Abstract
Anopheles stephensi invasion in Ethiopia poses a risk of increased malaria disease burden in the region. Thus, understanding the insecticide resistance profile and population structure of the recently detected An. stephensi population in Fiq, Ethiopia, is critical to inform vector control to stop the spread of this invasive malaria species in the country. Following entomological surveillance for An. stephensi in Fiq, Somali region, Ethiopia, we confirmed the presence of An. stephensi morphologically and molecularly in Fiq. Characterization of larval habitats and insecticide susceptibility tests revealed that Fiq An. stephensi is most often found in artificial containers and is resistant to most adult insecticides tested (organophosphates, carbamates, pyrethroids) except for pirimiphos-methyl and PBO-pyrethroids. However, the immature larval stage was susceptible to temephos. Further comparative genomic analyses with previous An. stephensi populations from Ethiopia using 1704 biallelic SNPs revealed genetic relatedness between Fiq An. stephensi and east-central Ethiopia An. stephensi populations, particularly Jigjiga An. stephensi. Our findings of the insecticide resistance profile, coupled with the likely source population of Fiq An. stephensi, can inform vector control strategies against this malaria vector in Fiq and Jigjiga to limit further spread out of these two locations to other parts of the country and continent.
Subject terms: Entomology, Population genetics, Comparative genomics
Introduction
Malaria is a major global health problem, with an estimated 247 million cases and 619,000 deaths reported in 20211. In Ethiopia, despite past successes in reducing the malaria burden due to the use of indoor residual spraying (IRS) and long-lasting insecticidal nets (LLINs) in malaria control and prevention strategies2, it remains a public health concern with an estimated 2 million cases and over 8000 deaths reported in 20211. The emergence of An. stephensi, a malaria vector commonly found in South Asia and parts of the Arabian Peninsula, in the Horn of Africa (HoA)3–5, also threatens any gains against malaria in Ethiopia6. Indeed, a recent An. stephensi-mediated malaria outbreak was reported in Dire Dawa, an urban hub in eastern Ethiopia7. Since the detection of An. stephensi in the HoA and subsequently, in Sudan (2019)8, Nigeria (2020)9, Yemen (2021)10,11, Kenya (2022)12, and Ghana (2023)13, the World Health Organization (WHO) has launched an initiative14 and released an updated vector alert in 202215 to recommend increased surveillance and research to determine the range of the invasion in order to stop the further spread of An. stephensi in Africa. In Ethiopia, An. stephensi was first detected in Kebridehar, Somali region, in 20164 and has subsequently been confirmed to be broadly distributed in eastern Ethiopia16. However, it was first detected in Fiq, Somali region in June 202117. Malaria cases in the Somali region of Ethiopia are relatively low compared to other regions of Ethiopia18. However, it remains understudied, and more research on malaria parasites and their vectors is needed.
Studies of the breeding sites and the ecology of mosquitoes are very crucial to inform mosquito control strategies such as mosquito larvicidal (temephos) and environmental control (larval habitat removal). Also, the WHO recommends larval source management as one of the immediate control strategies against An. stephensi in urban and peri-urban settings of invaded regions15. When larval source management or reduction is not feasible, such as a household or town water storage, larviciding may be considered. However, this vector control method can be costly when treating large numbers of larval habitats19. Thus, an alternative cost-effective approach is to target specific habitats that can produce large numbers of adult mosquitoes19. Thus, determining the Fiq An. stephensi susceptibility to larvicide such as temephos can help inform the decision making for this control method against the invasive malaria vector in Fiq town.
Moreover, genomic analyses can further inform control strategies against the newly detected Fiq An. stephensi. Specifically, assessing the genetic diversity and population structure of Fiq An. stephensi population in comparison to an established one in the region can give insights into its demographic history, dispersal patterns, and potential source populations.
Hence, we conducted an entomological survey after a year of the first detection of An. stephensi in Fiq town, Somali region, Ethiopia to first characterize larval habitats for An. stephensi and determine their insecticide susceptibility status, including susceptibility to the larvicide, temephos. After identifying the morphology, we confirmed it molecularly and used genomic approaches to analyze the demographic history and population structure of An. stephensi in Fiq. We compared the population structure with the previously detected An. stephensi populations in eastern Ethiopia to determine the extent of establishment in Fiq. We further assessed their genetic connectivity to those populations to uncover their potential source populations within the region.
Material and methods
Study setting
Fiq is a small town located in the Somali region of Ethiopia. It is situated at an altitude of 1200 m and 195 km west of Jigjiga, the capital city of the Somali Region. The town features sporadic mountains and small house constructions. It is common to see water stored in pits for building bricks for house construction.
Sample collection
Entomological survey was carried out in Fiq town during the rainy season from May to June 2022, by Jigjiga University. Adult mosquitoes were collected indoors and outdoors using pyrethrum spray collections (PSC) and CDC light traps (CDC LT), respectively, and Prokopack aspiration from animal shelters. Sampling of mosquito larvae was conducted from houses with water storage containers, particularly, cisterns and plastic sheet water storage. During each survey, a habitat was first visually inspected for the presence of mosquito larvae, and then twenty samples were taken with a soup ladle (350 ml capacity) from each breeding habitat (see Supplementary Fig. S1 online). The Anopheles larvae were separated from the Culicine larvae based on their position to the water surface. Collected Anopheles larvae were reared to the adult at a field laboratory for morphological species identification (see Supplementary Fig. S2 online).
Mosquito identification
Adult Anopheles were morphologically identified at the species level using the Afrotropical mosquitoes key20 (see Supplementary Fig. S2 online). Polymerase chain reaction (PCR) assays were conducted on a subset of An. stephensi specimens identified by morphology. These assays targeted portions of the mitochondrial cytochrome oxidase subunit I (COI) and the nuclear internal transcribed spacer 2 (ITS2) loci for molecular identification, as previously reported4. For further confirmation of species identification, we performed phylogenetic analysis with the generated COI sequences and those of previously detected An. stephensi from Ethiopia retrieved from GenBank (Accession# OK663483, OK663480, OK663484, OK663481, OK663479, OK663482)21. These accession numbers represent unique COI haplotypes of An. stephensi previously identified across eastern Ethiopia21. Phylogenetic relationships were inferred using a maximum likelihood approach with RAxML GUI22 using the GTR model of nucleotide substitutions, gamma model for rate of heterogeneity (GTRGAMMA option), and one thousand replicates in one run for bootstrap analysis. Anopheles maculatus was designated as an outgroup. The tree with the highest log likelihood was visualized and formatted in FigTree23.
WHO insecticide susceptibility tube tests
Insecticide susceptibility tests were conducted on adult female An. stephensi reared from wild larvae (see Supplementary Fig. S2 online) following standard procedures24. One hundred mosquitoes from each population were tested for each insecticide using the diagnostic concentration, and 50 mosquitoes were used for controls. The insecticides used were 0.1% bendiocarb, 0.25% pirimiphos-methyl, 0.05% alpha-cypermethrin, 0.05% deltamethrin, 0.75% permethrin, and 0.15% cyfluthrin. Based on the WHO mortality criteria, resistance was determined as follows: 98–100% mortality indicates susceptibility, 90–97% mortality indicates possible resistance (requires further investigation), and less than 90% mortality confirms resistance 19.
Piperonyl butoxide synergist assays
Piperonyl butoxide (PBO) synergist assays were conducted on An. stephensi against two pyrethroids (deltamethrin and permethrin). The synergist assays were conducted by pre-exposing mosquitoes to a 4% PBO paper for 60 min. Mosquitoes were then transferred to tubes with the pyrethroid of interest for 60 min and the susceptibility was determined based on the WHO mortality criteria24 described above.
Temephos susceptibility tests
Larval susceptibility tests were performed to assess the sensitivity of An. stephensi larvae to temephos, an organophosphate larvicide. According to WHO procedure25, conventional techniques were employed for the testing, and 312.5 mg/l, 62.5 mg/l, 12.5 mg/l, and 2.5 mg/l concentrations were utilized to generate a final concentration of 1.25 mg/l, 0.25 mg/l, 0.05 mg/l, and 0.01 mg/l when 1 ml of each concentration was added to 249 ml of normal water. To detect resistance, an estimated diagnostic dosage of 0.25 mg/l was employed26, and larvae mortality data were interpreted following the same WHO mortality criteria used for adult mosquitoes19. For each larvicide concentration, four duplicates of 25 larvae were employed, with two replicates serving as controls. To get 100 larvae examined per dosage, four beakers were utilized per dose. After 24 h all larvae without movement on the water surface were considered as dead.
Target-site insecticide resistance loci analysis
To analyze phenotypic and genotypic associations in observed pyrethroid-resistant An. stephensi, we sequenced the pyrethroid target site in the voltage-gated sodium channel (vgsc) gene and downstream intron to genotype any knockdown resistance mutation (kdr). Analyses were conducted using previously published protocols27,28. To genotype any kdr mutations, sequences were queried to NCBI BLAST to confirm correct kdr locus, then aligned to reference sequences from Samake et al.27 using CodonCode Aligner version 8 (CodonCode Corp., Centerville, MA, USA). We then generated an updated Ethiopian An. stephensi kdr mutation phylogenetic tree with kdr mutation sequences from Samake et al.27 also including the only non-African An. stephensi kdr mutation sequence available in Genbank (Accession# JF304952)28. Phylogenetic relationships were inferred using a maximum likelihood approach with RAxML GUI18 using the GTR model of nucleotide substitutions, gamma model for rate of heterogeneity (GTRGAMMA option), and one thousand replicates in one run for bootstrap analysis. The tree with the highest log likelihood was visualized and formatted in FigTree23.
Nuclear population structure and genetic diversity
To assess nuclear population structure and potential source populations, we first generated double digest restriction-site associated DNA (ddRAD) sequences of Fiq An. stephensi in addition to previously published raw ddRAD sequences of Ethiopian An. stephensi 29. Genomic DNA was extracted from adult An. stephensi mosquitoes using Qiagen DNeasy Blood and Tissue kit (Qiagen). DNA quality was assessed on a 1% agarose gel to ensure at least 10 kilobase DNA fragments and quantified using Nanodrop One Spectrophotometer (Thermo Fisher Scientific Inc.) to ensure a minimum concentration of 20 ng/μl. ddRAD-seq library preparation followed protocols outlined in Lavretsky et al.30 (also see Samake et al.29). In short, each genomic DNA was enzymatically fragmented using SbfI and EcoRI restriction enzymes and ligated with Illumina TruSeq compatible barcodes for demultiplexing purposes. Libraries were quantified using Qubit dsDNA BR Assay Kit (ThermoFisher Scientific, MA, USA), pooled in equimolar amounts, and sequenced using single-end chemistry sequencing on an Illumina HiSeq X at Novogene (Novogene CO., Ltd., Sacramento, CA, USA; see detailed methods in Supplementary Document S1 online). We then retrieved raw sequence reads of previously reported An. stephensi populations from 10 different sites across eastern Ethiopia (n = 183, BioProject PRJNA888109, Samake et al.29) to generate a combined bi-allelic SNPs dataset. Raw Illumina reads were demultiplexed, processed and SNP genotyped using the computational pipeline described in Lavretsky et al.31 (also see Samake et al.29). We used Trimmomatic32 to trim or discard poor-quality sequences using a Phred score of ≥ 30 to ensure only high-quality sequences were retained. We then used the Burrows-Wheeler Aligner33 (bwa) to align the remaining quality reads to the An. stephensi reference genome (Accession PRJNA661063; Chakraborty et al.34). Samples were sorted and indexed in Samtools and genotyped using the ‘mpileup’ function in BCFtools35.
We then identified variation among samples with a principal component analysis (PCA) using the–pca function in PLINK v.1.9 36 and visualized with the R package ggplot237. Next, individual maximum-likelihood population assignment probabilities were attained across samples using ADMIXTURE v.1.338. Each ADMIXTURE analysis was run with a tenfold cross validation (CV) and with a quasi-Newton algorithm to accelerate convergence39. To limit possible stochastic effects, each analysis was based on 1,000 bootstraps for each population K value of 1–10. The block relaxation algorithm for point estimation was used for each analysis and terminated once the log-likelihood of the point estimation increased by < 0.0001. The optimum population value was based on the average of CV-errors across the analyses per K value. ADMIXTURE assignment probability outputs were visualized using the R package ggplot237. Additionally, nucleotide diversity (π), counts of segregating SNPs (S), Tajima’s D, and pairwise estimates of fixation index (Fst) by site were calculated in the R package PopGenome40.
Genetic network
To further access potential source populations of the Fiq An. stephensi population, we performed a network analysis with the combined biallelic SNPs dataset from Fiq sequences (n = 20) and Genbank retrieved An. stephensi sequences from 10 different sites across eastern Ethiopia (n = 183, Samake et al.29). We used EDENetworks41, which allows network analyses based on genetic distance matrices without a prior assumption. The network consists of nodes representing populations connected by edges/links weighted by their Fst based Reynolds’ genetic distances (D)42 which provide the strength of connectivity between pairs of populations41. The thicker the edge/link, the stronger the genetic connectivity between the two populations. Moreover, node size is proportional to the cumulative weighted edge linkages for each population. Thus, the larger the node the higher the connectivity hub or sink. Statistical confidence of the nodes was evaluated using 1000 bootstrap replicates. Nodes that appear in the top 5 and top 1 lists of betweenness centrality (BC) values (number of shortest genetic paths passing through a node) can be considered as statistically significant43.
Results
A total of 221 adult mosquitoes were collected using CDC LT, PSC, and Prokopack from animal shelters. Of these, 219 adults Culex spp, and only two adult An. stephensi were collected using Prokopack aspiration (see Supplementary Table S1 online). No other Anopheles mosquitoes were collected using PSC and CDC LT.
Anopheles stephensi larval habitats
Thirty-one mosquito potential breeding sites were inspected. Twenty-six containers including eight plastic ones, fifteen cisterns, and three barrels were positive for mosquito larvae (see Supplementary Table S2 online). There were no mosquito larvae recovered from five containers. A total of 4103 larvae of mosquito were collected from these different breeding sites. Of these, 3713 Anopheles and 390 Culex were identified (see Supplementary Table S2 online). All of the Anopheles mosquitoes were reared to adults successfully and morphologically identified as An. stephensi.
Insecticide susceptibility and synergist assays
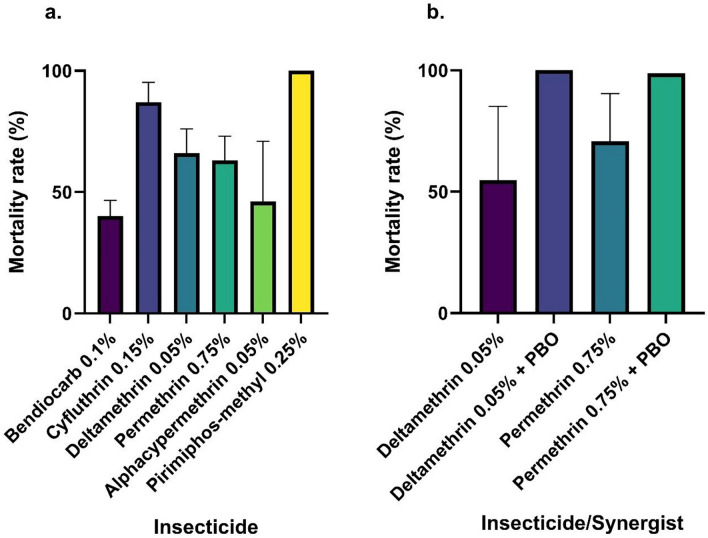
According to the WHO bioassay procedure, 1200 non-fed female An. stephensi were tested with various insecticide classes and PBO-pyrethroids. Based on the WHO mortality criteria, Fiq An. stephensi revealed resistance to all insecticides tested, except for pirimiphos-methyl which resulted in 100% mortality (Fig. 1a; also see Supplementary Table S3 online). Pre-exposure to PBO restored complete sensitivity to deltamethrin and permethrin in the synergist tests (Fig. 1b; also see Supplementary Table S3 online).
Fig. 1.
Mortality rate of An. stephensi exposed to different insecticides in Fiq, Ethiopia. Bars represent mean values (with 95% CIs) (a) Diagnostic dose assays. (b) Synergist assays.
Temephos susceptibility
The Temephos susceptibility test against Fiq An. stephensi larvae revealed that concentrations of 1.25 mg/l and 0.25 mg/l killed 100% of the An. stephensi larvae after 24 h (Table 1). However, mortality rates for the rest of the concentrations, such as 0.05 mg/l and 0.01 mg/l, were 94 (95% CI [79, 100]) and 16 (95% CI [4, 28]) percent, respectively, below the WHO mortality criteria of greater than 98 percent for susceptibility (Table 1).
Table 1.
Mortality rate of An. stephensi larvae against WHO standard concentrations of Temephos.
| Concentration | Final concentration | Mortality rate (%) | Mortality rate (%) mean [95% CI] | |||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | |||
| 312.5 mg/l | 1.25 mg/l | 100 | 100 | 100 | 100 | 100 |
| 62.5 mg/l | 0.25 mg/l | 100 | 100 | 100 | 100 | 100 |
| 12.5 mg/l | 0.05 mg/l | 100 | 96 | 80 | 100 | 94 [79, 100] |
| 2.5 mg/l | 0.01 mg/l | 12 | 8 | 20 | 24 | 16 [4, 28] |
NB: A total of 1000 adult An. stephensi identified and no other Anopheles species encountered to provide proof that the susceptibility test was done on An. stephensi larvae. R1-R4 are replicates.
Molecular identification
A subset of 154 morphologically identified An. stephensi collected from the various sites in Fiq were preserved with silica gel and sent to Baylor University for molecular and genomics analyses. Genomic DNA was extracted from 20 of those specimens for a comparative sample size with previous An. stephensi SNPs dataset from eastern Ethiopia29. The 20 samples were successfully confirmed as An. stephensi based on the presence of the characteristic band in the ITS2 endpoint assay (see Supplementary Fig. S3 online), and phylogenetic analysis of the cytochrome oxidase subunit I (COI) mitochondrial marker further confirmed An. stephensi. All the analyzed Fiq An. stephensi COI sequences clustered with the most prevalent Ethiopian An. stephensi COI haplotype (Hap2)21 (see Supplementary Fig. S4 online).
Kdr mutation population frequency
Of the 20 An. stephensi samples analyzed for pyrethroid target site phenotypic-genotypic association in observed resistant An. stephensi, one (5%) carried the kdr L1014F mutation with a heterozygote allele (see Supplementary Table S5 online). The Kdr L1014S was not observed. Phylogenetic analysis confirmed the Fiq kdr L1014F mutation, as the analyzed sequence clustered with previously published An. stephensi kdr L1014F mutation sequences from Ethiopia27 and India28 (bootstrap 100) (Fig. 2).
Fig. 2.

Phylogenetic tree of kdr L1014F mutation in Fiq An. stephensi and available global An. stephensi kdr mutation sequence. The evolutionary history was inferred by using the Maximum Likelihood method based on the General Time Reversible model. The tree with final ML optimization likelihood (− 146.34) and bootstrap values > 70 are shown.
Nuclear genetic diversity and population structure
For a comparative analysis between the Fiq studied population and the previously reported An. stephensi populations in 10 different sites across eastern Ethiopia29, genetic diversity and population structure assessments were based on a combined SNPs dataset of 1704 independent bi-allelic SNPs after filtering for linkage disequilibrium and non-biallelic SNPs. From the combined Ethiopian An. stephensi bi-allelic SNPs analyzed, the lowest nucleotide diversity was observed in Godey (π = 0.1979) in southeastern Ethiopia, and the highest nucleotide diversity was observed in Awash Sebat Kilo (π = 0.2300) in northeastern Ethiopia (see Supplementary Table S4 online). Fiq had the second lowest nucleotide diversity (π = 0.1986) (see Supplementary Table S4 online). Anopheles stephensi populations from southeastern Ethiopia and Fiq were also found to have the highest Tajima’s D values, 1.36, 1.48, 1.19, and 1.29 for Degehabur, Kebridehar, Godey, and Fiq, respectively, indicating a lack of rare variants relative to neutral expectations44 (see Supplementary Table S4 online).
Assessing population structure using the combined An. stephensi independent bi-allelic SNPs dataset and based on principal component analysis (PCA), we identified three semi-discrete genetic clusters (Fig. 3b and c). Plotting the first two principal components grouped An. stephensi populations regionally with Fiq An. stephensi mostly clustering with east central Ethiopia An. stephensi populations (Fig. 3). The ADMIXTURE analysis based on an optimum population K model 5 more specifically identified five main genetic similarities across An. stephensi populations that include (1) Erer Gota, Dire Dawa, and Jigjiga, (2) Fiq, (3) Bati and Semera, (4) Gewane and Awash Sebat Kilo, and (5) Degehabur, Kebridehar, and Godey (see Supplementary Fig. S5 online). From these genetic clusters, Fiq An. stephensi displayed the lowest admixture proportions as it is composed of a single ancestral lineage that is predominantly observed in Jigjiga admixed An. stephensi population (see Supplementary Fig. S5 online). The Fst pairwise estimates also showed Fiq An. stephensi being less differentiated from Jigjiga An. stephensi from east central Ethiopia populations (Fst = 0.07) and more differentiated from Bati An. stephensi from northeastern Ethiopia populations (Fst = 0.14) (see Supplementary Fig. S6 online).
Fig. 3.
(a) Anopheles stephensi sampling locations in eastern Ethiopia. Fiq data was generated in this present study. Data from all other sites were previously generated (Samake et al.29, 2023). Map was created with mapchart.net. (b) Principal component analysis (PCA) of individual An. stephensi population in eastern Ethiopia. The amounts of variation explained by each principal component (PC 1 on the x-axis, PC 2 on the y-axis) are given in percentages. The sample sites are Awash Sebat Kilo (AW18), Bati (BA18), Dire Dawa (DD18), Degehabur (DE18), Erer Gota (ER18), Fiq (FI22), Godey, (GD18), Gewane (GW18), Jigjiga (JJ18), Kebriderar (KB18), Semera (SM18). (c) Scatterplot of subgroup variation based on principal component analysis (PCA). The amounts of variation explained by each principal component (PC 1 on the x-axis, PC 2 on the y-axis) are given in percentages. Anopheles stephensi population subgroups are color-coded as described in the legend.
Genetic network
Network reconstruction was based on the same combined 1704 independent bi-allelic SNP dataset. Out of the ten Ethiopian An. stephensi populations analyzed, Fiq An. stephensi was found to be connected to two populations, Dire Dawa and Jigjiga, that were recovered as statistically significant nodes based on the bootstrapping test (Fig. 4, also see Supplementary Fig. S7 online). However, the network revealed higher genetic connectivity between Fiq and Jigjiga than Dire Dawa (Fig. 4a). This finding suggests Jigjiga is the potential source population for the studied Fiq An. stephensi.
Fig. 4.
(a) Genetic network of An. stephensi populations in eastern Ethiopia. Network nodes represent populations/hubs and links represent weighted genetic distances/genetic connectivities. The figure is produced by EDENetworks based on a genotype autosomal ddRAD-seq loci matrix by applying a single realization of bootstrapping with 0.85 percentage of nodes at each location and thresholded at 0.12. The colors and sizes of the links represent the strength of genetic connectivity from lowest (white) to highest (black). The colors and sizes of the nodes represent the cumulative weighted links from lowest (white) to highest (black). (b) Map showing Fiq road connections to other sites. The map was generated using ArcGIS Pro v 3.1 (Environmental Systems Research Institute (ESRI), Redlands, CA, USA).
Discussion
We report the presence of An. stephensi in abundance during a rainy season (May–June 2022) in Fiq, Somali region, Ethiopia. From the more than three thousand and five hundred Anopheles larvae collected, all were reared and morphologically identified as An. stephensi. Molecular identification of a subset for further molecular analyses also confirms the studied samples as An. stephensi. All An. stephensi larval habitats identified were artificial breeding sites such as plastic sheet water storages, covered and uncovered cisterns, and barrels which are consistent with other An. stephensi larval habitats reported in eastern Ethiopia45. The fact that no other Anopheles larvae were collected suggests that An. stephensi could persist during dry seasons15 in Fiq, characteristics typically distinct from An. arabiensis, the primary malaria vector in Ethiopia46,47. However, in Kenya, An. stephensi larvae were found in both artificial containers and a riverbed setting48, emphasizing the potential diversity of larval habitats of this invasive An. stephensi that is worth noting for future entomological surveillance of this invasive malaria vector in Ethiopia and Africa.
The newly detected Fiq An. stephensi were found to be resistant to bendiocarb (carbamate), cyfluthrin, deltamethrin, permethrin, and alphacypermethrin (pyrethroids), but susceptible to pirimiphos-methyl (organophosphate) according to the WHO criteria of 98—100% mortality indicating susceptibility24. However, restored susceptibility to deltamethrin and permethrin was observed following the synergist bioassay with piperonyl butoxide (PBO), which is a detoxifying enzyme inhibitor that bolsters the effects of pyrethroid insecticides, thus indicating a metabolic insecticide resistance mechanism49,50. This finding is consistent with previous reports of insecticide resistance among the Ethiopian An. stephensi populations51 and suggests that PBO plus pyrethroids may be more effective against adult An. stephensi in Fiq. Additionally, Fiq An. stephensi larvae were found to be susceptible to the diagnostic dose of temephos (0.25 mg/l) with a 100% mortality rate (Table 1), which is consistent with recent larvicide studies conducted on three other An. stephensi populations in eastern Ethiopia52,53. Thus, temephos may be an effective larvicide to control An. stephensi larvae in Fiq.
Also, our molecular analysis of the pyrethroid target site knockdown gene revealed the presence of the kdr mutation in a single pyrethroid-resistant Fiq sample (Fig. 2). The low kdr mutation frequency among the Fiq pyrethroid-resistant studied An. stephensi is consistent with previous low kdr mutation frequency in the Ethiopian An. stephensi from eastern Ethiopia27. This finding confirms that kdr mutations may not be a primary mechanism of pyrethroid resistance in the invasive An. stephensi27. Thus, the metabolic resistance mechanism again seems more relevant, as revealed by the positive synergist assays in pyrethroid-resistant An. stephensi in this study (Fig. 1), and previously reported in Balkew et al.54. However, the analysis of gene expression at metabolic resistance loci is needed to fully understand the underlying molecular mechanisms of insecticide resistance development in the invasive An. stephensi.
Furthermore, comparative population structure analyses of the newly detected An. stephensi with other Ethiopian An. stephensi revealed high genetic similarities between Fiq An. stephensi and east central An. stephensi (Fig. 3b and 3c) and the recentness of Fiq An. stephensi compared to them. The recency of Fiq An. stephensi was further revealed by their low genetic diversity and high Tajima’s D compared to the east-central Ethiopia An. stephensi populations. Thus, these findings suggest that Fiq An. stephensi population is from a recent founder event, such as a bottleneck event from east central Ethiopia An. stephensi populations. Further population pairwise Fst and genetic network analyses pinpoint Jigjiga as the potential source of the founder event in Fiq. Specifically, Fiq An. stephensi were less genetically differentiated from Jigjiga An. stephensi than the other analyzed Ethiopian An. stephensi and shared higher genetic connectivity with Jigjiga than other sites (Fig. 4a). Although Fiq is located 195 km away from Jigjiga, the genetic connectivity observed could be explained by the fact that they are connected by a major road (Fig. 4b). The same major road also connects both Jigjiga and Fiq to Dire Dawa, the site of a recent An. stephensi-mediated malaria outbreak7. Thus, our finding highlights the potential role of roads, perhaps through human and/or good transportation, in the expansion of An. stephensi into Fiq. The role that transports routes (marines, roads, etc.) play in invasive species incursion into new geographic areas has been well documented55–58. Thus, this potential mechanism of An. stephensi dispersal within the region warrants further investigation.
Conclusion
The present study revealed the high prevalence of the invasive An. stephensi in Fiq, its larval habitats, insecticide resistance status for both adult and immature stages, genetic diversity, population structure, and potential source populations. Our results showed that Fiq An. stephensi population is susceptible to pirimiphos-methyl, PBO-pyrethroids, and temephos.Q1 Thus, these insecticides may be effectively used in control strategies against this invasive malaria vector in Fiq. We also found that the Fiq An. stephensi population shares genetic connectivity with two major An. stephensi hubs (i.e., Jigjiga and Dire Dawa) in eastern Ethiopia, with a stronger connection to Jigjiga. Thus, heightened vector control in those areas could help prevent further An. stephensi incursions into Fiq and beyond. Overall, this study provides a comprehensive approach to investigate a recent An. stephensi expansion into a new geographical area to determine the extent of establishment, assess effectiveness of insecticides, and identify potential source populations to prevent further spread.
Supplementary Information
Acknowledgements
We are thankful to the field teams who collected and organized the mosquitoes. We thank Dr. Philip Lavretsky (University of Texas at El Paso) for the Illumina barcodes. We also thank Ms. Madison Follis, Ms. Amelia Wickham, and Ms. Nidhi Kotha (Baylor University) for their lab support and Mr. Joshua Been (Baylor Library Data and Digital Scholarship) for his support with the GIS road map.
Author contributions
JNS, SY, and TEC conceptualized the study. SY, MA, TEC, and SZ organized field collections. SY conducted the bioassays. JNS and TEC collected and analyzed the molecular and genomic data. JNS, SY, SZ, and TEC contributed to the writing of the manuscript. All authors reviewed and approved the final manuscript.
Funding
This research was funded by the Centers for Disease Control and Prevention. SZ was funded by the U.S. President’s Malaria Initiative. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Data availability
DNA sequences: Bioproject PRJNA1042829 and Genbank accessions SAMN38322421 – SAMN38322440.
Declarations
Competing interests
The author(s) declare no competing interests.
Disclaimer
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These author contributed equally: Jeanne N. Samake and Solomon Yared.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78072-4.
References
- 1.WHO. World malaria report 2022, https://www.who.int/publications/i/item/9789240040496 (2022).
- 2.Kenea, O. et al. Impact of combining indoor residual spraying and long-lasting insecticidal nets on Anopheles arabiensis in Ethiopia: results from a cluster randomized controlled trial. Malar. J.18, 182. 10.1186/s12936-019-2811-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali, S., Samake, J. N., Spear, J. & Carter, T. E. Morphological identification and genetic characterization of Anopheles stephensi in Somaliland. Parasit. Vectors15, 247. 10.1186/s13071-022-05339-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter, T. E. et al. First detection of Anopheles stephensi Liston, 1901 (Diptera: culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop.188, 180–186. 10.1016/j.actatropica.2018.09.001 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Faulde, M. K., Rueda, L. M. & Khaireh, B. A. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti Horn of Africa. Acta Trop.139, 39–43. 10.1016/j.actatropica.2014.06.016 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Hamlet, A. et al. The potential impact of Anopheles stephensi establishment on the transmission of Plasmodium falciparum in Ethiopia and prospective control measures. BMC Med.20, 135. 10.1186/s12916-022-02324-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emiru, T. et al. Evidence for a role of Anopheles stephensi in the spread of drug and diagnosis-resistant malaria in Africa. Nat. Med.10.1038/s41591-023-02641-9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed, A., Khogali, R., Elnour, M.-A.B., Nakao, R. & Salim, B. Emergence of the invasive malaria vector Anopheles stephensi in Khartoum State, Central Sudan. Parasit. Vectors14, 511. 10.1186/s13071-021-05026-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Malaria Threat Map, https://apps.who.int/malaria/maps/threats/?theme=invasive&mapType=invasive (2023).
- 10.Assada, M. et al. Molecular confirmation of Anopheles stephensi in the Al Hudaydah Governorate, Yemen, 2021–2022. bioRxiv, 2024.2002.2022.577782 (2024). 10.1101/2024.02.22.577782 [DOI] [PMC free article] [PubMed]
- 11.Allan, R. et al. Confirmation of the presence of Anopheles stephensi among internally displaced people’s camps and host communities in Aden city, Yemen. Malar. J.22, 1–8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochomo, E. O. et al. Detection of Anopheles stephensi Mosquitoes by Molecular Surveillance, Kenya. Emerg. Infect. Dis.29, 2498 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afrane, Y. A. et al. First detection of Anopheles stephensi in Ghana using molecular surveillance. Biorxiv, 2023.2012. 2001.569589 (2023).
- 14.WHO. WHO launches new initiative to stop the spread of invasive malaria vector in Africa, https://www.who.int/news-room/feature-stories/detail/mosquito-on-the-move (2022).
- 15.WHO. Vector alert: Anopheles stephensi invasion and spread in Africa and Sri Lanka, https://iris.who.int/bitstream/handle/10665/365710/9789240067714-eng.pdf?sequence=1 (2022).
- 16.Balkew, M. et al. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit. Vectors10.1186/s13071-020-3904-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PMI. PMI VectorLink Ethiopia Project final entomology report April 2021-March 2022, https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2023/02/Entomological-Monitoring-Report-Ethiopia-2022.pdf (2022).
- 18.Ethiopia Malaria Profile. 5 (U.S. President’s Malaria Initiative, 2023).
- 19.WHO. (2009).
- 20.Coetzee, M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar. J.19, 70. 10.1186/s12936-020-3144-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter, T. E. et al. Genetic diversity of Anopheles stephensi in Ethiopia provides insight into patterns of spread. Parasit. Vectors14, 602. 10.1186/s13071-021-05097-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edler, D., Klein, J., Antonelli, A. & Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol.12, 373–377. 10.1111/2041-210X.13512 (2021). [Google Scholar]
- 23.Rambaut, A. FigTree. Tree figure drawing tool. http://tree.bio.ed.ac.uk/software/figtree/ (2009).
- 24.WHO. Standard operating procedure for testing insecticide susceptibility of adult mosquitoes in WHO tube tests, https://iris.who.int/bitstream/handle/10665/352316/9789240043831-eng.pdf?sequence=1 (2022).
- 25.WHO. Guidelines for laboratory and field testing of mosquito larvicides, https://iris.who.int/bitstream/handle/10665/69101/WHO_CDS_WHOPES_GCDPP_2005.13.pdf?sequence=1 (2005).
- 26.WHO. Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides, https://iris.who.int/handle/10665/69615 (1981).
- 27.Samake, J. N. et al. Detection and population genetic analysis of kdr L1014F variant in eastern Ethiopian Anopheles stephensi. Infect. Genet. Evolut.99, 105235. 10.1016/j.meegid.2022.105235 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh, O. P., Dykes, C. L., Lather, M., Agrawal, O. P. & Adak, T. Knockdown resistance (kdr)-like mutations in the voltage-gated sodium channel of a malaria vector Anopheles stephensi and PCR assays for their detection. Malar. J.10, 59. 10.1186/1475-2875-10-59 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samake, J. N. et al. Population genomic analyses reveal population structure and major hubs of invasive Anopheles stephensi in the Horn of Africa. Mol. Ecol.32, 5695–5708. 10.1111/mec.17136 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Lavretsky, P. et al. Speciation genomics and a role for the Z chromosome in the early stages of divergence between Mexican ducks and mallards. Mol. Ecol.24, 5364–5378. 10.1111/mec.13402 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Lavretsky, P. et al. Assessing changes in genomic divergence following a century of human-mediated secondary contact among wild and captive-bred ducks. Mol. Ecol.29, 578–595. 10.1111/mec.15343 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics30, 2114–2120. 10.1093/bioinformatics/btu170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, H. & Durbin, R. Inference of human population history from individual whole-genome sequences. Nature475, 493–496. 10.1038/nature10231 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakraborty, M. et al. Author correction: Hidden genomic features of an invasive malaria vector, Anopheles stephensi, revealed by a chromosome-level genome assembly. BMC Biol.20, 96. 10.1186/s12915-022-01314-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics27, 2987–2993. 10.1093/bioinformatics/btr509 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purcell, S. et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet.81, 559–575. 10.1086/519795 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wickham, H. ggplot2: Elegant graphics for data analysis (Springer-Verlag, 2016). [Google Scholar]
- 38.Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome. Res.19, 1655–1664. 10.1101/gr.094052.109 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou, H., Alexander, D. & Lange, K. A quasi-Newton acceleration for high-dimensional optimization algorithms. Stat. Comput.21, 261–273. 10.1007/s11222-009-9166-3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfeifer, B., Wittelsbürger, U., Ramos-Onsins, S. E. & Lercher, M. J. PopGenome: An efficient Swiss army knife for population genomic analyses in R. Mol. Biol. Evol.31, 1929–1936. 10.1093/molbev/msu136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kivelä, M., Arnaud-Haond, S. & Saramäki, J. EDENetworks: A user-friendly software to build and analyse networks in biogeography, ecology and population genetics. Mol. Ecol. Resour.15, 117–122. 10.1111/1755-0998.12290 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Reynolds, J., Weir, B. S. & Cockerham, C. C. Estimation of the coancestry coefficient: basis for a short-term genetic distance. Genetics105, 767–779. 10.1093/genetics/105.3.767 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kivelä, M. et al. Multilayer networks. J. Complex Netw.2, 203–271. 10.1093/comnet/cnu016 (2014). [Google Scholar]
- 44.Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics123, 585–595. 10.1093/genetics/123.3.585 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balkew, M. et al. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit. Vectors13, 35. 10.1186/s13071-020-3904-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenea, O., Balkew, M. & Gebre-Michael, T. Environmental factors associated with larval habitats of anopheline mosquitoes (Diptera: Culicidae) in irrigation and major drainage areas in the middle course of the Rift Valley, central Ethiopia. J. Vector Borne Dis.48, 85–92 (2011). [PubMed] [Google Scholar]
- 47.Adugna, F., Wale, M. & Nibret, E. Review of Anopheles mosquito species, abundance, and distribution in Ethiopia. J. Trop. Med.2021, 1–7. 10.1155/2021/6726622 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ochomo, E. O. et al. Molecular surveillance leads to the first detection of Anopheles stephensi in Kenya (Research Square Platform LLC, 2023).
- 49.Chouaïbou, M., Zivanovic, G. B., Knox, T. B., Jamet, H. P. & Bonfoh, B. Synergist bioassays: A simple method for initial metabolic resistance investigation of field Anopheles gambiae s.l. populations. Acta Trop.130, 108–111. 10.1016/j.actatropica.2013.10.020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pasay, C. et al. The effect of insecticide synergists on the response of scabies mites to Pyrethroid Acaricides. PLoS Negl. Trop. Dis.3, e354. 10.1371/journal.pntd.0000354 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balkew, M. et al. An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018–2020. Malar. J.10.1186/s12936-021-03801-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muhammed, M. et al. Insecticidal effects of some selected plant extracts against Anopheles stephensi (Culicidae: Diptera). Malar. J.21, 295. 10.1186/s12936-022-04320-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teshome, A. et al. Laboratory-based efficacy evaluation of Bacillus thuringiensis var. israelensis and temephos larvicides against larvae of Anopheles stephensi in ethiopia. Malar. J.10.1186/s12936-023-04475-9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balkew, M. et al. An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018–2020. Malar. J.20, 263. 10.1186/s12936-021-03801-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahn, J., Sinka, M., Irish, S. & Zohdy, S. Modeling marine cargo traffic to identify countries in Africa with greatest risk of invasion by Anopheles stephensi. Sci. Rep.10.1038/s41598-023-27439-0 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ammar, S. E. et al. Intercepted mosquitoes at New Zealand’s ports of entry, 2001 to 2018: Current status and future concerns. Trop. Med. Infect. Dis.4, 101. 10.3390/tropicalmed4030101 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hulme, P. E. Trade, transport and trouble: managing invasive species pathways in an era of globalization. J. Appl. Ecol.46, 10–18. 10.1111/j.1365-2664.2008.01600.x (2009). [Google Scholar]
- 58.Swan, T. et al. A literature review of dispersal pathways of Aedes albopictus across different spatial scales: implications for vector surveillance. Parasit. Vectors15, 303. 10.1186/s13071-022-05413-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences: Bioproject PRJNA1042829 and Genbank accessions SAMN38322421 – SAMN38322440.