Abstract
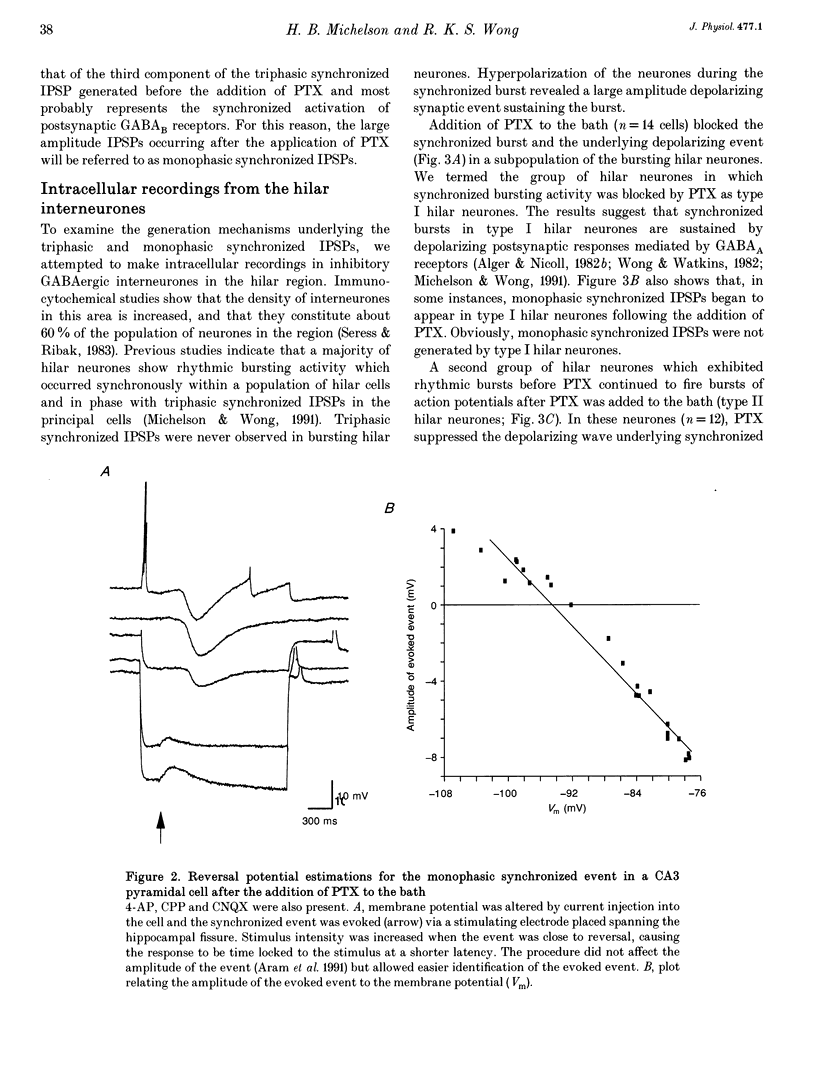
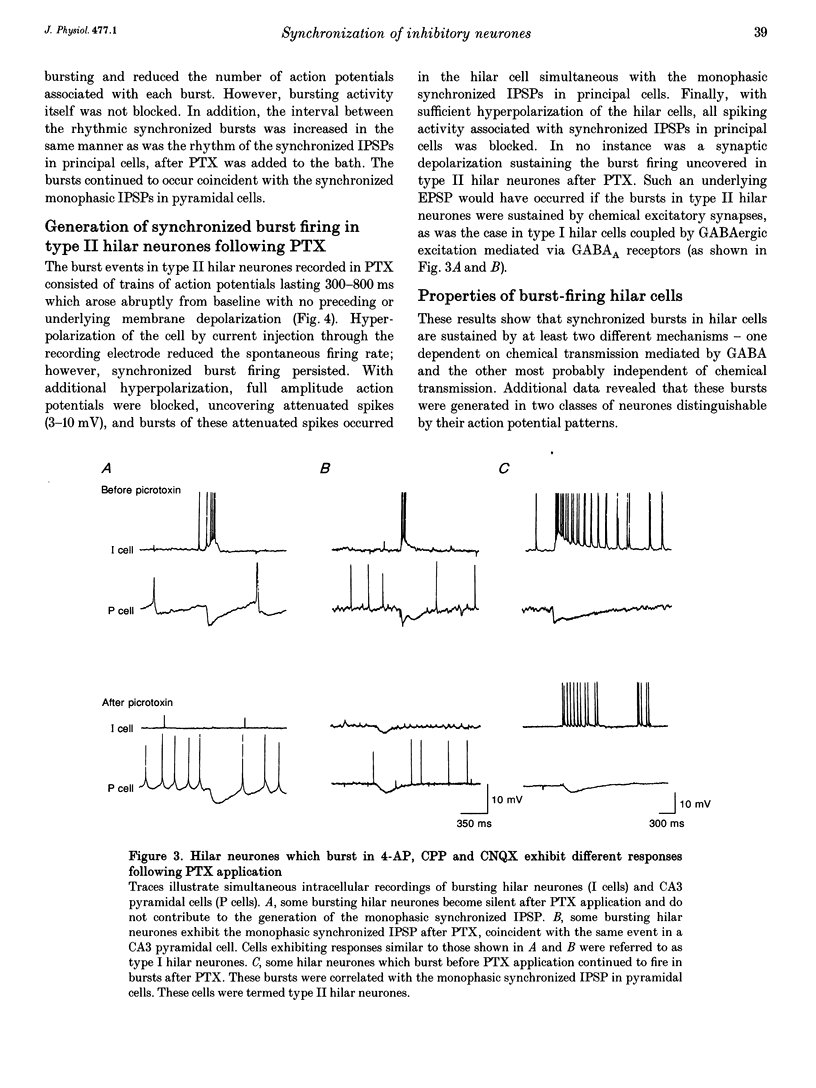
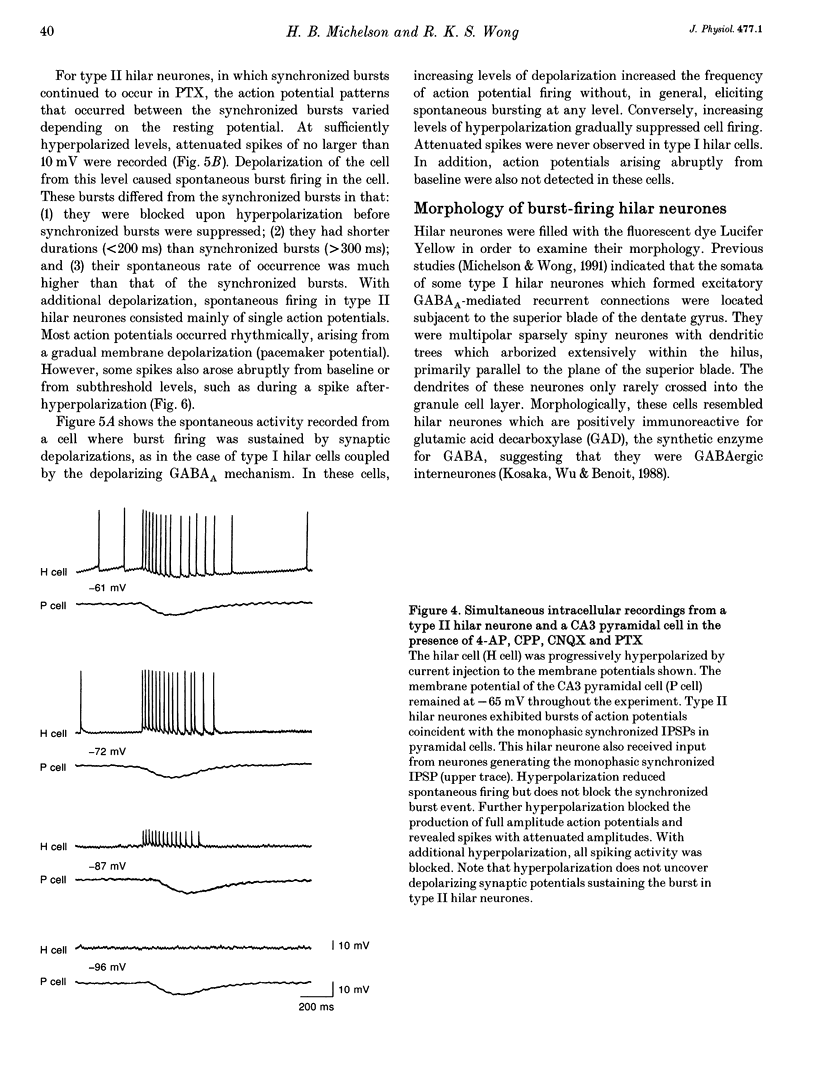
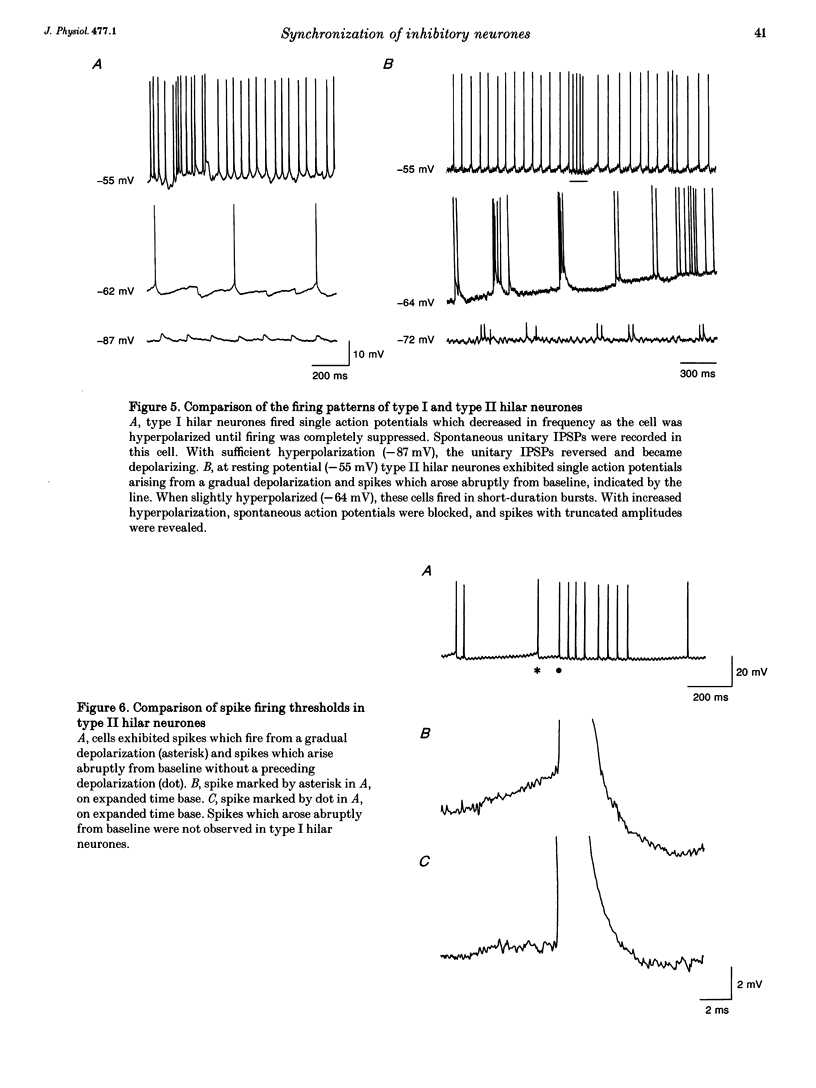
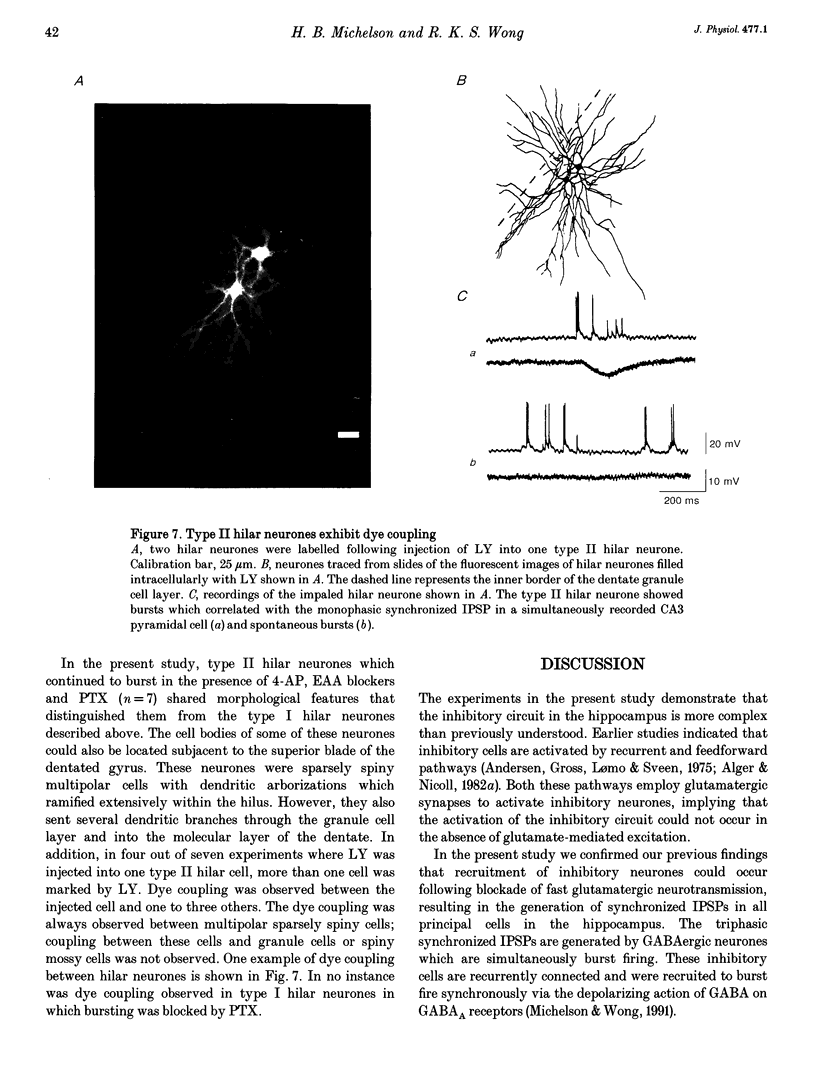
1. Intracellular recordings were obtained from pyramidal, granule and hilar cells in transverse slices of guinea-pig hippocampus to examine synaptic interactions between GABAergic neurones. 2. In the presence of the convulsant compound 4-aminopyridine (4-AP), after fast excitatory amino acid (EAA) neurotransmission was blocked pharmacologically, large amplitude inhibitory postsynaptic potentials (IPSPs) occurred rhythmically (every 4-8 s) and synchronously in all principal cell populations (triphasic synchronized IPSPs). In the presence of the GABAA receptor blocker picrotoxin (PTX), a large amplitude IPSP continued to occur spontaneously in all principal cells simultaneously (monophasic synchronized IPSP). 3. Burst firing occurred simultaneously in a group of hilar neurones (synchronized bursting neurones) coincident with triphasic synchronized IPSPs in principal cells. After PTX was added, the bursts and the underlying depolarizing synaptic potentials were completely suppressed in some of the synchronized bursting neurones (type I hilar neurones), while others (type II hilar neurones) continued to fire in bursts coincident with monophasic synchronized IPSPs in principal cells. Intense hyperpolarization blocked burst firing and revealed underlying attenuated spikes of less than 10 mV, but did not uncover any underlying depolarizing synaptic potentials. 4. In type II hilar neurones, during sufficient hyperpolarization, spontaneous activity consisted of attenuated spikes. With depolarization, the small spikes began to trigger full size action potentials. These data suggest the presence of electrotonically remote spike initiation sites. 5. The morphology of synchronized bursting neurones was revealed by intracellular injection of the fluorescent dye Lucifer Yellow. Attempts to inject dye into one type II hilar neurone often resulted in the labelling of two to four cells (dye coupling). Dye coupling was not observed in type I hilar neurones. 6. These findings indicate that excitatory interactions synchronizing the firing of GABAergic neurones can occur in the absence of fast EAA neurotransmission. GABAergic neurones can become synchronized via their recurrent collaterals through the depolarizing action of synaptically activated GABAA receptors. In addition, a subpopulation of GABAergic neurones can become synchronized by a mechanism probably involving electrotonic coupling.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D. G. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978 Dec 15;182(4 Pt 2):851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Andersen P., Blackstad T. W., Lömo T. Location and identification of excitatory synapses on hippocampal pyramidal cells. Exp Brain Res. 1966;1(3):236–248. doi: 10.1007/BF00234344. [DOI] [PubMed] [Google Scholar]
- Andersen P., Dingledine R., Gjerstad L., Langmoen I. A., Laursen A. M. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980 Aug;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aram J. A., Michelson H. B., Wong R. K. Synchronized GABAergic IPSPs recorded in the neocortex after blockade of synaptic transmission mediated by excitatory amino acids. J Neurophysiol. 1991 May;65(5):1034–1041. doi: 10.1152/jn.1991.65.5.1034. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Benardo L. S., Prince D. A. Coupling between neurons of the developing rat neocortex. J Neurosci. 1983 Apr;3(4):773–782. doi: 10.1523/JNEUROSCI.03-04-00773.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott P. L., Somogyi P. Quantitative distribution of GABA-immunoreactive neurons in the visual cortex (area 17) of the cat. Exp Brain Res. 1986;61(2):323–331. doi: 10.1007/BF00239522. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Schwark H. D., Jones E. G., Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987 May;7(5):1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Katsumaru H., Kosaka T., Heizmann C. W., Hama K. Gap junctions on GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus (CA1 region). Exp Brain Res. 1988;72(2):363–370. doi: 10.1007/BF00250257. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Hama K. Two subtypes of non-pyramidal cells in rat hippocampal formation identified by intracellular recording and HRP injection. Brain Res. 1987 May 12;411(1):190–195. doi: 10.1016/0006-8993(87)90700-1. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Hama K. Gap junctions between non-pyramidal cell dendrites in the rat hippocampus (CA1 and CA3 regions): a combined Golgi-electron microscopy study. J Comp Neurol. 1985 Jan 8;231(2):150–161. doi: 10.1002/cne.902310203. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Katsumaru H., Hama K., Wu J. Y., Heizmann C. W. GABAergic neurons containing the Ca2+-binding protein parvalbumin in the rat hippocampus and dentate gyrus. Brain Res. 1987 Sep 1;419(1-2):119–130. doi: 10.1016/0006-8993(87)90575-0. [DOI] [PubMed] [Google Scholar]
- Kosaka T. Neuronal gap junctions in the polymorph layer of the rat dentate gyrus. Brain Res. 1983 Oct 31;277(2):347–351. doi: 10.1016/0006-8993(83)90943-5. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Wu J. Y., Benoit R. GABAergic neurons containing somatostatin-like immunoreactivity in the rat hippocampus and dentate gyrus. Exp Brain Res. 1988;71(2):388–398. doi: 10.1007/BF00247498. [DOI] [PubMed] [Google Scholar]
- Lacaille J. C., Schwartzkroin P. A. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. II. Intrasomatic and intradendritic recordings of local circuit synaptic interactions. J Neurosci. 1988 Apr;8(4):1411–1424. doi: 10.1523/JNEUROSCI.08-04-01411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert N. A., Levitin M., Harrison N. L. Induction of giant depolarizing potentials by zinc in area CA1 of the rat hippocampus does not result from block of GABAB receptors. Neurosci Lett. 1992 Feb 3;135(2):215–218. doi: 10.1016/0304-3940(92)90439-e. [DOI] [PubMed] [Google Scholar]
- Michelson H. B., Wong R. K. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991 Sep 20;253(5026):1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol. 1986 Apr;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Latent synaptic pathways revealed after tetanic stimulation in the hippocampus. Nature. 1987 Oct 22;329(6141):724–726. doi: 10.1038/329724a0. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983 Nov 24;306(5941):371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Unitary inhibitory synaptic potentials in the guinea-pig hippocampus in vitro. J Physiol. 1984 Nov;356:97–113. doi: 10.1113/jphysiol.1984.sp015455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld U., Bijak M., Brunner H., Dembowsky K. K-dependent inhibition in the dentate-CA3 network of guinea pig hippocampal slices. J Neurophysiol. 1992 Nov;68(5):1548–1557. doi: 10.1152/jn.1992.68.5.1548. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Deisz R. A., Dodt H. U., Lux H. D. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986 Jun 13;232(4756):1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Müller W., Misgeld U. Picrotoxin- and 4-aminopyridine-induced activity in hilar neurons in the guinea pig hippocampal slice. J Neurophysiol. 1991 Jan;65(1):141–147. doi: 10.1152/jn.1991.65.1.141. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Obenaus A., Esclapez M., Houser C. R. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993 Oct;13(10):4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault P., Avoli M. 4-aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci. 1992 Jan;12(1):104–115. doi: 10.1523/JNEUROSCI.12-01-00104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault P., Avoli M. Effects of low concentrations of 4-aminopyridine on CA1 pyramidal cells of the hippocampus. J Neurophysiol. 1989 May;61(5):953–970. doi: 10.1152/jn.1989.61.5.953. [DOI] [PubMed] [Google Scholar]
- Perreault P., Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991 Apr;65(4):771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- Prince D. A. Neurophysiology of epilepsy. Annu Rev Neurosci. 1978;1:395–415. doi: 10.1146/annurev.ne.01.030178.002143. [DOI] [PubMed] [Google Scholar]
- Rutecki P. A., Lebeda F. J., Johnston D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol. 1987 Jun;57(6):1911–1924. doi: 10.1152/jn.1987.57.6.1911. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A. Characteristics of CA1 neurons recorded intracellularly in the hippocampal in vitro slice preparation. Brain Res. 1975 Mar 7;85(3):423–436. doi: 10.1016/0006-8993(75)90817-3. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Prince D. A. Penicillin-induced epileptiform activity in the hippocampal in vitro prepatation. Ann Neurol. 1977 May;1(5):463–469. doi: 10.1002/ana.410010510. [DOI] [PubMed] [Google Scholar]
- Segal M. Repetitive inhibitory postsynaptic potentials evoked by 4-aminopyridine in hippocampal neurons in vitro. Brain Res. 1987 Jun 30;414(2):285–293. doi: 10.1016/0006-8993(87)90008-4. [DOI] [PubMed] [Google Scholar]
- Seress L., Ribak C. E. GABAergic cells in the dentate gyrus appear to be local circuit and projection neurons. Exp Brain Res. 1983;50(2-3):173–182. doi: 10.1007/BF00239181. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987 Jan 2;235(4784):73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Voskuyl R. A., Albus H. Spontaneous epileptiform discharges in hippocampal slices induced by 4-aminopyridine. Brain Res. 1985 Sep 2;342(1):54–66. doi: 10.1016/0006-8993(85)91352-6. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Watkins D. J. Cellular factors influencing GABA response in hippocampal pyramidal cells. J Neurophysiol. 1982 Oct;48(4):938–951. doi: 10.1152/jn.1982.48.4.938. [DOI] [PubMed] [Google Scholar]
- Xie X. M., Smart T. G. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 1991 Feb 7;349(6309):521–524. doi: 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]



