Abstract
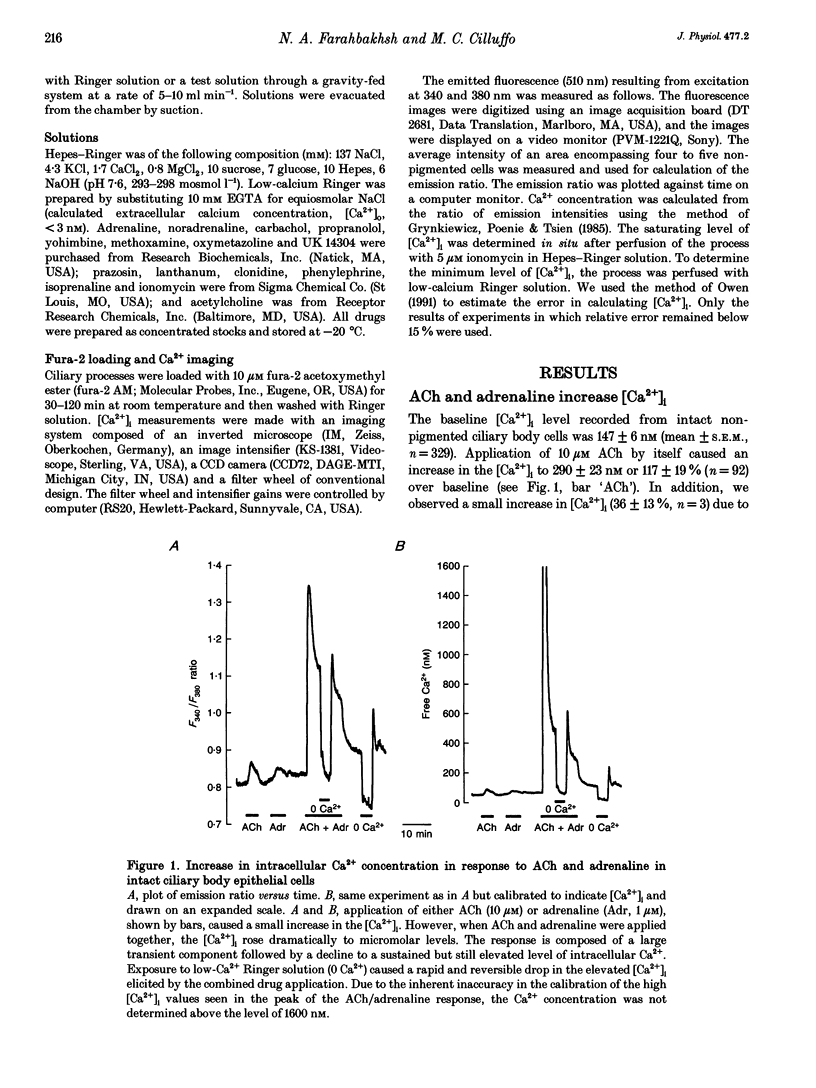
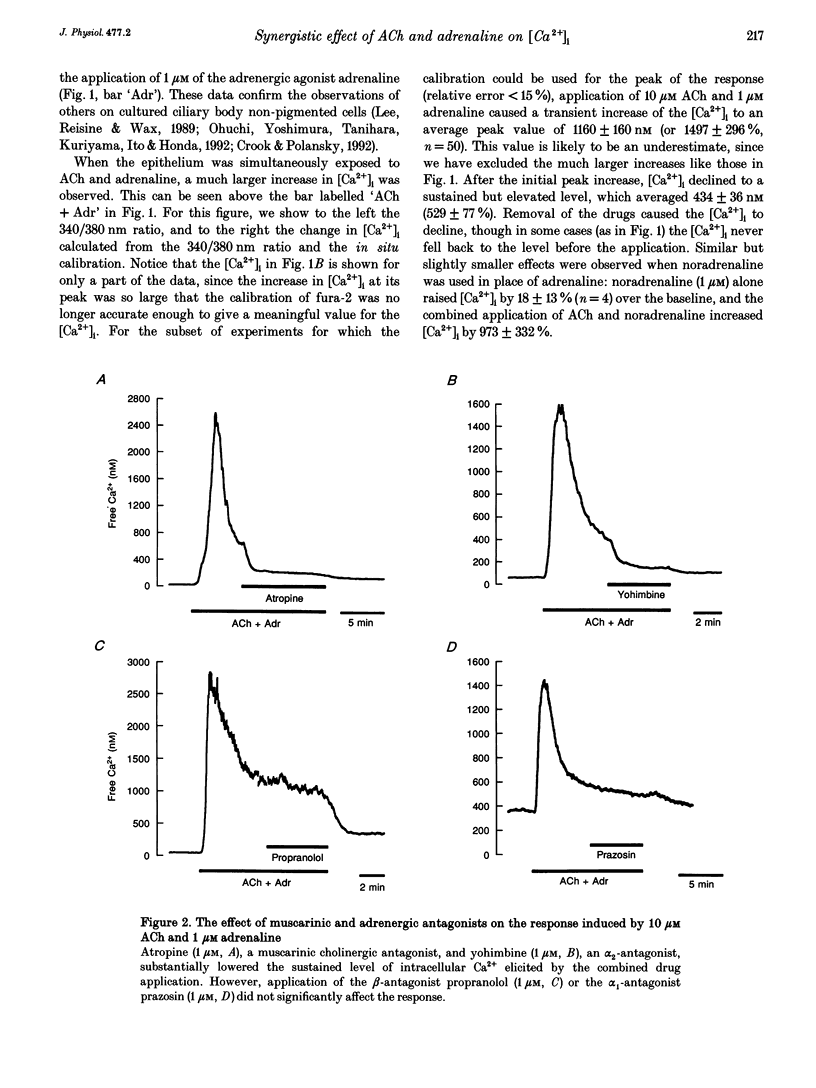
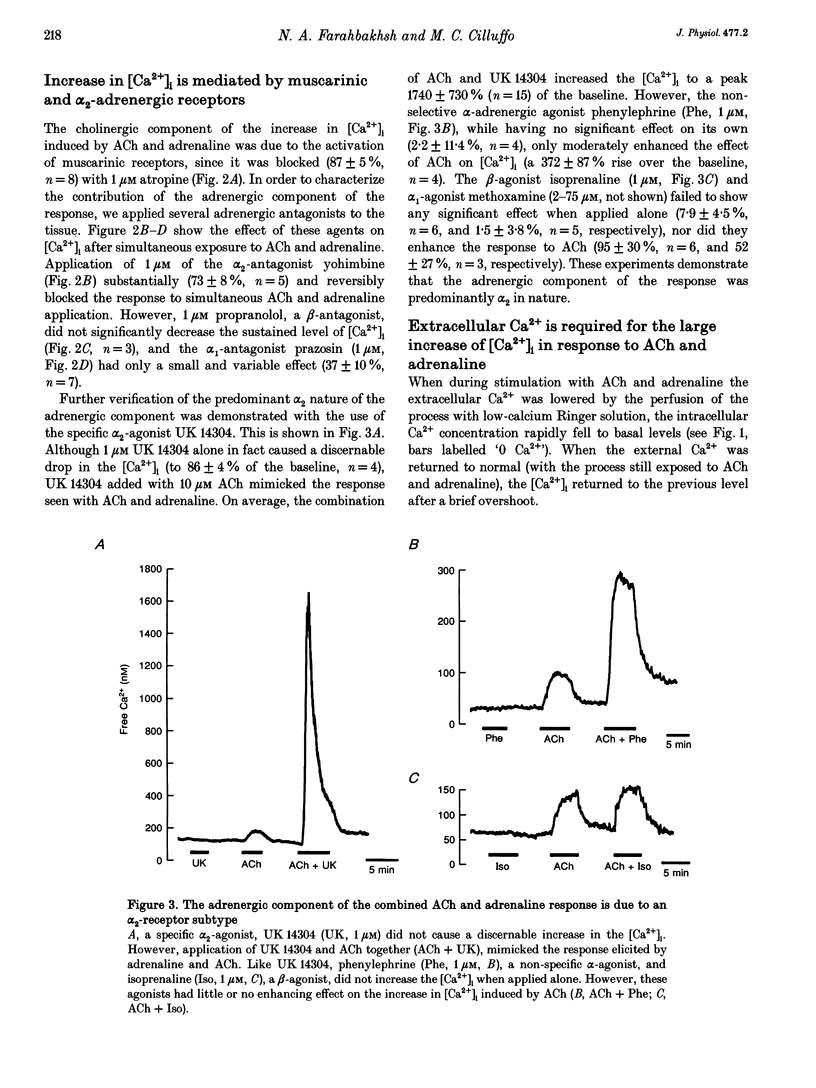
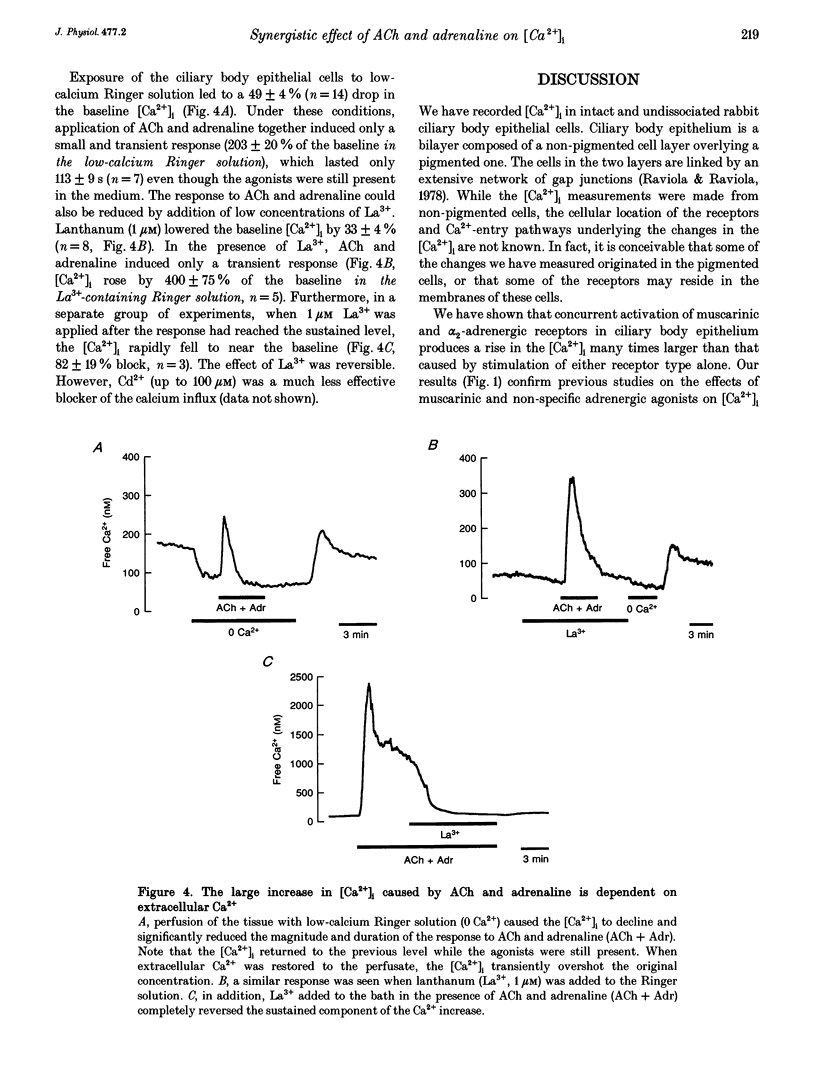
1. Changes in cytosolic free calcium concentration ([Ca2+]i) in response to cholinergic and adrenergic agents alone and in combination were investigated using fura-2 fluorescence imaging in intact non-pigmented epithelial cells of rabbit ciliary body. 2. Resting ('baseline') [Ca2+]i was 147 +/- 6 nM (mean +/- S.E.M.). Acetylcholine (ACh, 10 microM) doubled [Ca2+]i, and adrenaline (1 microM) increased it by about 36%. When ACh (10 microM) and adrenaline (1 microM) were applied together [Ca2+]i was transiently increased to 1160 +/- 160 nM, about 7 times the response induced by ACh alone. 3. Noradrenaline and 5-bromo-6-(2-imidazolin-2-yl-amino)-quinoxaline (UK 14304) had effects similar to adrenaline in enhancing the response to ACh. Phenylephrine (Phe) had a relatively smaller effect and none was observed for methoxamine and isoprenaline (Iso). 4. The response to ACh and adrenaline could be blocked by atropine (1 microM, 87 +/- 5%), yohimbine (1 microM, 73 +/- 8%), and to a lesser degree by prazosin (1 microM). Propranolol had no effect. 5. Lowering the extracellular calcium concentration to 3 nM dropped the baseline [Ca2+]i by half and reduced the response to ACh and adrenaline to a small and transient rise in [Ca2+]i. Addition of La3+ to Ca(2+)-containing solution also lowered [Ca2+]i and largely reduced the response. 6. We conclude that simultaneous activation of muscarinic and alpha 2-adrenergic receptors induces a large increase in [Ca2+]i, which is the result of both Ca2+ release and influx.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brubaker R. F. Flow of aqueous humor in humans [The Friedenwald Lecture]. Invest Ophthalmol Vis Sci. 1991 Dec;32(13):3145–3166. [PubMed] [Google Scholar]
- Crook R. B., Polansky J. R. Neurotransmitters and neuropeptides stimulate inositol phosphates and intracellular calcium in cultured human nonpigmented ciliary epithelium. Invest Ophthalmol Vis Sci. 1992 Apr;33(5):1706–1716. [PubMed] [Google Scholar]
- Elayan H., Kennedy B., Ziegler M. G. Epinephrine synthesis in the rat iris. Invest Ophthalmol Vis Sci. 1990 Apr;31(4):677–680. [PubMed] [Google Scholar]
- Farahbakhsh N. A., Cilluffo M. C., Chronis C., Fain G. L. Dihydropyridine-sensitive Ca2+ spikes and Ca2+ currents in rabbit ciliary body epithelial cells. Exp Eye Res. 1994 Feb;58(2):197–205. doi: 10.1006/exer.1994.1008. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Illek B., Fischer H., Machen T. E. Intracellular Ca2+ signalling is modulated by K+ channel blockers in colonic epithelial cells (HT-29/B6). Pflugers Arch. 1992 Oct;422(1):48–54. doi: 10.1007/BF00381512. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Reisine T. D., Wax M. B. Alterations of intracellular calcium in human non-pigmented ciliary epithelial cells of the eye. Exp Eye Res. 1989 Jun;48(6):733–743. doi: 10.1016/0014-4835(89)90060-2. [DOI] [PubMed] [Google Scholar]
- Minneman K. P. Alpha 1-adrenergic receptor subtypes, inositol phosphates, and sources of cell Ca2+. Pharmacol Rev. 1988 Jun;40(2):87–119. [PubMed] [Google Scholar]
- Mittag T. W., Tormay A. Adrenergic receptor subtypes in rabbit iris-ciliary body membranes: classification by radioligand studies. Exp Eye Res. 1985 Feb;40(2):239–249. doi: 10.1016/0014-4835(85)90009-0. [DOI] [PubMed] [Google Scholar]
- Negresku E. V., Baldenkov G. N., Grigorian G. Iu, Mazaev A. V., Tkachuk V. A. Biokhimicheskie osobennosti alpha2-adrenoretseptorov trombotsitov i ikh sviaz' s povysheniem kontsentratsii vnutrikletochnogo Ca2+. Biokhimiia. 1989 Jun;54(6):909–915. [PubMed] [Google Scholar]
- Ohuchi T., Yoshimura N., Tanihara H., Kuriyama S., Ito S., Honda Y. Ca2+ mobilization in nontransformed ciliary nonpigmented epithelial cells. Invest Ophthalmol Vis Sci. 1992 Apr;33(5):1696–1705. [PubMed] [Google Scholar]
- Owen C. S. Spectra of intracellular Fura-2. Cell Calcium. 1991 Jun;12(6):385–393. doi: 10.1016/0143-4160(91)90064-l. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Raviola G., Raviola E. Intercellular junctions in the ciliary epithelium. Invest Ophthalmol Vis Sci. 1978 Oct;17(10):958–981. [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Nichols A. J., Stadel J. M., Hieble J. P. Pharmacologic and therapeutic applications of alpha 2-adrenoceptor subtypes. Annu Rev Pharmacol Toxicol. 1993;33:243–279. doi: 10.1146/annurev.pa.33.040193.001331. [DOI] [PubMed] [Google Scholar]
- Stone R. A., Kuwayama Y., Laties A. M. Regulatory peptides in the eye. Experientia. 1987 Jul 15;43(7):791–800. doi: 10.1007/BF01945357. [DOI] [PubMed] [Google Scholar]
- Wallnöfer A., Cauvin C., Lategan T. W., Rüegg U. T. Differential blockade of agonist- and depolarization-induced 45Ca2+ influx in smooth muscle cells. Am J Physiol. 1989 Oct;257(4 Pt 1):C607–C611. doi: 10.1152/ajpcell.1989.257.4.C607. [DOI] [PubMed] [Google Scholar]


