Abstract
1. The effects of repeated injections of bacterial lipopolysaccharide (LPS) at 3 day intervals on abdominal temperature and systemic release of tumour necrosis factor alpha (TNF)-like and interleukin-6 (IL-6)-like activity were measured in guinea-pigs. 2. After the third injection of LPS the fever response was significantly attenuated. 3. TNF-like activity (peak 1 h after LPS injection) and IL-6-like activity (peak 3 h after LPS injection) in plasma changed correspondingly, both being significantly reduced after the third and subsequent injections of LPS. 4. The increase of IL-6-like activity in plasma after LPS injection correlated to the febrile change in body temperature. This correlation remained manifest throughout the whole time course of the development of endotoxin tolerance. 5. The reduced production of TNF-like activity after repeated injections of LPS correlated to the attenuation of the fever index, the integration of the thermal response after LPS application. 6. The results support the hypothesis that one component of the development of endotoxin tolerance is reduced production and release of cytokines in response to repeated injections of the same amount of LPS.
Full text
PDF


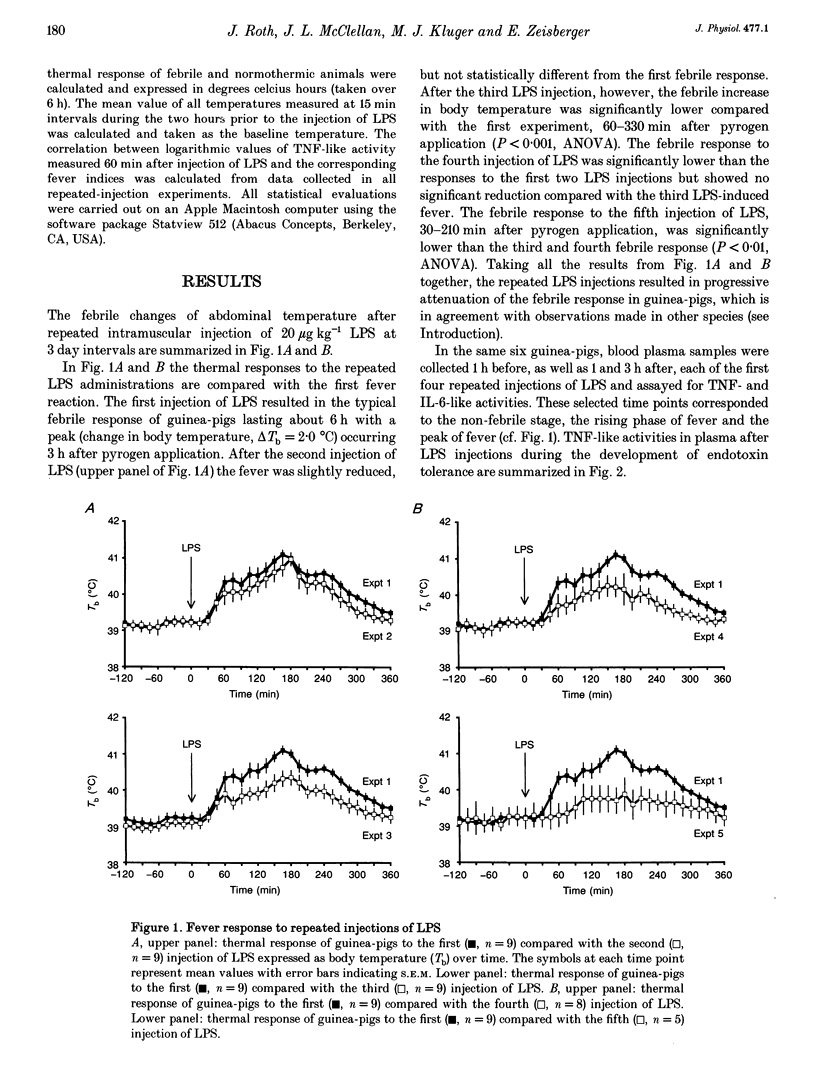
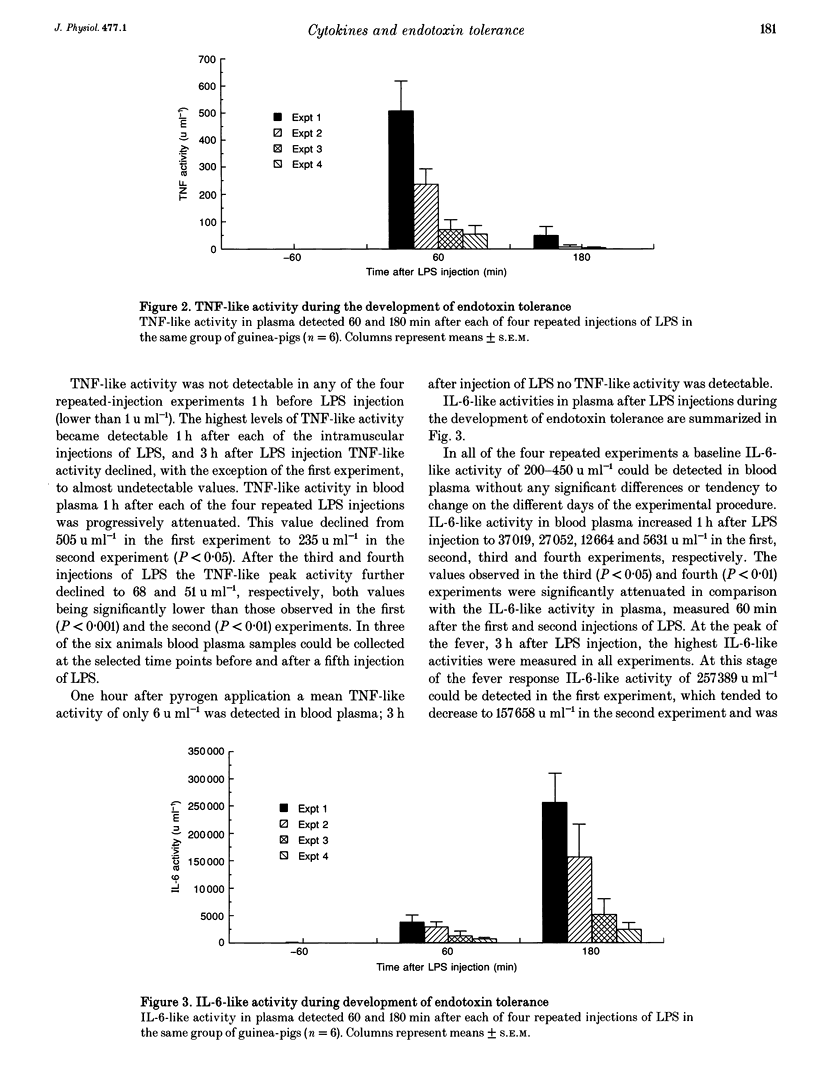
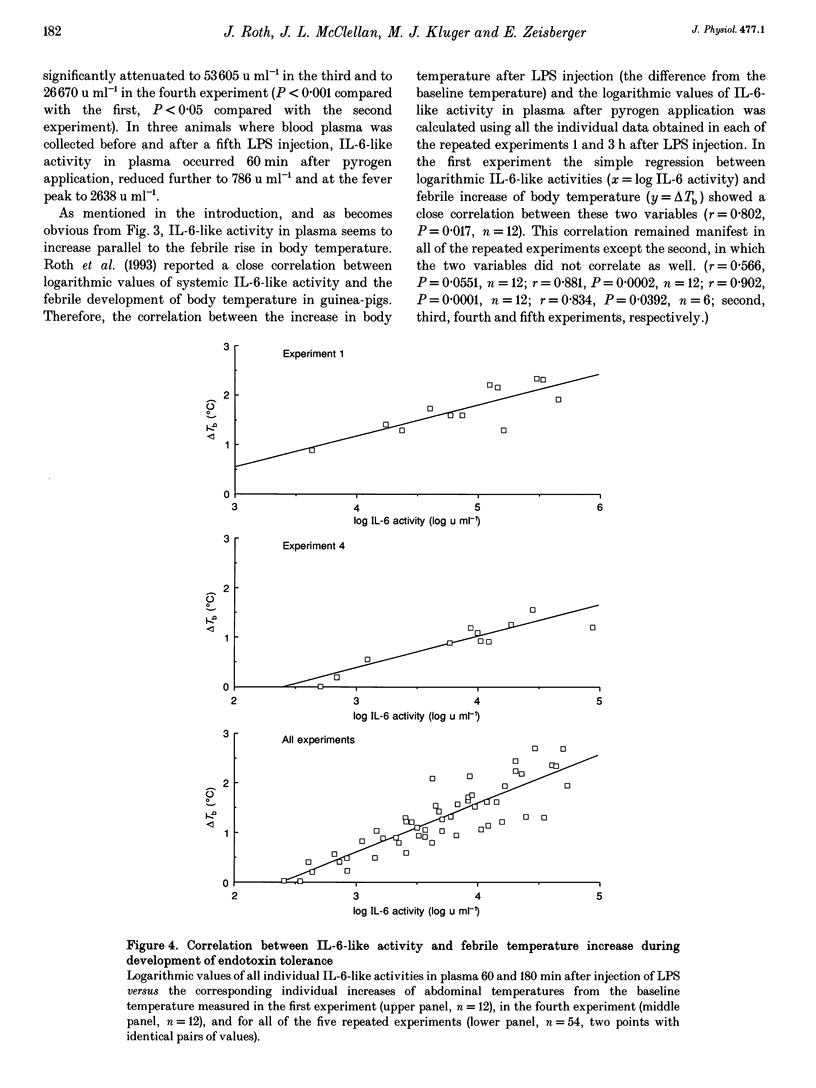
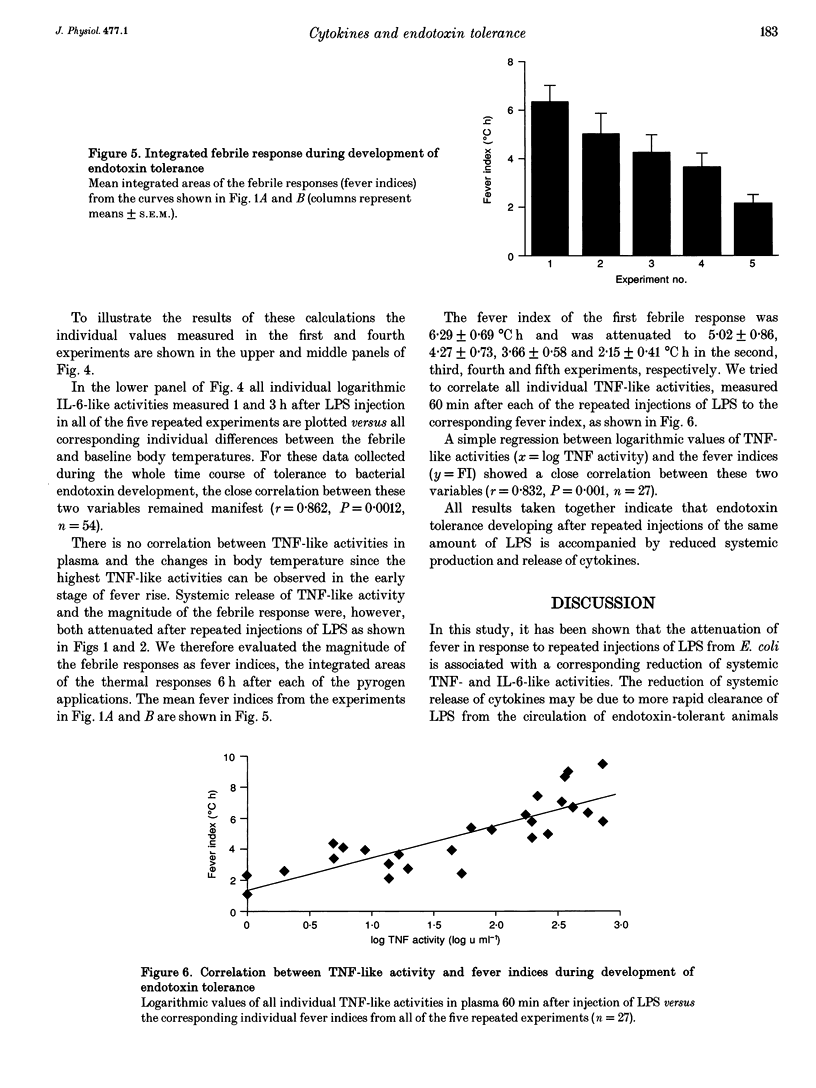


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINS E. Pathogenesis of fever. Physiol Rev. 1960 Jul;40:580–646. doi: 10.1152/physrev.1960.40.3.580. [DOI] [PubMed] [Google Scholar]
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Blatteis C. M. Neuromodulative actions of cytokines. Yale J Biol Med. 1990 Mar-Apr;63(2):133–146. [PMC free article] [PubMed] [Google Scholar]
- Blatteis C. M., Quan N., Xin L., Ungar A. L. Neuromodulation of acute-phase responses to interleukin-6 in guinea pigs. Brain Res Bull. 1990 Dec;25(6):895–901. doi: 10.1016/0361-9230(90)90185-3. [DOI] [PubMed] [Google Scholar]
- Cooper K. E., Blähser S., Malkinson T. J., Merker G., Roth J., Zeisberger E. Changes in body temperature and vasopressin content of brain neurons, in pregnant and non-pregnant guinea pigs, during fevers produced by Poly I:Poly C. Pflugers Arch. 1988 Aug;412(3):292–296. doi: 10.1007/BF00582511. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. The proinflammatory cytokines interleukin-1 and tumor necrosis factor and treatment of the septic shock syndrome. J Infect Dis. 1991 Jun;163(6):1177–1184. doi: 10.1093/infdis/163.6.1177. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Fischer E., Marano M. A., Van Zee K. J., Rock C. S., Hawes A. S., Thompson W. A., DeForge L., Kenney J. S., Remick D. G., Bloedow D. C. Interleukin-1 receptor blockade improves survival and hemodynamic performance in Escherichia coli septic shock, but fails to alter host responses to sublethal endotoxemia. J Clin Invest. 1992 May;89(5):1551–1557. doi: 10.1172/JCI115748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelst K., Laburn H. Response of body temperature and serum iron concentration to repeated pyrogen injection in rabbits. Pflugers Arch. 1991 Feb;417(6):558–561. doi: 10.1007/BF00372951. [DOI] [PubMed] [Google Scholar]
- He W., Fong Y., Marano M. A., Gershenwald J. E., Yurt R. W., Moldawer L. L., Lowry S. F. Tolerance to endotoxin prevents mortality in infected thermal injury: association with attenuated cytokine responses. J Infect Dis. 1992 May;165(5):859–864. doi: 10.1093/infdis/165.5.859. [DOI] [PubMed] [Google Scholar]
- Kluger M. J. Fever: role of pyrogens and cryogens. Physiol Rev. 1991 Jan;71(1):93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMay L. G., Otterness I. G., Vander A. J., Kluger M. J. In vivo evidence that the rise in plasma IL 6 following injection of a fever-inducing dose of LPS is mediated by IL 1 beta. Cytokine. 1990 May;2(3):199–204. doi: 10.1016/1043-4666(90)90016-m. [DOI] [PubMed] [Google Scholar]
- LeMay L. G., Vander A. J., Kluger M. J. Role of interleukin 6 in fever in rats. Am J Physiol. 1990 Mar;258(3 Pt 2):R798–R803. doi: 10.1152/ajpregu.1990.258.3.R798. [DOI] [PubMed] [Google Scholar]
- Long N. C., Kunkel S. L., Vander A. J., Kluger M. J. Antiserum against tumor necrosis factor enhances lipopolysaccharide fever in rats. Am J Physiol. 1990 Feb;258(2 Pt 2):R332–R337. doi: 10.1152/ajpregu.1990.258.2.R332. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Virca G. D., Wolfson E., Tobias P. S., Glaser K., Ulevitch R. J. Adaptation to bacterial lipopolysaccharide controls lipopolysaccharide-induced tumor necrosis factor production in rabbit macrophages. J Clin Invest. 1990 Apr;85(4):1108–1118. doi: 10.1172/JCI114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijsten M. W., de Groot E. R., ten Duis H. J., Klasen H. J., Hack C. E., Aarden L. A. Serum levels of interleukin-6 and acute phase responses. Lancet. 1987 Oct 17;2(8564):921–921. doi: 10.1016/s0140-6736(87)91413-9. [DOI] [PubMed] [Google Scholar]
- O'Reilly B., Vander A. J., Kluger M. J. Effects of chronic infusion of lipopolysaccharide on food intake and body temperature of the rat. Physiol Behav. 1988;42(3):287–291. doi: 10.1016/0031-9384(88)90084-4. [DOI] [PubMed] [Google Scholar]
- Rackow E. C., Astiz M. E. Pathophysiology and treatment of septic shock. JAMA. 1991 Jul 24;266(4):548–554. [PubMed] [Google Scholar]
- Raetz C. R., Ulevitch R. J., Wright S. D., Sibley C. H., Ding A., Nathan C. F. Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J. 1991 Sep;5(12):2652–2660. doi: 10.1096/fasebj.5.12.1916089. [DOI] [PubMed] [Google Scholar]
- Roth J., Conn C. A., Kluger M. J., Zeisberger E. Kinetics of systemic and intrahypothalamic IL-6 and tumor necrosis factor during endotoxin fever in guinea pigs. Am J Physiol. 1993 Sep;265(3 Pt 2):R653–R658. doi: 10.1152/ajpregu.1993.265.3.R653. [DOI] [PubMed] [Google Scholar]
- Shrader R. E., Everson G. J. Intravenous injection and blood sampling using cannulated guinea pigs. Lab Anim Care. 1968 Apr;18(2):214–219. [PubMed] [Google Scholar]
- Van Zee K. J., Kohno T., Fischer E., Rock C. S., Moldawer L. L., Lowry S. F. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4845–4849. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Knights C. V., Siber G. R. Neutralization and lipoprotein binding of lipopolysaccharides in tolerant rabbit serum. J Infect Dis. 1986 Nov;154(5):784–791. doi: 10.1093/infdis/154.5.784. [DOI] [PubMed] [Google Scholar]
- Wilkinson M. F., Kasting N. W. Centrally acting vasopressin contributes to endotoxin tolerance. Am J Physiol. 1990 Feb;258(2 Pt 2):R443–R449. doi: 10.1152/ajpregu.1990.258.2.R443. [DOI] [PubMed] [Google Scholar]


