Abstract
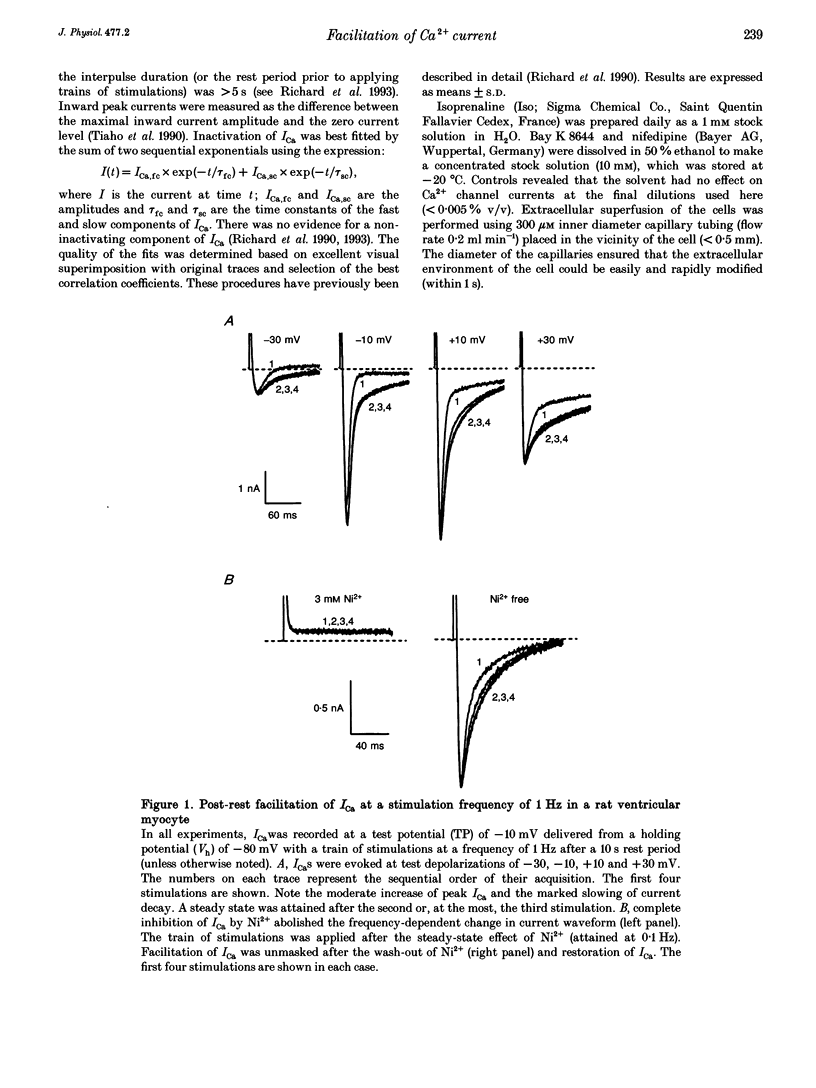
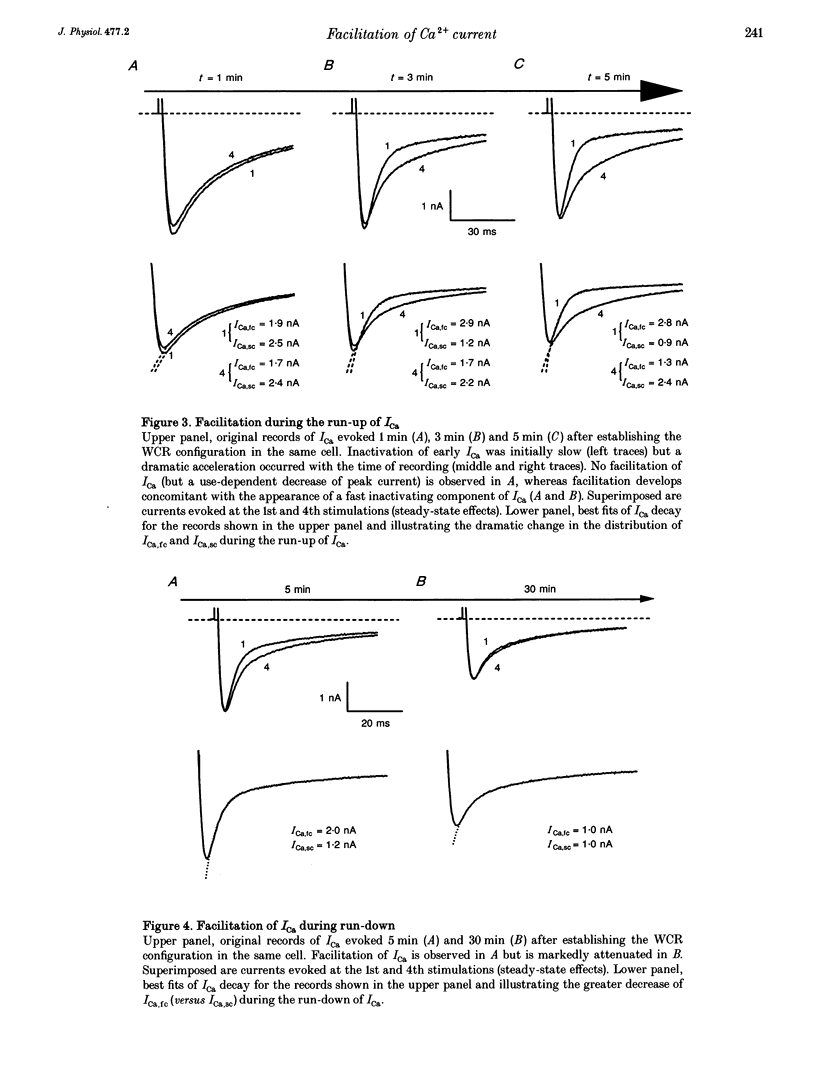
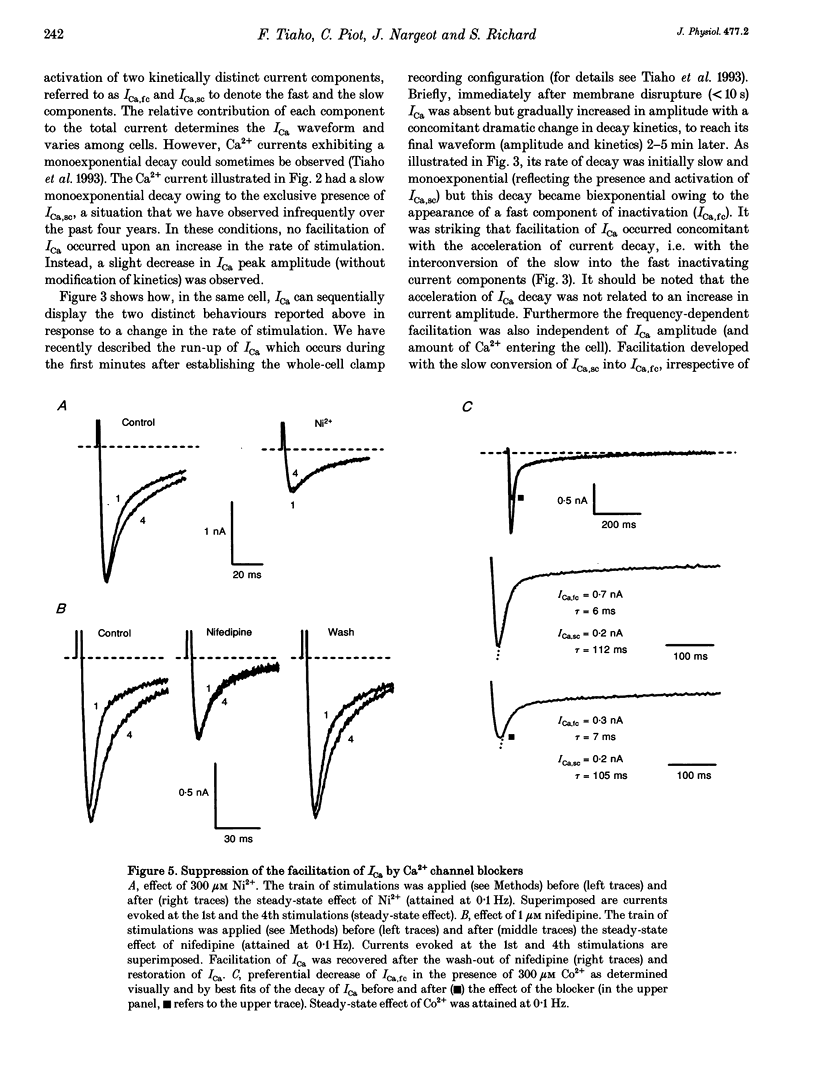
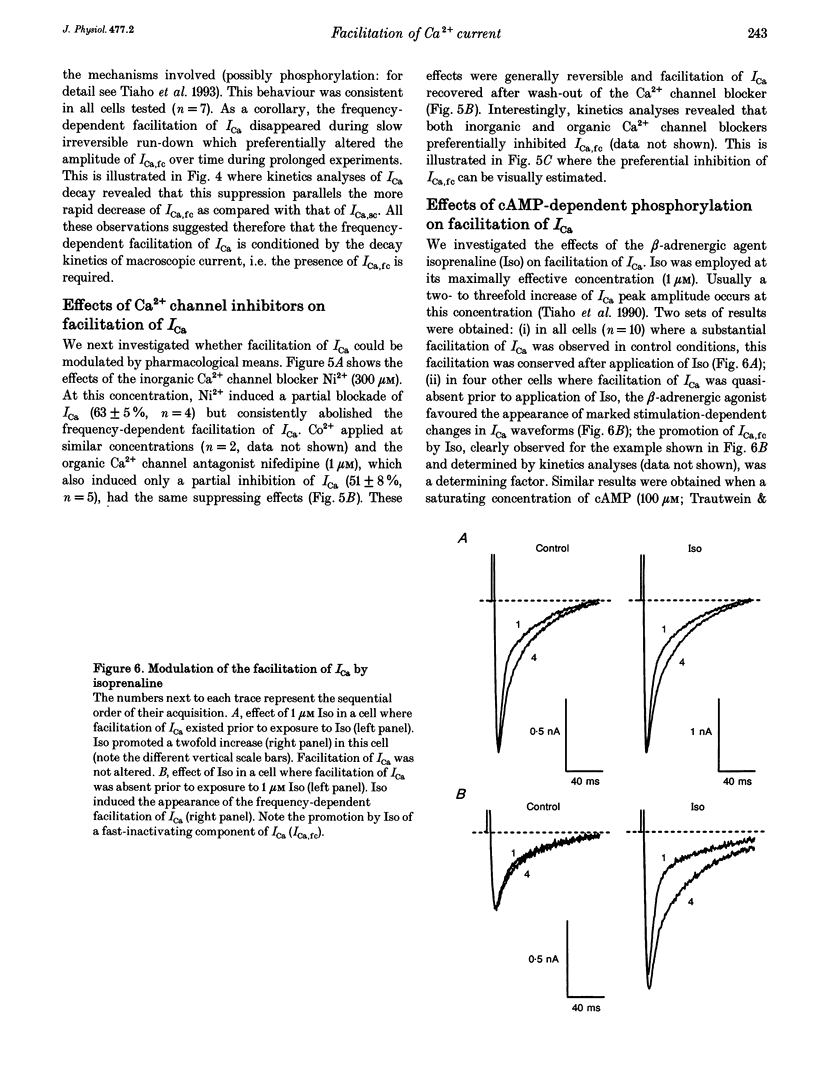
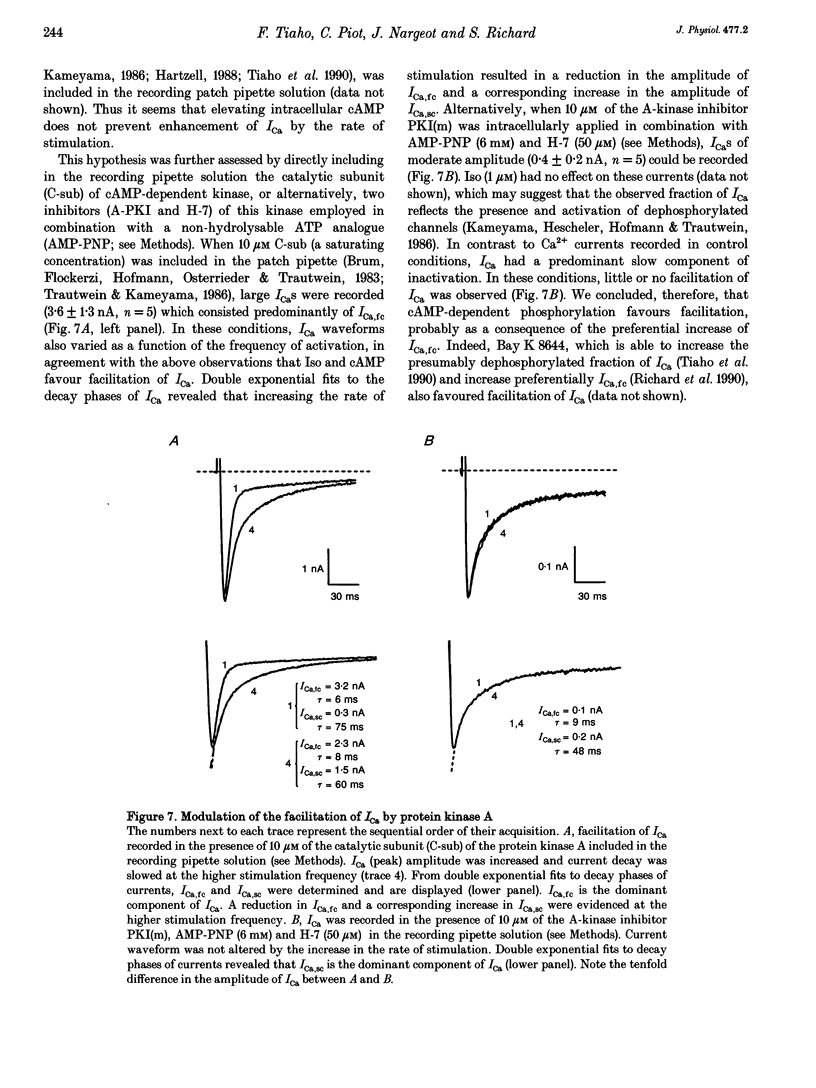
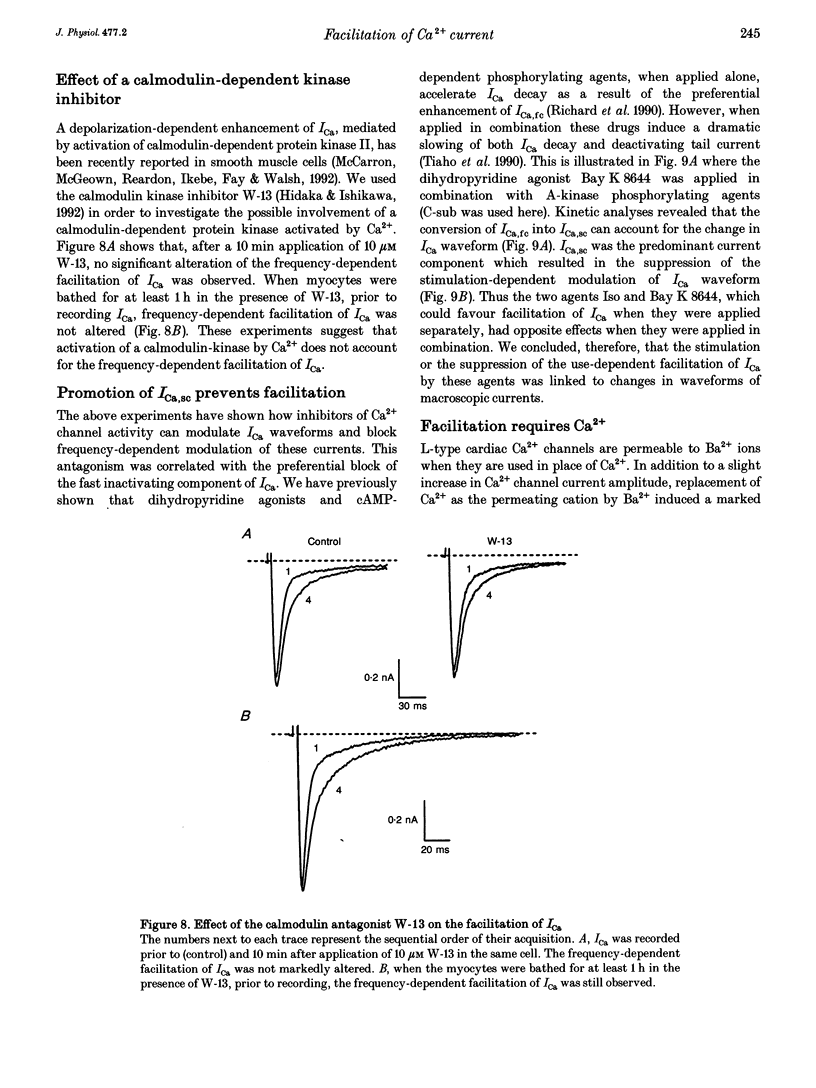
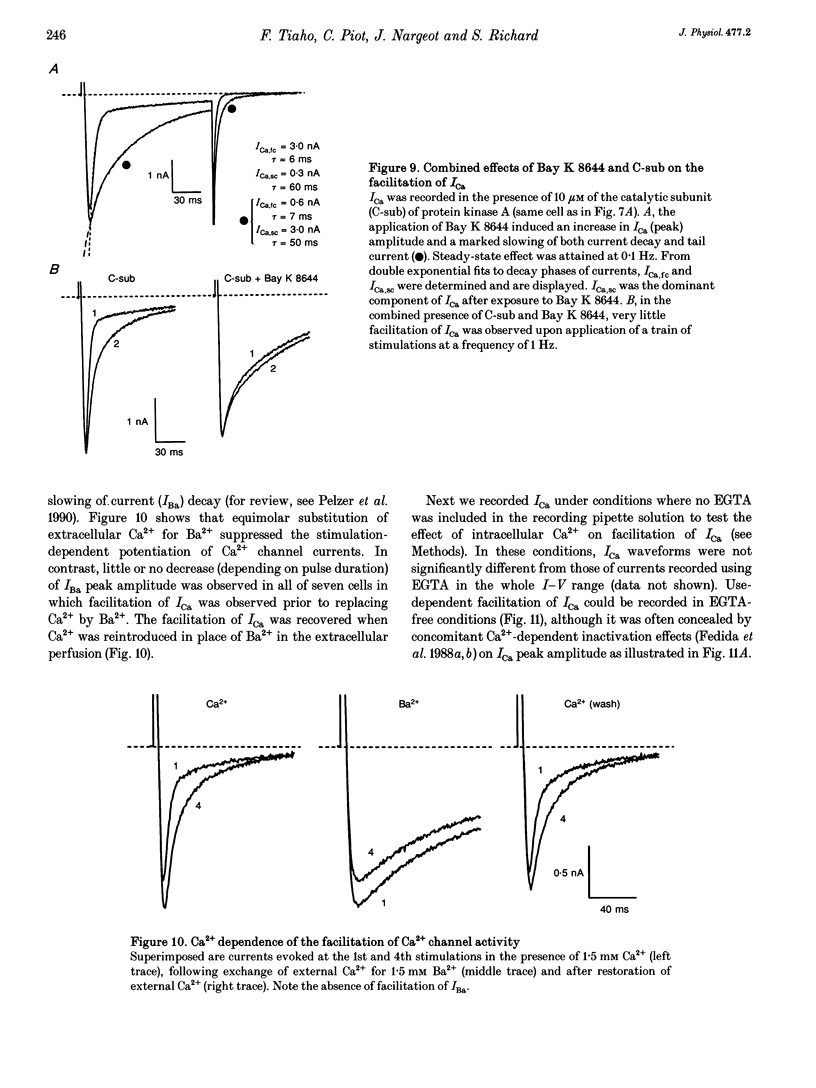
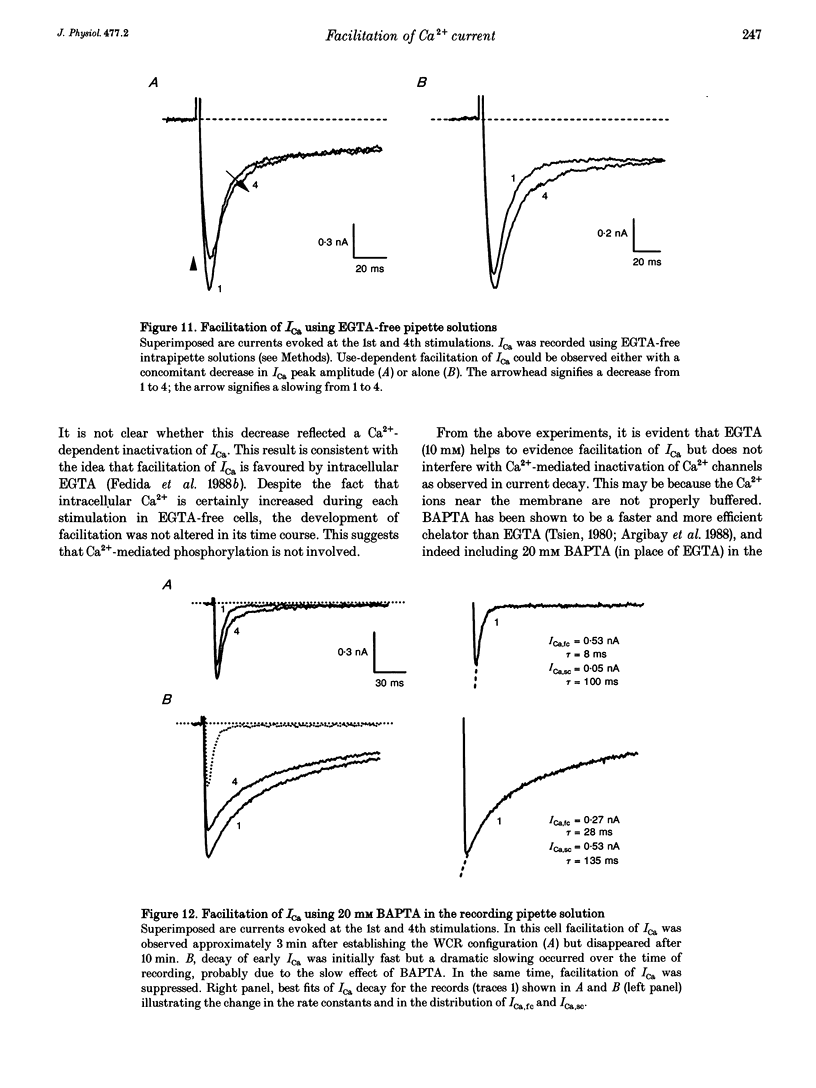
1. An increase in the rate of stimulation induces an augmentation of L-type Ca2+ currents (ICa) and concomitant slowing of current decay in rat ventricular cells. This facilitation is quasi immediate (1-3 s), graded with the rate of stimulation, and occurs only from negative holding potentials. We investigated this effect using trains of stimulation at 1 Hz and the whole-cell patch-clamp technique (18-22 degrees C). 2. The decay of ICa is normally bi-exponential and comprises fast and slow current components (ICa,fc and ICa,sc, respectively). Facilitation of ICa was observed only when ICa,fc was predominant. 3. Facilitation developed during the run-up of ICa with the interconversion of ICa,sc into ICa,fc, and vanished during the run-down of ICa with the loss of ICa,fc.Ni2+ (300 microM) and nifedipine (1 microM) suppressed facilitation owing to the preferential inhibition of ICa,fc. 4. Facilitation of ICa was not altered (when present) or favoured (when absent) by the cAMP-dependent phosphorylation of Ca2+ channels promoted by isoprenaline or by intracellular application of cAMP or of the catalytic subunit of protein kinase A (C-sub). A similar effect was observed when the dihydropyridine agonist Bay K 8644 was applied. In both cases, facilitation was linked to a preferential increase of ICa,fc. 5. Following intracellular application of inhibitors of protein kinase A in combination with a non-hydrolysable ATP analogue, ICa consisted predominantly of ICa,sc and no facilitation was observed. The calmodulin antagonist naphthalenesulphonamide had no effect on facilitation. 6. When Bay K 8644 was applied in combination with isoprenaline, cAMP or C-sub, the decay of ICa was slowed with the predominant development of ICa,sc, and facilitation of ICa was nearly abolished. Facilitation also depended on extracellular Ca2+, and was suppressed when Ba2+ replaced Ca2+ as the permeating ion. 7. When no EGTA was included in the patch pipette, facilitation was not further enhanced but a use-dependent decrease of ICa frequently occurred. When BAPTA was used in place of EGTA, the rate of inactivation of ICa was reduced and facilitation was abolished. 8. In conclusion, the facilitation of ICa that reflects a voltage-driven interconversion of ICa,fc into ICa,sc is also regulated by Ca2+ and by cAMP-dependent phosphorylation. The presence of the gating pattern typified by ICa,fc is required. Ca2+ may exert its effect near the inner pore of the Ca2+ channel protein and control the distribution between the closed states of the two gating pathways.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argibay J. A., Fischmeister R., Hartzell H. C. Inactivation, reactivation and pacing dependence of calcium current in frog cardiocytes: correlation with current density. J Physiol. 1988 Jul;401:201–226. doi: 10.1113/jphysiol.1988.sp017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo C. R., Ariano M. A., Perlman R. L., Fox A. P. Activation of facilitation calcium channels in chromaffin cells by D1 dopamine receptors through a cAMP/protein kinase A-dependent mechanism. Nature. 1990 Nov 15;348(6298):239–242. doi: 10.1038/348239a0. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Rossie S., Perlman R. L., Fox A. P. Voltage-dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature. 1992 Jul 2;358(6381):63–66. doi: 10.1038/358063a0. [DOI] [PubMed] [Google Scholar]
- Bechtel P. J., Beavo J. A., Krebs E. G. Purification and characterization of catalytic subunit of skeletal muscle adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Apr 25;252(8):2691–2697. [PubMed] [Google Scholar]
- Brum G., Flockerzi V., Hofmann F., Osterrieder W., Trautwein W. Injection of catalytic subunit of cAMP-dependent protein kinase into isolated cardiac myocytes. Pflugers Arch. 1983 Jul;398(2):147–154. doi: 10.1007/BF00581064. [DOI] [PubMed] [Google Scholar]
- Charnet P., Richard S., Gurney A. M., Ouadid H., Tiaho F., Nargeot J. Modulation of Ca currents in isolated frog atrial cells studied with photosensitive probes. Regulation by cAMP and Ca2+: a common pathway? J Mol Cell Cardiol. 1991 Mar;23(3):343–356. doi: 10.1016/0022-2828(91)90070-3. [DOI] [PubMed] [Google Scholar]
- Fedida D., Noble D., Spindler A. J. Mechanism of the use dependence of Ca2+ current in guinea-pig myocytes. J Physiol. 1988 Nov;405:461–475. doi: 10.1113/jphysiol.1988.sp017342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Noble D., Spindler A. J. Use-dependent reduction and facilitation of Ca2+ current in guinea-pig myocytes. J Physiol. 1988 Nov;405:439–460. doi: 10.1113/jphysiol.1988.sp017341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A., Mery J., Vandromme M., Basset M., Cavadore J. C., Lamb N. J. Effective intracellular inhibition of the cAMP-dependent protein kinase by microinjection of a modified form of the specific inhibitor peptide PKi in living fibroblasts. Exp Cell Res. 1991 Aug;195(2):468–477. doi: 10.1016/0014-4827(91)90398-e. [DOI] [PubMed] [Google Scholar]
- Gurney A. M., Charnet P., Pye J. M., Nargeot J. Augmentation of cardiac calcium current by flash photolysis of intracellular caged-Ca2+ molecules. Nature. 1989 Sep 7;341(6237):65–68. doi: 10.1038/341065a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Ishikawa T. Molecular pharmacology of calmodulin pathways in the cell functions. Cell Calcium. 1992 Jun-Jul;13(6-7):465–472. doi: 10.1016/0143-4160(92)90059-2. [DOI] [PubMed] [Google Scholar]
- Hryshko L. V., Bers D. M. Ca current facilitation during postrest recovery depends on Ca entry. Am J Physiol. 1990 Sep;259(3 Pt 2):H951–H961. doi: 10.1152/ajpheart.1990.259.3.H951. [DOI] [PubMed] [Google Scholar]
- Imredy J. P., Yue D. T. Submicroscopic Ca2+ diffusion mediates inhibitory coupling between individual Ca2+ channels. Neuron. 1992 Aug;9(2):197–207. doi: 10.1016/0896-6273(92)90159-b. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Lory P., Nargeot J. Cyclic AMP-dependent modulation of cardiac Ca channels expressed in Xenopus laevis oocytes. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1059–1065. doi: 10.1016/0006-291x(92)91839-i. [DOI] [PubMed] [Google Scholar]
- McCarron J. G., McGeown J. G., Reardon S., Ikebe M., Fay F. S., Walsh J. V., Jr Calcium-dependent enhancement of calcium current in smooth muscle by calmodulin-dependent protein kinase II. Nature. 1992 May 7;357(6373):74–77. doi: 10.1038/357074a0. [DOI] [PubMed] [Google Scholar]
- Mironov S. L., Lux H. D. Calmodulin antagonists and protein phosphatase inhibitor okadaic acid fasten the 'run-up' of high-voltage activated calcium current in rat hippocampal neurones. Neurosci Lett. 1991 Dec 9;133(2):175–178. doi: 10.1016/0304-3940(91)90563-9. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Influence of a change in stimulation rate on action potentials, currents and contractions in rat ventricular cells. J Physiol. 1985 Jul;364:113–130. doi: 10.1113/jphysiol.1985.sp015734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. Two types of calcium channels in guinea pig ventricular myocytes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5340–5344. doi: 10.1073/pnas.83.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot J., Nerbonne J. M., Engels J., Lester H. A. Time course of the increase in the myocardial slow inward current after a photochemically generated concentration jump of intracellular cAMP. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2395–2399. doi: 10.1073/pnas.80.8.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S., Shimoni Y. The calcium and frequency dependence of the slow inward current 'staircase' in frog atrium. J Physiol. 1981 Jan;310:57–75. doi: 10.1113/jphysiol.1981.sp013537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau N., Garnier D., Argibay J. A. Rate dependence of action potential duration and calcium current in isolated guinea-pig cardiocytes. Exp Physiol. 1992 Jul;77(4):615–625. doi: 10.1113/expphysiol.1992.sp003624. [DOI] [PubMed] [Google Scholar]
- Pelzer D., Pelzer S., McDonald T. F. Properties and regulation of calcium channels in muscle cells. Rev Physiol Biochem Pharmacol. 1990;114:107–207. doi: 10.1007/BFb0031019. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Hess P. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 1990 Aug 16;346(6285):651–655. doi: 10.1038/346651a0. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Hess P. Reversible uncoupling of inactivation in N-type calcium channels. Nature. 1991 Jun 20;351(6328):657–659. doi: 10.1038/351657a0. [DOI] [PubMed] [Google Scholar]
- Richard S., Charnet P., Nerbonne J. M. Interconversion between distinct gating pathways of the high threshold calcium channel in rat ventricular myocytes. J Physiol. 1993 Mar;462:197–228. doi: 10.1113/jphysiol.1993.sp019551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S., Tiaho F., Charnet P., Nargeot J., Nerbonne J. M. Two pathways for Ca2+ channel gating differentially modulated by physiological stimuli. Am J Physiol. 1990 Jun;258(6 Pt 2):H1872–H1881. doi: 10.1152/ajpheart.1990.258.6.H1872. [DOI] [PubMed] [Google Scholar]
- Rose W. C., Balke C. W., Wier W. G., Marban E. Macroscopic and unitary properties of physiological ion flux through L-type Ca2+ channels in guinea-pig heart cells. J Physiol. 1992 Oct;456:267–284. doi: 10.1113/jphysiol.1992.sp019336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scamps F., Mayoux E., Charlemagne D., Vassort G. Calcium current in single cells isolated from normal and hypertrophied rat heart. Effects of beta-adrenergic stimulation. Circ Res. 1990 Jul;67(1):199–208. doi: 10.1161/01.res.67.1.199. [DOI] [PubMed] [Google Scholar]
- Schouten V. J., Morad M. Regulation of Ca2+ current in frog ventricular myocytes by the holding potential, c-AMP and frequency. Pflugers Arch. 1989 Oct;415(1):1–11. doi: 10.1007/BF00373135. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992 Mar;13(3):183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- Tiaho F., Nargeot J., Richard S. Repriming of L-type calcium currents revealed during early whole-cell patch-clamp recordings in rat ventricular cells. J Physiol. 1993 Apr;463:367–389. doi: 10.1113/jphysiol.1993.sp019599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiaho F., Nargeot J., Richard S. Voltage-dependent regulation of L-type cardiac Ca channels by isoproterenol. Pflugers Arch. 1991 Dec;419(6):596–602. doi: 10.1007/BF00370301. [DOI] [PubMed] [Google Scholar]
- Tiaho F., Richard S., Lory P., Nerbonne J. M., Nargeot J. Cyclic-AMP-dependent phosphorylation modulates the stereospecific activation of cardiac Ca channels by Bay K 8644. Pflugers Arch. 1990 Sep;417(1):58–66. doi: 10.1007/BF00370769. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Kameyama M. Intracellular control of calcium and potassium currents in cardiac cells. Jpn Heart J. 1986 Nov;27 (Suppl 1):31–50. [PubMed] [Google Scholar]
- Tseng G. N. Calcium current restitution in mammalian ventricular myocytes is modulated by intracellular calcium. Circ Res. 1988 Aug;63(2):468–482. doi: 10.1161/01.res.63.2.468. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Xu X. P., Best P. M. Increase in T-type calcium current in atrial myocytes from adult rats with growth hormone-secreting tumors. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4655–4659. doi: 10.1073/pnas.87.12.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Backx P. H., Imredy J. P. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990 Dec 21;250(4988):1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Herzig S., Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990 Jan;87(2):753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt A. C., Maylie J. Stimulation-dependent facilitation of the high threshold calcium current in guinea-pig ventricular myocytes. J Physiol. 1990 Sep;428:653–671. doi: 10.1113/jphysiol.1990.sp018233. [DOI] [PMC free article] [PubMed] [Google Scholar]


