Abstract
Almost 50 years ago, scientists developed the bi-hormonal abnormality hypothesis, stating that diabetes is not caused merely by the impaired insulin signaling. Instead, the presence of inappropriate level of glucagon is a prerequisite for the development of type 1 diabetes (T1D). It is widely understood that the hormones insulin and glucagon, secreted by healthy β and α cells respectively, operate in a negative feedback loop to maintain the body’s blood sugar levels. Despite this fact, traditional T1D treatments rely solely on exogenous insulin injections. Furthermore, research on cell-based therapies and stem-cell derived tissues tends to focus on the replacement of β cells alone. In vivo, the pancreas is made up of 4 major endocrine cell types, that is, insulin-producing β cells, glucagon-producing α cells, somatostatin-producing δ cells, and pancreatic polypeptide-producing γ cells. These distinct cell types are involved synergistically in regulating islet functions. Therefore, it is necessary to produce a pancreatic islet organoid in vitro consisting of all these cell types that adequately replaces the function of the native islets. In this review, we describe the unique function of each pancreatic endocrine cell type and their interactions contributing to the maintenance of normoglycemia. Furthermore, we detail current sources of whole islets and techniques for their long-term expansion and culture. In addition, we highlight a vast potential of the pancreatic islet organoids for transplantation and diabetes research along with updated new approaches for successful transplantation using stem cell-derived islet organoids.
INTRODUCTION
According to the Center for Disease Control and Prevention, 1.9 million Americans have type 1 diabetes (T1D).1 There are several complications associated with diabetes including blindness, stroke, renal failure, heart attack, and lower limb amputation.2 Despite the gravity of these complications, the most common T1D treatment, injection of exogenous insulin, does not address them.3 Exogenous insulin injections, when used properly, have the potential to mitigate the high blood glucose associated with this disease. However, insulin injections are not a cure, as they do not address the changes in β and α cell number and function underlying the development of diabetes.
The development of T1D is rooted in improper secretion of both insulin and glucagon.4,5 This is due to the fact that the heterotypic interactions between glucagon-secreting α and insulin-secreting β cells in human islets is paramount in regulating glucose homeostasis.6,7 Therefore, it is logical to conclude that treatments based on the injection of exogenous insulin alone are insufficient to treat T1D or adequately replicate the non-diabetic state. One major source of concern associated with exogenous insulin injections for the treatment of T1D is hypoglycemia.8 Should insulin injections lower blood glucose levels below a healthy level (90—110 mg/dL), transport of glucose to the brain is severely limited.8,9 This is hazardous and may lead to death. Despite the dangers of hypoglycemia, patients with T1D experience symptomatic episodes of hypoglycemia an average of 2 times each week.8
Even ignoring the failure to address the α cell dysfunction associated with T1D, exogenous insulin treatments are unable to replicate the function of the endogenous hormone.10 As a matter of fact, patients who receive several insulin injections each day experience glycemic volatility, indicating that their blood glucose levels vary between hyperglycemic and hypoglycemic.10 This is due in part to the fact that injected insulin is unable to reach the islets with sufficiently high concentration.10 Therefore, glucagon secretion is not suppressed as a result of the exogenous insulin.10 A higher dose of injected insulin is necessary to overcome the subsequent low ratio of insulin to glucagon.10 However, this high insulin dose may lead to hypoglycemia.10 Maintenance of blood glucose levels in the non-diabetic body is a delicate balance between insulin and glucagon. The complex interplay between these hormones is difficult to recreate using exogenous hormone injections, leading to glycemic instability.
In light of each of these limitations, islet transplantation is preferable to exogenous insulin injections. However, there too are severe limitations in the availability of pancreatic islets for transplantation as a means of curing T1D. This is due to the wide gap between the available cadaveric donors and the number of T1D patients who would benefit from a transplant.11 Furthermore, there are many T1D patients who would benefit from a transplant that are not eligible for one. This is because insulin therapy is often used preferentially to transplantation. While pancreas and islet transplants are the only means for restoring endogenous insulin secretion and the fine-tuned glycemic control that results, these methods are limited to patients who experience additional renal failure or severe hypoglycemia and glycemic instability despite exogenous insulin treatment.12
An appealing solution to the organ donor shortage is the use of stem cell-derived pancreatic islet organoids as an alternative to cadaveric islets. Stem cells are capable of differentiating into multiple cell types. Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) are both commonly used for the development of pancreatic islet organoids.13 These cells may give rise to all of the major endocrine cell types within the islet of Langerhans: insulin-producing β cells, glucagon-producing α cells, somatostatin-producing δ cells, and pancreatic polypeptide-producing γ cells. Because each of these cell types plays an important role in metabolism and the establishment of normoglycemia in the non-diabetic state, it is critical that they are each present in organoids intended to treat T1D. In this review, we discuss the unique function of each pancreatic islet cell subset and their roles in maintaining normoglycemia in the body. We highlight the sources of whole islets, techniques for their long-term expansion and culture, and their utility for transplantation and research. In addition, we summarize updated new approaches for successful transplantation using stem cell-derived islet organoids. We will begin by highlighting the critical functions of each major endocrine cell type within the islet of Langerhans individually. Then, the effects that each cell type exhibits on its counterparts will be discussed.
ROLE OF HEALTHY BETA CELLS
β Cells are the most prevalent endocrine cell type located within the pancreas in the islet of Langerhans.14 In human islets, β cells, accounting for approximately 50%–75% of the volume of islets, are located in high amounts within the anterior head, body, and tail of the pancreas.14–16 Murine islets are generally arranged with a core of β cells surrounded by α, δ, and γ cells at the periphery.17 In contrast to this and in conflict with the islet architecture described in some textbooks, studies suggest that β cells within human islets are more randomly distributed among the other endocrine cell types.15,17,18
β Cells produce, store, and secrete the hormone insulin in response to changes in blood glucose levels.19 They secrete insulin in response to high ATP/ADP ratios within the cells. It is in this way that the β cells exhibit glucose responsive insulin secretions.20 When glycemic levels are high following a meal, there is a high concentration of glucose within the extracellular space of β cells.20 The glucose is transported into the cell by glucose transporters like GLUT1 in human and GLUT2 in rodent and then metabolized.21 Furthermore, glucokinase within the β cells phosphorylates glucose, leading to increased levels of ATP and decreased levels of ADP.20 Consequently, the ATP binds to potassium channels within the β cells to depolarize the cell which allows calcium to flow in through voltage-gated calcium channels, resulting in insulin exocytosis.20 In addition, insulin is an anti-hyperglycemic peptide hormone, which functions to lower blood glucose levels.19 It binds to receptors located on the cell-membrane of target cells such as liver, fat, and skeletal muscle cells.22,23 Insulin binding on receptors of liver cells promotes the receptor-mediated endocytosis of glucose and its subsequent conversion into glycogen thereby decreasing circulating blood glucose levels.23 In addition to triggering glucose uptake in target cells, insulin also antagonizes several hyperglycemic hormones such as glucagon, growth hormone, glucocorticosteroids, and epinephrine.19 In this way, insulin functions to maintain a healthy blood glucose level following postprandial spikes in blood sugar.
Several studies have investigated pancreatic development, with a focus on β cell differentiation.24–30 Through the use of animals, especially murine models, scientists have established the role that several transcription factors such as Pancreas/duodenum homeobox protein 1 (PDX1), Neurogenin-3 (NGN3), and V-maf musculoaponeurotic fibrosarcoma oncogene homolog A (MAFA) play in β cell development, survival, and function in vivo.25,31–38 MAFA is a β cell maturation marker, as it regulates the transcription of insulin gene in response to glucose levels.39 PDX1, expressed early on during development, is essential for the function and survival of β cells.25,32 Pdx1 null mice and humans with homozygous PDX1 mutations exhibit pancreatic agenesis.32 Furthermore, humans with heterozygous missense and frameshift PDX1 mutations are unable to secrete insulin and exhibit maturity onset diabetes of the young.32 In general, PDX1 is thought to regulate β cell development by activating genes associated with β cell identity and suppressing those critical to α cell identity.33
ROLE OF HEALTHY ALPHA CELLS
After β cells, the second most prevalent endocrine cell type within the pancreatic islets is the α cell.17 The percentage of α cells comprising the human islet is generally 20%–40%.15,18,40 These cells produce and secrete the hormone glucagon.41 Glucagon is released into the bloodstream in response to low blood glucose levels. Within α cells themselves, there are voltagegated Ca2+ channels, such as the P/Q-type Ca2+ channel, that open in regard to low blood glucose levels.41 As a result, there is an influx of Ca2+ into the cell, leading to glucagon exocytosis.41 This hormone increases the amount of glucose present in the blood by interacting with glucagon receptors (GCGR) on the liver and triggering both gluconeogenesis, the synthesis of glucose, and glycogenolysis, the catabolism of glycogen into glucose.41 The regulation of glucagon secretion is less understood than that of insulin, but it is thought to involve a combination of intrinsic and paracrine signals.41,42 The dysfunction of α cells directly contributes to T1D development.4,5 Further involvement of α cells in islet functionality and diabetes are described in the following sections.
ROLE OF HEALTHY DELTA, GAMMA, AND EPSILON CELLS
Another subtype of endocrine cells within the islet of Langerhans is the δ cell. The function and therapeutic potential of these cells are less widely understood compared to the more prevalent β and α cells. δ cells make up about 5%–10% of human islet cells, and they secrete the hormone somatostatin.17,43 Somatostatin, also referred to as growth hormone inhibiting hormone, is released by the gastrointestinal tract, the hypothalamus, and the central nervous system in addition to pancreatic δ cells.44 Somatostatin exists in 2 forms in the body: somatostatin-28 and somatostatin-14, the latter being secreted by pancreatic δ cells.43 δ cells influence β and α cells through paracrine interactions. They do so using long neurite-like projections that extend between them.43 Research also indicates that δ and β cells are electrically coupled, further suggesting their interactions.43 Somatostatin secretion from δ cells is induced by glucose concentrations, beginning at a concentration of 3 millimolar and increasing proportionately as glucose concentration increases.43 Somatostatin is an inhibitor of both insulin and glucagon.45 One study investigated this function using somatostatin-null (Sst−/−) mice.45 They demonstrated that Sst−/− mice had significantly higher plasma levels of both insulin and glucagon following intravenous administration of glucose or arginine compared to the wild type mice.45 A similar difference was apparent during ex vivo experiments done on isolated mouse islets.45 Islet isolated from Sst−/− mice secreted approximately twice as much insulin and glucagon in response to the glucose and arginine compared to the control.45 This suggests that the source of insulin and glucagon suppression is δ cell-derived somatostatin rather than gastrointestinal, neural, or neuroendocrine sources of the hormone.45 In β cell destruction models such as streptozotocin (STZ)-induced diabetic rats, there is an observed increase in the quantity of δ cells as well as an increase in pancreatic somatostatin.46
γ Cells, also referred to as pancreatic polypeptide (PP) cells, make up approximately 1%—2% of the islet of Langerhans.40 These cells secrete the hormone pancreatic polypeptide, a 36 amino acid peptide that is released after eating in a biphasic manner.47 The first phase of pancreatic polypeptide secretion is stimulated by the vagus nerve.47 This first phase is shorter than the second, which occurs in response to hormones including cholecystokinin.47 Levels of pancreatic polypeptide in the plasma remain elevated for up to 6 hours following eating.48 Its release is also stimulated by hypoglycemia and exercise via adrenergic stimulation.48 Pancreatic polypeptide inhibits gallbladder motility, pancreatic exocrine, and gastric acid secretions.48,49 It does so by binding to dorsal vagal complex receptors and causing changes in the vagovagal reflex arc.48 Additionally, the pancreatic polypeptide is thought to promote feelings of satiety following a meal via the vagus nerve.48,49 Studies show that transgenic mice that overexpress pancreatic polypeptide exhibit weight loss, decreased food intake, and decreased gastric emptying.48 Furthermore, when the pancreatic islets from lean mice were transplanted into their genetically obese littermates, their obesity was reversed.50 This suggests that decreased pancreatic polypeptide levels and sensitivity can contribute to obesity. Diabetes is sometimes associated with elevated pancreatic polypeptide levels, with about 10% of diabetes patients exhibiting high basal pancreatic polypeptide levels.47 However, more research on the differences between healthy and diabetic γ cells would be helpful to fully understand the function of this cell type.51
In addition, there is a small proportion of epsilon (ε) cells present in the islet of Langerhans. ε cells make up less than 1% of islets, and they were not discovered until the early 2000s.52 They produce the hormone ghrelin which is 28 amino acids in length.52 Ghrelin, also referred to as the hunger hormone, stimulates appetite.52 It also induces the secretion of growth hormones from the pituitary.52 Even though ε cells make up a very small percentage of islets, this type of cells are detectable in stem cell-derived pancreatic islet tissue. For example, researchers performing single-cell RNA sequencing may evaluate the expression of GHRL which encodes a precursor to ghrelin.53
DIABETES IS A BI-HORMONAL DISORDER
There are several changes in β cell survival and function as a result of the development of diabetes mellitus. T1D, also referred to as insulin-dependent diabetes, is associated with a decrease in the number of β cells.54 In T1D, the immune system attacks β cells, leading to decreased insulin production and a failure to maintain blood glucose levels.54 The abnormal β cell populations and their association with the development of diabetes mellitus often overshadow the role that α cells play in the disease. However, the importance of the hormone glucagon in diabetes cannot be ignored. In 1975, the bi-hormonal abnormality hypothesis replaced the previous unihormonal concept.4 This relatively newer hypothesis states that improper secretion or function of insulin alone is not responsible for the hyperglycemia associated with diabetes.4 Insufficient insulin levels must be coupled with the presence and hypersecretion of glucagon for the development of diabetes mellitus.4 This is supported by a study comparing the results of β cell destruction on glucagon receptor null (GCGR−/−) and wild type mice.55 In this study, 100 milligrams followed by 80 milligrams per kilogram of body weight of the chemical STZ was injected intravenously in order to induce the destruction of β cells.55 This process was performed in 2 groups of male mice, one with global glucagon receptor knockout and one wild type group.55 Only the wild type mice showed traditional symptoms of T1D, including hyperglycemia, hyperketonemia, polyuria, and cachexia.55 Through this study, researchers were able to recreate the conditions of T1D and demonstrate the critical role that glucagon plays in symptom development.55 These results were further supported through experiments restoring hepatic glucagon receptors in GCGR−/− mice.56 Once again, this study demonstrated that STZ-mediated β cell destruction only led to clinical symptoms such as hyperglycemia in wild type mice expressing glucagon receptors.56 After utilizing an adenovirus containing GCGR cDNA to restore the glucagon receptors, hyperglycemia developed in the mouse models, further suggesting that the action of glucagon is critical in T1D.56 Hence, T1D treatment using glucagon receptor inhibition has been investigated extensively.57–60
The interplay between the synergetic regulation of insulin/glucagon and hyperglycemia has been demonstrated by a recent report showing that insulin receptor substrate 1 plays a crucial role in the regulation of glucagon secretion. It changes glucagon transcription in response to exogenous insulin,61 which provides a mechanism underlying how insulin signaling regulates glucagon secretion.62 As T1D is characterized by insulin dysfunction combined with high levels of glucagon, a diabetes treatment that can restore both β and α cell function is highly desired. Okamoto et al. demonstrated that an insulin receptor antagonist, S961, induced symptoms of diabetes in mice, including insulin resistance, hyperglycemia, and ketonemia.60 When a monoclonal antibody was used to block the glucagon receptor, normoglycemia was restored. Interestingly, the mass of β cells doubled with glucagon receptor blocking compared to treatment with S961 alone, with a 5.8-fold increase in β cell mass compared to the control which received no monoclonal antibody or S961. Additionally, treatment with the monoclonal antibody increased α cell mass by 5.7-fold.60 This study poses a potential therapy for diabetes while demonstrating the essential role of glucagon in symptom development. In a clinical trial, a glucagon receptor antibody, REMD-477, has been studied to examine its efficacy for T1D treatment. The results indicated that this glucagon receptor antibody improves glycaemic control in patients with T1D and reduces insulin usage.63
ENDOCRINE CELL INTERACTIONS THAT INFLUENCE ISLET FUNCTION
In native pancreatic islets, there are complex interactions between each endocrine cell type to maintain healthy conditions within the body. In particular, β cells and α cells operate in a negative feedback loop to establish normoglycemia. In individuals without diabetes, the spike in blood sugar accompanying a meal leads to increased secretion of insulin from β cells and decreased glucagon secretion from α cells.64 These conditions lead to a decrease in plasma glucose levels. Should this level dip below the healthy level and hypoglycemia develop, α cells will begin secreting glucagon, thereby raising plasma glucose levels.64
Since human islets of Langerhans are predominantly composed of a heterogeneous mixture of β, α, δ, and γ cells, there is an ample opportunity for paracrine signaling between each cell type.65,66 As opposed to the cytoarchitecture of other species such as mice, human β cells are not arranged in a cluster, rather they are intermingled with other islet cell types.18,67,68 Studies show that 71% of human β cells are in contact with other endocrine cell types, further supporting the importance of paracrine signaling, especially with human β cells.18 These paracrine signals include hormones, peptides, neurotransmitters, and metabolites.65 The majority of β, α, and δ cells are located along blood vessels within the islet.18 However, the order of cells appears random, suggesting that the order of paracrine interactions between these cell types is not decided by the microcirculation.18 Paracrine interactions play an instrumental role in regulating the secretion of glucagon from α cells. Both insulin and somatostatin are released under hyperglycemic conditions, and these hormones are capable of inhibiting glucagon secretion.28 It is hypothesized that insulin inhibits the secretion of glucagon indirectly, through the action of somatostatin.69 Insulin secreted by β cells stimulates the secretion of somatostatin from δ cells in mouse islets.69 Furthermore, the ability of insulin to regulate glucagon release was lost in somatostatin-secreting δ cell insulin receptor knockout mice, suggesting that the action of insulin on α cells is indirect and requires the involvement of δ cells.69 In addition to insulin, several factors released by β cells are capable of inhibiting the secretion of glucagon by α cells including γ-aminobutyric acid (GABA), ATP, and zinc ions.70 The influence of paracrine signaling on glucagon regulation is apparent when α cells are studied separately from whole islets. For instance, a study using isolated primary rat α cells showed that glucose level regulates KATP-channel activity in the cells, resulting in sugar level responsive glucagon secretion. Such glucose level responsiveness can be interrupted by means of ion channel modulators, such as diazoxide, a potassium channel activator. The activated signal of potassium channel would come from tissue surrounding the α cells.71
Although α cells do not typically release high levels of the hormone glucagon-like peptide-1 (GLP-1), in circumstances such as following IL-6 treatment, β cell regeneration after STZ treatment in neonatal rats α cells produce GLP-1.70 This hormone binds with the G-protein coupled receptors present on β cell membranes, leading to increased insulin secretion.72 Furthermore, in vitro studies show that glucagon stimulates the secretion of insulin by β cells.70 Meanwhile, the pancreatic polypeptide secreted by γ cells agonizes neuropeptide Y4 receptors which are expressed by several somatostatin-containing cells including those in islets.73 The result of this agonism is decreased secretion of somatostatin by δ cells.73 Additionally, pancreatic polypeptide interacts with the pancreatic polypeptide receptor present on α cells to regulate the secretion of glucagon.74 At lower concentrations (1 nM), pancreatic polypeptide decreased glucagon secretion whereas it increased secretions at higher concentrations (100 nM) in rodent islets.74 The various paracrine interactions between β, α, δ, and γ cells are visualized in Fig 1.
Fig 1.
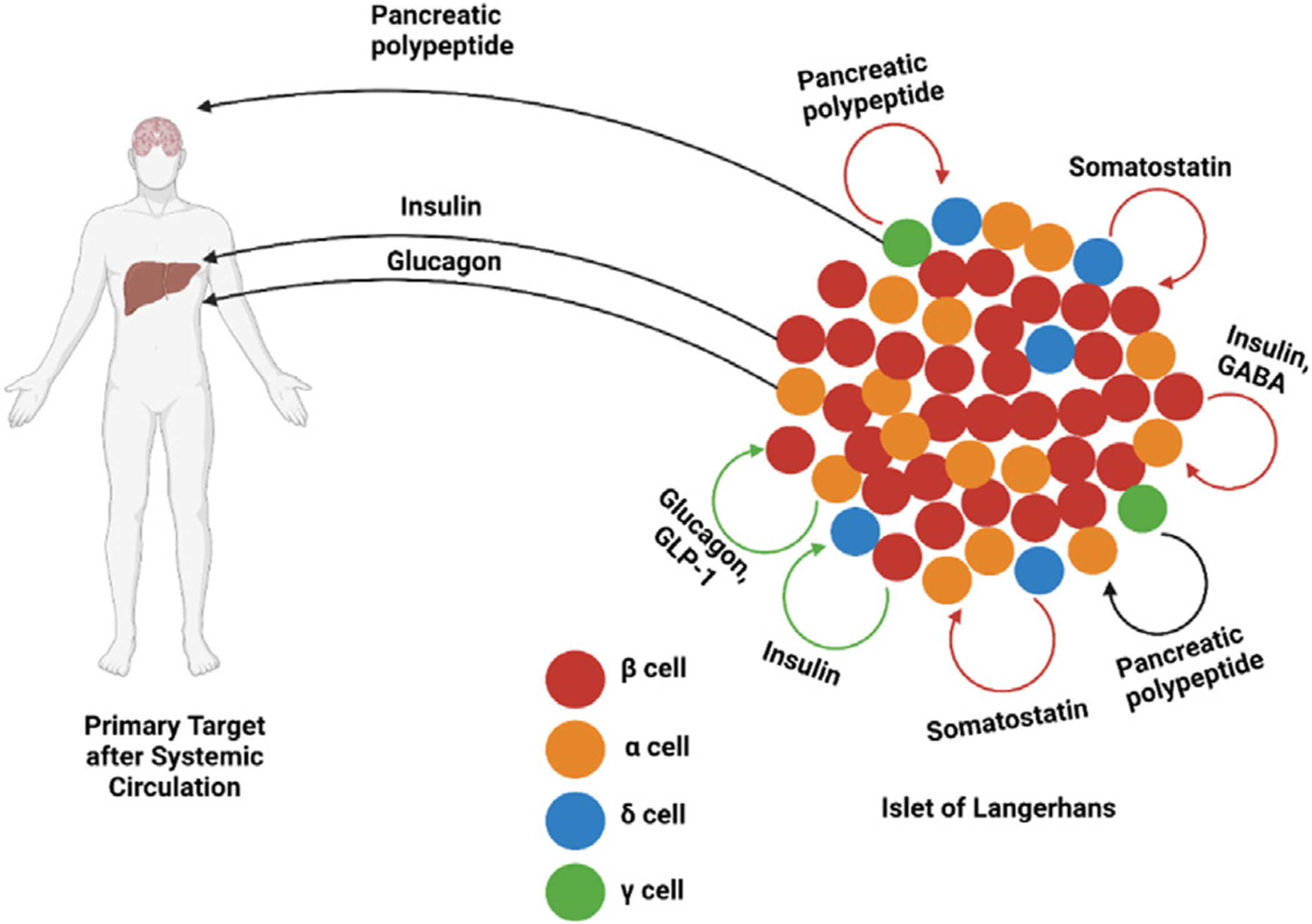
Paracrine and systemic targets of β-, α-, δ-, and γ-cell secretions, with red arrows indicating inhibitory paracrine interactions, green arrows indicating stimulatory paracrine interactions, and black arrows indicating concentration-dependent paracrine interactions. GABA: γ-aminobutyric acid. GLP-1: glucagon-like peptide 1. Created with BioRender.com.
SOURCES OF WHOLE ISLETS FOR CLINICAL APPLICATIONS
Because of the crucial role each islet cell type within pancreatic islets plays, it is important to obtain whole islets for transplantation, as well as for use in a lab setting performing drug testing, disease modeling, and pathophysiological study. These islets can be obtained from cadavers or differentiated from stem cells (Fig 2). Key procedures to obtain donor islets and stem cell-derived islet organoids are visualized in Fig 2. The first attempt at treating T1D through pancreas transplantation was performed in the year 1966 by Richard Lillehei and William Kelly at the University of Minnesota.75,76 In this surgery, a 28-year-old female was simultaneously given a kidney and a pancreas graft.75 Although exogenous insulin treatments were eventually necessary, this patient did not require insulin injections for an initial period of 6 days.75 Despite this modest success, the surgery eventually failed due to the extensive use of steroids to prevent rejection.75 Furthermore, the patient experienced pancreatitis due to the ligation of the duct, and the transplanted organs were subsequently removed.75 Since then, several advances have been made in the area of allogeneic pancreas transplantation. The success of subsequent pancreas transplants owe in part to the development of effective immunosuppressive drugs such as cyclosporin A.76
Fig 2.
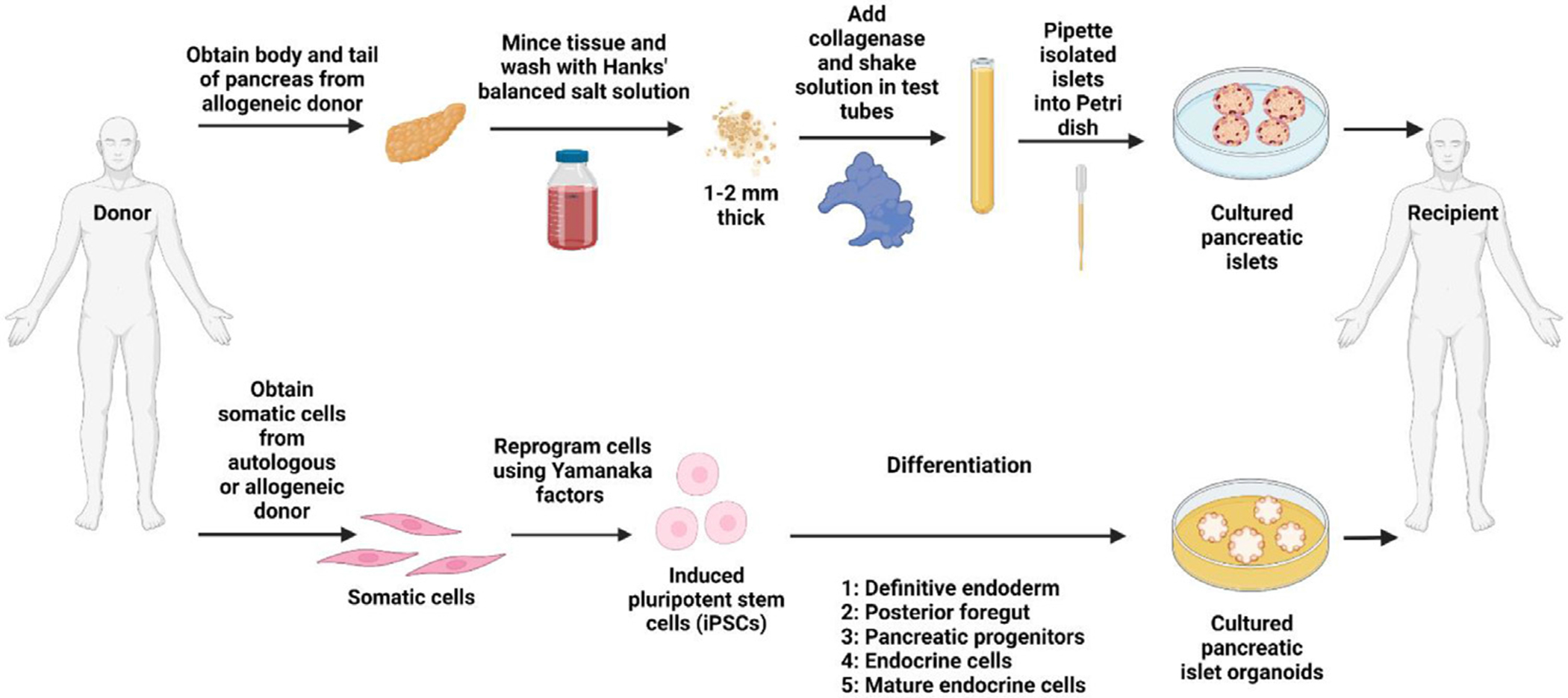
Key procedures to obtain donor islets and stem cell-derived islet organoids for transplantation. Somatic cells obtained from autologous or allogeneic sources are reprogrammed into pluripotent stem cells using cell reprogramming technologies. Pancreatic islet organoids can be generated from induced iPSC differentiation. Created with BioRender.com
Whole-organ pancreas transplantation is usually performed in conjunction with kidney transplantation.12 There exists a less invasive alternative for the treatment of T1D: islet transplantation.12 The procedure for islet transplantation, known as the Edmonton protocol, was first outlined in the New England Journal of Medicine in the year 2000.77 Before human islets can be transplanted, they must be digested using enzymes, purified, and cultured.78 After this process is carried out, the islets are infused via the portal vein.78 This is achieved by suspending the purified cells in transplant media containing heparin and infusing the solution using a catheter inserted into the skin.78 Compared to the surgery necessary for the whole-organ pancreas transplantation, there are lower risks associated with islet transplantation.12 However, islet transplants are limited in that they require more donors.12 It is recommended that a patient receive at least 5000 islet equivalents for each kilogram of their body weight.78 This further exacerbates the issue of donor limitations, as the number of cadaver pancreases available for transplantation is far less than the number of patients in need of a transplant.
Because of the severe limitations in donor availability, researchers have focused on the potential for stem cell-derived pancreatic islets for transplantation as well as diabetes research. While these tissues may originate from hESCs or iPSCs, pancreatic islet organoids derived from autologous iPSCs are advantageous in that they do not necessitate the lifelong use of immunosuppressants.79 Furthermore, iPSCs do not face the same ethical constraints as ESCs which require the destruction of an embryo.79,80 Studies have demonstrated that iPSCs and ESCs exhibited similar differentiation capacities for the generation of functional tissues or organs.68,81–84 Hence, iPSCs became an unlimited source for the generation of human tissues.80,85 Although there is often a focus on the differentiation of β cells, there exist protocols for the derivation of pancreatic islet organoids containing all or most of the major endocrine cell types, outlined in Table I.67,68,81,82,86–88 As mentioned previously, the development of these multi-cell type organoids is preferred for cell-based therapies rather than generating solely β cells.
Table I.
Studies obtaining pancreatic islet organoids composed of multiple islet cell types
| Cell Line | β cells | α cells | δ cells | γ cells | Novelty | Reference |
|---|---|---|---|---|---|---|
| iPSCs hIveNry | Detected | Detected | Detected | Not detected | SV40LT added to induce proliferation of endocrine progenitors and removed before differentiation | 86 |
| iPSCs TkDN4-M | Detected | Detected | Detected | Not detected | Suspension culture | 87 |
| hESCs HUES8, iPSC-1 and iPSC-2 | Detected | Detected | Detected | Not detected | Modulation of signaling pathways in each stage of differentiation using small molecules and growth factors | 88 |
| hESCs H9 | Detected | Detected | Detected | Detected | Collagen type I biomimetic scaffold | 67 |
| hESCs HUES8 | Detected | Detected | Detected | Not detected | Dynamical regulation of TGF-β signaling | 89 |
| hESCs H9 | Detected | Detected | Detected | Not detected | Isolation of CD177+ anterior definitive endoderm cells to inhibit Wnt signaling for further differentiation | 90 |
| iPSCs IMR90, hESCs H9 | Detected | Detected | Detected | Detected | Decellularized pancreatic extracellular matrix in coating substrates | 81 |
| iPSCs IMR90, hESCs H9 | Detected | Detected | Detected | Detected | Collagen type V presented in coating substrates | 68 |
| iPSCs IMR90 and DF4, hESCs H9 | Detected | Detected | Detected | Detected | Angiopoietin stimulation in the middle of stepwise differentiation | 82 |
Typically, the generation of islet organoids from pluripotent stem cells in vitro is carried out in a stepwise manner that replicates the development of the native endocrine cells.91 The stages of this stepwise protocol generally include the differentiation of stem cells into the definitive endoderm (Stage 1), followed by the posterior foregut (Stage 2), pancreatic progenitor cells (Stage 3), endocrine cells (Stage 4), and ending with the development of mature islet cells (Stage 5).91 This protocol is recreated at varying levels of completion for the treatment of T1D. Some approaches utilizing stem cell-derived products to treat diabetes involve the procurement of β cells in vitro and subsequent use in vivo,92–96 the injection of pancreatic progenitor cells for in vivo maturation,97,98 and the development of heterogeneous pancreatic islet organoids in vitro comprising some or all of the major pancreatic endocrine cell types.67,68,81,82,87–90,99,100
Although several studies focus on the development of insulin-producing β-like cells for diabetes cell therapies, there is an evidence that these cells are not fully mature as they often co-express both insulin and glucagon.98 A proposed alternative is the injection of pancreatic progenitor cells in vivo.98 Upon injection, these pancreatic progenitors continue to mature in vivo.98 One study optimized the differentiation of hESC line H1 into pancreatic endoderm and endocrine precursor cells that highly expressed the marker genes PDX1, NGN3, and NKX6.1.98 Populations of these cells were transplanted into mice that were previously given an intraperitoneal injection of STZ to model T1D.98 Following implantation, the endocrine precursor cells continued maturing. Instead of co-expressing insulin and glucagon, as 40% of cells did before implantation, they began to form islet-like clusters.98 Three months after implantation, 95% of these cells were monohormonal, including insulin-, glucagon-, and somatostatin-positive cells.98 The mice were weaned off of exogenous insulin initially used to treat their STZ-induced diabetes, and the hESC-derived cells were eventually able to secrete insulin, as measured by C-peptide, in response to both intraperitoneal and oral glucose.98
In addition to investigate the maturation of pancreatic and endocrine progenitor cells in vivo, researches aim to develop mature islet organoids in vitro that contain β, α, δ, and γ cells prior to implantation. Through temporal treatment with signaling molecules and 3D microenvironment, researchers have developed protocols for the differentiation of pluripotent stem cells into insulin-producing islet-like clusters (ILCs).67,101 For example, treatment of sodium butyrate and activin A leads to upregulation of definitive endoderm-associated genes such as SOX17, FOXA2 and HNF4α.101 Eventually, these pancreatic endoderm cells begin expressing higher levels of PDX1, PTF1a, NGN3, and NKX6.1, each of which is associated with the endocrine cells.101 After maturation, characterization of ILCs through immunocytochemical staining reveals that they are made up of insulin, glucagon, and somatostatin-positive cells.101 Furthermore, these hESC-derived ILCs showed glucose-responsive C-peptide and insulin secretions.101 Wang et al. showed that collagen-based 3D scaffolds could significantly improve the generation of ILCs with high-level expression of C-peptide and higher sensitivity to glucose levels compared with that of 2D culture microenvironment (Table I).67 Several research groups have developed protocols for the development of similar islet-like organoids, with variations in signaling molecules used or adding preselection through cell sorting technique (Table I).82,88–90,102
The goal of many studies is to identify important microenvironmental signals during the pancreatic development in vivo and recapitulate them in vitro so as to effectively develop islets for treatments, disease modeling, drug screening, and other applications. Bi et al. demonstrated the efficacy of decellularized pancreatic extracellular matrix (dpECM) proteins and collagen type V as critical microenvironmental cues in islet development, showing that their presentation in the matrix-coating substrates during differentiation of human iPSCs and hESCs leads to the generation of pancreatic islet organoids containing insulin-, glucagon-, somatostatin, and pancreatic polypeptide-secreting islet organoids.68,81 This differentiation protocol leads to the formation of islet organoids with glucose-responsive glucagon as well as insulin secretions (Table I).68,81 Organ-on-a-chip techniques have also been utilized to recreate the native pancreatic microenvironment, including the key cell-cell interactions and mechanical stimuli.103 Overall, stem cell-derived pancreatic cells and tissues offer a promising alternative to cadaveric islets for both research and clinical applications. However, a diverse array of chemical and molecular stimuli plays a critical role during in vivo development. More research is necessary to effectively recreate the native tissue microenvironment in vitro in order to improve differentiation protocols for the generation of mature islet organoids. Karanth et al. discovered that angiogenic factors, angiopoietin-1 or angiopoietin-2, facilitate the formation of islet organoids from induced iPSC differentiation with the elevated glucose responsiveness. The iPSC-derived islets generated under angiopoietin stimulation displayed glucose synchronized calcium ion influx in repetitive glucose challenges. Mechanistically, these islet organoids were able to regulate insulin exocytosis through actin-filament polymerization and depolymerization in response to glucose level change.82
STRATEGIES FOR LONG-TERM CULTURE AND PRESERVATION OF ISLETS
Before transplantation, cadaveric islets must be isolated and purified. There is often a period of time during which the isolated islets must be maintained in a tissue culture before use.104 In addition, due to the wide supply-demand gap for cadaveric islets, it requires the development of long-term culture and preservation techniques to maintain maximal function of pancreatic islets prior to clinical application. A plethora of investigations has been performed surrounding the best methods of culturing and preserving both cadaveric islets and pancreatic islet organoids for obtaining their flexible usage such as transplantation. Numerous studies have been done on pancreases obtained from animals such as mice, rats, and guinea pigs, as well as organoids derived from rodent stem cell lines.3,105–115 Additionally, several researchers have developed protocols for human islet preservation so as to best conserve β cell function. Importantly, some studies focus on the best means of culturing human stem cell-derived islet organoids.
As early as the 70s, research groups like Andersson et al. worked to develop protocols to maintain islets within a culture.104 By adequately purifying and culturing these islets, there is a greater potential for utilizing multiple donors for source tissue and avoiding complications and rejection.104 After pancreatic glands are isolated from cadavers and perfused with a chilled solution of Dextran 40 and electrolytes, the body and tail are injected with Hanks’ Balanced Salt Solution (HBSS) and cut into small pieces.104 The pieces are put into suspension and treated with collagenase.104 After collagenase digestion and fat removal through HBSS washing, the islets are added to petri dishes with culture medium changed every 2 days starting from day 3.104 In this protocol, the culture medium is supplemented with 20% calf serum and antibiotics.104 In more recent years, efforts have been made to identify molecules with which to supplement traditional culture media while still maintaining serum-free conditions.116 This helps avoid contamination, which is critical for transplanted tissues.117 Results of preliminary studies indicated that islets could be cultured in vitro for at least one week with no disturbances in β or α cell function.104
In order to best preserve pancreatic islet tissue and function, differing conditions have been studied including various media formulations, additional molecules provided, and environments on which the tissues are cultured. Islets may be cultured in suspension, on surfaces coated with ECM, encapsulated or embedded in biomaterials, on scaffolds, in bioreactors, or even in microphysiological systems.118,119 The simplest and the best understood culturing environment of islets and islet organoids is the suspension culture.118 As compared to an adherent culture, the suspension culture allows the islets to maintain their three-dimensional architecture.118 Several experiments have shown that supplementation with insulin,120 prolactin,121 sericin,122 human albumin,123 ethanolamine, phosphoethanolamine, transferrin, triiodothyronine, and insulin-like growth factor 1124 leads to better preservation of islet and β cell function. Alternative methods such as culture on ECM or in bioreactors restores the mechanical stimuli lost in suspension cultures such as the cell-cell and cell-ECM interactions and hydrodynamic shear stress.118 These methods prove to be more effective in maintaining islet cell phenotype.118
In cases where a longer period of time passes between obtaining and utilizing islets, cryopreservation is often used. This technique is useful in cases where islets are obtained from several donors. Cryopreservation of isolated and purified islets includes a supercooling process to around −40°C in ethanol after adding serum containing dimethyl sulfoxide (DMSO), a cryoprotectant that prevents cell injury caused by the development of intracellular and extracellular crystals.125,126 Then, the tissues are kept at low temperatures such as −196°C by submerging them in liquid nitrogen.125 Studies show that after 46.5 days, cryopreserved islets had similar insulin extraction values on the day of thawing compared to before freezing.125 When islet aliquots from cryopreserved islets and those that were cultured overnight were perfused with Krebs bicarbonate buffer containing basal (60 mg/dl) and high (300 mg/dl) concentrations of glucose, they showed similar primary insulin secretions.125 However, the islets thawed after cryopreservation showed lower secondary insulin secretions.125 Secondary insulin secretion follows the initial short spike in insulin concentrations.127 This second phase of insulin secretion is characterized by a 2—3-hour slow increase in insulin concentrations that eventually plateaus.127 While the difference in secondary insulin secretion between the 2 groups was not statistically significant, it reveals modest damage to pancreatic islets after cryopreservation.125 This is further supported by the idea that secondary insulin secretions are typically made up of the newly synthesized hormone.125
Variables commonly altered during the optimization of cryopreservation protocols include cryoprotectant chemicals and freezing rate.128,129 Although the most standard cryoprotectant used is DMSO, several research groups have investigated the effects of varying its concentration and utilizing other cryoprotectants alone or in combination with DMSO.128–130 Combining DMSO with chemicals such as hydroxyethyl starch decreases its inherent toxicity to islets.130 Another study found that using lower concentrations (1.5 M) of DMSO as a cryoprotectant shows better results than 2.0 M DMSO or various concentrations of ethylene glycol.128 This conclusion was made on the basis of significantly improved percentage of recovered islets and stimulation indices.128 Although the toxicity of DMSO makes its use as a cryoprotectant hotly debated, islets cryopreserved using DMSO have shown clinical success when transplanted after thawing.126,131 Warnock et al. demonstrated that a 36-year-old patient with T1D showed normoglycemia with the withdrawal of exogenous insulin injection following a simultaneous islet-kidney transplant using islets cryopreserved with DMSO.131
As previously mentioned, one approach for the treatment of diabetes is to use pancreatic or endocrine progenitor cells generated in vitro and allow their maturation in vivo or in vitro. Tanaka et al. developed a protocol for the expansion of iPSC-derived pancreatic islet progenitors in culture by introducing and subsequently removing the proliferation gene SV40LT.86 Introducing this gene to a population of endocrine progenitor cells expressing NGN3 via lentiviral vector led to cell proliferation while inhibiting their differentiation into cells expressing NGN3, NEUROD1 and NKX2.2.86 After the SV40LT gene was removed using Cre recombinase, the proliferation ceased, and the progenitor cells differentiated into β, α, and δ cells (Table I).86 This protocol could be applied in the future to efficiently expand endocrine progenitors for the eventual generation of mature islet cells and pancreatic islet organoids both in vitro and in vivo.
UTILITY OF ISLET ORGANOIDS
The therapeutic potential of pancreatic islet organoids as a source for transplantable islets is clear, and it is highlighted in several papers.13,132–136 The utility of islet organoids in addressing T1D has been demonstrated in animal models.98,102,137 Protocols exist for the transplantation of islet organoids that hold the potential to remedy current challenges such as limited availability of cadaveric islets, need for immunosuppressive drugs, and even engraftment failure.137 In addition to the potential benefits of implanting pancreatic islet organoids to treat diabetes and restore endogenous levels of insulin, glucagon, and other pancreatic hormones in patients, there are other applications for these organoids in vitro. Much of the research devoted to the potential of pancreatic organoids in diabetes disease modeling.138–144 By using islet organoids derived from human stem cells, researchers can investigate the mechanisms of disease progression and identify therapeutics without relying on animal models.138 This is a valuable way to more accurately study human physiology instead of generalizing between different species.138 Furthermore, by recapitulating the disease state in a laboratory setting, researchers can easily investigate the ability of drug candidates to ameliorate symptoms.139 Moreover, this could be applied to patient-specific personalized medicine should autologous cells be used for organoids. By using pancreatic islet organoids on microphysiological systems, researchers can also investigate the interactions between the pancreas and other organs, like the liver, in a more precise, controlled manner.138,139 Multi-organ microphysiological systems create a unique opportunity to examine the interactions and crosstalk between organs at a level that is more complex than a single cell culture or organoid yet simpler than in vivo studies. For example, Tao et al. utilized microfluidic organ-on-a-chip systems to model the liver-pancreatic islet axis.138 The pancreatic islet organoids for this study expressed markers associated with β, α, δ, and γ cells, and the microfluidic device used allowed for the exchange of media and metabolites between the islet and liver organoids.138 They found that this system was effective in modeling the ability of β cells to stimulate uptake of glucose by the liver in response to elevated glucose level.138 This represents one of many studies that makes use of pancreatic organoids to model healthy and diseased islet function. Overall, pancreatic islet organoids are valuable beyond organ transplantation in their utility in disease progression studies and drug discovery.
CONCLUDING REMARKS
There is an urgent need to identify alternative sources for pancreatic islets as a means to treat diabetes. Islets derived from pluripotent stem cells, including iPSCs, are an appealing solution to the current organ donor shortage. However, β cell treatments, like exogenous insulin injections, fail to address the role of glucagon in the development of diabetes. Furthermore, each endocrine cell type in the islet of Langerhans plays its own critical role in normal physiology, both in the production and the secretion of their respective hormone and in their complex interactions regulating one another. For this reason, whole intact islets or islet organoids are preferred to reestablish healthy physiological conditions. However, there are many challenges currently facing the generation and transplantation of heterocellular pancreatic islet organoids.
One key challenge in the effective generation and isolation of pancreatic islet organoids in vitro is the absence of instrumentation and markers to reliably distinguish between islet organoids and other cell aggregates after differentiation.68 Because the desired product is multicellular and heterogenous, flow cytometry cannot be used for purification. This leads to decreased efficiency in islet organoid generation. In contrast to the generation of uniform populations of β cells, preselection cannot be performed for the generation of pancreatic islet organoids composed of several endocrine cell types. During the development of monohormonal insulin-producing cells, preselection is often used to increase the final cell purity. For example, Rezania et al. demonstrated that selection of hESC-derived pancreatic progenitor cells that highly express both PDX1 and NKX6.1 leads to more efficient maturation into insulin-secreting cells in vivo.102 Pancreatic progenitor cells with high PDX1 and NKX6.1 expression were more effective in reversing diabetes in mice when transplanted subcutaneously in macroencapsulation devices compared to pancreatic progenitor cells with the low NKX6.1 expression.102 This preselection often requires using a reporter stem cell line allowing for single cell sorting through flow cytometry.145–147 For instance, reporter cell lines inserting green fluorescence protein into the INS locus for subsequent isolation148 and transfecting cells with a plasmid containing both NKX6.1 promoter and neomycin-resistance genes for the subsequent selection of NKX6.1-positive cells149 have been used as enrichment methods. However, increased heterogeneity and a lack of consistent cell surface markers for islet organoids makes these methods inapplicable to increase the purity and the efficiency for the generation of pancreatic islet organoids. Furthermore, there are resulting difficulties in quantifying the relative amounts of each endocrine cell type within islet organoids.68 Cryo-sectioning, immunostaining, and fluorescence microscopy may be utilized to measure the percentage of β, α, δ, and γ cells, but the presence of non-islet cell types leads to significant challenges.68
Despite the current shortcomings in the efficient generation of pure islet organoids containing β, α, δ, and γ cells, there are many promising areas of further research. For example, gene editing may be used to address limitations in islet organoid purity.150 One major risk of using pluripotent stem cell-derived tissues for transplantation is the potential for teratoma formation from undifferentiated cells.150 This risk is high upon implantation of pancreatic islet organoids due to the aforementioned challenges in organoid purification and characterization. By selectively expressing a suicide gene in undifferentiated stem cells, this risk can be mitigated.150 Wu et al. accomplished this by inserting the suicide gene inducible caspase-9, designated as iC9, into the endogenous SOX2 locus of the hESC line H1 using CRISPR-Cas9 technology (Fig 3).150 In the resulting cell line, undifferentiated cells underwent apoptosis following treatment with an apoptotic drug AP1903 whereas differentiated cells were unaffected.150 This approach poses a potential solution to eliminate undifferentiated stem cells. However, it is still challenging to apply this method to remove multiple progenitors or non-islet organoids due to the nature of heterogeneity during organoid differentiation.
Fig 3.
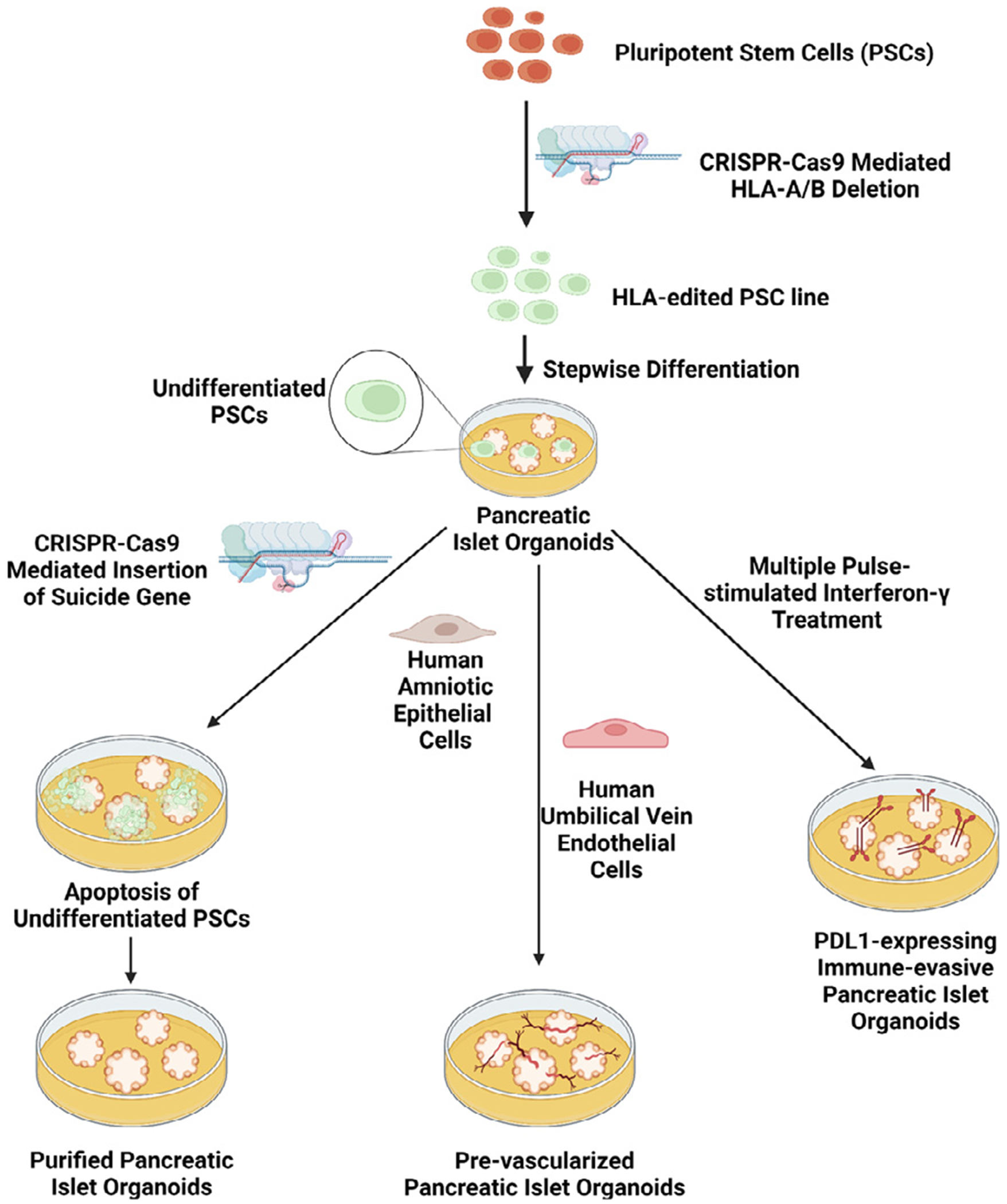
Novel strategies to enhance the efficacy of using pancreatic islet organoids in vivo. Created with BioRender.com
Another significant challenge in the clinical use of pancreatic islet organoids is their limited survival following transplantation. Due to the shortage of oxygen at the transplantation site, organoids survival rate in vivo without the promotion of angiogenesis is low. In order to quickly establish a vascular network to provide nutrients and the exchange of oxygen and carbon dioxide to pancreatic islet organoids, efforts have been made focusing on using encapsulation devices that are able to generate oxygen in site or the formation of prevascularized islets before transplant.133,137,151–155 Wang et al. designed a scaffold which provides internal air supply continuously to deliver oxygen to the hydrogel encapsulated cells to support long-term function of transplanted cells.155 This encapsulation device is promising for transplantation requiring high cell payloads. As an alternative approach, prevascularized islets, could be achieved by fusing islet cells with microvascular fragments137 or with multiple cell types including human amniotic epithelial cells (hAECs), and human umbilical vein endothelial cells (HUVECs) (Fig 3).133,153,154 Nalbach et al. developed prevascularized pancreatic islet organoids by co-culture of pancreatic islet cells and microvascular fragments at an optimal ratio, allowing the formation of microvessels before transplantation.137 These microvascular fragments were isolated by mechanic and enzymatic digestion of epididymal fat pads of mice, followed by filtration through a 500 μm filter. These organoids demonstrate high angiogenic capabilities and restore normoglycemia in STZ-treated mice faster than freshly isolated islets.137 Although the organoids developed in this study were derived from primary murine islet cells, a similar protocol could be applied in the future with autologous pluripotent stem cells and liposuction derived microvascular fragments.137 The prevascularization leads to favorable engraftment, increased angiogenesis, and enhanced function of the islet organoids following implantation in murine models.133,137,154. Crosstalk between islet cells, hAECs, and HUVECs leads to the upregulation of angiogenesis genes such as vascular endothelial growth factor A as well as genes associated with proper β cell function like PDX1.154
In addition, avoiding the immune system attacking transplanted organoids is critical for graft survival. Methods for a successful islet transplantation have been well discussed elsewhere.156–159 A commonly used approach is encapsulation of therapeutic cells prior to the transplant. Researchers have developed biocompatible microcapsules for cell encapsulation that can mitigate the buildup of fibrotic tissue to improve function of transplanted cells.160–163 Alagpulinsa et al. found that encapsulating hESC-derived β cells into microcapsules containing high concentration of CXCL12 supports β cell function and protects against the immune response and rejection.162 This study indicates that supplementation of immune-cell-repelling protein CXCL12 improves the likelihood of a successful transplantation. Another approach developed to protect stem cell-derived cells from immune recognition is to induce the expression of immune checkpoint protein programmed death-ligand 1 (PD-L1) (Fig 3).164,165 Treatment of human islets with interferon-γ (IFN-γ) led to a 20-fold increase in PD-L1 expression after 20 hours.164 Furthermore, when IFN-γ was presented through multiple short exposures, the islets exhibited no changes in glucose-stimulated insulin secretions.164 In vivo testing demonstrated that PD-L1+ islet organoids showed more prolonged effects in diabetic mice compared to organoids without PD-L1 expression.164 Fig 3 outlines some critical methods of improving pancreatic islet organoids for use in vivo.
Another challenge using iPSC-derived organoids for cell-based therapy is immunological rejection if allogeneic stem cells were used, which requires the use of immunosuppressants to prevent rejection. While this can be avoided through the use of autologous stem cells as a source for pancreatic islet organoids, it too has its set of shortcomings. The process of reprogramming an individual’s autologous cells into iPSCs is time-consuming and expensive. At the same time, the requirement for donor-recipient HLA compatibility is a major challenge for use of an off-the-shelf iPSC line.166 The use of HLA-edited iPSCs may overcome this challenge (Fig 3).166,167 Using CRISPR-Cas9, Koga et al. demonstrated that HLA-A/B-knockout iPSCs are not rejected by donors with HLA-C-matched natural killer and T cells.166 Furthermore, 12 HLA-C alleles were capable of 95% of the global population.166 Therefore, these 12 HLA-edited iPSC lines could serve as a semi-universal source for stem cell-derived tissues.166 These cells could be used as an unlimited source for pancreatic islet organoids while still avoiding immune rejection following implantation. Castro-Gutierrez et al. established a PD-L1 inducible expression and HLA class I knockout hPSC line by genome editing. Islet β-like cells generated from this genome edited, PD-L1 overexpressing cell line revoked CD8 T cell activation characterized by T cell stimulation assay.165 It should be pointed out that disadvantages in the development of immune-evasive tissues are the loss of the capability of immune surveillance and the risk of tumorigenesis.168 The use of HLA-editing inherently hinders the ability of the immune system to monitor tissues for the development of cancer, thereby impacting its ability to be applied clinically.167 Wuputra et al. summarized approaches to manage and avoid tumorigenesis developed from iPSCs.168 Taken together, this review emphasizes the importance of multiple pancreatic islet cell types in islets and the generation of the multicellular islet organoids from stem cells, particularly with updated new strategies to improve cell-based therapies using stem cell-derived islet organoids.
ACKNOWLEDGMENTS
This work is partially supported by the National Institute of Health 1R15EB027391 and National Science Foundation CBET1928855 and CBET1919830.
Abbreviations:
- DMSO
dimethyl sulfoxide
- GABA
γ-aminobutyric acid
- GCGR
glucagon receptor
- GLP-1
glucagon-like peptide-1
- hAECs
human amniotic epithelial cells
- HBSS
Hanks’ Balanced Salt Solution
- hESCs
human embryonic stem cells
- HLA
human leukocyte antigen
- HUVECs
human umbilical vein endothelial cells
- IFN-γ
interferon-γ
- iPSCs
induced pluripotent stem cells
- ILCs
islet-like clusters
- LepR
leptin receptor
- MAFA
V-maf musculoaponeurotic fibrosarcoma oncogene homolog A
- NGN3
Neurogenin-3
- PDX1
Pancreas/duodenum homeobox protein 1
- STZ
streptozotocin
Footnotes
Conflicts of Interest: All authors have read the journal’s policy on disclosure of potential conflicts of interest. The authors have no conflicts of interest to declare.
Contributor Information
EMMA S. HEATON, Department of Biomedical Engineering, Thomas J. Watson School of Engineering and Applied Sciences, State University of New York at Binghamton, Binghamton, New York
SHA JIN, Center of Biomanufacturing for Regenerative Medicine, State University of New York at Binghamton, Binghamton, New York.
REFERENCES
- 1.National Diabetes Statistics Report. Centers for Disease Control and Prevention. February 15, 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html. [Google Scholar]
- 2.Diabetes. World Health Organization. 2021. https://www.who.int/news-room/fact-sheets/detail/diabetes. [Google Scholar]
- 3.Dolezalova N, Gruszczyk A, Barkan K, et al. Accelerating cryoprotectant diffusion kinetics improves cryopreservation of pancreatic islets. Sci Rep 2021;11:10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975;1:14–6. [DOI] [PubMed] [Google Scholar]
- 5.Burcelin R, Knauf C, Cani PD. Pancreatic alpha-cell dysfunction in diabetes. Diabetes Metab 2008;34(Suppl 2):S49–55. [DOI] [PubMed] [Google Scholar]
- 6.Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol 2003;5:330–5. [DOI] [PubMed] [Google Scholar]
- 7.Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 2013;75:155–79. [DOI] [PubMed] [Google Scholar]
- 8.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26:1902–12. [DOI] [PubMed] [Google Scholar]
- 9.Yatabe T, Yamazaki R, Kitagawa H, et al. The evaluation of the ability of closed-loop glycemic control device to maintain the blood glucose concentration in intensive care unit patients. Crit Care Med 2011;39:575–8. [DOI] [PubMed] [Google Scholar]
- 10.Lee YH, Wang MY, Yu XX, Unger RH. Glucagon is the key factor in the development of diabetes. Diabetologia 2016;59:1372–5. [DOI] [PubMed] [Google Scholar]
- 11.Punch JD, Hayes DH, LaPorte FB, McBride V, Seely MS. Organ donation and utilization in the United States, 1996-2005. Am J Transplant 2007;7:1327–38. [DOI] [PubMed] [Google Scholar]
- 12.Bellin MD, Dunn TB. Transplant strategies for type 1 diabetes: whole pancreas, islet and porcine beta cell therapies. Diabetologia 2020;63:2049–56. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L, Shen Y, Liu Y, Zhang L, Jiang W. Making human pancreatic islet organoids: Progresses on the cell origins, biomaterials and three-dimensional technologies. Theranostics 2022;12:1537–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher S Beta cells : functions, pathology, and research. Cell biology research progress. New York: Nova Science Publishers; 2011:1 online resource. [Google Scholar]
- 15.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005;53:1087–97. [DOI] [PubMed] [Google Scholar]
- 16.Pisania A, Weir GC, O’Neil JJ, et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab Invest 2010;90:1661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets 2010;2:135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006;103:2334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchetti P, Bugliani M, De Tata V, Suleiman M, Marselli L. Pancreatic Beta Cell Identity in Humans and the Role of Type 2 Diabetes. Front Cell Dev Biol 2017;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fridlyand LE, Philipson LH. Glucose sensing in the pancreatic beta cell: a computational systems analysis. Theor Biol Med Model 2010;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vos A, Heimberg H, Quartier E, et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest 1995;96:2489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev 2018;98:2133–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Meyts P The Insulin Receptor and Its Signal Transduction Network. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al. , eds. South Dartmouth (MA): Endotext; 2000. [PubMed] [Google Scholar]
- 24.Piper K, Brickwood S, Turnpenny LW, et al. Beta cell differentiation during early human pancreas development. J Endocrinol 2004;181:11–23. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Liu Q, Zhou Z, Ikeda Y. PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration. Stem Cell Res Ther 2017;8:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murtaugh LC. Pancreas and beta-cell development: from the actual to the possible. Development 2007;134:427–38. [DOI] [PubMed] [Google Scholar]
- 27.Castaing M, Duvillie B, Quemeneur E, Basmaciogullari A, Scharfmann R. Ex vivo analysis of acinar and endocrine cell development in the human embryonic pancreas. Dev Dyn 2005;234:339–45. [DOI] [PubMed] [Google Scholar]
- 28.Eliasson L, Esguerra JL. Role of non-coding RNAs in pancreatic beta-cell development and physiology. Acta Physiol (Oxf) 2014;211:273–84. [DOI] [PubMed] [Google Scholar]
- 29.Al-Masri M, Krishnamurthy M, Li J, et al. Effect of forkhead box O1 (FOXO1) on beta cell development in the human fetal pancreas. Diabetologia 2010;53:699–711. [DOI] [PubMed] [Google Scholar]
- 30.Stoffers DA. The development of beta-cell mass: recent progress and potential role of GLP-1. Horm Metab Res 2004;36:811–21. [DOI] [PubMed] [Google Scholar]
- 31.Shao S, Fang Z, Yu X, Zhang M. Transcription factors involved in glucose-stimulated insulin secretion of pancreatic beta cells. Biochem Biophys Res Commun 2009;384:401–4. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto K, Polonsky KS. Pdx1 and other factors that regulate pancreatic beta-cell survival. Diabetes Obes Metab 2009;11 (Suppl 4):30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao T, McKenna B, Li C, et al. Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metab 2014;19:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baeyens L, Bonne S, German MS, Ravassard P, Heimberg H, Bouwens L. Ngn3 expression during postnatal in vitro beta cell neogenesis induced by the JAK/STAT pathway. Cell Death Differ 2006;13:1892–9. [DOI] [PubMed] [Google Scholar]
- 35.Aguayo-Mazzucato C, Koh A, El Khattabi I, et al. Mafa expression enhances glucose-responsive insulin secretion in neonatal rat beta cells. Diabetologia 2011;54:583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaneto H, Matsuoka TA, Kawashima S, et al. Role of MafA in pancreatic beta-cells. Adv Drug Deliv Rev 2009;61:489–96. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Brun T, Kataoka K, Sharma AJ, Wollheim CB. MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia 2007;50:348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura W, Takahashi S, Yasuda K. MafA is critical for maintenance of the mature beta cell phenotype in mice. Diabetologia 2015;58:566–74. [DOI] [PubMed] [Google Scholar]
- 39.Hang Y, Stein R. MafA and MafB activity in pancreatic beta cells. Trends Endocrinol Metab 2011;22:364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Da Silva Xavier G The Cells of the Islets of Langerhans. J Clin Med 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briant L, Salehi A, Vergari E, Zhang Q, Rorsman P. Glucagon secretion from pancreatic alpha-cells. Ups J Med Sci 2016;121:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker JN, Ramracheya R, Zhang Q, Johnson PR, Braun M, Rorsman P. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes Metab 2011;13 (Suppl 1):95–105. [DOI] [PubMed] [Google Scholar]
- 43.Rorsman P, Huising M. The somatostatin-secreting pancreatic delta-cell in health and disease. Nat Rev Endocrinol 2018;14:404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Toole TJ, Sharma S. Physiology, Somatostatin. Treasure Island (FL): StatPearls; 2022. [Google Scholar]
- 45.Hauge-Evans AC, King AJ, Carmignac D, et al. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 2009;58:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerich JE. Somatostatin and diabetes. Am J Med 1981;70:619– 26. [DOI] [PubMed] [Google Scholar]
- 47.Lonovics J, Devitt P, Watson LC, Rayford PL, Thompson JC. Pancreatic polypeptide. A review. Arch Surg 1981;116:1256–64. [DOI] [PubMed] [Google Scholar]
- 48.Kojima S, Ueno N, Asakawa A, et al. A role for pancreatic polypeptide in feeding and body weight regulation. Peptides 2007;28:459–63. [DOI] [PubMed] [Google Scholar]
- 49.Hazelwood RL. The pancreatic polypeptide (PP-fold) family: gastrointestinal, vascular, and feeding behavioral implications. Proc Soc Exp Biol Med 1993;202:44–63. [DOI] [PubMed] [Google Scholar]
- 50.Katsuura G, Asakawa A, Inui A. Roles of pancreatic polypeptide in regulation of food intake. Peptides 2002;23:323–9. [DOI] [PubMed] [Google Scholar]
- 51.Maxwell JE, O’Dorisio TM, Bellizzi AM, Howe JR. Elevated pancreatic polypeptide levels in pancreatic neuroendocrine tumors and diabetes mellitus: causation or association? Pancreas 2014;43:651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakata N, Yoshimatsu G, Kodama S. Development and Characteristics of Pancreatic Epsilon Cells. Int J Mol Sci 2019;20:1867. 10.3390/ijms20081867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balboa D, Barsby T, Lithovius V, et al. Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells. Nat Biotechnol 2022. 10.1038/s41587-022-01219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pipeleers D, Ling Z. Pancreatic beta cells in insulin-dependent diabetes. Diabetes Metab Rev 1992;8:209–27. [DOI] [PubMed] [Google Scholar]
- 55.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 2011;60:391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee Y, Berglund ED, Wang MY, Fu X, Yu X, Charron MJ, et al. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc Natl Acad Sci U S A 2012;109:14972–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okamoto H, Kim J, Aglione J, et al. Glucagon receptor blockade with a human antibody normalizes blood glucose in diabetic mice and monkeys. Endocrinology 2015;156:2781–94. [DOI] [PubMed] [Google Scholar]
- 58.Yan H, Gu W, Yang J, et al. Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. J Pharmacol Exp Ther 2009;329:102–11. [DOI] [PubMed] [Google Scholar]
- 59.Gu W, Yan H, Winters KA, et al. Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. J Pharmacol Exp Ther 2009;331:871–81. [DOI] [PubMed] [Google Scholar]
- 60.Okamoto H, Cavino K, Na E, et al. Glucagon receptor inhibition normalizes blood glucose in severe insulin-resistant mice. Proc Natl Acad Sci U S A 2017;114:2753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takatani T, Shirakawa J, Shibue K, et al. Insulin receptor substrate 1, but not IRS2, plays a dominant role in regulating pancreatic alpha cell function in mice. J Biol Chem 2021;296:100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawamori D, Kurpad AJ, Hu J, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab 2009;9:350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pettus J, Reeds D, Cavaiola TS, et al. Effect of a glucagon receptor antibody (REMD-477) in type 1 diabetes: A randomized controlled trial. Diabetes Obes Metab 2018;20:1302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goke B Islet cell function: alpha and beta cells–partners towards normoglycaemia. Int J Clin Pract Suppl 2008;62:2–7. [DOI] [PubMed] [Google Scholar]
- 65.Hartig SM, Cox AR. Paracrine signaling in islet function and survival. J Mol Med (Berl) 2020;98:451–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leung YM, Ahmed I, Sheu L, et al. Insulin regulates islet alpha-cell function by reducing KATP channel sensitivity to adenosine 5’-triphosphate inhibition. Endocrinology 2006;147:2155–62. [DOI] [PubMed] [Google Scholar]
- 67.Wang W, Jin S, Ye K. Development of islet organoids from H9 human embryonic stem cells in biomimetic 3D scaffolds. Stem Cells Dev 2017;26:394–404. [DOI] [PubMed] [Google Scholar]
- 68.Bi H, Ye K, Jin S. Proteomic analysis of decellularized pancreatic matrix identifies collagen V as a critical regulator for islet organogenesis from human pluripotent stem cells. Biomaterials 2020;233:119673. [DOI] [PubMed] [Google Scholar]
- 69.Vergari E, Knudsen JG, Ramracheya R, et al. Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nat Commun 2019;10:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moede T, Leibiger IB, Berggren PO. Alpha cell regulation of beta cell function. Diabetologia 2020;63:2064–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olsen HL, Theander S, Bokvist K, Buschard K, Wollheim CB, Gromada J. Glucose stimulates glucagon release in single rat alpha-cells by mechanisms that mirror the stimulus secretion coupling in beta-cells. Endocrinology 2005;146:4861–70. [DOI] [PubMed] [Google Scholar]
- 72.Carlessi R, Chen Y, Rowlands J, et al. GLP-1 receptor signalling promotes beta-cell glucose metabolism via mTOR-dependent HIF-1alpha activation. Sci Rep 2017;7:2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim W, Fiori JL, Shin YK, et al. Pancreatic polypeptide inhibits somatostatin secretion. FEBS Lett 2014;588:3233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aragon F, Karaca M, Novials A, Maldonado R, Maechler P, Rubi B. Pancreatic polypeptide regulates glucagon release through PPYR1 receptors expressed in mouse and human alpha-cells. Biochim Biophys Acta 2015;1850:343–51. [DOI] [PubMed] [Google Scholar]
- 75.Squifflet JP, Gruessner RW, Sutherland DE. The history of pancreas transplantation: past, present and future. Acta Chir Belg 2008;108:367–78. [DOI] [PubMed] [Google Scholar]
- 76.Casanova D en nombre de Grupo Espanol de Trasplante de P. Pancreas transplantation: 50 years of experience. Cir Esp 2017;95:254–60. [DOI] [PubMed] [Google Scholar]
- 77.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–30. [DOI] [PubMed] [Google Scholar]
- 78.Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol 2017;13:268–77. [DOI] [PubMed] [Google Scholar]
- 79.Medvedev SP, Shevchenko AI, Zakian SM. Induced pluripotent stem cells: problems and advantages when applying them in regenerative medicine. Acta Naturae 2010;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 80.Yefroyev DA, Jin S. Induced pluripotent stem cells for treatment of Alzheimer’s and Parkinson’s diseases. Biomedicines 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bi H, Karanth SS, Ye K, Stein R, Jin S. Decellularized tissue matrix enhances self-Assembly of islet organoids from pluripotent stem cell differentiation. ACS Biomater Sci Eng 2020;6:4155–65. [DOI] [PubMed] [Google Scholar]
- 82.Karanth SS, Sun S, Bi H, Ye K, Jin S. Angiopoietins stimulate pancreatic islet development from stem cells. Sci Rep 2021;11:13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marei HE, Althani A, Lashen S, Cenciarelli C, Hasan A. Genetically unmatched human iPSC and ESC exhibit equivalent gene expression and neuronal differentiation potential. Sci Rep 2017;7:17504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wen Y, Jin S. Production of neural stem cells from human pluripotent stem cells. J Biotechnol 2014;188:122–9. [DOI] [PubMed] [Google Scholar]
- 85.Glicksman MA. Induced Pluripotent Stem Cells: The Most Versatile Source for Stem Cell Therapy. Clin Ther 2018;40:1060–5. [DOI] [PubMed] [Google Scholar]
- 86.Tanaka A, Watanabe A, Nakano Y, Matsumoto M, Okazaki Y, Miyajima A. Reversible expansion of pancreatic islet progenitors derived from human induced pluripotent stem cells. Genes Cells 2020;25:302–11. [DOI] [PubMed] [Google Scholar]
- 87.Yabe SG, Fukuda S,Nishida J, Takeda F, Nashiro K, Okochi H. Induction of functional islet-like cells from human iPS cells by suspension culture. Regen Ther 2019;10:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014;159:428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Velazco-Cruz L, Song J, Maxwell KG, et al. Acquisition of dynamic function in human stem cell-derived beta cells. Stem Cell Reports 2019;12:351–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mahaddalkar PU, Scheibner K, Pfluger S, et al. Generation of pancreatic beta cells from CD177(+) anterior definitive endoderm. Nat Biotechnol 2020;38:1061–72. [DOI] [PubMed] [Google Scholar]
- 91.Huang H, Bader TN, Jin S. Signaling molecules regulating pancreatic endocrine development from pluripotent stem cell differentiation. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Russ HA, Parent AV, Ringler JJ, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J 2015;34:1759–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vegas AJ, Veiseh O, Gurtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016;22:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Basford CL, Prentice KJ, Hardy AB, et al. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia 2012;55:358–71. [DOI] [PubMed] [Google Scholar]
- 95.Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes 2001;50:1691–7. [DOI] [PubMed] [Google Scholar]
- 96.Soria B, Roche E, Berna G, Leon-Quinto T, Reig JA, Martin F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 2000;49:157–62. [DOI] [PubMed] [Google Scholar]
- 97.Bruin JE, Rezania A, Xu J, et al. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia 2013;56:1987–98. [DOI] [PubMed] [Google Scholar]
- 98.Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012;61:2016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006;24:1392–401. [DOI] [PubMed] [Google Scholar]
- 100.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 2001;292:1389–94. [DOI] [PubMed] [Google Scholar]
- 101.Jiang J, Au M, Lu K, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 2007;25:1940–53. [DOI] [PubMed] [Google Scholar]
- 102.Rezania A, Bruin JE, Xu J, et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells 2013;31:2432–42. [DOI] [PubMed] [Google Scholar]
- 103.Tao T, Wang Y, Chen W, et al. Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip 2019;19:948–58. [DOI] [PubMed] [Google Scholar]
- 104.Andersson A, Borg H, Groth CG, et al. Survival of isolated human islets of Langerhans maintained in tissue culture. J Clin Invest 1976;57:1295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Broutier L, Andersson-Rolf A, Hindley CJ, et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc 2016;11:1724–43. [DOI] [PubMed] [Google Scholar]
- 106.Cornelius JG, Tchernev V, Kao KJ, Peck AB. In vitro-generation of islets in long-term cultures of pluripotent stem cells from adult mouse pancreas. Horm Metab Res 1997;29:271–7. [DOI] [PubMed] [Google Scholar]
- 107.Hyon SH, Kim DH. Long-term preservation of rat pancreatic islets under physiological conditions. J Biotechnol 2001;85:241–6. [DOI] [PubMed] [Google Scholar]
- 108.Kawasaki F, Matsuda M, Kanda Y, Inoue H, Kaku K. Structural and functional analysis of pancreatic islets preserved by pioglitazone in db/db mice. Am J Physiol Endocrinol Metab 2005;288:E510–8. [DOI] [PubMed] [Google Scholar]
- 109.Ferguson J, Allsopp RH, Taylor RM, Johnston ID. Isolation and long term preservation of pancreatic islets from mouse, rat and guinea pig. Diabetologia 1976;12:115–21. [DOI] [PubMed] [Google Scholar]
- 110.Nakagawara G, Kobayashi T, Kimura T, Yamaguchi A, Shimada H. Preservation of pancreatic islets in rodent models. Pancreas 1998;16:392–5. [DOI] [PubMed] [Google Scholar]
- 111.Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia 1978;14:397–404. [DOI] [PubMed] [Google Scholar]
- 112.Kaiser N, Corcos AP, Tur-Sinai A, Ariav Y, Cerasi E. Mono-layer culture of adult rat pancreatic islets on extracellular matrix: long term maintenance of differentiated B-cell function. Endocrinology 1988;123:834–40. [DOI] [PubMed] [Google Scholar]
- 113.Weber CJ, Hardy MA, Pi-Sunyer F, Zimmerman E, Reemtsma K. Tissue culture preservation and intramuscular transplantation of pancreatic islets. Surgery 1978;84:166–74. [PubMed] [Google Scholar]
- 114.Foreman J, Moriya H, Taylor MJ. Effect of cooling rate and its interaction with pre-freeze and post-thaw tissue culture on the in vitro and in vivo function of cryopreserved pancreatic islets. Transpl Int 1993;6:191–200. [DOI] [PubMed] [Google Scholar]
- 115.Nakayama-Iwatsuki K, Hirabayashi M, Hochi S. Fabrication of functional rat pseudo-islets after cryopreservation of pancreatic islets or dispersed islet cells. J Tissue Eng Regen Med 2021;15:686–96. [DOI] [PubMed] [Google Scholar]
- 116.Rush BT, Fraga DW, Kotb MY, et al. Preservation of human pancreatic islet in vivo function after 6-month culture in serum-free media. Transplantation 2004;77:1147–54. [DOI] [PubMed] [Google Scholar]
- 117.Nuttall PA, Luther PD, Stott EJ. Viral contamination of bovine foetal serum and cell cultures. Nature 1977;266:835–7. [DOI] [PubMed] [Google Scholar]
- 118.Daoud J, Rosenberg L, Tabrizian M. Pancreatic islet culture and preservation strategies: advances, challenges, and future outlook. Cell Transplant 2010;19:1523–35. [DOI] [PubMed] [Google Scholar]
- 119.Patel SN, Ishahak M, Chaimov D, et al. Organoid microphysiological system preserves pancreatic islet function within 3D matrix. Sci Adv 2021;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Clayton H, Turner J, Swift S, James R, Bell P. Supplementation of islet culture medium with insulin may have a beneficial effect on islet secretory function. Pancreas 2001;22:72–4. [DOI] [PubMed] [Google Scholar]
- 121.Yamamoto T, Mita A, Ricordi C, et al. Prolactin supplementation to culture medium improves beta-cell survival. Transplantation 2010;89:1328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ogawa A, Terada S, Kanayama T, et al. Improvement of islet culture with sericin. J Biosci Bioeng 2004;98:217–9. [DOI] [PubMed] [Google Scholar]
- 123.Lee RH, Carter J, Szot GL, Posselt A, Stock P. Human albumin preserves islet mass and function better than whole serum during pretransplantation islet culture. Transplant Proc 2008;40:384–6. [DOI] [PubMed] [Google Scholar]
- 124.Clark SA, Chick WL. Islet cell culture in defined serum-free medium. Endocrinology 1990;126:1895–903. [DOI] [PubMed] [Google Scholar]
- 125.Kneteman NM, Alderson D, Scharp DW, Lacy PE. Long-term cryogenic storage of purified adult human islets of Langerhans. Diabetes 1989;38:386–96. [DOI] [PubMed] [Google Scholar]
- 126.Awan M, Buriak I, Fleck R, et al. Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen Med 2020;15:1463–91. [DOI] [PubMed] [Google Scholar]
- 127.Gerich JE. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes 2002;51(Suppl. 1):S117–21. [DOI] [PubMed] [Google Scholar]
- 128.Lakey JR, Anderson TJ, Rajotte RV. Novel approaches to cryo-preservation of human pancreatic islets. Transplantation 2001;72:1005–11. [DOI] [PubMed] [Google Scholar]
- 129.Wang S, Yuan X, Zhou J, Jin J, Zuo Q, Li B. Comparison of the effects of three cryoprotectants on the cryopreservation of mouse subcutaneous tissue under different conditions. Exp Ther Med 2020;20:3285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kenmochi T, Asano T, Maruyama M, et al. Cryopreservation of human pancreatic islets from non-heart-beating donors using hydroxyethyl starch and dimethyl sulfoxide as cryoprotectants. Cell Transplant 2008;17:61–7. [DOI] [PubMed] [Google Scholar]
- 131.Warnock GL, Kneteman NM, Ryan E, Seelis RE, Rabinovitch A, Rajotte RV. Normoglycaemia after transplantation of freshly isolated and cryopreserved pancreatic islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1991;34:55–8. [DOI] [PubMed] [Google Scholar]
- 132.Akolpoglu MB, Inceoglu Y, Bozuyuk U, et al. Recent advances in the design of implantable insulin secreting heterocellular islet organoids. Biomaterials 2021;269:120627. [DOI] [PubMed] [Google Scholar]
- 133.Lebreton F, Lavallard V, Bellofatto K, et al. Insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Nat Commun 2019;10:4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Soltanian A, Ghezelayagh Z, Mazidi Z, et al. Generation of functional human pancreatic organoids by transplants of embryonic stem cell derivatives in a 3D-printed tissue trapper. J Cell Physiol 2019;234:9564–76. [DOI] [PubMed] [Google Scholar]
- 135.Wassmer CH, Lebreton F, Bellofatto K, Bosco D, Berney T, Berishvili E. Generation of insulin-secreting organoids: a step toward engineering and transplanting the bioartificial pancreas. Transpl Int 2020;33:1577–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang D, Wang J, Bai L, et al. Long-term expansion of pancreatic islet organoids from resident procr(+) progenitors. Cell 2020;180:1198–1211.e19. [DOI] [PubMed] [Google Scholar]
- 137.Nalbach L, Roma LP, Schmitt BM, et al. Improvement of islet transplantation by the fusion of islet cells with functional blood vessels. EMBO Mol Med 2021;13:e12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tao T, Deng P, Wang Y, et al. Microengineered multi-organoid system from hiPSCs to recapitulate human liver-islet axis in normal and type 2 diabetes. Adv Sci (Weinh) 2022;9:e2103495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bauer S, Wennberg Huldt C, Kanebratt KP, et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: towards a novel human ex vivo type 2 diabetes model. Sci Rep 2017;7:14620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kawser Hossain M, Abdal Dayem A, Han J, et al. Recent advances in disease modeling and drug discovery for diabetes mellitus using induced pluripotent stem cells. Int J Mol Sci 2016;17:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Balboa D, Iworima DG, Kieffer TJ. Human pluripotent stem cells to model islet defects in diabetes. Front Endocrinol (Lausanne) 2021;12:642152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hindley CJ, Cordero-Espinoza L, Huch M. Organoids from adult liver and pancreas: stem cell biology and biomedical utility. Dev Biol 2016;420:251–61. [DOI] [PubMed] [Google Scholar]
- 143.Tsakmaki A, Fonseca Pedro P, Bewick GA. Diabetes through a 3D lens: organoid models. Diabetologia 2020;63:1093–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang X, Ma Z, Song E, Xu T. Islet organoid as a promising model for diabetes. Protein Cell 2021;13:239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gupta SK, Wesolowska-Andersen A, et al. NKX6.1 induced pluripotent stem cell reporter lines for isolation and analysis of functionally relevant neuronal and pancreas populations. Stem Cell Res 2018;29:220–31. [DOI] [PubMed] [Google Scholar]
- 146.Lee Y, Choi HY, Kwon A, et al. Generation of a PDX1-EGFP reporter human induced pluripotent stem cell line, KSCBi005-A-3, using the CRISPR/Cas9 system. Stem Cell Res 2019;41:101632. [DOI] [PubMed] [Google Scholar]
- 147.Lee NS, Rohan JG, Zitting M, et al. A novel dual-color reporter for identifying insulin-producing beta-cells and classifying heterogeneity of insulinoma cell lines. PLoS One 2012;7:e35521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Micallef SJ, Li X, Schiesser JV, et al. INS(GFP/w) human embryonic stem cells facilitate isolation of in vitro derived insulin-producing cells. Diabetologia 2012;55:694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leon-Quinto T, Jones J, Skoudy A, Burcin M, Soria B. In vitro directed differentiation of mouse embryonic stem cells into insulin-producing cells. Diabetologia 2004;47:1442–51. [DOI] [PubMed] [Google Scholar]
- 150.Wu Y, Chang T, Long Y, Huang H, Kandeel F, Yee JK. Using Gene Editing to Establish a Safeguard System for Pluripotent Stem-Cell-Based Therapies. iScience 2019;22:409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.An D, Wang LH, Ernst AU, et al. An atmosphere-breathing refillable Biphasic Device for cell replacement therapy. Adv Mater 2019;31:e1905135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang LH, Ernst AU, Flanders JA, et al. An inverse-breathing encapsulation system for cell delivery. Sci Adv 2021;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takahashi Y, Sekine K, Kin T, Takebe T, Taniguchi H. Self-condensation culture enables vascularization of tissue fragments for efficient therapeutic transplantation. Cell Rep 2018;23:1620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wassmer CH, Lebreton F, Bellofatto K, et al. Bio-Engineering of Pre-Vascularized Islet Organoids for the Treatment of Type 1 Diabetes. Transpl Int 2021;35:10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang LH, Ernst AU, An D, et al. A bioinspired scaffold for rapid oxygenation of cell encapsulation systems. Nat Commun 2021;12:5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang X, Brown NK, Wang B, et al. Local immunomodulatory strategies to prevent Allo-Rejection in transplantation of insulin-producing cells. Adv Sci (Weinh) 2021;8:e2003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Razavi M, Ren T, Zheng F, et al. Facilitating islet transplantation using a three-step approach with mesenchymal stem cells, encapsulation, and pulsed focused ultrasound. Stem Cell Res Ther 2020;11:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bochenek MA, Veiseh O, Vegas AJ, et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat Biomed Eng 2018;2:810–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang X, Moore DJ, Ketchum RJ, et al. Resolving the conundrum of islet transplantation by linking metabolic dysregulation, inflammation, and immune regulation. Endocr Rev 2008;29:603–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu W, Flanders JA, Wang LH, et al. A safe, fibrosis-mitigating, and scalable Encapsulation Device supports long-term function of insulin-producing cells. Small 2022;18:e2104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.An D, Chiu A, Flanders JA, et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc Natl Acad SciUSA 2018;115:E263–E72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Alagpulinsa DA, Cao JJL, Driscoll RK, et al. Alginate-microencapsulation of human stem cell-derived beta cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. Am J Transplant 2019;19:1930–40. [DOI] [PubMed] [Google Scholar]
- 163.Boettler T, Schneider D, Cheng Y, et al. Pancreatic tissue transplanted in theraCyte Encapsulation Devices is protected and prevents hyperglycemia in a mouse model of immune-mediated diabetes. Cell Transplant 2016;25:609–14. [DOI] [PubMed] [Google Scholar]
- 164.Yoshihara E, O’Connor C, Gasser E, et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature 2020;586:606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Castro-Gutierrez R, Alkanani A, Mathews CE, Michels A, Russ HA. Protecting stem cell derived pancreatic beta-like cells from diabetogenic T cell recognition. Front Endocrinol (Lausanne) 2021;12:707881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Koga K, Wang B, Kaneko S. Current status and future perspectives of HLA-edited induced pluripotent stem cells. Inflamm Regen 2020;40:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu H, Wang B, Ono M, et al. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with enhanced immune compatibility. Cell Stem Cell 2019;24:566–578.e7. [DOI] [PubMed] [Google Scholar]
- 168.Wuputra K, Ku CC, Wu DC, Lin YC, Saito S, Yokoyama KK. Prevention of tumor risk associated with the reprogramming of human pluripotent stem cells. J Exp Clin Cancer Res 2020;39:100. [DOI] [PMC free article] [PubMed] [Google Scholar]


