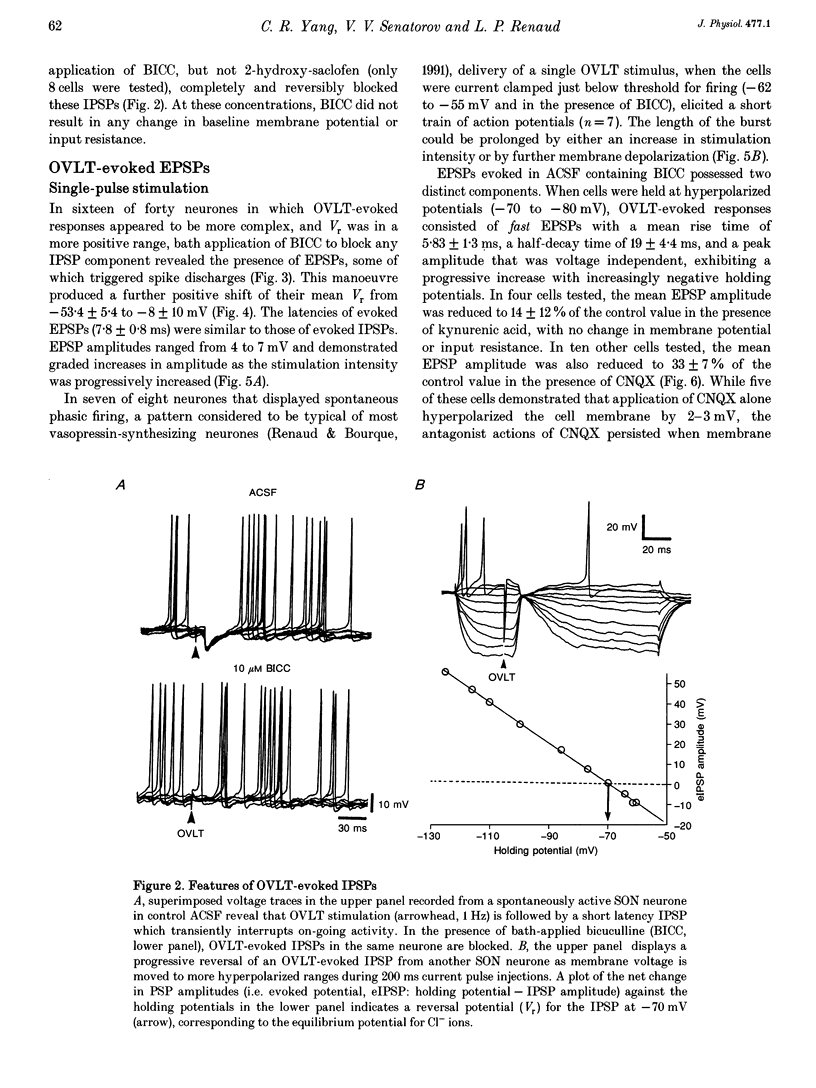
Abstract
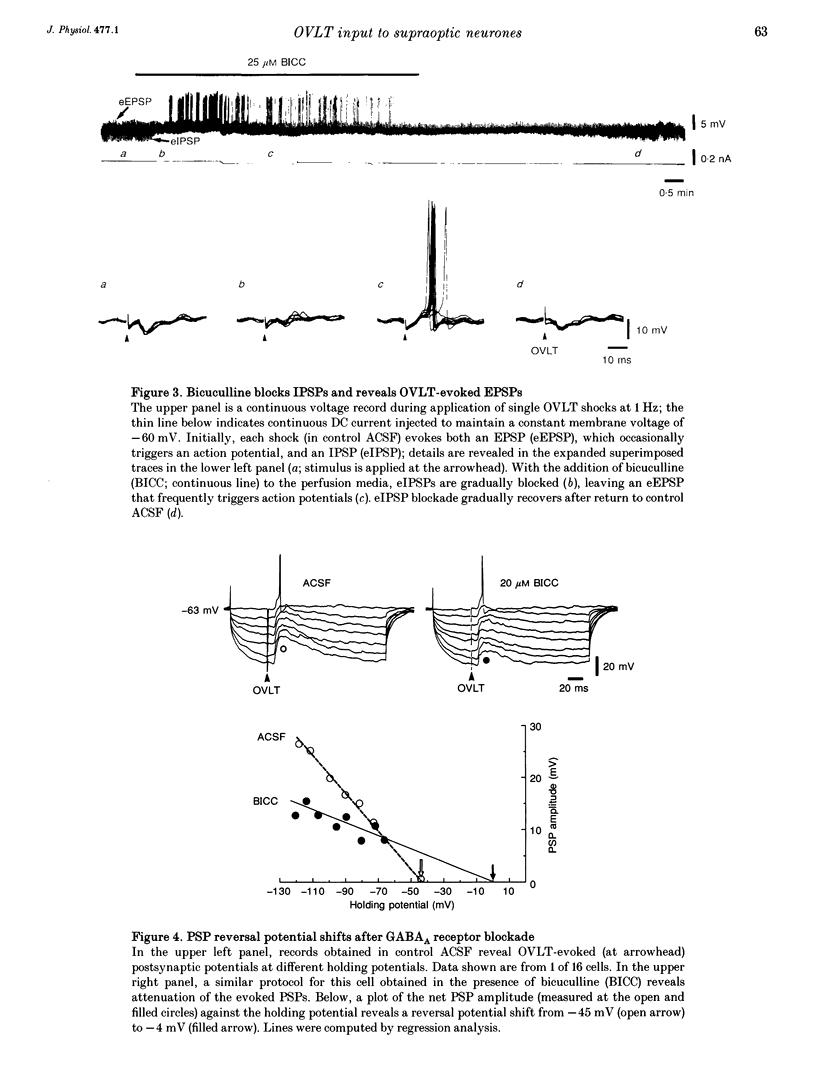
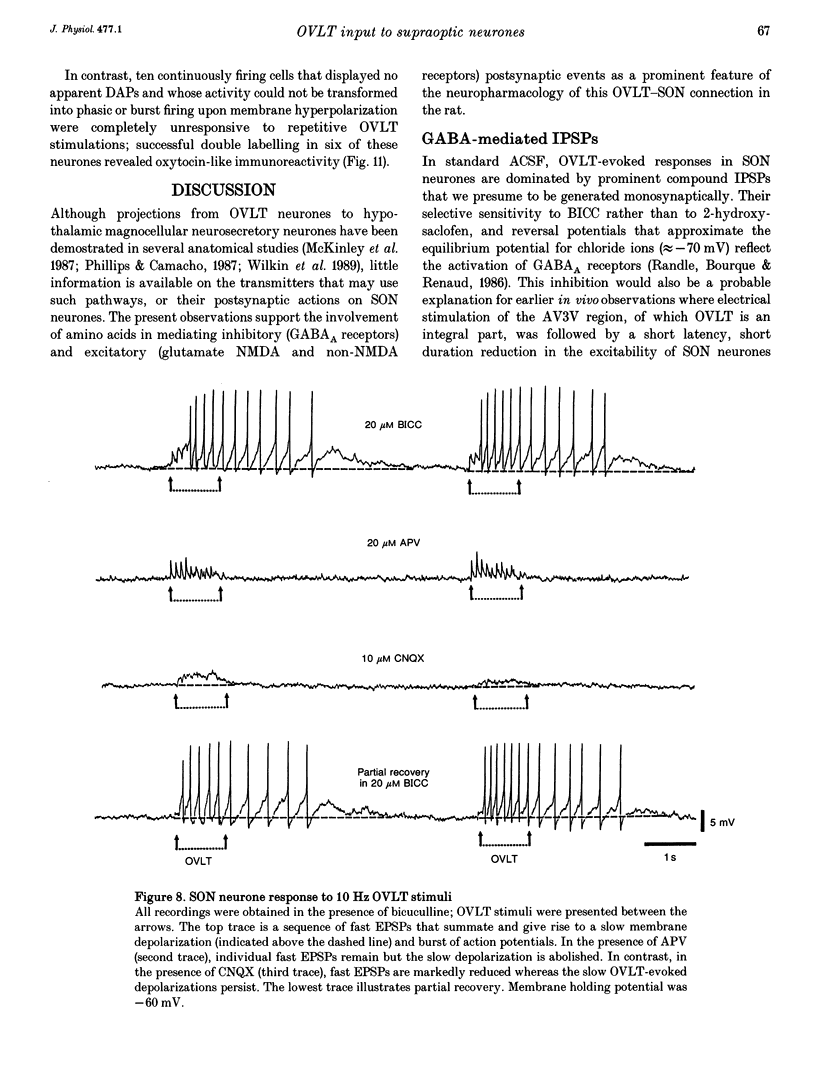
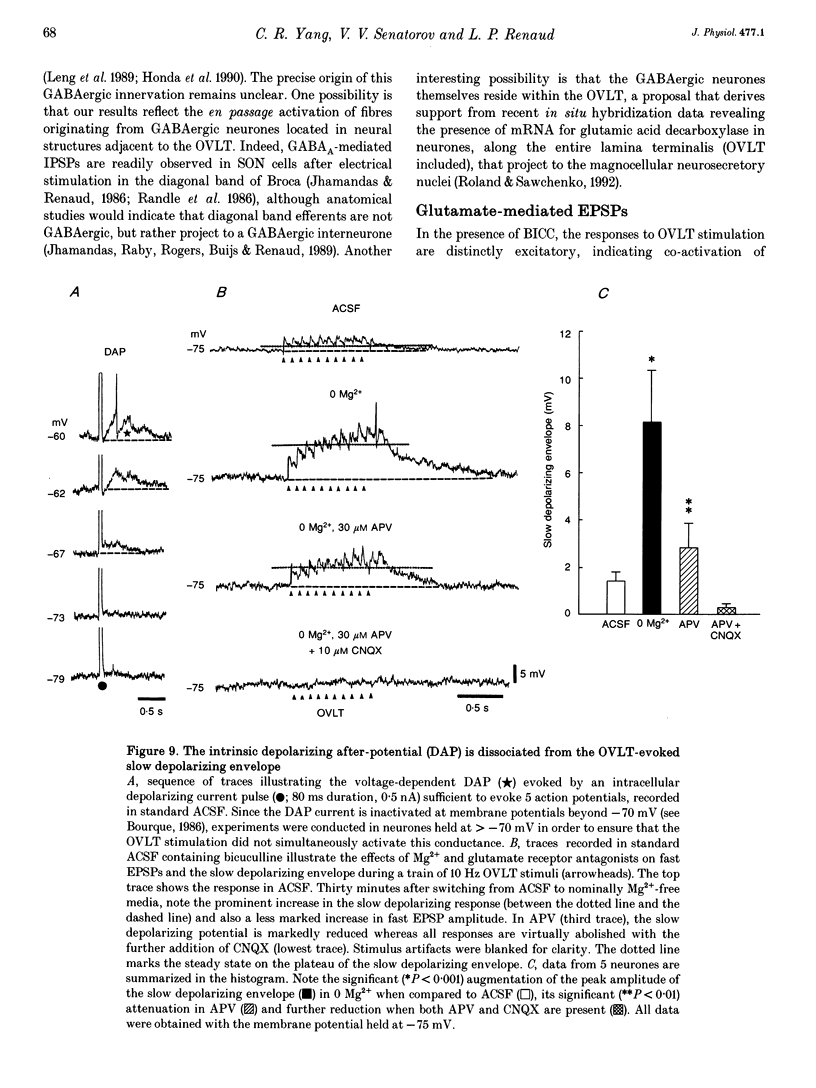
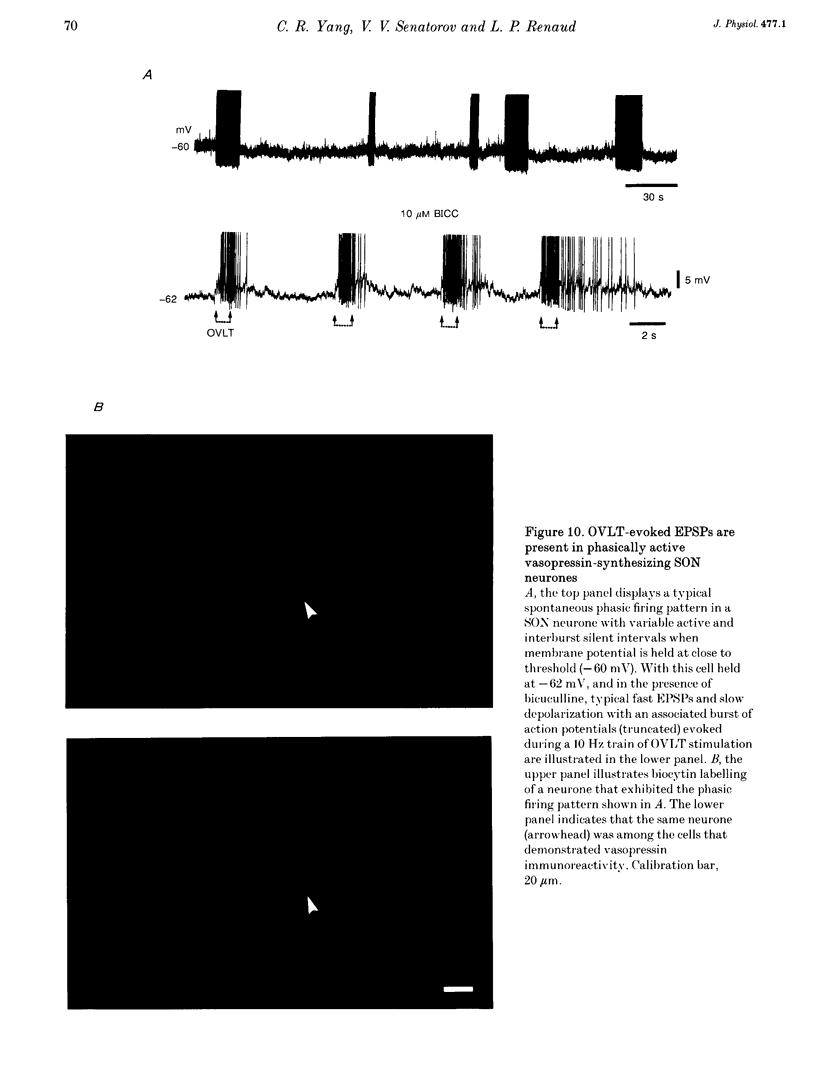
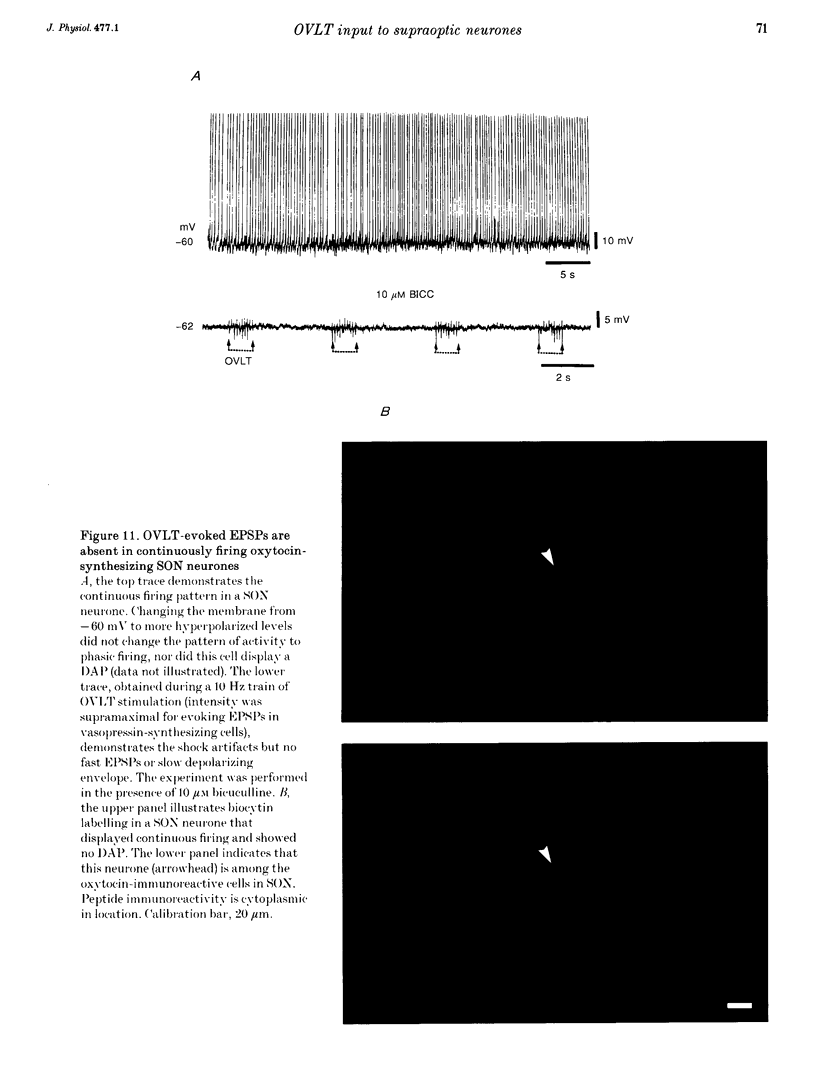
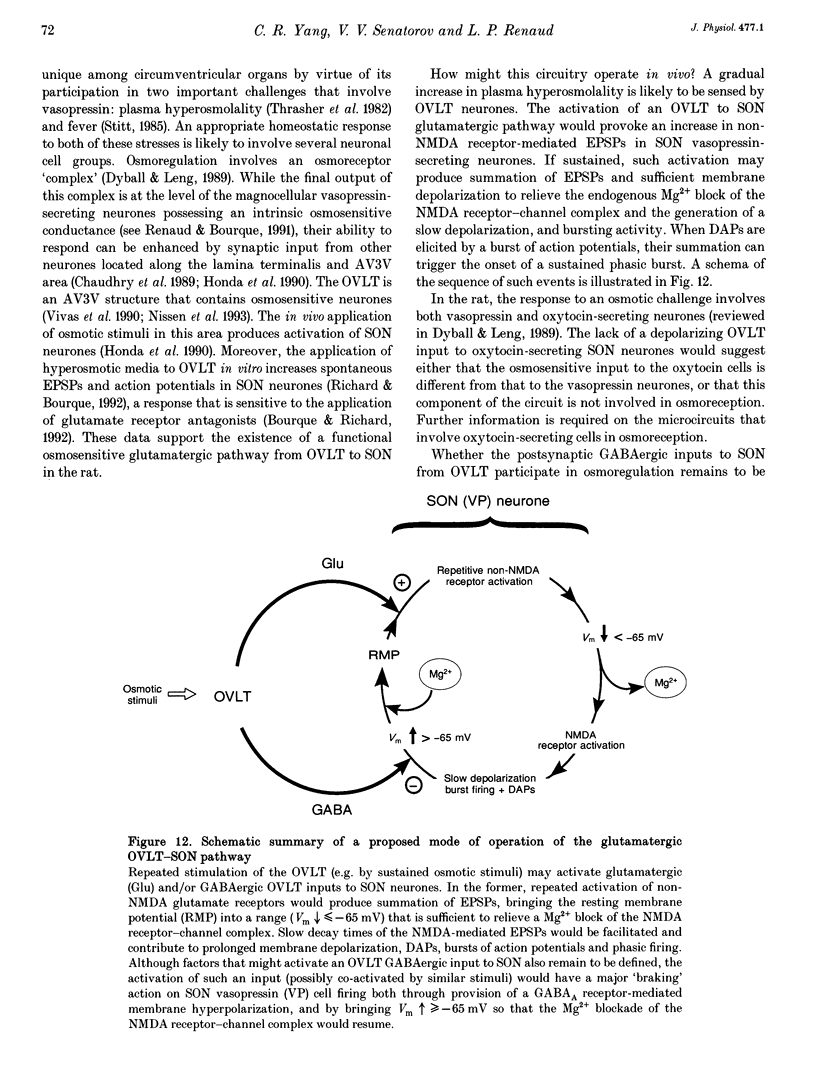
1. To characterize the organum vasculosum lamina terminalis (OVLT) innervation of hypothalamic supraoptic nucleus (SON) neurones, current clamp recordings were obtained in SON cells in superfused rat hypothalamic explants. Stimulation of 1 Hz evoked 5-10 mV bicuculline-sensitive IPSPs in forty out of forty-six SON neurones, including both phasic (vasopressin immunoreactive) and continuously firing (oxytocin immunoreactive) cells. 2. In twenty-four cells, mean IPSP latency was 8.7 +/- 1 ms (+/- S.D.) and reversal potentials (Vr) ranged between -60 and -75 mV. In the other sixteen cells, Vr ranged between -20 and -55 mV and the addition of bicuculline revealed underlying EPSPs (latency, 7.8 +/- 0.8 ms; mean Vr, -8 +/- 10 mV) with two components: (a) fast (rise and half-decay times of 5.83 +/- 1.3 ms and 19 +/- 4.4 ms respectively), with reversible blockade by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX); (b) slow (4- to 5-fold increase in rise and half-decay time), with reversible reduction by (-)-aminophosphonovaleric acid (APV). 3. During 10 Hz stimulation, EPSPs summated into 3-7 mV depolarizing envelopes lasting 1.5-3.0 s and sustaining action potential bursts. Depolarizing envelopes displayed voltage dependence, and were enhanced after removal of extracellular magnesium, diminished by APV and completely abolished by APV and CNQX together. 4. Thus, non-NMDA receptors probably mediate fast EPSPs whereas NMDA receptors mediate slow EPSPs and depolarizing envelopes. OVLT-evoked EPSPs were only seen in vasopressin-immunoreactive neurones. 5. These observations indicate converging inhibitory and target-selective excitatory amino acid-mediated inputs from OVLT to SON; the latter may modulate the excitability of SON vasopressin neurones to a hyperosmotic challenge.
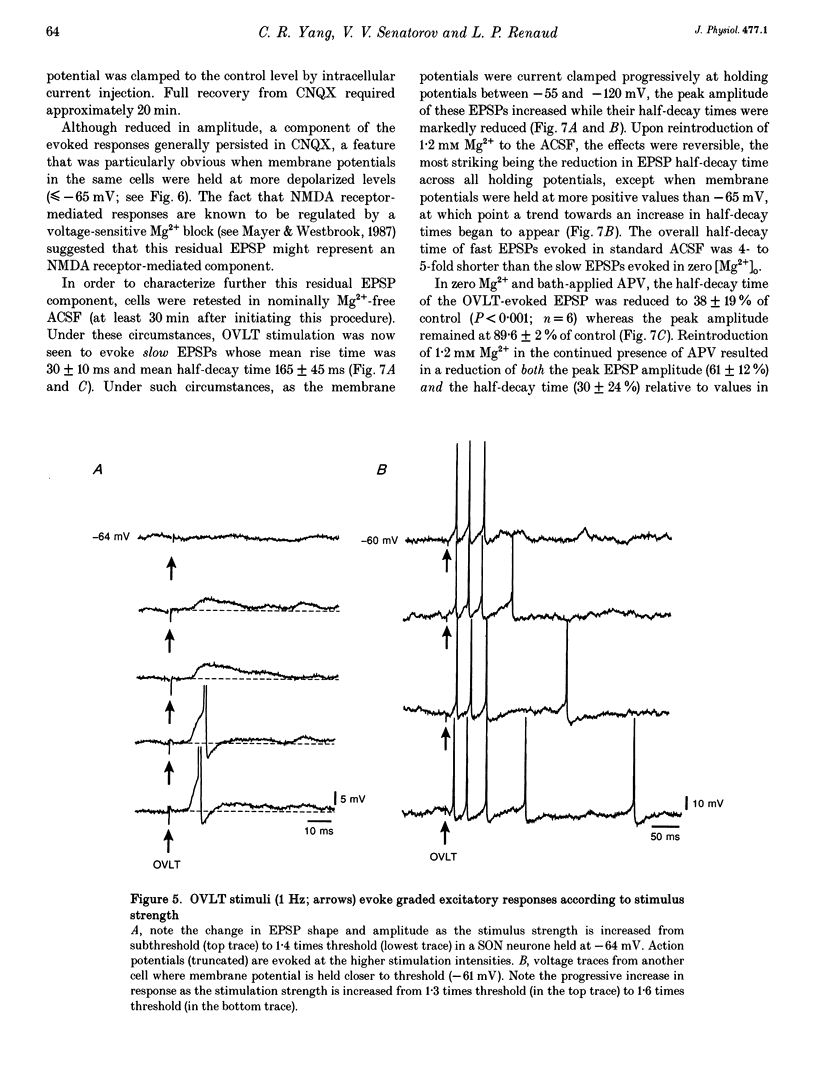
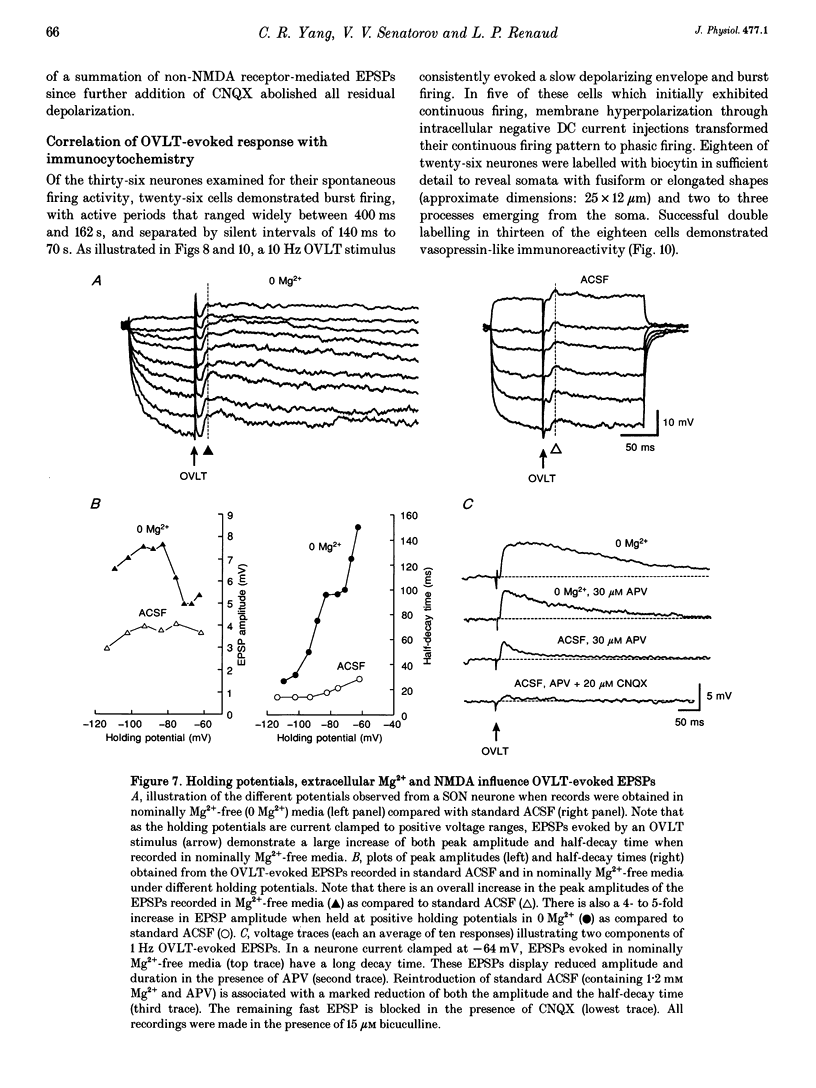
Full text
PDF


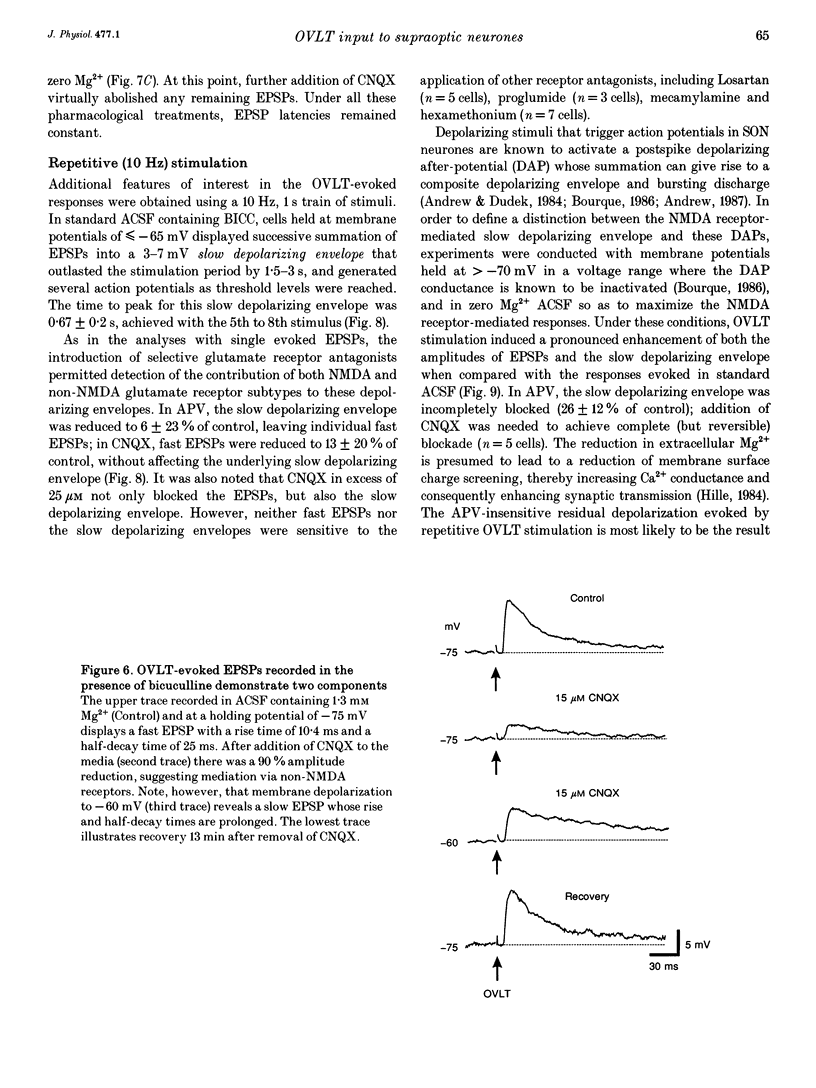



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew R. D., Dudek F. E. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol. 1984 Mar;51(3):552–566. doi: 10.1152/jn.1984.51.3.552. [DOI] [PubMed] [Google Scholar]
- Andrew R. D. Endogenous bursting by rat supraoptic neuroendocrine cells is calcium dependent. J Physiol. 1987 Mar;384:451–465. doi: 10.1113/jphysiol.1987.sp016463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn R. E., Leng G., Russell J. A. Control of magnocellular oxytocin neurones by the region anterior and ventral to the third ventricle (AV3V region) in rats. J Endocrinol. 1987 Aug;114(2):253–261. doi: 10.1677/joe.0.1140253. [DOI] [PubMed] [Google Scholar]
- Bourque C. W. Calcium-dependent spike after-current induces burst firing in magnocellular neurosecretory cells. Neurosci Lett. 1986 Oct 8;70(2):204–209. doi: 10.1016/0304-3940(86)90464-7. [DOI] [PubMed] [Google Scholar]
- Bourque C. W. Ionic basis for the intrinsic activation of rat supraoptic neurones by hyperosmotic stimuli. J Physiol. 1989 Oct;417:263–277. doi: 10.1113/jphysiol.1989.sp017800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry M. A., Dyball R. E., Honda K., Wright N. C. The role of interconnection between supraoptic nucleus and anterior third ventricular region in osmoregulation in the rat. J Physiol. 1989 Mar;410:123–135. doi: 10.1113/jphysiol.1989.sp017524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 1988 May;399:301–312. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. R., Ronnekleiv O. K., Kelly M. J. Electrophysiology of guinea-pig supraoptic neurones: role of a hyperpolarization-activated cation current in phasic firing. J Physiol. 1993 Jan;460:407–425. doi: 10.1113/jphysiol.1993.sp019478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff V. K., Dudek F. E. Effects of excitatory amino acid antagonists on synaptic responses of supraoptic neurons in slices of rat hypothalamus. J Neurophysiol. 1990 Jan;63(1):60–71. doi: 10.1152/jn.1990.63.1.60. [DOI] [PubMed] [Google Scholar]
- Gribkoff V. K. Electrophysiological evidence for N-methyl-D-aspartate excitatory amino acid receptors in the rat supraoptic nucleus in vitro. Neurosci Lett. 1991 Oct 14;131(2):260–262. doi: 10.1016/0304-3940(91)90628-7. [DOI] [PubMed] [Google Scholar]
- Herron C. E., Lester R. A., Coan E. J., Collingridge G. L. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986 Jul 17;322(6076):265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Honda K., Negoro H., Dyball R. E., Higuchi T., Takano S. The osmoreceptor complex in the rat: evidence for interactions between the supraoptic and other diencephalic nuclei. J Physiol. 1990 Dec;431:225–241. doi: 10.1113/jphysiol.1990.sp018328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas J. H., Raby W., Rogers J., Buijs R. M., Renaud L. P. Diagonal band projection towards the hypothalamic supraoptic nucleus: light and electron microscopic observations in the rat. J Comp Neurol. 1989 Apr 1;282(1):15–23. doi: 10.1002/cne.902820103. [DOI] [PubMed] [Google Scholar]
- Jhamandas J. H., Renaud L. P. A gamma-aminobutyric-acid-mediated baroreceptor input to supraoptic vasopressin neurones in the rat. J Physiol. 1986 Dec;381:595–606. doi: 10.1113/jphysiol.1986.sp016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. K. The periventricular anteroventral third ventricle (AV3V): its relationship with the subfornical organ and neural systems involved in maintaining body fluid homeostasis. Brain Res Bull. 1985 Dec;15(6):595–601. doi: 10.1016/0361-9230(85)90209-6. [DOI] [PubMed] [Google Scholar]
- Krisch B., Leonhardt H., Buchheim W. The functional and structural border between the CSF- and blood-milieu in the circumventricular organs (organum vasculosum laminae terminalis, subfornical organ, area postrema) of the rat. Cell Tissue Res. 1978 Dec 29;195(3):485–497. doi: 10.1007/BF00233891. [DOI] [PubMed] [Google Scholar]
- Lester R. A., Quarum M. L., Parker J. D., Weber E., Jahr C. E. Interaction of 6-cyano-7-nitroquinoxaline-2,3-dione with the N-methyl-D-aspartate receptor-associated glycine binding site. Mol Pharmacol. 1989 May;35(5):565–570. [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- McAllen R. M., Pennington G. L., McKinley M. J. Osmoresponsive units in sheep median preoptic nucleus. Am J Physiol. 1990 Sep;259(3 Pt 2):R593–R600. doi: 10.1152/ajpregu.1990.259.3.R593. [DOI] [PubMed] [Google Scholar]
- McKinley M. J., Denton D. A., Coghlan J. P., Harvey R. B., McDougall J. G., Rundgren M., Scoggins B. A., Weisinger R. S. Cerebral osmoregulation of renal sodium excretion--a response analogous to thirst and vasopressin release. Can J Physiol Pharmacol. 1987 Aug;65(8):1724–1729. doi: 10.1139/y87-271. [DOI] [PubMed] [Google Scholar]
- Nissen R., Bourque C. W., Renaud L. P. Membrane properties of organum vasculosum lamina terminalis neurons recorded in vitro. Am J Physiol. 1993 Apr;264(4 Pt 2):R811–R815. doi: 10.1152/ajpregu.1993.264.4.R811. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Bourque C. W., Renaud L. P. Characterization of spontaneous and evoked inhibitory postsynaptic potentials in rat supraoptic neurosecretory neurons in vitro. J Neurophysiol. 1986 Dec;56(6):1703–1717. doi: 10.1152/jn.1986.56.6.1703. [DOI] [PubMed] [Google Scholar]
- Renaud L. P., Bourque C. W. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Prog Neurobiol. 1991;36(2):131–169. doi: 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- Richard D., Bourque C. W. Synaptic activation of rat supraoptic neurons by osmotic stimulation of the organum vasculosum lamina terminalis. Neuroendocrinology. 1992 May;55(5):609–611. doi: 10.1159/000126174. [DOI] [PubMed] [Google Scholar]
- Sibbald J. R., Hubbard J. I., Sirett N. E. Responses from osmosensitive neurons of the rat subfornical organ in vitro. Brain Res. 1988 Oct 4;461(2):205–214. doi: 10.1016/0006-8993(88)90251-x. [DOI] [PubMed] [Google Scholar]
- Stitt J. T. Evidence for the involvement of the organum vasculosum laminae terminalis in the febrile response of rabbits and rats. J Physiol. 1985 Nov;368:501–511. doi: 10.1113/jphysiol.1985.sp015872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher T. N., Keil L. C., Ramsay D. J. Lesions of the organum vasculosum of the lamina terminalis (OVLT) attenuate osmotically-induced drinking and vasopressin secretion in the dog. Endocrinology. 1982 May;110(5):1837–1839. doi: 10.1210/endo-110-5-1837. [DOI] [PubMed] [Google Scholar]
- Vivas L., Chiaraviglio E., Carrer H. F. Rat organum vasculosum laminae terminalis in vitro: responses to changes in sodium concentration. Brain Res. 1990 Jun 11;519(1-2):294–300. doi: 10.1016/0006-8993(90)90091-o. [DOI] [PubMed] [Google Scholar]
- Wilkin L. D., Mitchell L. D., Ganten D., Johnson A. K. The supraoptic nucleus: afferents from areas involved in control of body fluid homeostasis. Neuroscience. 1989;28(3):573–584. doi: 10.1016/0306-4522(89)90006-7. [DOI] [PubMed] [Google Scholar]
- Yang C. R., Phillips M. I., Renaud L. P. Angiotensin II receptor activation depolarizes rat supraoptic neurons in vitro. Am J Physiol. 1992 Dec;263(6 Pt 2):R1333–R1338. doi: 10.1152/ajpregu.1992.263.6.R1333. [DOI] [PubMed] [Google Scholar]