Abstract
Asymptomatic IgM gammopathy encompasses IgM monoclonal gammopathy of undetermined significance (MGUS) and asymptomatic Waldenström macroglobulinemia (AWM), both having a risk of progression to symptomatic disease. Here, we assessed the risk of progression and the mortality of 956 patients with asymptomatic IgM gammopathy across 25 Spanish centers. After a median follow‐up of 5.7 years, 156 patients progressed, most of them to symptomatic WM (SWM). The cumulative incidence of progression was 13% and 20% at 5 and 10 years, respectively. The serum IgM ≥10 g/L, bone marrow (BM) infiltration ≥20%, β2‐microglobulin ≥3 mg/L, and albumin <4 g/dL were the most potent predictors of disease progression in a multivariate Cox regression model, allowing the identification of three risk categories. The probability of progression to symptomatic disease at 5 years was 4.5%, 15.7%, and 42.8% for low‐, intermediate‐, and high‐risk groups, respectively. In patients without a BM evaluation, the presence of none or 1 risk factor and 2 or 3 risk factors conferred a progression risk of 6% and 27% at 5 years, respectively. The model was independent of the presence of MYD88 L265P, which conferred a negative impact only in AWM patients. The relative survival (RS) ratio at 5 years of asymptomatic patients was similar to the Spanish population, which contrasted with the 0.76 5‐year RS of SWM patients. Overall, the Spanish Multicenter Model comprehensively describes the risk of progression of asymptomatic patients and shows that the excess mortality is increased only in the symptomatic stage of the disease.
INTRODUCTION
The association of a monoclonal immunoglobulin M (IgM) in serum with diverse lymphoproliferative disorders has been extensively reported, being IgM monoclonal gammopathy of undetermined significance (MGUS) and Waldenström macroglobulinemia (WM) the two most well characterized. The prevalence of IgM MGUS increases with age and could eventually progress to a B cell or plasma cell malignancy. 1 The spectrum of disease progression includes predominantly WM, followed by diffuse large B cell lymphoma (DLBCL), AL amyloidosis, chronic lymphocytic leukemia (CLL), and multiple myeloma (MM), among others. 2 , 3 Meanwhile, WM is a B cell lymphoplasmacytic lymphoma that is most of the time preceded by IgM MGUS. This relationship has long been studied, as both share the same cell of origin in terms of morphological, immunophenotypic, and genomic features. 4 , 5 , 6 , 7
In this sense, there has been great interest to model disease progression for both IgM MGUS and asymptomatic WM (AWM), as they also share similar biomarkers of disease progression. 8 , 9 , 10 For instance, the largest study of patients with AWM showed that the progression rate was 30.8% at 2 years, 9 which was modeled by the presence of risk factors, such as low albumin, high serum IgM, high bone marrow (BM) infiltration by biopsy, and high β2‐microglobulin. Its applicability is of great importance in AWM; however, many patients who lack a BM biopsy or have less tumor burden are still not well represented. More recently, the addition of the MYD88 mutation as a potential predictor of disease progression has been reported, and its validation on large series is still pending. 11 , 12 These issues, added to the low incidence 13 and the clinical heterogeneity of disease progression, challenge an appropriate risk stratification.
Regarding survival trends, it is even more challenging to model the excess mortality in asymptomatic IgM gammopathy patients. Population‐based studies have reported that lymphoplasmacytic lymphoma/WM patients had an excess mortality, regardless of whether they were asymptomatic or not. 14 , 15
To better understand the natural history of asymptomatic IgM gammopathy in terms of progression risks and survival trends, we gathered a large cohort of patients with asymptomatic IgM monoclonal gammopathies from 25 Spanish hospitals with real‐world data. We retrospectively validated previous risk models and proposed a revised, comprehensive, and easily applicable model to the clinic for patients with and without a BM evaluation, an examination not so frequent in standard clinical practice. We also showed how different are the mortality outcomes of these patients compared to a group of patients with symptomatic WM in the same country.
MATERIALS AND METHODS
Study design
This is a retrospective and multicenter study from 25 hospitals from the Programa de Registro de Macroglobulinemia de Waldenström en España (PRAME). We included 956 patients diagnosed with asymptomatic IgM gammopathy (encompassing both IgM MGUS and AWM) as the main group of interest, while patients with symptomatic WM served as controls for the analysis of the excess mortality. The study involved patients diagnosed between 1979 and 2021. The analysis of predictors' performance and relative survival was performed in the whole set of patients (N = 956). Data from patients who were available for the analysis of the risk of progression (N = 679) were then divided for those who had a BM evaluation (N = 495) and those who did not (N = 184) (Figure 1). The diagnostic criteria followed local institutional protocols of each center and included the Mayo Clinic 16 , 17 or the International Consensus criteria. 18 , 19 Treatment initiation followed the criteria from the International Consensus, and this was used to define symptomatic WM patients. 20 Patients who required treatment because of IgM‐related disorders or IgM monoclonal gammopathy of clinical significance were excluded. The study was approved by the local institutional review boards from all the centers involved. More details can be found in the Supporting Information Material.
Figure 1.
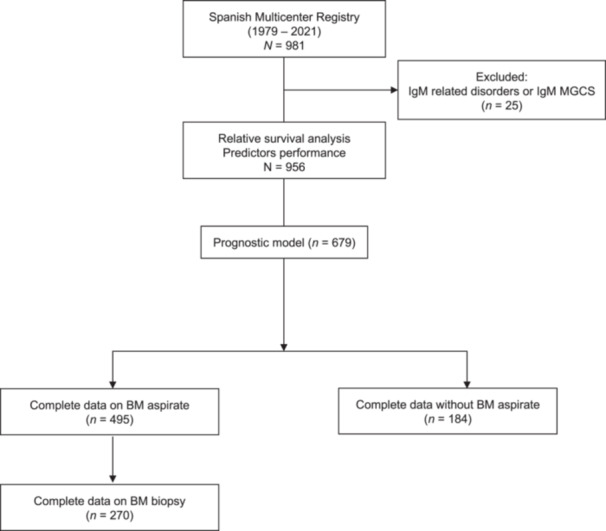
Flowchart and design of the study of the Spanish registry of asymptomatic IgM monoclonal gammopathy patients. n, number of patients.
Data collection and endpoints
Clinical and laboratory information was collected from individual registries and consisted of serum IgM/IgG/IgA levels, serum monoclonal protein (M‐protein), isotype of light chain, BM infiltration either by aspirate (N = 495) or by biopsy (N = 270), MYD88 L265P mutation, serum albumin, β2‐microglobulin, lactate dehydrogenase (LDH), and blood counts (red blood cell, lymphocyte, and platelet counts). The MYD88 L265P mutation was assessed using either allele‐specific polymerase chain reaction (AS‐PCR) or droplet digital PCR (ddPCR) on whole bone marrow cells. Immunoparesis was defined as a reduction below the normal range in the serum IgG (less than 6 g/L) and/or IgA (less than 0.6 g/L). 10 , 17 , 21 Quantitative assessment of variables at the time of progression was available in patients who presented progression during follow‐up. Progression‐free survival (PFS) was defined as the time from diagnosis to the time of disease progression that required treatment.
Statistical analyses
The Gönen and Heller k‐statistic was used to evaluate the performance of risk models that were designed for both IgM MGUS and AWM patients, including the one reported by the Italian group in 2005, 8 the Dana Farber Cancer Institute (DFCI) in 2019, 9 and the Barcelona group in 2021. 10 The log‐rank test was used to calculate the significance of the difference between survival curves.
To analyze the predictors in our series, we iteratively calculated the hazard ratios (HRs) and the C‐index using a Cox proportional hazards regression for each potential cutoff, as previously reported. 22 To find the best predictors, we used a backward stepwise Cox regression followed by bootstrap with 1000 replications. We then assigned one point for each biomarker after visual inspection of the observed and predicted survival probabilities for each point to cluster and generate risk groups. The prognostic model was also analyzed by considering all‐cause deaths before progression as competing events. The Wilcoxon matched‐pairs signed‐rank test was used to analyze the differences between the predictors as continuous at diagnosis and at disease progression. Logistic regression was used to assess the receiver operating characteristic (ROC) between risk models to discriminate disease progression at 5 years. To analyze the relative survival (RS) in our cohort, we leveraged data from the Human Mortality Database and matched the Spanish population in terms of year, age, and sex, with a cutoff date in 2020. The Ederer II method was used to analyze the RS rates. 23 Poisson regression was used to analyze the excess mortality according to each diagnosis. All the analyses were performed in Stata version 18 (StataCorp LLC).
RESULTS
Patients' characteristics
The clinical and laboratory characteristics of patients are summarized in Table 1. About 74% of patients had a kappa light chain isotype by immunofixation, and the median serum M‐protein and serum IgM were 9.0 and 7.6 g/L, respectively. The median lymphocyte and plasma cell infiltration rates were 14% and 2%, respectively. The MYD88 L265P analysis was only available in 453 patients, being positive in 68.4% of the entire cohort. Around 136 (14.2%) and 820 (85.8%) patients were diagnosed before and after the year 2005, respectively (Supporting Information S1: Figure 1). About 440 (47%) patients had a BM infiltration less than 10% or a serum IgM less than 5 g/L. Missing data were not imputed as they were associated with the lack of BM assessment (Supporting Information Material).
Table 1.
Clinical and laboratory characteristics of patients.
| Patients' characteristics | N | |
|---|---|---|
| Age, median (IQR) | 956 | 69.4 (60.5–77.1) |
| Sex, female (%) | 956 | 411 (43.0) |
| Laboratory values, median (IQR) | ||
| Hemoglobin, mg/dL | 920 | 13.5 (12.2–14.6) |
| Platelets, K/µL | 916 | 236.0 (183.0–288.0) |
| Albumin, g/dL | 864 | 4.2 (3.9–4.5) |
| β2‐microglobulin mg/L | 727 | 2.3 (1.8–3.1) |
| Serum M‐protein, g/L | 727 | 9.0 (4.0–14.7) |
| Serum IgM, g/L | 912 | 7.6 (3.5–16.2) |
| Serum IgG, g/L | 901 | 8.7 (6.7–10.7) |
| Serum IgA, g/L | 899 | 1.5 (0.9–2.3) |
| LDH, UI/L | 857 | 249 (175.0–328.0) |
| Bone marrow, % | ||
| Mature lymphocytes | 601 | 14 (8–25) |
| Plasma cells | 570 | 2 (1–4) |
| IgM <5 g/L or bone marrow infiltration <10%, (%) | 926 | 440 (47.5) |
| kappa light chain, (%) | 737 | 545 (74.0) |
| MYD88 L265P mutation, (%) | 453 | 310 (68.4) |
Abbreviations: IQR, interquartile range; LDH, lactate dehydrogenase.
Biomarkers of disease progression
After a median follow‐up of 5.7 years (interquartile range [IQR]: 2.8–9.6), 156 patients progressed to a lymphoproliferative/plasma cell disorder that required treatment (144 to SWM, 5 cases of DLBCL, 3 IgM AL amyloidosis, 1 CLL, 1 IgM MM, and 2 follicular lymphoma). The cumulative probability of progression was 13.1% (95% confidence interval [CI]: 10.9–15.7) and 19.5% (95% CI: 16.4–23.2) at 5 and 10 years, respectively (Figure 2A). No differences in PFS were observed between patients diagnosed before or after 2005 (log‐rank p = 0.956).
Figure 2.
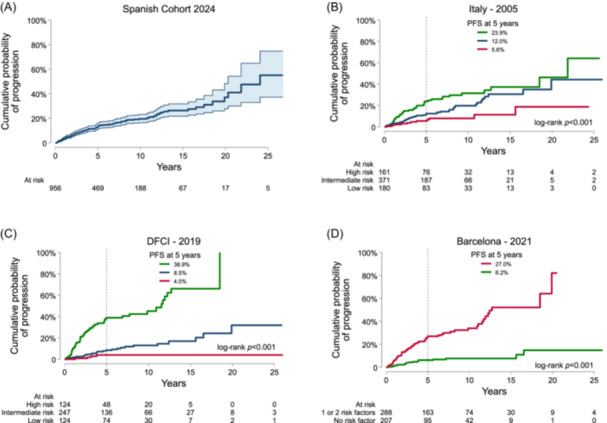
Progression‐free survival (PFS) in the Spanish multicenter registry and according to each prognostic risk model. Each plot reflects a Kaplan–Meier survival curve of the progression risk in the Spanish Cohort (A), the Italian group (B), the Dana Farber Cancer Institute (DFCI) group (C), and the Barcelona group (D).
We then analyzed the performance in our series of three prognostic models that took into account patients diagnosed with asymptomatic IgM gammopathy. First, we evaluated the risk model proposed by the Italian group considering the M‐protein size (<7.0, 7.0–13.9, 14.0–20.9, ≥21 g/L), hemoglobin (≥15.0, 13–14.9, 11.0–12.9, <11.0 g/dL), and sex (female as low risk). 8 The Gönen and Heller k‐statistic was 0.61 (N = 712), and the probability of progression at 5 years in our series was 5.6%, 12.0%, and 23.9% for low, intermediate, and high risk, respectively (p < 0.001) (Figure 2B). Second, we evaluated the model proposed by the DFCI, considering serum IgM, BM infiltration rate, β2‐microglobulin, and albumin. In this model, the risk was categorized according to the quartile distribution of the predicted HR generating three groups. 9 The Gönen and Heller k‐statistic was 0.72 (N = 495), and the probability of progression at 5 years was 4.0%, 8.5%, and 38.9% for low, intermediate, and high risk, respectively (p < 0.001) (Figure 2C). More recently, the Barcelona group developed a two‐risk model considering immunoparesis and a BM infiltration cutoff point of 20%. 10 Here, the Gönen and Heller k‐statistic was 0.67 (N = 495), and the probability of progression at 5 years was 6.2% and 27.0% for low and high risk, respectively (Figure 2D).
Considering that the categorization of variables is dependent on many factors such as the center‐specific protocol for patient follow‐up or how each variable was measured, we aimed to investigate whether there were any other potential risk factors that could help to improve the aforementioned models. To do that, we first analyzed the impact on progression of the four predictors for which cutoff points were difficult to establish. For these variables, we iteratively calculated the predicted HR and Harrell's C‐index for each value.
Accordingly, the best cutoff points were a serum IgM between 10 and 15 g/L, a BM infiltration rate of 20%, a β2‐microglobulin between 2.5 and 3 mg/L, and an albumin between 4 and 4.5 g/dL. The cumulative incidence of progression at 5 years was 22.4% for IgM ≥10 g/L, 26.2% for bone marrow infiltration ≥20%, 25.8% for β2‐microglobulin ≥3 mg/L, and 22.1% for serum albumin <4 g/dL (Supporting Information S1: Figures 2 and 3).
As a biomarker, the MYD88 L265P mutation (N = 453) was associated with a shorter PFS (HR: 3.5, CI: 1.8–7.1, p < 0.001). To explore the impact of MYD88 on the type of diagnosis in patients who had a BM biopsy, we observed a higher risk of progression in AWM compared to IgM MGUS (Supporting Information S1: Figure 4).
Prognostic risk model
Subsequently, we analyzed the independent impact of the risk factors from previous risk models. Here, we did not consider MYD88 because only less than half of the study population were diagnosed before the universal setup of the technique, neither sex (male vs. female, HR: 0.9, CI: 0.7–1.3, p = 0.734) nor age (≥65 vs. <65, HR: 0.9, CI: 0.7–1.4, p = 0.885) because of their lack of impact on disease progression. Table 2 summarizes the univariate analysis of each biomarker on a Cox proportional hazards model. The backward stepwise method selected serum IgM ≥10 g/L, BM infiltration ≥20%, β2‐microglobulin ≥3 mg/L, and albumin <4 g/dL. The 1000‐bootstrap replicates of this model showed comparable findings with a p < 0.10 selection cutoff (Supporting Information S1: Table 1). In order to build a parsimonious and easy‐to‐use model, we assigned one point to each biomarker and clustered the patients based on the observed and predicted HRs (Supporting Information S1: Figure 5). Consequently, patients with either no points or 1 point were categorized as being in the low‐risk group, whereas those with two points or three to four points were classified into the intermediate‐ and high‐risk groups, respectively (Figure 3A). The 5‐year probability of progression was 4.5%, 15.7%, and 42.8% in the low‐, intermediate‐, and high‐risk groups (log‐rank p < 0.001). The Gönen and Heller k‐statistic of this model was 0.72 (N = 495). With this, we developed a model that can be easily abbreviated as the “10‐20‐3‐4” related to numbers from the cutoff values for each biomarker. After fitting a logistic regression model to evaluate the discrimination between the DFCI model versus ours, we observed that the ROC area was 0.76 versus 0.79 (p = 0.025) at 5 years of follow‐up, respectively. In a competing risk framework, the risk categories behaved similarly (high risk vs. low risk—subhazard ratio [SHR]: 10.03, 95% CI: 5.47–18.42, p < 0.001; intermediate risk vs. low risk—SHR 5.20, 95% CI: 2.76–9.78) (Supporting Information S1: Figure 6).
Table 2.
Impact of biomarkers included in previous prognostic models.
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | N | HR | 95% CI | p Value | N | HR | 95% CI | p Value |
|
IgM ≥10 g/L |
912 | 4.41 | 2.96–6.57 | <0.001 | 495 | 2.16 | 1.23–3.78 | 0.007 |
|
BM lymphocytes ≥20% |
601 | 3.79 | 2.50–5.73 | <0.001 | 495 | 3.47 | 2.04–5.93 | <0.001 |
|
β2‐microglobulin ≥3 mg/L |
727 | 2.38 | 1.64–3.46 | <0.001 | 495 | 1.90 | 1.17–3.09 | 0.010 |
|
Albumin <4 g/dL |
864 | 2.59 | 1.82–3.69 | <0.001 | 495 | 1.58 | 0.97–2.56 | 0.060 |
|
IgA <0.6 g/L |
899 | 3.14 | 2.14–4.63 | <0.001 | 495 | ‐ | ‐ | ‐ |
|
IgG <6 g/L |
901 | 1.51 | 1.05–2.17 | 0.025 | 495 | ‐ | ‐ | ‐ |
|
Hemoglobin <11.5 g/dL |
920 | 2.56 | 1.69–3.87 | <0.001 | 495 | ‐ | ‐ | ‐ |
|
LDH ≥450 UI/L |
857 | 1.37 | 0.51–3.72 | 0.533 | 495 | ‐ | ‐ | ‐ |
Abbreviations: BM, bone marrow; CI, confidence interval; HR, hazard ratio; LDH, lactate dehydrogenase; N, number of patients for each biomarker.
Figure 3.
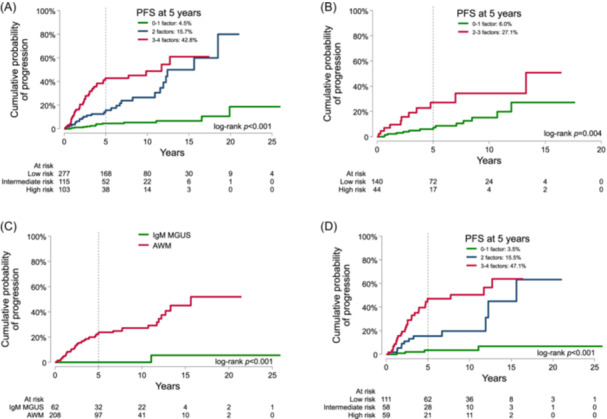
The new proposed model of the risk of progression in the entire series of patients with complete data with (A) and without a bone marrow aspirate (B). Below are depicted the 270 patients with an available bone marrow biopsy, allowing to study the different risks of progression of IgM monoclonal gammopathy of undetermined significance (MGUS) and asymptomatic Waldenström macroglobulinemia (AWM) (C), and the performance of the proposed model based on bone marrow aspirate in patients with bone marrow biopsy (D). PFS, progression‐free survival.
In patients who did not have a BM evaluation by aspiration, the same model (IgM ≥10 g/L, β2‐microglobulin ≥3 mg/L, and albumin <4 g/dL) was able to predict the risk of progression. The cohort (N = 184) was divided into two risk groups according to the presence of none or 1 risk factor (low risk) and 2 or 3 risk factors (high risk). The cumulative incidence of progression at 5 years was 6.0% and 27.1%, respectively, in this BM‐free model (Figure 3B).
In addition, we only identified 270 patients (62 IgM MGUS and 208 AWM patients) who have both a BM aspirate and BM biopsy, respectively (Supporting Information S1: Table 2), following local guidelines and clinical criteria. Only one patient with IgM MGUS progressed after 10 years of follow‐up, while the cumulative incidence of progression of AWM patients at 5 years was 23.8% (95% CI: 18.0–31.0) (Figure 3C). The diagnostic performance of the patients with full criteria using BM biopsy was quite similar to the prognostic model of those with only a BM aspirate (Figure 3D).
We further analyzed the additional impact of the MYD88 mutation on the prognostic model. The MYD88 mutation was only associated with higher BM infiltration rates and serum IgM values (Supporting Information S1: Table 3). In patients who had complete data on all predictors (N = 327), the MYD88 mutation (HR: 1.83, 95% CI: 0.75–4.45; p = 0.180) did not change the prognostic model score predictions (HR: 2.53, 95% CI: 1.93–3.31; p < 0.001) after fitting a multivariate Cox regression analysis. After adjusting the prognostic model with the type of diagnosis based on BM biopsy, AWM (HR: 7.27; 95% CI: 0.97–53.9; p = 0.052) was not an independent factor (Supporting Information S1: Table 4).
Refining the low risk
Given that there is also great interest in identifying patients who will not progress during the entire follow‐up, we reanalyzed the whole set of patients and plotted the distribution of the two most important risk factors (serum IgM and BM infiltration) across the low, intermediate, and high risk of progression groups to discriminate a new subgroup of patients with very low tumor burden characterized by a serum IgM less than 5 g/L and a BM infiltration less than 10%. After a graphical inspection of the series, 83 patients had a serum IgM less than 5 g/L and a BM infiltration less than 10%. These patients were followed for a median of 6 years and had an even lower probability of progression compared to the low‐risk group mentioned above. The cumulative incidence of progression was 1.6% (95% CI: 0.23–10.74) at 5 years (Supporting Information S1: Figure 7).
Predictors during progression
For patients who experienced progression, we had access to information about various potential biomarkers and observed how they changed over time. In this sense, we analyzed the 4 predictors from the model. Each individual subject was analyzed in two‐time points showing an increase in IgM, β2‐microglobulin, and BM infiltration; meanwhile, a decrease was seen in the case of albumin (Figure 4A–D).
Figure 4.
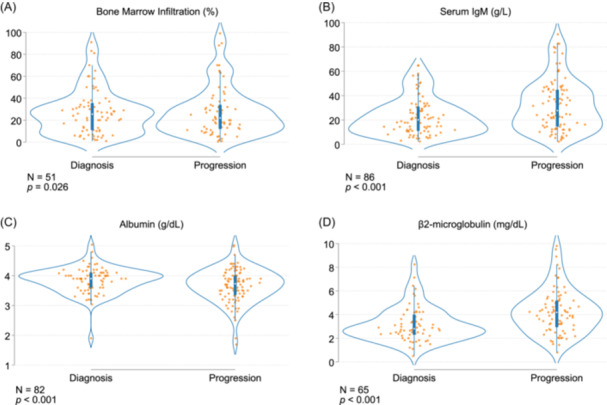
Longitudinal performance of the prognostic biomarkers. Each dot represents a separate time point per subject for the bone marrow infiltration by aspirate (A), serum IgM (B), albumin (C), and β2‐microglobulin (D). N, number of subjects.
Mortality trends
Two hundred and forty‐seven patients died during follow‐up. Among them, 127 (51.5%) died because of solid neoplasms, 30 (12.1%) from age‐related comorbidities, 23 (9.3%) from disease progression or complications related to the treatment received, and 67 (27.1%) were missing. Compared to the remaining healthy Spanish population, asymptomatic patients had a 5‐, 10‐, 15‐, and 20‐year RS rates of 0.99, 0.97, 0.90, and 0.86, respectively (Table 3). So, during the first 5 to 10 years from diagnosis, the mortality remained similar to the Spanish population in asymptomatic patients (p = 0.940) (Figure 5A). To give more insights into the survival trends of patients with IgM gammopathy, we gathered data from a cohort of 448 patients with symptomatic WM from our registry. We identified 227 deaths, from whom 61 (26.9%) were directly linked to the disease either because of progression or treatment complications, another 61 (26.9%) deaths were because of concomitant solid neoplasms, 22 (9.7%) because of other age‐related comorbidities, and 83 (36.5%) were missing. The RS rates at 5‐, 10‐, 15‐, and 20‐year were 0.76, 0.66, 0.44, and 0.36, respectively (Figure 5B). Thus, symptomatic WM exhibited a higher excess mortality compared to the Spanish population in each timespan (p < 0.001). Overall, patients with IgM gammopathy showed a trend to worse survival trends in the symptomatic stage.
Table 3.
Relative survival (RS) rates in patients with asymptomatic IgM monoclonal gammopathy and symptomatic Waldenström macroglobulinemia.
| Diagnosis | Start | End | RS | CI |
|---|---|---|---|---|
| Asymptomatic IgM gammopathy | 0 | 5 | 0.99 | 0.96–1.00 |
| 5 | 10 | 0.97 | 0.92–1.00 | |
| 10 | 15 | 0.90 | 0.81–0.90 | |
| 15 | 20 | 0.86 | 0.70–0.90 | |
| Symptomatic Waldenström macroglobulinemia | 0 | 5 | 0.76 | 0.70–0.81 |
| 5 | 10 | 0.66 | 0.59–0.74 | |
| 10 | 15 | 0.44 | 0.34–0.54 | |
| 15 | 20 | 0.36 | 0.25–0.49 |
Abbreviation: CI, 95% confidence interval.
Figure 5.
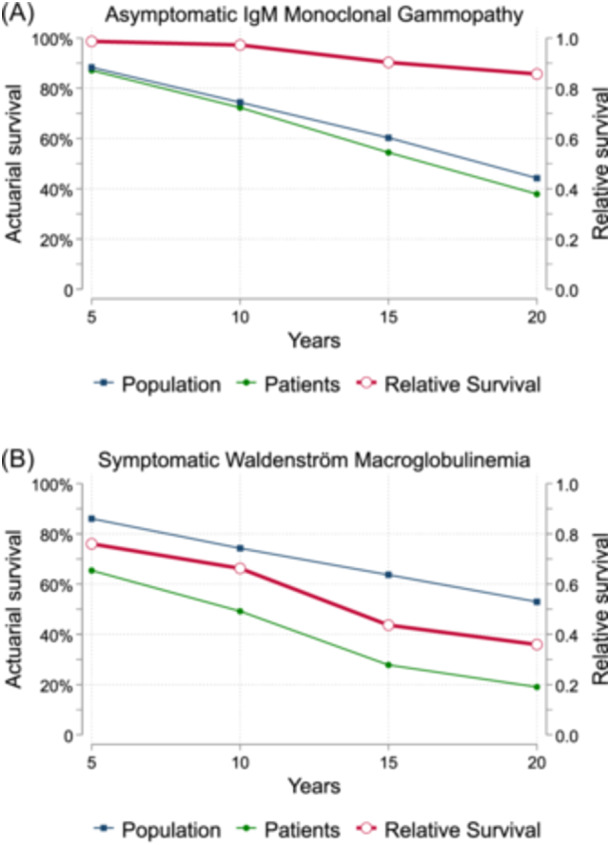
Relative survival rates compared with the Spanish Health records from the Human Mortality Database. Each plot reflects the relative survival rate (in red), the Spanish population actuarial survival (in blue), and the patients' actuarial survival (in green) (A). Symptomatic Waldenström macroglobulinemia patients showed a survival curve below the Spanish population since diagnosis (B). CI, confidence interval.
DISCUSSION
We have assembled a large cohort of patients diagnosed with asymptomatic IgM monoclonal gammopathy, allowing us to describe its progression risk and give more insights into its natural history, so far needed. Many factors challenge a comprehensive evaluation of the progression risk in asymptomatic IgM monoclonal gammopathies, such as the low incidence, the high dependency on single‐center registries, the arbitrary inclusion of biomarkers in multivariate analyses, and the difficulty of assessing the risk in patients with low tumor burden or those who lack a BM biopsy and do not fit in any diagnostic criteria. Although not yet analyzed, the latter situation is very common in routine practice. In fact, low‐risk IgM MGUS patients usually lack a BM biopsy and are diagnosed based on serum biomarkers. 3 This inevitably might lead to misclassification in certain cases, as it has been reported that the BM lymphocytic infiltration does not correlate well with the monoclonal serum IgM. 19
To surpass these issues, we designed a multicenter approach to blindly analyze patients with a monoclonal IgM in serum that were asymptomatic at the time of diagnosis. In our study, as in many European centers, 6 , 8 , 10 , 24 we had data based on the BM aspirate given its easier applicability, while the biopsy is usually performed in patients with high tumor burden or there is suspicion of disease progression or other lymphoproliferative condition.
We first observed that the cumulative incidence of progression from our series (20% at 10 years) was lower compared to that proposed by the DFCI 9 (close to 80% at 10 years). Although several explanations can arise underlying this phenomenon, we consider the inclusion of more patients with low tumor burden in our series as the most important argument. The DFCI series reported a minority of patients with low tumor burden (for instance, those with less than 10% of BM infiltration), while in our study they represented half of the entire series based on the BM aspirate. In fact, our results were similar to the cumulative incidence of progression of 15% and 34% at 10 years reported by Baldini et al. in patients with IgM MGUS and AWM, respectively. 8
With our data, we validated previous risk models that took into account asymptomatic IgM gammopathy patients. We showed that the models proposed by the Italian group, the DFCI, and the Barcelona group classified patients into separate risk categories; however, there was great variation between intermediate‐ and low‐risk groups, probably due to the low number of patients with less tumor burden that were included in each study. Another important issue was related to the inclusion of predictors, as they varied across all three studies or were not reported in multivariate analyses. As a result, we improved the accuracy of the risk classification by incorporating all previous biomarkers. We proposed the “10‐20‐3‐4” Spanish Multicenter Model, a new and easy‐to‐handle model that showed common variables such as 10 g/L of serum IgM, 20% of BM infiltration, 3 mg/L of β2‐microglobulin, and 4 g/dL of albumin. The predictors had different cutoff points compared to other studies, 8 , 9 , 11 which reflects the inclusion of patients with low tumor burden. The predictors also allowed a clear separation of the risk groups at 5 years, each of them having three times the estimated probability of progression of the previous risk category. In patients who had a BM biopsy and were able to be further classified into IgM MGUS and AWM by the International Consensus Criteria, 19 , 25 we observed that only one patient with IgM MGUS progressed, while AWM represented the majority of the population whose progression risk was modeled. This prompted us to identify a very low‐risk group characterized by having a serum IgM less than 5 g/L and a BM infiltration by aspirate less than 10%, which resembled the progression risk of patients with less than 10% of BM infiltration by biopsy reported by the DFCI. 9
We showed that the BM aspirate is a reliable surrogate marker of disease infiltration and that the risk of progression of “true” IgM MGUS was lower than that reported by other studies, 3 and therefore, a BM evaluation can help to precisely model the risk of progression and to avoid potential misclassification in this subset of patients. However, we highlight that performing BM biopsies on patients with a suspicion of IgM MGUS can be delayed in certain cases, as previously reported, given its very low risk of progression. 3 , 26 In this context, we also applied our new proposed model in patients without a BM assessment and were able to differentiate the risk into two categories by using the same biomarkers, allowing a “BM‐free” system for stratification to be explored. These patients were selected by their physicians as low risk before the decision of not to undergo a BM study. Overall, we consider that patients without a BM study but who have other high‐risk features such as low albumin, high β2‐microglobulin, and high IgM according to our model should undergo a BM biopsy/aspirate, as an important proportion of them progress during follow‐up. In patients with low or intermediate risk, the BM aspirate could be an excellent tool especially in the presence of other comorbidities. This strategy can help to discriminate better those patients who are unlikely to progress in the next 5 years. Patients who have a BM infiltration less than 10% and a serum IgM less than 5 g/L may not require further studies with a hematologist and may continue their follow‐up with their primary care team. Thus, we provided a clinically relevant prognostic model, fitting different types of scenarios that are usually present in real clinical practice.
Another novelty of our study was to include the largest series of asymptomatic patients from whom the analysis of the MYD88 mutation was available. We observed that there was a negative impact on PFS in the whole series, a finding that has been reported previously. 12 In patients with available BM biopsy, this pattern was only observed in AWM. Our results differed from other studies that showed a negative impact in MYD88 wild‐type patients. 9 This can be due to technical differences and the number of patients included. As a retrospective multicenter study, sample processing might differ from each center, or the PCR assay may change throughout time. In fact, we used now either AS‐PCR or ddPCR to detect the mutation. Regardless of the sensitivity, our results resembled more the biological continuum of progression in patients with IgM gammopathy, as the MYD88 mutation has recently been even identified in early precursors of lymphoplasmacytic clones, 7 followed by an increased mutation tumor burden in each state of the disease. 5 , 12 After adjusting the prognostic model with the mutation status, the MYD88 did not add an extra value, probably due to its association with the BM disease burden. We showed that the predictive ability of MYD88 in the risk of progression was lower compared to common clinical biomarkers. As stated by others, 11 , 12 , 27 the data from our study served as another attempt to model disease progression based on molecular biomarkers in IgM monoclonal gammopathy. However, we consider that efforts to standardize the MYD88 testing, inclusion of other somatic mutations such as CXCR4 using high‐throughput techniques, and longer follow‐up can be further exploited to precisely and uniformly assess the risk of progression based on the MYD88 L265P status prospectively.
Finally, we analyzed the mortality of asymptomatic IgM gammopathy. Previous studies have focused the analysis on the diagnosis of lymphoplasmacytic lymphoma or WM without the distinction of those who required treatment or not. In fact, a 5‐year RS was reported to be between 0.6 and 0.8 with an improving trend for each decade, 14 and another recent population‐based study reported a 5‐year conditional RS of 84% in WM patients. 15 We observed that the RS rate of asymptomatic patients resembled that of the rest of the Spanish population during the first 5 to 10 years from diagnosis, being the development of solid neoplasms the most frequent cause of death. However, we acknowledge that asymptomatic patients can benefit from early access to the healthcare system and, in some cases, the long time that takes to develop disease progression as potential causes for our findings. 28 When compared to a cohort of symptomatic WM from our registry, the differences were much more marked, showing an excess of mortality in patients who were diagnosed in the symptomatic stage; in them, the most frequent causes of death were disease progression or treatment complications.
Our study has several strengths, such as the high number of centers and patients included, the validation of other risk models, the analysis of all previous biomarkers in a single framework to comprehensively build a new prognostic model, the inclusion of patients with very low risk of progression, and the analysis of the relative survival of asymptomatic patients in contrast to symptomatic patients. Although we relied more on the BM aspirate instead of the biopsy as recommended in the consensus, 19 , 25 and that a bias might have been introduced in the models, the high number of patients allowed us to use the prognostic model in those with a BM evaluation either by aspirate or biopsy, reflecting the clinical practice in many countries. Thus, our study intended to give a full picture of the clinical course of patients with IgM monoclonal gammopathies. The limitations of our study are related to its multicenter approach. In our study, the MYD88 impact in IgM MGUS patients was challenging to assess, since none of them progressed. Other potential predictors such as the CXCR4 mutations were not analyzed, as they were not routinely assessed. Another limitation is the shorter follow‐up time compared to others, 3 , 8 , 9 explained by the large proportion of patients diagnosed in the last decade, especially for those with very low tumor burden. However, we showed similar progression rates in this subgroup of patients compared to studies with longer follow‐up. 3 Regarding the new prognostic model, we acknowledge that its performance on risk prediction needs to undergo continued evaluation and validation in another national database to determine the real advantage in front of the DFCI model.
In summary, we described the risk of progression of patients with asymptomatic IgM gammopathy and proposed a prognostic model available worldwide for patients with or without a BM evaluation, highlighting the presence of a very low‐risk group. In addition, we demonstrated that the relative mortality of these patients was different from those with symptomatic WM. Our results may help in the algorithm on how to follow these patients, with social and economic impact.
AUTHOR CONTRIBUTIONS
David F. Moreno, Cristina Jiménez, Elham Askari, Ramón García‐Sanz, and Carlos Fernández de Larrea designed the research, wrote the manuscript, and analyzed the data. David F. Moreno, Cristina Jiménez, Elham Askari, Marta Castellanos‐Alonso, Ramón García‐Sanz, Fernando Escalante, Mario Arnao, Ángela Heredia, Miguel Á. Canales, Magdalena Alcalá, Arancha Bermúdez, Ana Saus Carreres, María Casanova, Luis Palomera, Cristina Motlló, Ricarda García‐Sánchez, Pablo Ríos Rull, and Carlos Fernández de Larrea collected the information, reviewed the clinical histories, and wrote the manuscript. All authors revised the article and gave approval of the final version to be published.
CONFLICT OF INTEREST STATEMENT
David F. Moreno received travel grants and honoraria from Janssen. Carlos Fernández de Larrea consulted and received honoraria from GSK, Sanofi, Pfizer, BeiGene, Amgen, BMS, and Janssen and received research funding from GSK, Amgen, and Janssen. Mario Arnao consulted and was on the speakers bureau for Janssen, Sanofi, and Amgen and consulted for BMS/Celgene. Ángela Heredia was on the speakers bureau for Janssen and NovoNordisk. Ricarda García‐Sánchez was on the speakers bureau and consulted for BMS/Celgene, Janssen‐Cilag, and GSK; consulted for Amgen and Takeda; and was on the advisory board member for Janssen‐Cilag, BMS/Celgene, Amgen, GSK, Takeda, and Beigene. Pablo Ríos Rull consulted, received honoraria, and was on the speakers bureau for GSK, AMGEN, and Sanofi; received honoraria from Celgene and Takeda; consulted for Beigene; has participated in medical meetings for GSK, Janssen, Celgene, Takeda, Amgen, Novartis, and Sanofi; and received research funding from BMS/Celgene. Ramón García‐Sanz received research support from the Asociación Española Contra el Cáncer, Takeda, Gilead, Incyte, Janssen; received honoraria from Beigene, Amgen, Takeda, Janssen, Incyte, BMS; and was on the speakers bureau for Beigene, Takeda, Janssen. Fernando Escalante consulted for Janssen Oncology, Amgen, GlaxoSmithKline, BeiGene, Sanofi; was on the speakers bureau for Janssen Oncology, GlaxoSmithKline; and received travel grants from BeiGene, Janssen Oncology, Amgen. Miguel Á. Canales consulted for Beigene, BMS, Incyte, Janssen, Karyopharm, Kite, Kyowa, Lilly, Roche, Takeda; and was part of the speakers bureau for Incyte, Janssen, Kite, Kyowa, Roche, Takeda. Luis Palomera received honoraria for lectures and advisory boards from Janssen, Amgen, Sanofi, GSK. The remaining authors declare no competing conflicts of interests.
FUNDING
The Spanish Society of Hematology and Hemotherapy received an unrestricted grant from Janssen to support the registry that provided the data for this study.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We are indebted to the Emili Letang Grant from Hospital Clínic de Barcelona, the Spanish Society of Hematology and Hemotherapy (Amanda López, María Molina, and Teresa Pérez) for the support to carry out a multicenter registry in Spain, and all the people involved who participated in this study: Isabel García Cabrera (Hospital San Cecilio, Granada), Eugenia Abella (Hospital del Mar, Barcelona), Joan Bargay (Hospital Son Llatzer, Palma de Mallorca), Paz Ribas (Hospital Dr. Peset, Valencia), María Belén Navarro (Hospital Puerta de Hierro, Madrid), Mercedes Gironella (Hospital Vall d'Hebrón, Barcelona), Miguel Ángel Álvarez (Hospital Reina Sofía, Córdoba), Antonio García Guiñón (Hospital Arnau de Vilanova, Lleida), Laura Abril Sabater (Instituto Catalán de Oncología, Badalona), and María Jesús Blanchard (Hospital Ramón y Cajal, Madrid).
Contributor Information
Ramón García‐Sanz, Email: rgarcias@usal.es.
Carlos Fernández de Larrea, Email: cfernan1@clinic.cat.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362‐1369. [DOI] [PubMed] [Google Scholar]
- 2. Kyle RA, Benson JT, Larson DR, et al. Progression in smoldering Waldenström macroglobulinemia: long‐term results. Blood. 2012;119:4462‐4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kyle RA, Larson DR, Therneau TM, et al. Long‐term follow‐up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378:241‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström's macroglobulinemia. N Engl J Med. 2012;367:826‐833. [DOI] [PubMed] [Google Scholar]
- 5. Xu L, Hunter ZR, Tsakmaklis N, et al. Clonal architecture of CXCR4 WHIM‐like mutations in Waldenström Macroglobulinaemia. Br J Haematol. 2016;172:735‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paiva B, Corchete LA, Vidriales MB, et al. The cellular origin and malignant transformation of Waldenström macroglobulinemia. Blood. 2015;125:2370‐2380. [DOI] [PubMed] [Google Scholar]
- 7. Rodriguez S, Celay J, Goicoechea I, et al. Preneoplastic somatic mutations including MYD88 L265P in lymphoplasmacytic lymphoma. Sci Adv. 2022;8:eabl4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baldini L, Goldaniga M, Guffanti A, et al. Immunoglobulin M monoclonal gammopathies of undetermined significance and indolent Waldenström's macroglobulinemia recognize the same determinants of evolution into symptomatic lymphoid disorders: proposal for a common prognostic scoring system. J Clin Oncol. 2005;23:4662‐4668. [DOI] [PubMed] [Google Scholar]
- 9. Bustoros M, Sklavenitis‐Pistofidis R, Kapoor P, et al. Progression risk stratification of asymptomatic waldenström macroglobulinemia. J Clin Oncol. 2019;37:1403‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moreno DF, Pereira A, Tovar N, et al. Defining an ultra‐low risk group in asymptomatic IgM monoclonal gammopathy. Cancers. 2021;13:2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Varettoni M, Zibellini S, Boveri E, et al. A risk‐stratification model based on the initial concentration of the serum monoclonal protein and myd88 mutation status identifies a subset of patients with IgM monoclonal gammopathy of undetermined significance at high risk of progression to Waldenström macroglobulinaemia or other lymphoproliferative disorders. Br J Haematol. 2019;187:441‐446. [DOI] [PubMed] [Google Scholar]
- 12. Moreno DF, López‐Guerra M, Paz S, et al. Prognostic impact of myd88 and cxcr4 mutations assessed by droplet digital polymerase chain reaction in igm monoclonal gammopathy of undetermined significance and smouldering Waldenström macroglobulinaemia. Br J Haematol. 2023;200:187‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McMaster ML. The epidemiology of Waldenström macroglobulinemia. Sem Hematol. 2023;60:65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kristinsson SY, Eloranta S, Dickman PW, et al. Patterns of survival in lymphoplasmacytic lymphoma/waldenström macroglobulinemia: a population‐based study of 1,555 patients diagnosed in Sweden from 1980 to 2005. Am J Hematol. 2013;88:60‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amaador K, Kersten MJ, Visser O, et al. Conditional relative survival in Waldenström's macroglobulinaemia: a population‐based study in The Netherlands. Br J Haematol. 2022;196:1205‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gertz MA. Waldenström macroglobulinemia: 2023 update on diagnosis, risk stratification, and management. Am J Hematol. 2023;98:348‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kyle RA, Therneau TM, Rajkumar SV, et al. A long‐term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564‐569. [DOI] [PubMed] [Google Scholar]
- 18. Treon SP, Tedeschi A, San‐Miguel J, et al. Report of consensus Panel 4 from the 11th International Workshop on Waldenstrom's macroglobulinemia on diagnostic and response criteria. Sem Hematol. 2023;60:97‐106. [DOI] [PubMed] [Google Scholar]
- 19. Owen RG, Treon SP, Al‐Katib A, et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30:110‐115. [DOI] [PubMed] [Google Scholar]
- 20. Dimopoulos MA, Kastritis E, Owen RG, et al. Treatment recommendations for patients with Waldenström macroglobulinemia (WM) and related disorders: IWWM‐7 consensus. Blood. 2014;124:1404‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pérez‐Persona E, Vidriales MB, Mateo G, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586‐2592. [DOI] [PubMed] [Google Scholar]
- 22. Evens AM, Danilov A, Jagadeesh D, et al. Burkitt lymphoma in the modern era: real‐world outcomes and prognostication across 30 US cancer centers. Blood. 2021;137:374‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dickman PW, Coviello E. Estimating and modeling relative survival. Stata J. 2015;15:186‐215. [Google Scholar]
- 24. Paiva B, Montes MC, García‐Sanz R, et al. Multiparameter flow cytometry for the identification of the Waldenström's clone in IgM‐MGUS and Waldenström's Macroglobulinemia: new criteria for differential diagnosis and risk stratification. Leukemia. 2014;28:166‐173. [DOI] [PubMed] [Google Scholar]
- 25. Campo E, Jaffe ES, Cook JR, et al. The International Consensus Classification of mature lymphoid neoplasms: a report from the clinical advisory committee. Blood. 2022;140:1229‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538‐e548. [DOI] [PubMed] [Google Scholar]
- 27. Treon SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenström macroglobulinemia. Blood. 2014;123:2791‐2796. [DOI] [PubMed] [Google Scholar]
- 28. Marcadis AR, Marti JL, Ehdaie B, Hakimi AA, Davies L, Morris LGT. Characterizing relative and disease‐specific survival in early‐stage cancers. JAMA Intern Med. 2020;180:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


