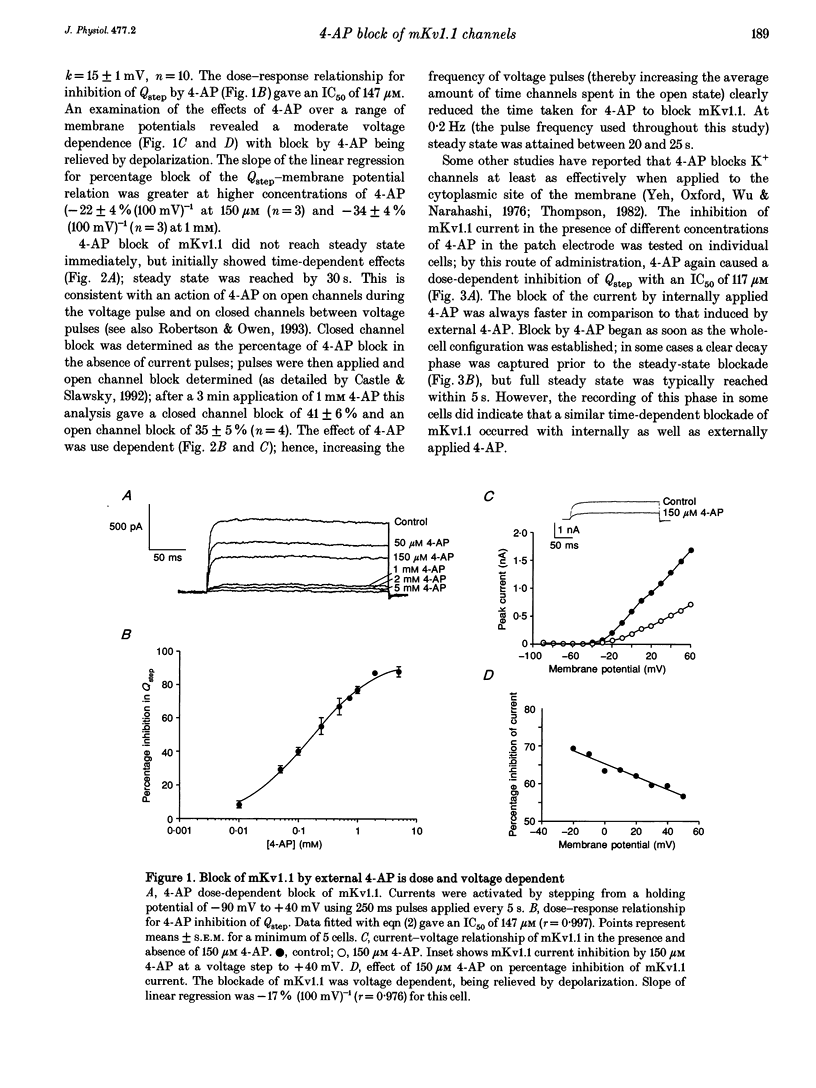
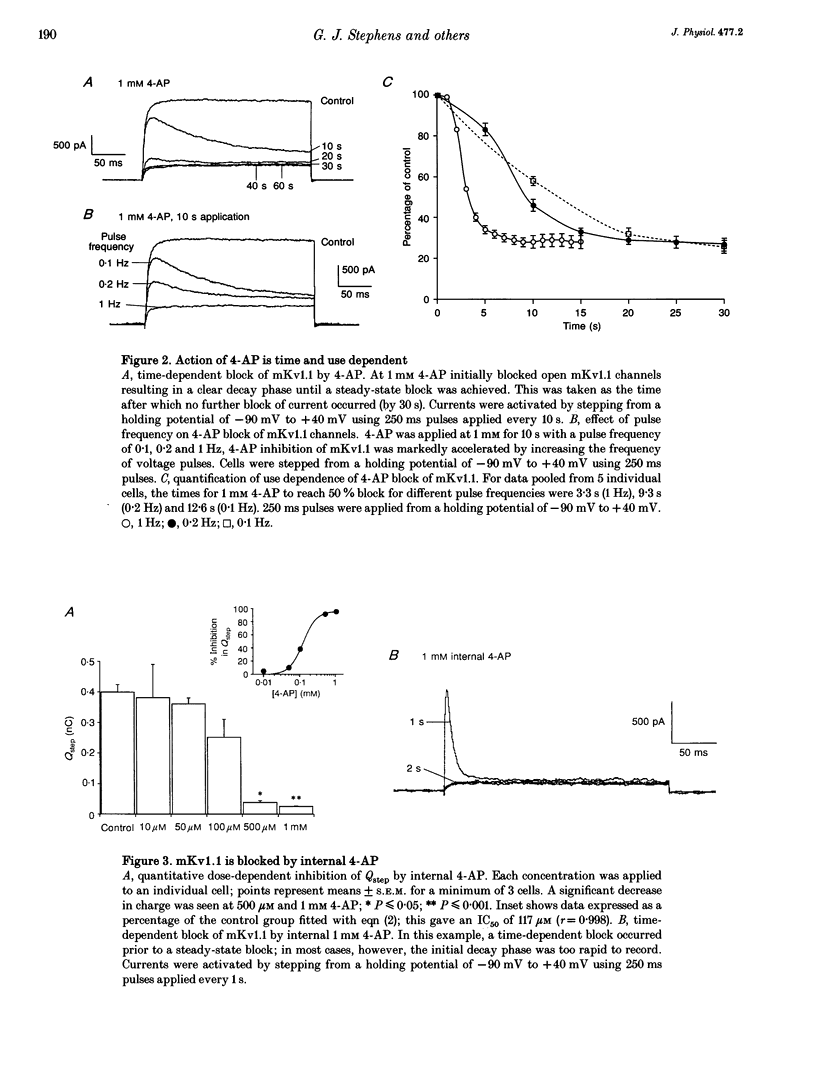
Abstract
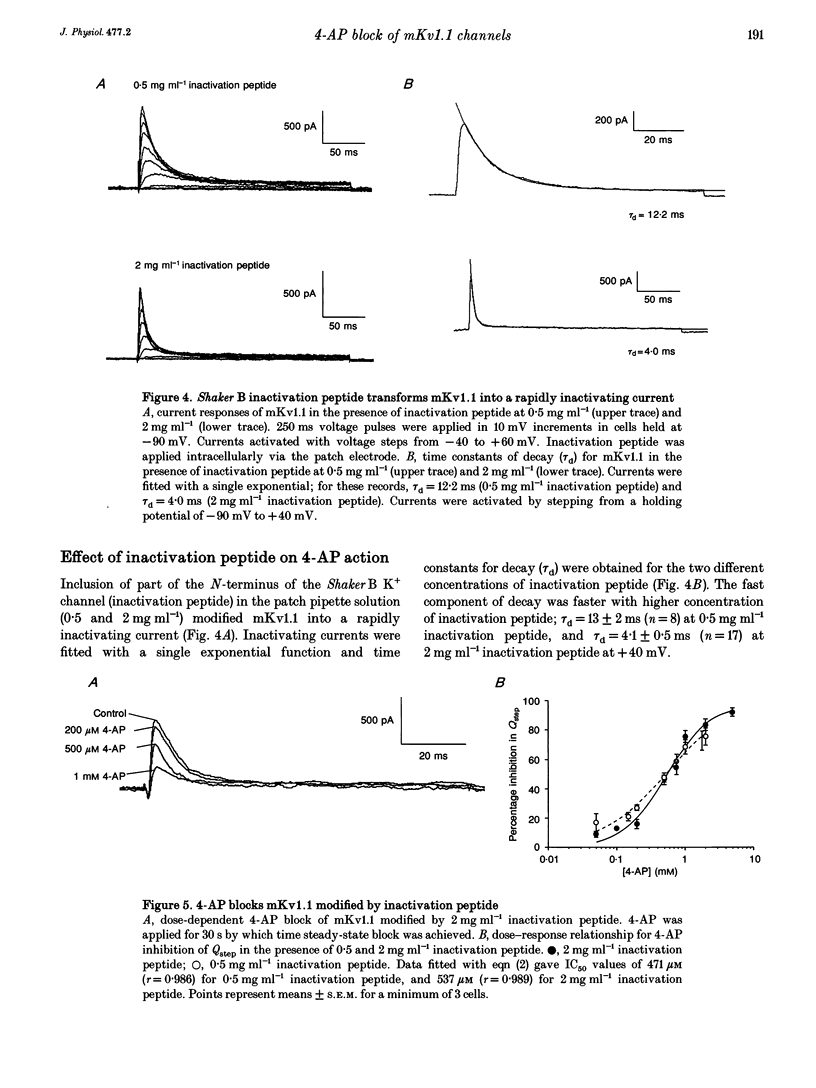
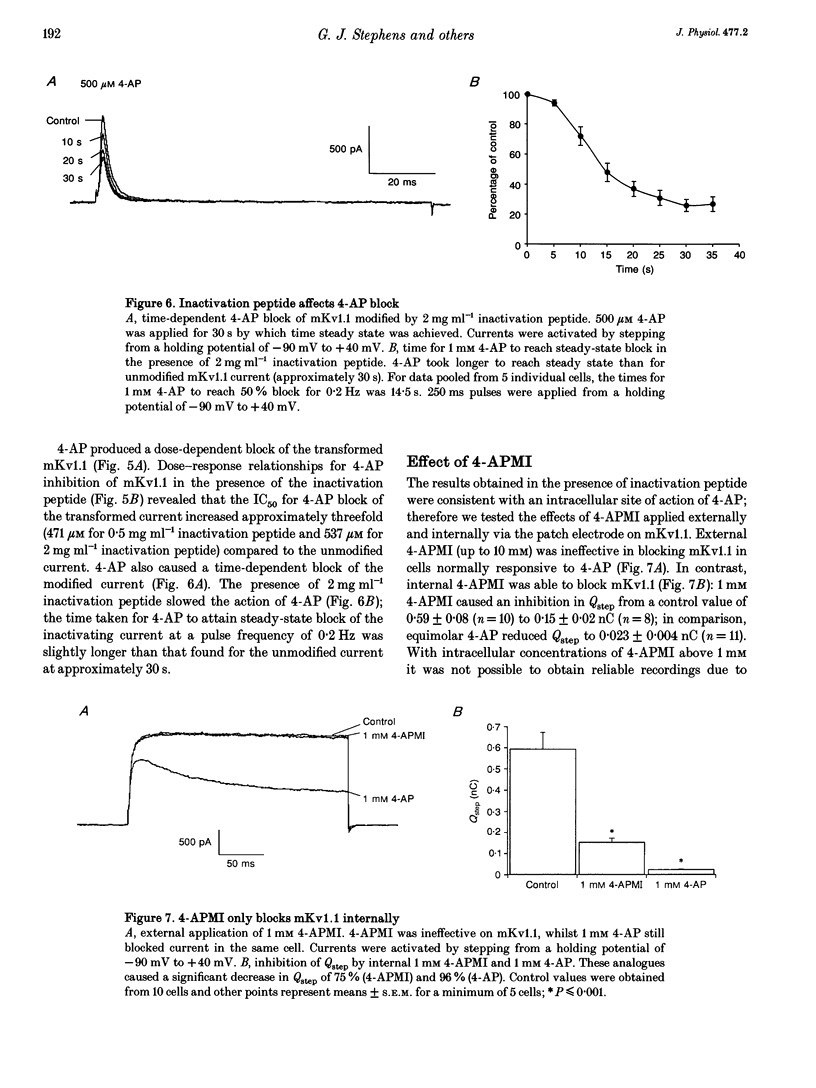
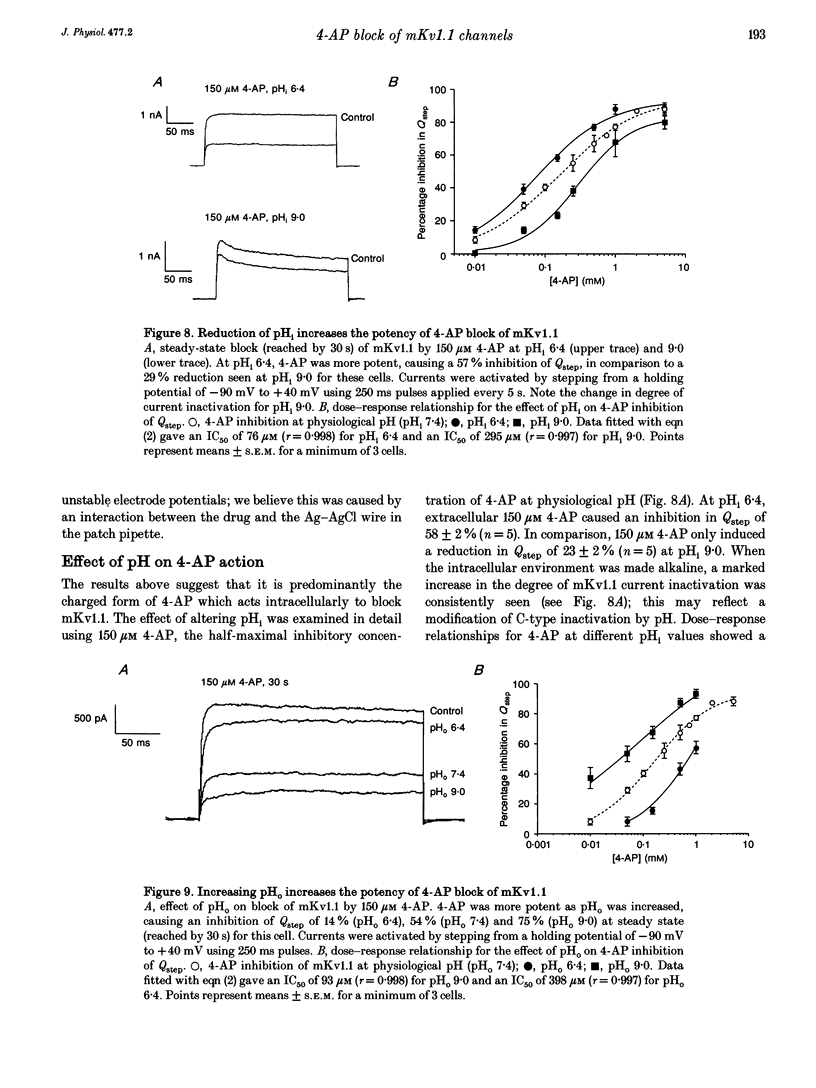
1. This study used the whole-cell patch clamp technique to investigate the mechanism of action of the K+ channel blocker 4-aminopyridine (4-AP) on the cloned K+ channel mouse Kv1.1 (mKv1.1) expressed in Chinese hamster ovary cells. 2. Cells transfected with mKv1.1 expressed a non-inactivating, delayed rectifier-type K+ current. 4-AP induced a dose-, voltage- and use-dependent block of mKv1.1. 3. 4-AP blockade of mKv1.1 was similar whether 4-AP was administered extracellularly (IC50 = 147 microM) or intracellularly (IC50 = 117 microM). 4. Inclusion of the first twenty amino acids of the N-terminus sequence of the Shaker B K+ channel ('inactivation peptide') in the patch electrode transformed mKv1.1 into a rapidly inactivating current. The time constant of decay for the modified current was dependent on the concentration of inactivation peptide, and under these conditions extracellular 4-AP had a reduced potency (IC50 values of 471 and 537 microM for 0.5 and 2 mg ml-1 inactivation peptide, respectively). 5. A permanently charged analogue of 4-AP, 4-aminopyridine methiodide (4-APMI), was found to block mKv1.1 when applied inside the cell, but was without effect when administered externally. 6. Decreasing the intracellular pH (pHi) to 6.4 caused an increase in 4-AP potency (IC50 = 76 microM), whereas at pHi 9.0, the 4-AP potency fell (IC50 = 295 microM). Conversely, increasing extracellular pH (pHo) to 9.0 caused an increase in 4-AP potency (IC50 = 93 microM), whereas at pHo 6.4, 4-AP potency decreased (IC50 = 398 microM). 7. Taken together, these findings support the hypotheses that the uncharged form of 4-AP crosses the membrane, and that it is predominantly the cationic form which acts on mKv1.1 channels intracellularly, possibly at or near to the binding site for the inactivation peptide.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M., Howe J. R., Ritchie J. M. Two types of 4-aminopyridine-sensitive potassium current in rabbit Schwann cells. J Physiol. 1993 May;464:321–342. doi: 10.1113/jphysiol.1993.sp019637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. L., Mossman C., Aubé J., Yellen G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 1993 Mar;10(3):533–541. doi: 10.1016/0896-6273(93)90340-w. [DOI] [PubMed] [Google Scholar]
- Choquet D., Korn H. Mechanism of 4-aminopyridine action on voltage-gated potassium channels in lymphocytes. J Gen Physiol. 1992 Feb;99(2):217–240. doi: 10.1085/jgp.99.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M. J., Adelman J. P., Douglass J., North R. A. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989 Apr 14;244(4901):221–224. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Deutsch C., Lee S. C. Modulation of K+ currents in human lymphocytes by pH. J Physiol. 1989 Jun;413:399–413. doi: 10.1113/jphysiol.1989.sp017660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. I., Hutter O. F. Proceedings: The actions of 4-aminopyridine on the delayed potassium current in skeletal muscle fibres. J Physiol. 1975 Nov;252(2):70P–71P. [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of 4-aminopyridine on potassium currents in a molluscan neuron. J Gen Physiol. 1981 Jul;78(1):63–86. doi: 10.1085/jgp.78.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Ritchie J. M. On the active form of 4-aminopyridine: block of K+ currents in rabbit Schwann cells. J Physiol. 1991 Feb;433:183–205. doi: 10.1113/jphysiol.1991.sp018421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Putative receptor for the cytoplasmic inactivation gate in the Shaker K+ channel. Nature. 1991 Sep 5;353(6339):86–90. doi: 10.1038/353086a0. [DOI] [PubMed] [Google Scholar]
- Kamb A., Weir M., Rudy B., Varmus H., Kenyon C. Identification of genes from pattern formation, tyrosine kinase, and potassium channel families by DNA amplification. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4372–4376. doi: 10.1073/pnas.86.12.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl S. J. 4-Aminopyridine causes a voltage-dependent block of the transient outward K+ current in rat melanotrophs. J Physiol. 1990 Dec;431:515–528. doi: 10.1113/jphysiol.1990.sp018344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Narahashi T. Site of action and active form of aminopyridines in squid axon membranes. J Pharmacol Exp Ther. 1983 Jul;226(1):174–179. [PubMed] [Google Scholar]
- Klumpp D. J., Farber D. B., Bowes C., Song E. J., Pinto L. H. The potassium channel MBK1 (Kv1.1) is expressed in the mouse retina. Cell Mol Neurobiol. 1991 Dec;11(6):611–622. doi: 10.1007/BF00741449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Pichon Y. The effect of internal and external 4-aminopyridine on the potassium currents in intracellularly perfused squid giant axons. J Physiol. 1977 Jun;268(2):511–532. doi: 10.1113/jphysiol.1977.sp011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B., Owen D. G. Pharmacology of a cloned potassium channel from mouse brain (MK-1) expressed in CHO cells: effects of blockers and an 'inactivation peptide'. Br J Pharmacol. 1993 Jul;109(3):725–735. doi: 10.1111/j.1476-5381.1993.tb13634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M., Stühmer W., Wittka R., Wang X., Müller R., Ferrus A., Pongs O. Alternative Shaker transcripts express either rapidly inactivating or noninactivating K+ channels. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8903–8907. doi: 10.1073/pnas.87.22.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Stocker M., Sakmann B., Seeburg P., Baumann A., Grupe A., Pongs O. Potassium channels expressed from rat brain cDNA have delayed rectifier properties. FEBS Lett. 1988 Dec 19;242(1):199–206. doi: 10.1016/0014-5793(88)81015-9. [DOI] [PubMed] [Google Scholar]
- Tempel B. L., Jan Y. N., Jan L. Y. Cloning of a probable potassium channel gene from mouse brain. Nature. 1988 Apr 28;332(6167):837–839. doi: 10.1038/332837a0. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. Aminopyridine block of transient potassium current. J Gen Physiol. 1982 Jul;80(1):1–18. doi: 10.1085/jgp.80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner P. K., Oxford G. S. Aminopyridines block an inactivating potassium current having slow recovery kinetics. Biophys J. 1990 Dec;58(6):1481–1489. doi: 10.1016/S0006-3495(90)82493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., Castle N. A., Wang G. K. Identification of RBK1 potassium channels in C6 astrocytoma cells. Glia. 1992;5(2):146–153. doi: 10.1002/glia.440050209. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z. A pharmacological approach to the structure of the Na channel in squid axon. Prog Clin Biol Res. 1982;79:17–49. [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990 Oct 26;250(4980):568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]