Barnes maze trials over training days for aged (26~27-month-old) mice that were treated with vehicle or TAC for 6 months (n = 6 per group, two-way ANOVA with Sidak’s multiple comparisons test). (I) Discrimination index for vehicle- or TAC-treated aged (26~27-month-old) mice in the novel-location recognition test (n = 6 per group, two-way ANOVA with Tukey’s multiple comparisons test). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001. See also Figure S5.
Chronic neuroinflammation is a pervasive feature of the aging brain and is thought to contribute to diminished brain function. Microglia, the resident immune cells of the brain, are the primary players in neuroinflammation and microglial activation is considered a hallmark of neuroinflammation seen in most brain aging and neurodegenerative conditions.37,38 TAC administration significantly attenuated the levels of IBA1-positive activated microglia in the brains of aging mice relative to vehicle-treated controls (Figure 4F). TAC-treated animals displayed a prominent reduction in the cell density and soma size of IBA1-positive microglia in the hippocampus. Consistent with a crucial role for activated microglia as the primary source of pro-inflammatory cytokines in the brain,39 the age-related increases in expression of pro-inflammatory cytokines including Il-1β, Il-6, and Tnf-α were also attenuated in TAC-treated hippocampus relative to vehicle-treated controls (Figure 4G).
TAC-induced neurogenesis and reduced neuroinflammation prompted us to assess the role of TAC in modulating memory in aged mice approaching end of life. Naturally aged (20~21-month-old) C57BL/6 mice were randomized into TAC and vehicle treatment groups. Following a six-month treatment course (i.p. injection of either vehicle or 6 mg/kg/day of TAC, three times a week), 26~27-month-old (aged) mice treated with TAC showed improved performance on three hippocampal-dependent cognitive tests – Barnes maze, Y-maze and novel-location recognition tasks (Figures 4H, 4I, S5F and S5G). In addition, TAC-treated aged mice showed improved rotarod performance and grip strength (Figures S5H and S5I, respectively), which are known to decline with advancing age,40 consistent with improved motor coordination and muscle strength. Together, these findings demonstrated that the modest TAC-induced increase in endogenous TERT levels improves not only hippocampal-dependent cognitive function but also enhances neuromuscular function in aged mice without any overt adverse consequences.
DISCUSSION
This study highlights the significant regenerative capacity of aging organ systems as well as the ability to pharmacologically modulate aging hallmarks during natural aging. We report the discovery of a novel small molecule telomerase activator that induces the physiological expression of TERT in both human and mouse somatic tissues. Our findings reinforce the view that TERT exerts anti-aging activity not only by preserving telomere integrity but also by modulating gene expression and cellular signaling pathways governing cellular survival, senescence, neurogenesis, stress resistance, among other processes. TERT levels are tightly regulated in normal adult somatic cells and further repressed in aged tissues and in the Alzheimer’s Disease brain.16 The age-associated repression of TERT is reminiscent of other key longevity-associated genes, where epigenetic alterations altering chromatin structure and accessibility accumulate in the cis-regulatory sequences during aging, leading changes in the transcriptional regulation of genes governing aging.41,42 TAC effectively counteracts the epigenetic silencing of the TERT gene in adult somatic cells, thereby influencing the expression of genes governing aging hallmarks. Notably, TAC treatment significantly reduces the presence of senescent cells in diverse tissues along with DNMT3B-mediated repression of the master cellular mortality gene p16Ink4a, in a TERT-dependent manner. Additionally, long-term administration of brain-penetrant TAC in aged mice reduces neuroinflammation and inflammatory cytokines, enhances rejuvenation-associated gene signatures, promotes adult neurogenesis and preserves cognitive function without overt side effects. Thus, physiological TERT activation hold promise in assuaging aging phenotypes during natural aging in mice (Figure 5).
Figure 5. Schematic illustration of TAC/TERT-driven anti-aging effects.
The expression of TERT, a catalytic subunit of telomerase, is tightly suppressed in normal somatic cells. A novel small molecule TAC can trigger transcriptional activation of somatic TERT expression via activation of MEK/ERK/AP-1 signaling cascade. Somatic TERT induction not only reduces tissue senescence by silencing p16INK4a through promoter hypermethylation via DNMT3B and inflammation but also enhances adult neurogenesis and cognitive function by promoting hippocampal transcriptomic signatures.
In the setting of aging and chronic disease, small molecule therapeutics may offer advantages over biologics due to their lower immunogenic and cost-effectiveness.43,44 TAC possesses a low molecular weight (<400 Da) and lipophilicity that favor drug uptake across all tissues, including the CNS.45 Particularly encouraging is TAC’s ability to alleviate age-associated increase in proinflammatory cytokines (Il-1β, Il-6 and Tnf-α) and induction of the key neurotrophic factors (BDNF and GDNF) governing synaptic plasticity and memory. This encourages clinical testing of TAC as a neuroprotective agent in normal aging and in neurodegenerative diseases, such as Alzheimer’s Disease, or as a countermeasure for chemotherapy-induced neurological dysfunction. Indeed, our recent study provided additional genetic evidence that somatic TERT maintenance restrains the pathological hallmarks of Alzheimer’s Disease, including amyloid pathology, dendritic spine deficits and cognitive decline.16 In addition, our current analyses provide novel insights into the mechanism by which TAC and TERT impact the regulation of pivotal aging genes. Our biochemical and cell-based assays show that TAC fine-tunes MEK/ERK/FOS signaling pathway, resulting in transient upregulation of TERT expression in the physiological range. Along these lines, it is intriguing that ERK signaling has been linked to the cognitive-enhancing effects of plant secondary metabolites, such as curcumin and apigenin.46,47 Although sustained hyperactivation of MEK/ERK signaling is a prerequisite for malignant transformation or progression, the fine-tuning of this pathway activation by natural bioactive compounds, such as flavonoids, appears to promote physiological responses of gene transcription/translation and cell growth/survival and, in turn, advance human health as neuroprotectants and cognitive enhancers.48–51 And, it cannot be ruled out that additional molecular mediator(s) might be involved in TAC-driven TERT transactivation since most biologically active small molecules exhibit polypharmacology (that is, simple molecules are more likely to bind to multiple protein targets).52 Future studies are warranted to identify the precise target(s) of TAC using chemical proteomics and subsequent structural and functional validation studies. Nevertheless, our findings provide ample evidence that the ERK/AP-1 cascade functions as a prime genetic switch regulating TAC-induced somatic TERT expression and that fine-tuning of the ERK-AP-1-TERT signaling axis induces beneficial effects on biological aging.
Somatic TERT de-repression via TAC administration confers cellular and organismal benefits via canonical and non-canonical telomerase functions. Physiological activation of somatic TERT expression and activity not only sustains cellular replicative potential via telomere maintenance but also enhances tissue-level physiology by modulating multiple aging-relevant pathophysiological processes, including senescence and inflammation. The multifaceted organismal and functional benefits may arise from a combination of TERT’s canonical and non-canonical functions. To discern the specific contributions of these functions, further experiments using cells or animals deficient in telomerase enzymatic activity such as telomerase RNA component (TERC) knockout models will be necessary.
Long-term anti-aging treatments necessitate ensuring safety in the chronic settings. While it is encouraging that long-term TAC treatment was well-tolerated without any overt or histological adverse effects including carcinogenesis. Nonetheless, additional safety studies in non-human primates are warranted. Along these lines, it is worth noting that TAC may reduce cancer incidence given that insufficient telomerase and resultant telomere dysfunction, coupled with loss of p53-dependent DNA checkpoint control, is a major driver of chromosomal instability and cancer genesis in the aged.53 Correspondingly, TERT reactivation in aged inducible-TERT mice was shown to reverse aging phenotypes without causing an increase in cancer.15 Together, these observations strengthen the case for the testing of small molecule TERT activators capable of inducing transient physiological up-regulation of TERT expression. Moreover, while the half-life of TAC is short, TERT protein half-life is 2–3 hours and its actions can be more enduring via such actions as DNMT3B-mediated silencing of the p16INK4a promoter. In this light, the engagement of diverse aging mechanisms by TERT makes TAC treatment a viable strategy for aging per se and for specific associated age-related diseases, particularly those characterized by impaired telomerase/telomere function.
Limitations of the study
The study identifies a TERT activating compound (TAC) capable of restoring physiological TERT levels via activation of MEK/ERK signaling on a conserved AP-1 binding element in the TERT promoter. TAC modulates key aging-related regulatory factors such as p16INK4a and DNMT3b, mitigates aging phenotypes such as senescence and inflammation, and stimulates neurogenesis with improved cognition and neuromuscular function. Despite these encouraging findings, the study faces several limitations. Firstly, while TAC offers a variety of health benefits, its potential impact on lifespan extension remains to be determined. Secondly, although TAC exhibits favorable drug-like properties without evident toxicity, the study did not explore dose optimization, dosing schedules, nor determine the maximum tolerated dose. Further, the drug’s metabolites and their potential liabilities were not assessed. Thirdly, while prolonged TAC administration was well-tolerated in mice, differences in drug metabolism across species necessitate thorough toxicity studies in non-human primates and human subjects to assess safety, potentially requiring further drug modifications and formulation development. Lastly, although the maintenance of TERT levels in the physiological range transiently would preserve telomere function and reduce cancer incidence, it would be prudent to carefully monitor cancer incidence in clinical trials assessing sustained TAC administration.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for reagents may be directed to, and will be fulfilled by the corresponding author Ronald A. DePinho (RDePinho@mdanderson.org).
Materials Availability
The unique reagents generated in this study are available from the Lead Contact Ronald A. DePinho (RDePinho@mdanderson.org)
Data and Code Availability
All sequencing data generated in this paper can be accessed from GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-TERT | Abcam | Cat#: ab32020 RRID: AB_778296 |
| anti-H3K9me3 | Abcam | Cat#: ab8898 RRID: AB_306848 |
| anti-H3K27ac | Abcam | Cat#: ab4729 RRID: AB_2118291 |
| anti-pERK | Cell Signaling Tech | Cat#: 9101 RRID: AB_331646 |
| anti-ERK | Cell Signaling Tech | Cat#: 4695 RRID: AB_390779 |
| anti-FOS | Cell Signaling Tech | Cat#: 2250 RRID: AB_2247211 |
| anti-p16 | Abcam | Cat#: ab211542 RRID: AB_2891084 |
| anti-DCX | Abcam | Cat#: ab18723 RRID: AB_732011 |
| anti-DCX | Santa Cruz Biotech | Cat#: sc-271390 RRID: AB_10610966 |
| anti-PSA-NCAM | EMD Millipore | Cat#: MAB5324 RRID: AB_95211 |
| anti-IBA1 | Wako | Cat#: 019-19741 RRID: AB_839504 |
| anti-IL-1β | Cell Signaling Tech | Cat#: 12242 RRID: AB_2715503 |
| anti-IL-6 | Protein Tech | Cat#: 21865-1-AP RRID: AB_11142677 |
| anti-Tubulin | Sigma | Cat#: T5168 RRID: AB_477579 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Dulbecco's phosphate-buffered saline | GIBCO | 14190144 |
| Hanks' balanced salt solution | GIBCO | 14170112 |
| poly-D-lysine | Sigma | P6407 |
| poly-L-ornithine | EMD Millipore | A-004-C |
| Laminin | Sigma | L2020 |
| Eagle’s MEM | ATCC | 30-2003 |
| Fetal Bovine Serum | GIBCO | 10082147 |
| Penicillin/Streptomycin | GIBCO | 15140122 |
| Neurobasal medium | GIBCO | 12348-017 |
| B-27 Supplement | GIBCO | 35050-061 |
| N-2 Supplement | GIBCO | 17502-048 |
| GlutaMAX Supplement | GIBCO | 35050-061 |
| MEM Non-Essential Amino Acids | GIBCO | 11140-050 |
| DMEM/F12 | GIBCO | 11330-032 |
| Human BDNF | Peprotech | 450-02 |
| Human GDNF | Peprotech | 450-10 |
| Dibutyryl cyclic AMP | Sigma | D0627 |
| L-Ascorbic acid | Sigma | A4544 |
| NSC maintenance media | EMD Millipore | SCM005 |
| Recombinant human FGF-basic | Peprotech | AF-100-18B |
| ChIP-Grade Protein A/G Plus Agarose | Thermo Scientific | 26159 |
| Halt protease and phosphatase inhibitor cocktail | Thermo Scientific | 78442 |
| X-tremeGENE HP DNA Transfection reagent | Millipore Sigma | 6366236001 |
| Ficoll-Paque premium | Sigma | GE17-5446-02 |
| TRIzol Reagent | Invitrogen | 15596026 |
| SuperScript III First-Strand Synthesis System | Invitrogen | 18080-051 |
| SYBR Green PCR Master Mix | Invitrogen | 4309155 |
| Trametinib | Selleck Chemicals | S2673 |
| T-5224 | Selleck Chemicals | S8966 |
| Critical Commercial Assays | ||
| EZ DNA Methylation-Gold™ kit | Zymo Research | ZD5005 |
| Human phospho-kinase array kit | R&D Systems | ARY003B |
| Dual-luciferase reporter assay system | Promega | E1910 |
| Senescence β-Galactosidase staining kit | Cell Signaling Tech | 9860 |
| Mature BDNF mouse rapid ELISA assay | Biosensis | BEK-2211 |
| Protein synthesis assay kit | Cayman Chemical | 601100 |
| Deposited Data | ||
| RNA-seq data of WT and Tert−/− mouse brains | Shim et al., 2021 | GSE163524 |
| RNA-seq and ChIP-seq data | This paper |
GSE231454, GSE231455 |
| Experimental Models: Cell Lines | ||
| Mouse: Primary ear fibroblasts from hTERT-Rluc transgenic mouse | Jia et al., 2011 | N/A |
| Mouse: Primary cardiomyocytes | This paper | N/A |
| Mouse: Primary microglia cells | This paper | N/A |
| Mouse: Primary neuronal cells | This paper | N/A |
| Human: MRC-5 fibroblasts | ATCC | Cat#: CCL-171 |
| Human: Werner syndrome (WS) fibroblasts | Coriell Institute | Cat#: AG03141 |
| Human: NPCs derived from NDC's iPSCs | Raja et al., 2016 | N/A |
| Experimental Models: Organisms/Strains | ||
| C57BL/6 | The Jackson Laboratory | JAX: 000664 |
| LSL-mTERT (B6 background) | Chakravarti et al., 2020 | N/A |
| hTERT-Rluc transgenic mouse | Jia et al., 2011 | N/A |
| Recombinant DNA | ||
| pGL4-hTERT-promoter-4kb-WT | This study | N/A |
| pGL4-hTERT-promoter-4kb-ΔAP1-#1 | This study | N/A |
| pGL4-hTERT-promoter-4kb-ΔAP1-#2 | This study | N/A |
| pGL4-hTERT-promoter-4kb-ΔAP1-#1 | This study | N/A |
| Software and Algorithms | ||
| ImageJ v1.53a | NIH | |
| GraphPad Prism v8 | GraphPad Software | |
| htseq-count package | Anders et al., 2015 | |
| DESeq2 package | Love et al., 2014 | |
| Bowtie v1.2.2 | Langmead et al., 2009 | |
| SAMtools v1.9 | Li et al., 2009 | |
| MACS v1.4.2 | Zhang et al., 2008 | |
| GSEA v4.1.0 | Broad Institute | |
| BioRender | BioRender | |
| Other | ||
| Barnes Maze apparatus (basic mouse) | Panlab Harvard Apparatus | Cat#: 989999 |
| Y-maze apparatus (with door, mouse) | Panlab Harvard Apparatus | Cat#: 760079 |
| Rotarod (mouse) | Panlab Harvard Apparatus | Cat#: 760770 |
| Grip strength meter (mouse) | Panlab Harvard Apparatus | Cat#: 761068 |
| Bioruptor Pico sonication system | Diagenode | |
| HiSeq 4000 | Illumina |
This study did not generate any original code.
All other data are available upon request for Lead Contact/corresponding author.
Experimental Model and Study Participant Details
Animals
All animal procedures used in this study were reviewed and approved by the University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee. C57BL/6 mice (stock #000664) were purchased from the Jackson Laboratory. Tert deficient (LSL-mTert) and hTERT-Rluc transgenic mice were generated as previously described.20,54 Age-matched male mice were used in this study as these have been widely used for the aging studies and shown to correspond more closely to the phenotype of human aging55,56 and were ten to twenty seven months of age as described in the corresponding figure legends. All animals were housed in pathogen-free, ambient temperature (21–23 °C), 45–55% humidity, and 12-h dark/light cycle conditions, and cared for in accordance with the International Association for Assessment and Accreditation of Laboratory Animal Care policies and certification.
Human fibroblast culture
Human MRC-5 (CCL-171, ATCC) fibroblasts were maintained in EMEM (ATCC) supplemented with 10% FBS and 1x penicillin/streptomycin (Invitrogen). WS fibroblasts (AG03141, Coriell Institute) were maintained in EMEM (ATCC) supplemented with 15% FBS and 1x penicillin/streptomycin (Invitrogen).
Human iPSC-derived NPC and neuronal culture
For human neural culture, neural progenitor cells (NPCs)57 derived from non-demented control individual were used and maintained in neural stem cell maintenance medium (Millipore) supplemented with 20 ng/ml bFGF (Peprotech). For neuronal differentiation as previously described,58,59 NPCs were plated onto poly-L-ornithine (PLO)/laminin-coated plates, and incubated in neural differentiation media (DMEM/F12 (Invitrogen), 1x GlutaMax (Invitrogen), 1x N2 (Invitrogen), 1x B27 (Invitrogen), 20 ng/ml BDNF (Peprotech), 20 ng/ml GDNF (Peprotech), 1 μM dibutyryl-cyclic AMP (Sigma), and 0.2 μM ascorbic acid (Sigma). iPSCs-derived neurons were differentiated for 1~4 months.
Mouse primary cell culture
Mouse primary ear fibroblasts were prepared from adult hTERT-Rluc transgenic mouse, as previously described60,61 and maintained in fibroblast culture medium (DMEM supplemented with 10% FBS, 1x nonessential amino acids, and 1x penicillin/streptomycin (all from Invitrogen)). Mouse primary cardiomyocytes were isolated from 1- to 3-day-old neonatal C57BL/6 mouse heart using an enzymatic mixture of trypsin and collagenase type II, plated on laminin-coated plates and maintained in cardiomyocyte culture medium (DMEM/F12 supplemented with 10% FBS, 1x nonessential amino acids, 2 mM L-glutamine, 3 mM sodium pyruvate and 1x penicillin/streptomycin (all from Invitrogen)).62,63 Mouse primary microglia cells were isolated from the cortices and hippocampi of neonatal C57BL/6 mouse brain by trypsinization and Percoll density gradient (Sigma), plated on PDL-coated plates and maintained in microglia culture medium (DMEM/F12 supplemented with 10% FBS and 1x penicillin/streptomycin (all from Invitrogen)).64,65 Mouse primary neuronal cells were isolated from the cortices and hippocampi of 1- to 3-day-old neonatal C57BL/6 mouse brain by combined enzymatic and mechanical trituration (TrypLE treatment followed by mechanical trituration with a fire-polished glass Pasteur pipette), plated on PDL-coated plates and cultured in neuronal maintenance medium (Neurobasal medium supplemented with 1x B-27, 1x GlutaMax and 1x penicillin/streptomycin (all from Invitrogen)).66,67
METHOD DETAILS
High-throughput compound screening and hits confirmation
For primary high-throughput screening, 8,000 primary ear fibroblasts derived from hTERT-Rluc transgenic reporter mice were seeded into 1536-well plates (Greiner) in 6 μL of growth medium in wells pre-spotted with DMSO stocks of each screening compound such that each well contained a final concentration of 5 μM of each test article. 1 μM SAHA was used as a positive stimulation control. After 24 hours, 3 μL of Renilla-Glo reagent (Promega) was dispensed per well. Plates were shaken and then read on an Envision plate reader (Perkin Elmer).
Measurements of Renilla Luciferase activity in adult mouse tissues
The mouse tissues were freshly isolated from vehicle- or TAC-treated hTERT-Rluc transgenic male mice for the indicated times and the tissue lysates were prepared in Luciferase Lysis Buffer (Promega). Renilla luciferase activity reflecting changes in transcriptional activity of transgenic hTERT promoter was determined by using Renilla-Glo Luciferase Assay system (Promega) as described in the manufacturer’s protocol. The protein concentrations of the cell lysates were determined using Pierce BCA Protein Assay Kit (Thermo Fisher) and the bioluminescence was normalized to protein contents.
Human phospho-kinase array
A human phospho-kinase array (ARY003B, R&D Systems) was used to detect the relative phosphorylation levels of human kinases in vehicle- or TAC-treated MRC-5 cells according to the manufacturer’s instruction. Phosphorylation levels were detected using SuperSignal Chemiluminescent Substrate (Pierce) and quantitative densitometric analysis of each signal was carried out using ImageJ software.
RNA isolation and quantitative real-time PCR
Total RNA was isolated using TRIzol Reagent (Invitrogen), reverse-transcribed with SuperScript III First-Strand Synthesis System (Invitrogen) and amplified with SYBR Green PCR Master Mix (Invitrogen), according to the manufacturers’ protocols. The expression levels were normalized with mouse Hprt1 or human HPRT1 mRNA in each sample. The primer sequences are: human TERT F: GCC CTC AGA CTT CAA GAC CA; R: GCT GCT GGT GTC TGC TCT C, mouse p16 F: CCC AAC GCC CCG AAC T; R: GCA GAA GAG CTG CTA CGT GAA, mouse p21 F: GCA GAT CCA CAG CGA TAT CCA; R: AAC AGG TCG GAC ATC ACC AG, mouse Il-1α F: AGG GAG TCA ACT CAT TGG CG; R: TGG CAG AAC TGT AGT CTT CGT, mouse Il-1β F: TGC CAC CTT TTG ACA GTG ATG; R: TGA TGT GCT GCT GCG AGA TT, mouse Il-6 F: GCT ACC AAA CTG GAT ATA ATC AGG A; R: CCA GGT AGC TAT GGT ACT CCA GAA, mouse Mmp-3 F: CAA AAC ATA TTT CTT TGT AGA GGA CAA; R: TTC AGC TAT TTG CTT GGG AAA, mouse Tnf-α F: TAG CCA GGA GGG AGA ACA GA; R: TTT TCT GGA GGG AGA TGT GG, mouse Vegf F: AGC ACA GCA GAT GTG AAT GC; R: TTT CTT GCG CTT TCG TTT TT, mouse Dnmt1 F: TGA GGA AGG CTA CCT GGC TA; R: GTC TGC CAT TTC TGC TCT CC, mouse Dnmt3a F: ACC AGG CCA CCT ACA ACA AG; R: TTG TTC TGC ACT TCC ACA GC, mouse Dnmt3b F: ACT TGG TGA TTG GTG GAA GC; R: CCA GAA GAA TGG ACG GTT GT, mouse Bmi1 F: AGA AGA GAT TTT TAT GCA GCT CA; R: CAA CTT CTC CTC GGT CTT CA, mouse Yy1 F: ACC CTA AGC AAC TGG CAG AA; R: GGT GTG CAG ATG CTT TCT CA, mouse Id1 F: CAT GAA CGG CTG CTA CTC AC; R: GAC TCC GAG TTC AGC TCC AG.
RNA-sequencing
Total RNA was extracted from collected samples using TRIzol Reagent (Invitrogen), according to the manufacturer’s protocol. For mouse PBMC samples, middle-aged mice were treated with vehicle or TAC (daily i.p. injection of 6 mg/kg/day) for 1 week, and PBMCs were separated from peripheral blood using Ficoll (GE17–5446-02, Sigma) density gradient centrifugation. For mouse hippocampus samples, middle-aged mice were treated with vehicle or TAC (daily i.p. injection of 6 mg/kg/day) for 3 weeks, and hippocampal tissues were isolated from whole brains. For human samples, human neurons differentiated from iPSC-derived NPCs or primary fibroblasts were treated with vehicle or TAC (0.5 μM) for 1 hr. RNA quantity was determined to be optimal for each sample before further processing. For each RNA seq, purified RNA was amplified to construct Illumina sequencing libraries using standard mRNA-seq guide (the PE protocol), and the libraries were sequenced on Illumina HiSeq 4000 instrument. Gene-level quantification was implemented with htseqcount package.68 Data normalization and differential expression analysis were conducted using DESeq2 package.69 Gene set enrichment analysis (GSEA) on gene ontology biological processes were done using GSEA java package (gsea2–2.2.1.jar) from the Broad Institute.70,71
Human TERT reporter constructs and luciferase assay
A 4-kb fragment upstream of the human TERT transcription start site was amplified from human BAC clone (RP11–117B23) and cloned into pGL4.10 luciferase vector (Promega). Mutations which lack the AP-1 binding sequences were generated using QuikChange II XL Site-Directed Mutagenesis kit (Cat# 200521, Agilent). The mutations were confirmed by sequencing. The wild-type or mutant reporter plasmids were transiently transfected into MRC-5 cells using X-tremeGENE HP DNA Transfection reagent (Cat# 6366236001, Millipore Sigma), and the cells were treated with vehicle or TAC. Relative luciferase activities in cell lysates were normalized to control Renilla luciferase activity and measured using a Dual-Luciferase Reporter Assay System (Promega).
ChIP-sequencing and ChIP-quantitative PCR
Cells were fixed and cross-linked with 1.42% formaldehyde. The cross-linking was quenched with 125 mM glycine, and cells were washed and collected with ice-cold PBS containing 1x protease & phosphatase inhibitor (Pierce). The nuclei were isolated by lysing the cell pellets in RIPA buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1x protease and phosphatase inhibitor cocktail [Pierce]). The chromatin was then sheared by sonication using Bioruptor Pico (Diagenode). The lysates were cleared by centrifugation.
For chromatin immunoprecipitation, the lysates were pre-cleared by incubation with Protein A/G Plus Agarose (Pierce). Clear lysates were incubated overnight at 4 ºC with appropriate primary antibodies. The antibody complexes were obtained with ChIP-Grade Protein A/G Plus Agarose (Pierce) and washed five times with RIPA buffer. The DNA was recovered as described.72 For ChIP-seq, control immunoprecipitation was done in parallel without antibodies. Raw fastq reads for all experiments were processed using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), low-quality reads were removed with trimmomatic (DOI: 10.1093/bioinformatics/btu170) v0.33 with SLIDINGWINDOW:4:30 and quality reads were aligned to the mm9 (mouse) and hg19 (human) reference genome using Bowtie version 1.2.273 with the following criteria: --best --chunkmbs 320. To directly compare ChIP-seq between samples, uniquely mapped reads for each mark were normalized by total reads per condition, sorted, and indexed using SAMtools version 1.9.74 Model-based analysis of ChIP-seq (MACS version 1.4.2)75 was used to identify antibody-IP enrichment over input background. MACS2 was used to identify the differential binding of each protein in different conditions with the following criteria: bdgdiff –g 60 –l 120. To visualize ChIP-seq libraries on the IGV browser, we used deepTools version 2.7.15 to generate bigwig files by scaling the bam files to reads per kilobase per million (RPKM) using the following criteria: bamCoverage –b–normalizeUsing RPKM–smoothLength 300–binSize 30–extendReads 200 –o. For ChIP-qPCR, sonicated chromatin was subjected to ChIP by using specific antibodies or normal rabbit IgG and qPCR analysis was performed using target specific primers (hTERTp-3725-F: TGA AGA GGA ACA TGC CGT TT, R: GCC ATG GGG TGA AAT TCT TT; hTERTp-1672-F: TGT GGT GTT TTA AGC CAA TGA, R: CCC CAG TAT TAT GGG TGC AG).
Immunoprecipitation and immunoblot
Immunoprecipitation and immunoblotting were carried out as previously described76 with a minor modification. For coimmunoprecipitation, the differentiated neuronal cells were lysed on ice in a modified RIPA buffer. The cell supernatant was incubated at 4 °C overnight with appropriate primary antibodies, and ChIP-Grade Protein A/G Plus Agarose (Pierce) was then added and incubated for an additional hour at 4 °C. Immunoprecipitants were washed five times with RIPA buffer, recovered with SDS sample buffer, and subjected to immunoblot analysis. Protein samples were subjected to NuPAGE (Invitrogen), transferred to PVDF membrane (Bio-Rad), and probed with primary antibodies. Immunoreactivity was visualized with appropriate HRP-conjugated secondary antibodies (Cell Signaling Tech) followed by SuperSignal Chemiluminescent Substrate (Pierce). Quantitative densitometric analysis of select gel band intensities was carried out using ImageJ software.
Mouse p16Ink4a promoter DNA methylation
The DNA methylation levels of mouse p16Ink4a promoter region were measured by bisulfite modification of genomic DNA and subsequent methylation-specific PCR, as previously described.28,77 Briefly, 1 μg genomic DNA isolated from fresh mouse tissues was bisulfite treated, desulfonated and recovered using EZ DNA Methylation Gold kit (Zymo Research). Bisulfite-modified DNA was amplified with the methylated/unmethylated p16-specific primer sets using real-time methylation-specific PCR as described previously.28,29
ELISA analysis
The tissue lysates of the hippocampus freshly isolated from the mice treated with DMSO or TAC were prepared in RIPA buffer and mature BDNF were quantified with sandwich enzyme-linked immunosorbent assay (ELISA) kit (BEK-2211, Biosensis) according to the manufacturer’s instructions and normalized to total protein levels determined by BCA assay (Pierce).
Immunohistochemistry and immunofluorescence
Mice under anesthesia were transcardially perfused with saline and then with 4% paraformaldehyde in PBS (pH 7.4). For IHC, the tissues were post-fixed in the same fixative and embedded in paraffin. Subsequently, the tissue sections were deparaffinized, hydrated, and blocked with Biocare blocking reagent (Biocare Medical) according to standard procedures. After the epitope unmasking, the sections were incubated with primary antibodies, followed by secondary Envision plus antibodies with HRP-labeled polymer (Dako). The antigen was visualized using DAB chromogen system (Dako). Quantitative analysis of immunohistochemical staining intensities was carried out using ImageJ software. For IF, the staining was performed on paraffin-embedded tissue sections as described previously.78
For TIF analysis, the cells treated with vehicle or TAC were plated on glass culture slides. Cells were fixed in 4% paraformaldehyde for 10 min at room temperature, washed in PBS, and permeabilized with 0.5% NP-40 for 10 min. Next, cells were washed three times with PBS and then blocked in 10% goat serum and 1% BSA for 30 min. Following blocking, cells were incubated with primary antibodies in blocking solution overnight at 4°C. Cells were then washed with PBS and incubated with secondary Alexa Flour-conjugated antibodies for 1 hr at room temperature. Following five 5 min washed with PBS, slides were mounted in Prolong Glass antifade Mountant with NucBlue (Thermo Fisher Scientific). The confocal images were acquired using a Zeiss LSM800 equipped with an Airyscan detector (Zeiss).
Senescence-associated β-galactosidase (SA-β-gal) staining
SA-β-gal staining was performed as previously described.79 Fresh tissues from vehicle- or TAC-treated mice (26~27-month-old) mice were fixed in 10% neutral buffered formalin (NBF) for 4 hr and then transferred to 30% sucrose overnight. Tissues were then embedded in OCT and cryosectioned at 6 μm. SA-β-gal staining (pH 5.8–6.0) of tissue sections was performed at 37°C for 16–24 hr in SA-β-gal staining solution (#9860, Cell Signaling Technology), according to manufacturer’s protocol. Images were captured using bright-field microscopy at 20x magnification.
Telomere measurement by FISH and quantitative real-time PCR
Telomere length was analyzed by FISH and qPCR-based methods, as previously described.80 For telomere FISH, Werner syndrome (WS) fibroblasts were treated with DMSO or TAC (0.5 μM), and the telomere FISH was performed by using Cy5-labeled peptide nucleic acid (PNA) probe from Agilent (Cat# K532711–8) as previously described.81 Telomere intensity was measured from 50 cells from each group. For real-time qPCR assay, genomic DNA isolated from vehicle- or TAC-treated WS fibroblasts was analyzed using telomeric primers, primers for the reference control gene (mouse 36B4 single copy gene) and PCR settings as previously described.82,83
Protein synthesis measurement using OPP
The protein synthesis assay was performed following the manufacturer’s instruction using Protein Synthesis Assay kit (Cayman Chemical). In brief, cells were collected following treatment with O-propargyl-puromycin (OPP) for the last 1 hr at 37 °C. The cells were then fixed in 3.7% formaldehyde for 15 min, and permeabilized in 0.1% Triton X-100 for 15 min. OPP was then fluorescently labelled with 5 fluorescein-azide and the fluorescence intensities of protein synthesis were then assessed with a fluorescent plate reader.
Mouse behavioral tests
The evaluation of learning and memory in mice was carried out using Barnes Maze, Y-maze and Novel Location Recognition tasks as previously reported.16,84,85 For the Barnes maze task, the mice were given 180 s for each trial to identify and enter the escape hole on the apparatus consisted of a clear grey circular open disk (92 cm diameter) with 20 circular holes (5 cm diameter) (Panlab Harvard Apparatus). The holes were equally spaced around the perimeter and located 2 cm from the edge of the maze. A black escape box (20 cm x 9 cm x 9 cm) was positioned beneath one of these holes, and distinct visual cues were placed at three points around the maze. The mice received three trials per day with a 15-min inter-trial interval on four consecutive days during the acquisition and retention phases. The latency to enter the escape hole was recorded. For the Y-maze test, the Y-maze apparatus consisted of three arms (30 cm length, 15 cm height, and 6 cm width; Panlab Harvard Apparatus) with an angle of 120° between each other was placed in a quiet and illuminated room. During the training phase, one arm was blocked by a removable door and the mice were positioned in the start arm, facing the center of the maze and allowed to explore only two arms (start and other arms) for 15 min. The test was performed 1 hr later with the door blocking the novel arm removed and the animal were placed again in the start arm, facing the center of the maze and allowed to freely explore all three arms for 5 min. The number of entries and the time spent in each arm were measured. For the novel location recognition test, it is based on the ability of mice to show preference for novel versus familiar objects. On day 1, mice were individually habituated to two objects. In the recognition session (day 2), mice were placed back in the same arena in which one of the objects was replaced, and the exploration behavior was assessed by quantifying the time spent exploring each object. Between each trial, the maze and all objects were thoroughly cleaned with 70% ethanol to remove any other clues that might affect performance in subsequent trials.
The motor coordination and muscle strength were assessed using the rotarod and grip strength tests. For the rotarod test, the mice were given a habituation trial on day 1 where they were placed on the rotarod (Panlab Harvard Apparatus) at a constant speed (4 r.p.m.) and had to remain on the rotarod for 1 min. On the testing day, mice were placed on the accelerating rotarod (4–40 r.p.m. over 5 min) and the speed at which mice fell off the rod was recorded during three trials (30-min rest period between each trial). For the grip strength test, the mice were allowed to grasp the center of the grid of the grip strength meter (Panlab Harvard Apparatus) with forelimbs and were then gradually pulled backwards in a horizontal plane until they lost their grip. The force applied to the grid was measured and normalized to body weight. Three trials were taken with 1 min between each trial.
Quantification and Statistical Analysis
No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications.15,24,86 All data distributions were assumed to be normal, but this was not formally tested. The mice and cells were randomly assigned to control or experimental groups, except in experiments that required specific genotypes and ages. The investigators were generally not blinded to allocation during experiments and outcome assessment. No animal or sample was excluded from the analysis. All statistical analyses were performed using GraphPad Prism 8; the statistic tests and p values are described in the indicated figure legends.
Supplementary Material
Figure S1. Compound screening in hTERT-Rluc transgenic mouse and human fibroblasts, related to Figure 1. (A) mTert and hTERT mRNA levels in compound-treated primary ear fibroblasts isolated from adult hTERT-Rluc transgenic mice. (B) hTERT mRNA expression levels in compound-treated MRC-5 human fibroblasts. (C) Left, Immunoblots for the indicated endogenous proteins in vehicle- or TAC-treated MRC-5 fibroblasts. A tubulin was used as a loading control. Right, quantitative comparison of TERT levels in the immunoblots. The values were normalized to control band intensity (n = 3 per group, two-tailed unpaired t-test). (D-F) Relative Tert mRNA levels in primary mouse cardiomyocytes (D), microglia (E), and neurons (F) (n = 3~4 per group, two-tailed unpaired t-test). (G) Quantification of immunoblots in Figure 1G. The values were normalized to control band intensity (n = 3 per group, two-tailed unpaired t-test). (H) Left, representative images of confocal sections of TIFs in nuclei of WS fibroblasts treated with vehicle or TAC for 3 months. Right, quantification of TIFs in nuclei of vehicle- or TAC-treated WS fibroblasts (n = 20 nuclei per group, two-tailed unpaired t-test). (I) Cumulative growth curve of vehicle- or TAC-treated WS fibroblasts (n = 3~4 per group; two-tailed unpaired t-test). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure S2. Impact of TAC on global translation levels and quantification of immunoblots shown in Figure 2, related to Figure 2. (A) Quantification of immunoblots in Figure 2C. The values were normalized to control band intensity (n = 3 per group, two-tailed unpaired t-test). (B) Protein synthesis measurement of MRC-5 treated with vehicle or TAC for the indicated time, following incorporation of OPP (n = 4 per group; two-tailed unpaired t-test). (C) Quantification of immunoblots in Figure 2K. The values were normalized to control band intensity (n = 3 per group, two-tailed unpaired t-test). Data are mean ± s.e.m. ***P < 0.001, ****P < 0.0001.
Figure S3. The pharmacokinetic profiles of TAC in mice, related to Figure 3. (A) Pharmacokinetic assessment of TAC after i.p. administration to mouse. Pharmacokinetic values are the mean of three animals per dose route. (B) Plasma concentration, (C) brain concentration, and (D) brain/plasma ratio of TAC in adult male CD1 mice (n = 3 per group). Data are mean ± s.e.m.
Figure S4. The correlation of TERT with DNMT3b and p16INK4a, related to Figure 3. (A) Left, Immunoblots for the indicated endogenous proteins in mouse PBMCs of adult C57BL/6 mice treated with vehicle or TAC (i.p. injection of 6 mg/kg). A tubulin was used as a loading control. Right, quantitative comparison of TERT levels in the immunoblots. The values were normalized to control band intensity (n = 3 per group, two-tailed unpaired t-test). (B) p21Cip1 mRNA levels in the multiple tissues of TAC-treated wildtype mice relative to each control group (n = 4 per group; 10~12-month-old). (C,D) Representative images and quantification of p16INK4a immunostaining in the indicated tissues of vehicle- or TAC-treated mice (n = 4, 10~12-month-old, two-tailed unpaired t-test). Scale bar, 100 μm. (E,F) The mRNA levels of epigenetic regulators (E) and transcription factors (F), which are known to regulate p16Ink4a expression, in wild-type (WT) and Tert−/− (G1) mouse (n = 3 per group, two-tailed unpaired t-test). (G-J) Representative images and quantification of IL-1β (G,I) and IL-6 (H,J) immunostaining in the indicated tissues of vehicle- or TAC-treated mice (n = 4, 26~27-month-old, two-tailed unpaired t-test). Data are mean ± s.e.m. **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant.
Figure S5. TAC-induced adult hippocampal neurogenesis of naturally aged mice, related to Figure 4. (A) Immunoblots of mature BDNF in mouse hippocampus of middle-aged C57BL/6 mice (10~12-month-old) treated with vehicle or TAC for 1 week. (B) Mature BDNF levels measured by ELISA in vehicle- or TAC-treated mouse hippocampal lysates (n = 6 per group, two-tailed unpaired t-test). (C) Left, Immunoblots for the indicated endogenous proteins in mouse hippocampus of adult C57BL/6 mice treated with vehicle or TAC (i.p. injection of 6 mg/kg). A tubulin was used as a loading control. Right, quantitative comparison of TERT levels in the immunoblots. The values were normalized to control band intensity (n = 3 per group, two-tailed unpaired t-test). (D) Representative images of PSA-NCAM+ DCX+ cells in the DG of middle-aged mice (arrows) treated with vehicle or TAC for 1 month (n = 4 per group). Scale bar, 25 μm. (E) Body weight in mice after TAC dosing (n = 4 per group). (F-I) Graphs representing the percentage of time spent in the novel arm (F) and entries into the novel arm in Y-maze (G), maximal walking speed on rotarod (H), and grip strength (I) of aged C57BL/6 mice treated with vehicle or TAC for 6 months (n = 7 mice per group, two-tailed unpaired t-test). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
Highlights.
TERT has been linked directly or indirectly to all hallmarks of aging.
TERT gene is epigenetically repressed with onset of aging markers in all tissues.
TAC restores TERT levels to promote telomere maintenance and reprogram gene expression.
TAC in aged mice reduces senescence/inflammaging and increases neurogenesis/cognition.
ACKNOWLEDGEMENTS
The authors thank all the members of R.A.D laboratory for discussions and constructive suggestions for this project; and Derrick Sek Tong Ong, Li Cai, Deepavali Chakravarti, Matthew M Hamilton and Philip Jones for helpful suggestions and technical support. This work was supported by the National Institutes of Health (R01 CA084628 to R.A.D.), the Mathers Foundation (R.A.D) and a generous gift from Robert and Renee Belfer to the Neurodegeneration Consortium (R.A.D.). This study made use of the following MD Anderson core facilities: the Advanced Technology Genomics Core, the Research Animal Support Facility, the Cytogenetics and Cell Authentication Core, and the Advanced Microscopy Core, all supported by an NIH Cancer Center Support Grant (P30 CA016672 and S10 RR029552).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests specifically related to this work. R.A.D. is a founder, advisor and/or director of Tvardi Therapeutics, Inc., Nirogy Therapeutics, Inc., Stellanova Therapeutics, Inc., Sporos Bioventures, LLC., Bectas Therapeutics, Inc. and Asylia Therapeutics, Inc. which are focused on therapies for cancer and fibrosis and bear no direct relevance to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, and Kroemer G. (2013). The hallmarks of aging. Cell 153, 1194–1217. 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakravarti D, LaBella KA, and DePinho RA (2021). Telomeres: history, health, and hallmarks of aging. Cell 184, 306–322. 10.1016/j.cell.2020.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmona JJ, and Michan S. (2016). Biology of Healthy Aging and Longevity. Rev Invest Clin 68, 7–16. [PubMed] [Google Scholar]
- 4.Frenk S, and Houseley J. (2018). Gene expression hallmarks of cellular ageing. Biogerontology 19, 547–566. 10.1007/s10522-018-9750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth LN, and Brunet A. (2016). The Aging Epigenome. Mol Cell 62, 728–744. 10.1016/j.molcel.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benayoun BA, Pollina EA, and Brunet A. (2015). Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat Rev Mol Cell Biol 16, 593–610. 10.1038/nrm4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin E, and DePinho RA (2010). Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520–528. 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpless NE, and DePinho RA (2007). How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 8, 703–713. 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 9.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, and Dokal I. (2001). The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature 413, 432–435. 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, and Young NS (2005). Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med 352, 1413–1424. 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 11.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA 3rd, et al. (2007). Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 356, 1317–1326. 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 12.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, and Wright WE (1998). Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352. 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 13.Tomas-Loba A, Flores I, Fernandez-Marcos PJ, Cayuela ML, Maraver A, Tejera A, Borras C, Matheu A, Klatt P, Flores JM, et al. (2008). Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell 135, 609–622. 10.1016/j.cell.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, and Blasco MA (2012). Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med 4, 691–704. 10.1002/emmm.201200245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, et al. (2011). Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469, 102–106. 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shim HS, Horner JW, Wu CJ, Li J, Lan ZD, Jiang S, Xu X, Hsu WH, Zal T, Flores II, et al. (2021). Telomerase Reverse Transcriptase Preserves Neuron Survival and Cognition in Alzheimer's Disease Models. Nat Aging 1, 1162–1174. 10.1038/s43587-021-00146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernardes de Jesus B, Schneeberger K, Vera E, Tejera A, Harley CB, and Blasco MA (2011). The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 10, 604–621. 10.1111/j.1474-9726.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eitan E, Tichon A, Gazit A, Gitler D, Slavin S, and Priel E. (2012). Novel telomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis. EMBO Mol Med 4, 313–329. 10.1002/emmm.201200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan T, Weir EJ, Johnson M, Korolchuk VI, and Saretzki GC (2021). Increased telomerase improves motor function and alpha-synuclein pathology in a transgenic mouse model of Parkinson's disease associated with enhanced autophagy. Prog Neurobiol 199, 101953. 10.1016/j.pneurobio.2020.101953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia W, Wang S, Horner JW, Wang N, Wang H, Gunther EJ, DePinho RA, and Zhu J. (2011). A BAC transgenic reporter recapitulates in vivo regulation of human telomerase reverse transcriptase in development and tumorigenesis. FASEB J 25, 979–989. 10.1096/fj.10-173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyllie FS, Jones CJ, Skinner JW, Haughton MF, Wallis C, Wynford-Thomas D, Faragher RG, and Kipling D. (2000). Telomerase prevents the accelerated cell ageing of Werner syndrome fibroblasts. Nat Genet 24, 16–17. 10.1038/71630. [DOI] [PubMed] [Google Scholar]
- 22.Angel P, and Karin M. (1991). The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta 1072, 129–157. 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 23.Aikawa Y, Morimoto K, Yamamoto T, Chaki H, Hashiramoto A, Narita H, Hirono S, and Shiozawa S. (2008). Treatment of arthritis with a selective inhibitor of c-Fos/activator protein-1. Nat Biotechnol 26, 817–823. 10.1038/nbt1412. [DOI] [PubMed] [Google Scholar]
- 24.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, et al. (2011). Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365. 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brauning A, Rae M, Zhu G, Fulton E, Admasu TD, Stolzing A, and Sharma A. (2022). Aging of the Immune System: Focus on Natural Killer Cells Phenotype and Functions. Cells 11. 10.3390/cells11061017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE, Cui H, Feinberg AP, Lengauer C, Kinzler KW, et al. (2002). DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature 416, 552–556. 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 27.Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, et al. (2009). Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460, 66–72. 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, Wu EA, Horner JW, and DePinho RA (2001). Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413, 86–91. 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 29.Lo YM, Wong IH, Zhang J, Tein MS, Ng MH, and Hjelm NM (1999). Quantitative analysis of aberrant p16 methylation using real-time quantitative methylation-specific polymerase chain reaction. Cancer Res 59, 3899–3903. [PubMed] [Google Scholar]
- 30.Christian KM, Song H, and Ming GL (2014). Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci 37, 243–262. 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flavell SW, and Greenberg ME (2008). Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci 31, 563–590. 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glebova NO, and Ginty DD (2005). Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci 28, 191–222. 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- 33.Hevner RF, Hodge RD, Daza RA, and Englund C. (2006). Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res 55, 223–233. 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Grassi ES, and Pietras A. (2022). Emerging Roles of DLK1 in the Stem Cell Niche and Cancer Stemness. J Histochem Cytochem 70, 17–28. 10.1369/00221554211048951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schafer MJ, and LeBrasseur NK (2019). The influence of GDF11 on brain fate and function. Geroscience 41, 1–11. 10.1007/s11357-019-00054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, and Ross OA (2018). Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front Immunol 9, 586. 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Bernhardi R, Eugenin-von Bernhardi L, and Eugenin J. (2015). Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci 7, 124. 10.3389/fnagi.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leng F, and Edison P. (2021). Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 17, 157–172. 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 39.Lynch MA (2009). The multifaceted profile of activated microglia. Mol Neurobiol 40, 139–156. 10.1007/s12035-009-8077-9. [DOI] [PubMed] [Google Scholar]
- 40.Bellantuono I, de Cabo R, Ehninger D, Di Germanio C, Lawrie A, Miller J, Mitchell SJ, Navas-Enamorado I, Potter PK, Tchkonia T, et al. (2020). A toolbox for the longitudinal assessment of healthspan in aging mice. Nat Protoc 15, 540–574. 10.1038/s41596-019-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraga MF, and Esteller M. (2007). Epigenetics and aging: the targets and the marks. Trends Genet 23, 413–418. 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Cavalli G, and Heard E. (2019). Advances in epigenetics link genetics to the environment and disease. Nature 571, 489–499. 10.1038/s41586-019-1411-0. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Jeldres T, Tyler CJ, Boyer JD, Karuppuchamy T, Yarur A, Giles DA, Yeasmin S, Lundborg L, Sandborn WJ, Patel DR, and Rivera-Nieves J. (2019). Targeting Cytokine Signaling and Lymphocyte Traffic via Small Molecules in Inflammatory Bowel Disease: JAK Inhibitors and S1PR Agonists. Front Pharmacol 10, 212. 10.3389/fphar.2019.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olivera P, Danese S, and Peyrin-Biroulet L. (2017). Next generation of small molecules in inflammatory bowel disease. Gut 66, 199–209. 10.1136/gutjnl-2016-312912. [DOI] [PubMed] [Google Scholar]
- 45.Bharate SS, Mignani S, and Vishwakarma RA (2018). Why Are the Majority of Active Compounds in the CNS Domain Natural Products? A Critical Analysis. J Med Chem 61, 10345–10374. 10.1021/acs.jmedchem.7b01922. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Fang Y, Xu Y, Lian Y, Xie N, Wu T, Zhang H, Sun L, Zhang R, and Wang Z. (2015). Curcumin Improves Amyloid beta-Peptide (1–42) Induced Spatial Memory Deficits through BDNF-ERK Signaling Pathway. PLoS One 10, e0131525. 10.1371/journal.pone.0131525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venigalla M, Gyengesi E, and Munch G. (2015). Curcumin and Apigenin - novel and promising therapeutics against chronic neuroinflammation in Alzheimer's disease. Neural Regen Res 10, 1181–1185. 10.4103/1673-5374.162686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F, Mazzarino MC, et al. (2011). Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 3, 192–222. 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, Basecke J, Stivala F, Donia M, Fagone P, et al. (2011). Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget 2, 135–164. 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deschenes-Simard X, Kottakis F, Meloche S, and Ferbeyre G. (2014). ERKs in cancer: friends or foes? Cancer Res 74, 412–419. 10.1158/0008-5472.CAN-13-2381. [DOI] [PubMed] [Google Scholar]
- 51.Ayaz M, Sadiq A, Junaid M, Ullah F, Ovais M, Ullah I, Ahmed J, and Shahid M. (2019). Flavonoids as Prospective Neuroprotectants and Their Therapeutic Propensity in Aging Associated Neurological Disorders. Front Aging Neurosci 11, 155. 10.3389/fnagi.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jalencas X, and Mestres J. (2013). On the origins of drug polypharmacology. Medchemcomm 4, 80–87. 10.1039/c2md20242e. [DOI] [Google Scholar]
- 53.Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, and DePinho RA (2000). Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406, 641–645. 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 54.Chakravarti D, Hu B, Mao X, Rashid A, Li J, Li J, Liao WT, Whitley EM, Dey P, Hou P, et al. (2020). Telomere dysfunction activates YAP1 to drive tissue inflammation. Nat Commun 11, 4766. 10.1038/s41467-020-18420-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yanai S, and Endo S. (2021). Functional Aging in Male C57BL/6J Mice Across the Life-Span: A Systematic Behavioral Analysis of Motor, Emotional, and Memory Function to Define an Aging Phenotype. Front Aging Neurosci 13, 697621. 10.3389/fnagi.2021.697621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graber TG, Kim JH, Grange RW, McLoon LK, and Thompson LV (2015). C57BL/6 life span study: age-related declines in muscle power production and contractile velocity. Age (Dordr) 37, 9773. 10.1007/s11357-015-9773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raja WK, Mungenast AE, Lin YT, Ko T, Abdurrob F, Seo J, and Tsai LH (2016). Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer's Disease Phenotypes. PLoS One 11, e0161969. 10.1371/journal.pone.0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. (2011). Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225. 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu BY, and Zhang SC (2009). Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc 4, 1295–1304. 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan M, and Gasser S. (2016). Generating Primary Fibroblast Cultures from Mouse Ear and Tail Tissues. J Vis Exp. 10.3791/53565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore CB, and Allen IC (2013). Primary ear fibroblast derivation from mice. Methods Mol Biol 1031, 65–70. 10.1007/978-1-62703-481-4_8. [DOI] [PubMed] [Google Scholar]
- 62.Sreejit P, Kumar S, and Verma RS (2008). An improved protocol for primary culture of cardiomyocyte from neonatal mice. In Vitro Cell Dev Biol Anim 44, 45–50. 10.1007/s11626-0079079-4. [DOI] [PubMed] [Google Scholar]
- 63.Li D, Wu J, Bai Y, Zhao X, and Liu L. (2014). Isolation and culture of adult mouse cardiomyocytes for cell signaling and in vitro cardiac hypertrophy. J Vis Exp. 10.3791/51357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee JK, and Tansey MG (2013). Microglia isolation from adult mouse brain. Methods Mol Biol 1041, 17–23. 10.1007/978-1-62703-520-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lian H, Roy E, and Zheng H. (2016). Protocol for Primary Microglial Culture Preparation. Bio Protoc 6. 10.21769/BioProtoc.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beaudoin GM 3rd, Lee SH, Singh D, Yuan Y, Ng YG, Reichardt LF, and Arikkath J. (2012). Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc 7, 1741–1754. 10.1038/nprot.2012.099. [DOI] [PubMed] [Google Scholar]
- 67.Kaech S, and Banker G. (2006). Culturing hippocampal neurons. Nat Protoc 1, 2406–2415. 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- 68.Anders S, Pyl PT, and Huber W. (2015). HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Love MI, Huber W, and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, and Mesirov JP (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550. 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, et al. (2003). PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34, 267–273. 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 72.Terranova C, Tang M, Orouji E, Maitituoheti M, Raman A, Amin S, Liu Z, and Rai K. (2018). An Integrated Platform for Genome-wide Mapping of Chromatin States Using High-throughput ChIP-sequencing in Tumor Tissues. J Vis Exp. 10.3791/56972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langmead B, Trapnell C, Pop M, and Salzberg SL (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10, R25. 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, and Genome Project Data Processing, S. (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, and Liu XS (2008). Model-based analysis of ChIP-Seq (MACS). Genome Biol 9, R137. 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shim HS, Wei M, Brandhorst S, and Longo VD (2015). Starvation Promotes REV1 SUMOylation and p53-Dependent Sensitization of Melanoma and Breast Cancer Cells. Cancer Res 75, 1056–1067. 10.1158/0008-5472.Can-14-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sauer J, Jang H, Zimmerly EM, Kim KC, Liu Z, Chanson A, Smith DE, Mason JB, Friso S, and Choi SW (2010). Ageing, chronic alcohol consumption and folate are determinants of genomic DNA methylation, p16 promoter methylation and the expression of p16 in the mouse colon. Br J Nutr 104, 24–30. 10.1017/S0007114510000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zaqout S, Becker LL, and Kaindl AM (2020). Immunofluorescence Staining of Paraffin Sections Step by Step. Front Neuroanat 14, 582218. 10.3389/fnana.2020.582218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yousefzadeh MJ, Zhao J, Bukata C, Wade EA, McGowan SJ, Angelini LA, Bank MP, Gurkar AU, McGuckian CA, Calubag MF, et al. (2020). Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 19, e13094. 10.1111/acel.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herbert BS, Hochreiter AE, Wright WE, and Shay JW (2006). Nonradioactive detection of telomerase activity using the telomeric repeat amplification protocol. Nat Protoc 1, 1583–1590. 10.1038/nprot.2006.239. [DOI] [PubMed] [Google Scholar]
- 81.Chakravarti D, Lee R, Multani AS, Santoni A, Keith Z, Hsu WH, Chang K, Reyes L, Rashid A, Wu CJ, et al. (2021). Telomere dysfunction instigates inflammation in inflammatory bowel disease. Proc Natl Acad Sci U S A 118. 10.1073/pnas.2024853118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cawthon RM (2002). Telomere measurement by quantitative PCR. Nucleic Acids Res 30, e47. 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cawthon RM (2009). Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37, e21. 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yuan J, Chang SY, Yin SG, Liu ZY, Cheng X, Liu XJ, Jiang Q, Gao G, Lin DY, Kang XL, et al. (2020). Two conserved epigenetic regulators prevent healthy ageing. Nature 579, 118–122. 10.1038/s41586-020-2037-y. [DOI] [PubMed] [Google Scholar]
- 85.Kraeuter AK, Guest PC, and Sarnyai Z. (2019). The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol Biol 1916, 105–111. 10.1007/978-1-49398994-2_10. [DOI] [PubMed] [Google Scholar]
- 86.Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, et al. (2016). Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540, 230–235. 10.1038/nature20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Compound screening in hTERT-Rluc transgenic mouse and human fibroblasts, related to Figure 1. (A) mTert and hTERT mRNA levels in compound-treated primary ear fibroblasts isolated from adult hTERT-Rluc transgenic mice. (B) hTERT mRNA expression levels in compound-treated MRC-5 human fibroblasts. (C) Left, Immunoblots for the indicated endogenous proteins in vehicle- or TAC-treated MRC-5 fibroblasts. A tubulin was used as a loading control. Right, quantitative comparison of TERT levels in the immunoblots. The values were normalized to control band intensity (n = 3 per group, two-tailed unpaired t-test). (D-F) Relative Tert mRNA levels in primary mouse cardiomyocytes (D), microglia (E), and neurons (F) (n = 3~4 per group, two-tailed unpaired t-test). (G) Quantification of immunoblots in Figure 1G. The values were normalized to control band intensity (n = 3 per group, two-tailed unpaired t-test). (H) Left, representative images of confocal sections of TIFs in nuclei of WS fibroblasts treated with vehicle or TAC for 3 months. Right, quantification of TIFs in nuclei of vehicle- or TAC-treated WS fibroblasts (n = 20 nuclei per group, two-tailed unpaired t-test). (I) Cumulative growth curve of vehicle- or TAC-treated WS fibroblasts (n = 3~4 per group; two-tailed unpaired t-test). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure S2. Impact of TAC on global translation levels and quantification of immunoblots shown in Figure 2, related to Figure 2. (A) Quantification of immunoblots in Figure 2C. The values were normalized to control band intensity (n = 3 per group, two-tailed unpaired t-test). (B) Protein synthesis measurement of MRC-5 treated with vehicle or TAC for the indicated time, following incorporation of OPP (n = 4 per group; two-tailed unpaired t-test). (C) Quantification of immunoblots in Figure 2K. The values were normalized to control band intensity (n = 3 per group, two-tailed unpaired t-test). Data are mean ± s.e.m. ***P < 0.001, ****P < 0.0001.
Figure S3. The pharmacokinetic profiles of TAC in mice, related to Figure 3. (A) Pharmacokinetic assessment of TAC after i.p. administration to mouse. Pharmacokinetic values are the mean of three animals per dose route. (B) Plasma concentration, (C) brain concentration, and (D) brain/plasma ratio of TAC in adult male CD1 mice (n = 3 per group). Data are mean ± s.e.m.
Figure S4. The correlation of TERT with DNMT3b and p16INK4a, related to Figure 3. (A) Left, Immunoblots for the indicated endogenous proteins in mouse PBMCs of adult C57BL/6 mice treated with vehicle or TAC (i.p. injection of 6 mg/kg). A tubulin was used as a loading control. Right, quantitative comparison of TERT levels in the immunoblots. The values were normalized to control band intensity (n = 3 per group, two-tailed unpaired t-test). (B) p21Cip1 mRNA levels in the multiple tissues of TAC-treated wildtype mice relative to each control group (n = 4 per group; 10~12-month-old). (C,D) Representative images and quantification of p16INK4a immunostaining in the indicated tissues of vehicle- or TAC-treated mice (n = 4, 10~12-month-old, two-tailed unpaired t-test). Scale bar, 100 μm. (E,F) The mRNA levels of epigenetic regulators (E) and transcription factors (F), which are known to regulate p16Ink4a expression, in wild-type (WT) and Tert−/− (G1) mouse (n = 3 per group, two-tailed unpaired t-test). (G-J) Representative images and quantification of IL-1β (G,I) and IL-6 (H,J) immunostaining in the indicated tissues of vehicle- or TAC-treated mice (n = 4, 26~27-month-old, two-tailed unpaired t-test). Data are mean ± s.e.m. **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant.
Figure S5. TAC-induced adult hippocampal neurogenesis of naturally aged mice, related to Figure 4. (A) Immunoblots of mature BDNF in mouse hippocampus of middle-aged C57BL/6 mice (10~12-month-old) treated with vehicle or TAC for 1 week. (B) Mature BDNF levels measured by ELISA in vehicle- or TAC-treated mouse hippocampal lysates (n = 6 per group, two-tailed unpaired t-test). (C) Left, Immunoblots for the indicated endogenous proteins in mouse hippocampus of adult C57BL/6 mice treated with vehicle or TAC (i.p. injection of 6 mg/kg). A tubulin was used as a loading control. Right, quantitative comparison of TERT levels in the immunoblots. The values were normalized to control band intensity (n = 3 per group, two-tailed unpaired t-test). (D) Representative images of PSA-NCAM+ DCX+ cells in the DG of middle-aged mice (arrows) treated with vehicle or TAC for 1 month (n = 4 per group). Scale bar, 25 μm. (E) Body weight in mice after TAC dosing (n = 4 per group). (F-I) Graphs representing the percentage of time spent in the novel arm (F) and entries into the novel arm in Y-maze (G), maximal walking speed on rotarod (H), and grip strength (I) of aged C57BL/6 mice treated with vehicle or TAC for 6 months (n = 7 mice per group, two-tailed unpaired t-test). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
Data Availability Statement
All sequencing data generated in this paper can be accessed from GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-TERT | Abcam | Cat#: ab32020 RRID: AB_778296 |
| anti-H3K9me3 | Abcam | Cat#: ab8898 RRID: AB_306848 |
| anti-H3K27ac | Abcam | Cat#: ab4729 RRID: AB_2118291 |
| anti-pERK | Cell Signaling Tech | Cat#: 9101 RRID: AB_331646 |
| anti-ERK | Cell Signaling Tech | Cat#: 4695 RRID: AB_390779 |
| anti-FOS | Cell Signaling Tech | Cat#: 2250 RRID: AB_2247211 |
| anti-p16 | Abcam | Cat#: ab211542 RRID: AB_2891084 |
| anti-DCX | Abcam | Cat#: ab18723 RRID: AB_732011 |
| anti-DCX | Santa Cruz Biotech | Cat#: sc-271390 RRID: AB_10610966 |
| anti-PSA-NCAM | EMD Millipore | Cat#: MAB5324 RRID: AB_95211 |
| anti-IBA1 | Wako | Cat#: 019-19741 RRID: AB_839504 |
| anti-IL-1β | Cell Signaling Tech | Cat#: 12242 RRID: AB_2715503 |
| anti-IL-6 | Protein Tech | Cat#: 21865-1-AP RRID: AB_11142677 |
| anti-Tubulin | Sigma | Cat#: T5168 RRID: AB_477579 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Dulbecco's phosphate-buffered saline | GIBCO | 14190144 |
| Hanks' balanced salt solution | GIBCO | 14170112 |
| poly-D-lysine | Sigma | P6407 |
| poly-L-ornithine | EMD Millipore | A-004-C |
| Laminin | Sigma | L2020 |
| Eagle’s MEM | ATCC | 30-2003 |
| Fetal Bovine Serum | GIBCO | 10082147 |
| Penicillin/Streptomycin | GIBCO | 15140122 |
| Neurobasal medium | GIBCO | 12348-017 |
| B-27 Supplement | GIBCO | 35050-061 |
| N-2 Supplement | GIBCO | 17502-048 |
| GlutaMAX Supplement | GIBCO | 35050-061 |
| MEM Non-Essential Amino Acids | GIBCO | 11140-050 |
| DMEM/F12 | GIBCO | 11330-032 |
| Human BDNF | Peprotech | 450-02 |
| Human GDNF | Peprotech | 450-10 |
| Dibutyryl cyclic AMP | Sigma | D0627 |
| L-Ascorbic acid | Sigma | A4544 |
| NSC maintenance media | EMD Millipore | SCM005 |
| Recombinant human FGF-basic | Peprotech | AF-100-18B |
| ChIP-Grade Protein A/G Plus Agarose | Thermo Scientific | 26159 |
| Halt protease and phosphatase inhibitor cocktail | Thermo Scientific | 78442 |
| X-tremeGENE HP DNA Transfection reagent | Millipore Sigma | 6366236001 |
| Ficoll-Paque premium | Sigma | GE17-5446-02 |
| TRIzol Reagent | Invitrogen | 15596026 |
| SuperScript III First-Strand Synthesis System | Invitrogen | 18080-051 |
| SYBR Green PCR Master Mix | Invitrogen | 4309155 |
| Trametinib | Selleck Chemicals | S2673 |
| T-5224 | Selleck Chemicals | S8966 |
| Critical Commercial Assays | ||
| EZ DNA Methylation-Gold™ kit | Zymo Research | ZD5005 |
| Human phospho-kinase array kit | R&D Systems | ARY003B |
| Dual-luciferase reporter assay system | Promega | E1910 |
| Senescence β-Galactosidase staining kit | Cell Signaling Tech | 9860 |
| Mature BDNF mouse rapid ELISA assay | Biosensis | BEK-2211 |
| Protein synthesis assay kit | Cayman Chemical | 601100 |
| Deposited Data | ||
| RNA-seq data of WT and Tert−/− mouse brains | Shim et al., 2021 | GSE163524 |
| RNA-seq and ChIP-seq data | This paper |
GSE231454, GSE231455 |
| Experimental Models: Cell Lines | ||
| Mouse: Primary ear fibroblasts from hTERT-Rluc transgenic mouse | Jia et al., 2011 | N/A |
| Mouse: Primary cardiomyocytes | This paper | N/A |
| Mouse: Primary microglia cells | This paper | N/A |
| Mouse: Primary neuronal cells | This paper | N/A |
| Human: MRC-5 fibroblasts | ATCC | Cat#: CCL-171 |
| Human: Werner syndrome (WS) fibroblasts | Coriell Institute | Cat#: AG03141 |
| Human: NPCs derived from NDC's iPSCs | Raja et al., 2016 | N/A |
| Experimental Models: Organisms/Strains | ||
| C57BL/6 | The Jackson Laboratory | JAX: 000664 |
| LSL-mTERT (B6 background) | Chakravarti et al., 2020 | N/A |
| hTERT-Rluc transgenic mouse | Jia et al., 2011 | N/A |
| Recombinant DNA | ||
| pGL4-hTERT-promoter-4kb-WT | This study | N/A |
| pGL4-hTERT-promoter-4kb-ΔAP1-#1 | This study | N/A |
| pGL4-hTERT-promoter-4kb-ΔAP1-#2 | This study | N/A |
| pGL4-hTERT-promoter-4kb-ΔAP1-#1 | This study | N/A |
| Software and Algorithms | ||
| ImageJ v1.53a | NIH | |
| GraphPad Prism v8 | GraphPad Software | |
| htseq-count package | Anders et al., 2015 | |
| DESeq2 package | Love et al., 2014 | |
| Bowtie v1.2.2 | Langmead et al., 2009 | |
| SAMtools v1.9 | Li et al., 2009 | |
| MACS v1.4.2 | Zhang et al., 2008 | |
| GSEA v4.1.0 | Broad Institute | |
| BioRender | BioRender | |
| Other | ||
| Barnes Maze apparatus (basic mouse) | Panlab Harvard Apparatus | Cat#: 989999 |
| Y-maze apparatus (with door, mouse) | Panlab Harvard Apparatus | Cat#: 760079 |
| Rotarod (mouse) | Panlab Harvard Apparatus | Cat#: 760770 |
| Grip strength meter (mouse) | Panlab Harvard Apparatus | Cat#: 761068 |
| Bioruptor Pico sonication system | Diagenode | |
| HiSeq 4000 | Illumina |
This study did not generate any original code.
All other data are available upon request for Lead Contact/corresponding author.