ABSTRACT
In recent years, the diagnostic accuracy of Alzheimer’s disease has been enhanced by the development of different types of biomarkers that indicate the presence of neuropathological processes. In addition to improving patient selection for clinical trials, biomarkers can assess the effects of new treatments on pathological processes. However, there is concern about the indiscriminate and poorly supported use of biomarkers, especially in asymptomatic individuals or those with subjective cognitive decline. Difficulties interpreting these tests, high costs, and unequal access make this scenario even more challenging in healthcare. This article presents the recommendations from the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology (Departamento Científico de Neurologia Cognitiva e Envelhecimento da Academia Brasileira de Neurologia) regarding the rational use and interpretation of Alzheimer’s disease biomarkers in clinical practice. The clinical diagnosis of cognitive-behavioral syndrome is recommended as the initial step to guide the request for biomarkers.
Keywords: Alzheimer Disease, Cognitive Dysfunction, Diagnosis, Biomarkers, Neuroimaging
RESUMO
Nos últimos anos, a precisão diagnóstica da doença de Alzheimer tem sido aprimorada pelo desenvolvimento de diferentes tipos de biomarcadores que indicam a presença dos processos neuropatológicos. Além de melhorar a seleção de pacientes para ensaios clínicos, os biomarcadores podem avaliar os efeitos de novos tratamentos nos processos patológicos. No entanto, há preocupação com o uso indiscriminado e mal fundamentado de biomarcadores, especialmente em indivíduos assintomáticos ou com declínio cognitivo subjetivo. As dificuldades na interpretação desses testes, os altos custos e o acesso desigual tornam esse cenário ainda mais desafiador no cuidado assistencial. Neste artigo, são apresentadas as recomendações do Departamento Científico de Neurologia Cognitiva e Envelhecimento da Academia Brasileira de Neurologia quanto ao uso racional e interpretação de biomarcadores da doença de Alzheimer na prática clínica. O diagnóstico clínico da síndrome cognitivo-comportamental é recomendado como o passo inicial para orientar a solicitação de biomarcadores.
Palavras-chave: Doença de Alzheimer, Disfunção Cognitiva, Diagnóstico, Biomarcadores, Neuroimagem
INTRODUCTION
In recent years, the diagnostic accuracy of Alzheimer’s disease (AD) has been enhanced by the development of different types of biological markers (biomarkers) that indicate the presence of the neuropathological processes associated with AD. Beyond being very important for the research of new therapies, by improving the selection of patients for clinical trials, biomarkers are able to access the effects of new treatments on pathological processes much earlier than neuropsychological and functional evaluations 1 .
Since 2011, the use of biomarkers as a tool to increase the diagnostic accuracy of the symptomatic phases of AD in clinical practice has been debated. Subsequently, biomarkers began to be used to optimize the selection of patients and asymptomatic individuals (pre-clinical phase) for clinical trials and research. In 2018, biomarkers were definitively incorporated into research (biological diagnosis). In recent years, much progress has been achieved in molecular neuroimaging methods 2 and in the techniques for measuring biomolecules in body fluids — cerebrospinal fluid (CSF) and plasma 3 . More recently, in 2024, a workgroup from the Alzheimer’s Association (AA) updated the 2011 and 2018 diagnostic criteria, reaffirming that the diagnosis of AD should be biological, based on biomarkers, besides having recommended biomarkers for biological staging of the disease 4 .
However, there is concern about the indiscriminate and poorly founded use of biomarkers, especially in asymptomatic individuals or those with subjective cognitive decline (SCD). The development of plasma biomarkers and greater access to these tests make it even more necessary to exercise caution in requesting these biomarkers, as they will become increasingly available. Besides the intrinsic difficulties in interpreting these tests, this scenario is also challenging due to their high costs and unequal access in Brazil 5 and even in high-income countries 6 . Concerned about the inappropriate use of biomarkers, a European consensus established criteria for their application in clinical practice for mild cognitive impairment (MCI) and mild dementia 7 .
Given the recent advances in the field of AD biomarkers, the main objective of this article was to establish recommendations from the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology (Departamento Científico de Neurologia Cognitiva e Envelhecimento da Academia Brasileira de Neurologia) regarding the rational use and interpretation of AD biomarkers in clinical practice. These recommendations may serve as a guide for physicians who assist patients with cognitive impairment and dementia. Therefore, the diagnosis of the patient’s cognitive-behavioral syndrome through anamnesis and cognitive, behavioral, and functional clinical evaluations should always be the initial step to guide the request for biomarkers.
METHODS
The recommendations were prepared by a group of medical specialists and researchers in AD and other dementias (neurologists, geriatricians, psychiatrists, nuclear medicine physicians, and clinical pathologists). A non-systematic review of the literature was conducted from searches in the MEDLINE, Scopus, SciELO, and LILACS databases until July 2024, using the descriptors “Alzheimer’s disease” or “biomarkers”. We selected mainly articles published in the last ten years as well as relevant older publications. The authors then met several times for discussion, and consensus points were included in the recommendations.
DEFINITION OF BIOMARKERS
Biomarkers in medicine can be defined as measures or indicators of normal or pathological physiological processes or responses to a therapeutic intervention 8 . The ideal biomarker for AD should reflect the neuropathological characteristics of the disease; demonstrate diagnostic accuracy in pathologically confirmed cases; have a sensitivity of at least 85%; have specificity to differentiate AD from cognitively healthy individuals and other diseases causing cognitive impairment of at least 75%; indicate the real presence of AD and not merely an increased risk; allow monitoring of disease severity or progression; indicate the effectiveness of the therapeutic intervention; be non-invasive; and be economically accessible 9,10 .
GENESIS OF BIOMARKERS - WHERE DO THEY COME FROM?
The neuropathological signature of AD includes amyloid plaques formed by extracellular deposits of amyloid beta (Aβ) peptides and neurofibrillary tangles (NFTs) formed by intracellular aggregates of hyperphosphorylated tau proteins (p-tau), inflammatory reaction, astrocyte and microglial activation, as well as synaptic and neuronal loss 11,12,13,14 .
The amyloid cascade hypothesis
Amyloid plaques, composed of amyloid fibrils in a beta-sheet conformation, are considered the primary cause of AD according to the “amyloid cascade hypothesis” 15,16,17 . This theory suggests that altered metabolism of the amyloid precursor protein (APP) by secretases (α, β, γ) leads to the release and aggregation of Aβ peptides (mainly Aβ40 and Aβ42), causing microglial activation, astrocytic reactivity, neuroinflammation, oxidative stress, p-tau hyperphosphorylation, and NFTs 18,19,20 . The hypothesis explains the genetic forms of AD, but not the far more common sporadic forms.
The amyloid beta peptide and amyloid plaques
Aβ peptides initially appear as monomers that can form various aggregates, from small oligomers to larger protofibrils and fibrils. While amyloid fibrils are insoluble and form amyloid plaques characteristic of AD, oligomers are soluble and can spread throughout the brain. Under physiological conditions, most Aβ peptides are Aβ40, and less than 10% are Aβ42, which is more prone to aggregation and is associated with the formation of amyloid oligomers, fibrils, and plaques 21 . Aβ production is balanced by degradation, clearance, transport, and deposition processes. Around 10–15% of Aβ may enter the CSF and bloodstream from the brain 22 .
Amyloid fibrils aggregate with other molecules, forming plaques in the brain’s extracellular space, predominantly near synapses, in three types: diffuse, dense-core, and neuritic plaques 11,13,14,23 . Plaques gradually grow in the brain’s interstitial space from continuous extracellular deposition of Aβ peptides at “seed” sites.
Neurofibrillary pathology and phosphorylated tau protein
Neurofibrillary pathology in AD includes NFTs, neuropil threads, and dystrophic neurites 14,24,25 . NFTs are abnormal bundles of p-tau in neurons 24 .
There are six tau isoforms in the adult human brain 26,27 , with 3R and 4R isoforms present in approximately equal levels 28,29 . In AD, NFTs contain both 3R and 4R isoforms. Other human tauopathies present pathological aggregates of either 3R or 4R tau isoforms 27,30,31 . P-tau, primarily found in neurons, stabilizes microtubules 27,31 and is regulated by phosphorylation. Pathological phosphorylation of tau reduces its microtubule affinity, leading to cytoskeletal destabilization 32,33 .
P-tau can be released into interstitial fluid, CSF, and blood, where it can be detected as indirect evidence of AD 32,34 . Pathological p-tau can be phosphorylated at several specific amino acids, such as 181, 217, and 231.
CLINICAL AND BIOLOGICAL DIAGNOSES OF ALZHEIMER’S DISEASE: THE EVOLUTION OF DIAGNOSTIC CRITERIA
Although the description of the disease that bears his name was published in 1907 by the German neuropsychiatrist and neuropathologist Dr. Alois Alzheimer 35 , the first diagnostic criteria were proposed only in 1984 by McKhann et al. 36 . At that time, they considered only the amnestic presentation of AD and admitted diagnosis only in the dementia phase. In 2011, new criteria for diagnosing AD were published by the National Institute on Aging and the Alzheimer’s Association (NIA-AA) 37,38,39 . In contrast to previous recommendations, the 2011 NIA-AA criteria began to admit prodromal (i.e., pre-dementia) phases and the possibility of non-amnestic variants of AD 37,39 . The 2011 criteria anticipated the use of biomarkers for the etiological diagnosis of dementia syndrome 37 or MCI 38 , proposing the terms “dementia due to AD” 37 and “MCI due to AD” 38 . Subsequent studies showed that these 2011 clinical criteria, without biomarkers, had a sensitivity of 70.9–87.3% and a low specificity of 44.3–70.8% when compared to pathological diagnosis 40 . In another study, the use of biomarkers led to a change in diagnosis in 36% of dementia cases with uncertain diagnosis 41 . It is estimated that in specialized memory centers, diagnostic errors range from 25–30% when biomarkers are not used 42 .
Subsequently, in 2018, the NIA-AA proposed a new conceptual model for the biological definition of AD based on biomarkers according to the Amyloid-Tau-Neurodegeneration - AT(N) system. In this 2018 model, the presence or absence of symptoms would not be necessary for AD diagnosis, with biomarkers A and T being sufficient. According to this model, AD is biologically defined by evidence of disease-related pathology through biomarkers, independent of the clinical syndrome. The goal was to improve AD diagnosis specificity and apply it in clinical research, while the 2011 criteria would remain valid for use in clinical care 43,44 .
According to this proposal, AD is defined by the positivity of biomarkers for amyloid (A+) and p-tau (T+), regardless of the presence or absence of neurodegeneration (N+ or N-). Practically, the biological diagnosis of AD would require low concentrations of Aβ42 and high concentrations of p-tau in the CSF or positivity on amyloid-PET (positron emission tomography) and tau-PET (Table 1). When there is only the presence of Aβ42 (A+) without evidence of tau pathology (T-), the term used is “Alzheimer’s pathologic change,” representing the initial stage of the AD pathological spectrum according to the amyloid cascade hypothesis. Conversely, when there is positivity for p-tau (T+) and/or neurodegeneration (N+), but negativity for amyloid pathology (A), the diagnosis of Alzheimer’s pathology should be excluded, and it would be termed “suspected non-Alzheimer’s pathophysiology” (SNAP).
Table 1. Diagnostic categories defined by the Amyloid-Tau-Neurodegeneration system within the continuum of the biological definition of Alzheimer’s disease, according to the 2018 National Institute on Aging and the Alzheimer’s Association Research Framework 43,44 .
| ATN | Category according to biomarkers profile |
|---|---|
| A-T-N- | Normal Alzheimer’s disease biomarkers |
| A+T-N- | Alzheimer’s disease pathological changes |
| A+T+N- | Alzheimer’s disease (without neurodegeneration) |
| A+T+N+ | Alzheimer’s disease (with neurodegeneration) |
| A+T-N+ | Alzheimer’s disease pathological changes + non-Alzheimer pathology |
| A-T+N- | Suspected non-Alzheimer’s pathophysiology (SNAP) |
| A-T-N+ | Suspected non-Alzheimer’s pathophysiology (SNAP) |
| A-T+N+ | Suspected non-Alzheimer’s pathophysiology (SNAP) |
Abbreviations: ATN, Amyloid-Tau-Neurodegeneration.
Although the 2018 criteria consider only biomarkers for AD diagnosis and not for the clinical syndrome, the model recognizes that AD can manifest along a continuum from an asymptomatic phase, through stages of SCD and MCI, to the dementia phase. In 2018, the authors of this diagnostic consensus made it clear that this biological definition, independent of the presence of cognitive symptoms, should only be used in research and not in clinical practice due to the lack of effective treatments for preclinical diagnoses of the disease.
In 2024, an AA workgroup published an update to the 2018 criteria; before being published, an initial draft of the article was made public for scientific contributions for a few months 4 . The motivations for updating the 2018 criteria were:
Approval of anti-amyloid drugs by regulatory agencies, which can only be used in patients with a confirmed AD diagnosis by biomarkers and in the early stages of the disease;
Recent advances in the development of plasma biomarkers; and
Evidence that fluid and molecular neuroimaging biomarkers are not equivalent and constitute different categories.
The goal of the new criteria is to establish AD diagnosis by incorporating new advances in biomarkers, reinforcing the idea that the definition of AD is biological and that symptoms are not necessary for its diagnosis. Another proposed update is that biomarkers be used not only for diagnosis but also for staging and monitoring treatment response 4 . Although the authors state that these new criteria can inform diagnosis in both research and clinical care, they caution that these criteria are not intended to be step-by-step guidelines for clinical practice or treatment protocols. The authors also recognize that these new criteria may not be operationalizable in many medical centers, even in high-income countries 4 .
The 2024 AA workgroup criteria suggest dividing biomarkers into three categories (Table 2):
Core (or specific) biomarkers of AD (biomarkers of amyloid and tau proteinopathies);
Non-specific biomarkers involved in the pathogenesis of AD (biomarkers of neurodegeneration and inflammation); and
Biomarkers of common co-pathologies associated with AD (e.g., alpha-synuclein and cerebrovascular disease) 4 .
Table 2. New categorization of Alzheimer’s disease fluid and molecular neuroimaging biomarkers according to the 2024 Alzheimer’s Association Workgroup criteria 4 .
| Core AD biomarkers (or specific AD biomarkers) | |
|---|---|
| Core 1 | |
| A | Aβ proteinopathy: Aβ42 (CSF or plasma) and amyloid PET |
| T1 | Soluble (or secreted) phosphorylated tau proteinopathy: p-tau 181, p-tau 217, p-tau 231(CSF or plasma) |
| Core 2 | |
| T2 | Insoluble (or aggregated) phosphorylated tau proteinopathy: p-tau205, MTBR-243 (CSF or plasma), and tau PET |
| Non-specific biomarkers involved in the pathogenesis of AD | |
| N | Neurodegeneration: NfL (CSF or plasma), anatomic MRI, and FDG PET |
| I | Astrocytic inflammation and reactivity: GFAP (CSF or plasma) |
| Common AD associated co-pathologies biomarkers | |
| V | Vascular (cerebrovascular disease): infarction or WMH on MRI (or CT) |
| S | Alfa-synuclein proteinopathy: αSyn-SAA (CSF) |
Abbreviations: AD, Alzheimer’s disease; Aβ, amyloid beta; αSyn-SAA, alpha-synuclein seed amplification assay; CSF, cerebrospinal fluid; CT, computed tomography; FDG, fluorodeoxyglucose; GFAP, glial fibrillary acidic protein; MRI, magnetic resonance imaging; MTBR, microtubule-binding region; NfL, neurofilament light chain; PET, positron emission tomography; p-tau, ; WMH, white matter hyperintensity.
Tau biomarkers are divided into two categories: T1 and T2. This division is being proposed due to the chronological difference in the alterations of tau biomarkers 4 . Some p-tau isoforms (181, 217, 231) change earlier, around the same time that amyloid PET becomes positive 45,46,47 . Although these soluble p-tau analytes in CSF and plasma are tau biomarkers, they may indicate that the patient already has moderate/frequent amyloid plaques (high CERAD score, Consortium to Establish a Registry for Alzheimer’s Disease) and most of these cases are also in Braak stages III to VI, meeting the criteria for intermediate/high AD neuropathological change 4 .
On the other hand, tau PET and other tau analytes, such as p-tau205 and MTBR-tau243 (microtubule-binding region of tau-containing residue 243) change later, when fibrillar tauopathy is at Braak stages IV to VI. Hence, these biomarkers are termed T2, indicating insoluble (or aggregated) phosphorylated tau proteinopathy 46,47 48 . Thus, amyloid and T1 biomarkers are considered core 1 biomarkers, while T2 biomarkers are core 2. Core 1 biomarkers change in the early stages of AD (still in an asymptomatic phase), while core 2 biomarkers become abnormal later, when the first symptoms begin.
The 2024 AA workgroup criteria propose that an abnormality in one of the specific core 1 biomarkers (or a combination of them) is sufficient for the diagnosis of AD: amyloid PET, CSF Aβ42/40, CSF p-tau 181/Aβ42, or CSF t-tau/Aβ42 (Table 2). The workgroup raises the possibility that plasma biomarkers could be used for diagnosis in the future, as long as they have an accuracy equivalent to already approved CSF biomarkers or amyloid PET. Core 2 biomarkers alone are not sufficient for diagnosis, but the combination of core 1 and core 2 biomarkers can be used for AD biological staging 4 . The AA workgroup currently recommends against diagnostic testing in asymptomatic individuals outside the context of research studies.
These criteria were criticized by the scientific community, particularly by the European group, the International Working Group (IWG), which proposed a parallel diagnostic criterion 49 . The IWG does not consider the presence of biomarkers as a sole diagnosis of AD because many people have positive biomarkers but never develop clinical symptoms 49 . Additionally, the IWG argues that the presence of biomarkers is a risk factor for AD, but alone, does not allow for a diagnosis of the disease, in contrast to the criteria suggested by the AA in 2024. These differences reflect some of the criticisms about the AA’s proposed criteria, including the lack of access to biomarkers in resource-limited settings, leading to disparities in diagnostic access, uncertainty regarding the prognosis of “asymptomatic” disease, and difficulties in managing the AD nomenclature. Still, there is a significant stigma associated with the name “Alzheimer’s disease” due to its invariably progressive and irreversible nature once symptoms begin. Moreover, the impact of such a diagnosis on asymptomatic individuals or those in the early stages of the disease is unknown. Finally, despite the existence of anti-amyloid therapies that show some clinical effect, these drugs have modest efficacy, potentially serious and fatal adverse events, and high costs, which currently do not justify screening for AD in the population, as is done with other diseases like diabetes mellitus, colon or cervical cancer, that can be diagnosed in asymptomatic individuals.
BIOMARKERS IN CEREBROSPINAL FLUID
The most widely validated and studied CSF biomarkers are the proteins that characterize AD: the Aβ42 peptide and p-tau. In CSF, AD is characterized by a reduction in Aβ42 and an increase in p-tau. This pattern is known as the AD pathology signature in CSF 50 .
Recently, Aβ40 has been included in the diagnostic biomarker panel for AD, as evidence shows that the Aβ42/Aβ40 ratio outperforms Aβ42 dosage alone, demonstrating better concordance with amyloid PET positivity. The Aβ40 peptide has high concentration levels in CSF and does not vary in AD, unlike Aβ42. This causes the Aβ42/Aβ40 ratio in CSF to gradually decrease as the disease progresses 51 .
A meta-analysis of 131 studies revealed that CSF concentrations of Aβ42 in AD patients decrease by about 56% from normal levels 52 . A steeper decline in CSF Aβ42 levels occurs at the onset of the disease’s pathological process, followed by relative stability as the disease evolves. In contrast, p-tau levels change slowly over the course of the disease 53 .
Most studies showed approximately 90% concordance in comparing amyloid PET and CSF Aβ42 levels in LCR 51 . In a longitudinal study with older individuals without dementia, Palmqvist et al. observed that those with low CSF Aβ42 levels and negative amyloid PET had a higher rate of PET positivity over time than individuals with normal Aβ42 levels 54 . This finding suggests that CSF changes precede amyloid PET changes, explaining why the concordance between the two biomarkers is below 100%. It is important to note that these studies involved asymptomatic individuals. In patients at the dementia stage, a higher concordance between amyloid PET results and CSF Aβ42 levels is observed.
The accumulation of p-tau is the other component that defines AD besides Aβ peptide. The main assays used to measure p-tau quantify p-tau181, which presented the best accuracy in differentiating AD from cognitively healthy controls or other degenerative dementias. Assays using p-tau231 and p-tau199 also demonstrated good accuracy 51 . The ratio of p-tau/Aβ42 levels has also proven useful in AD diagnosis, with a sensitivity of 91.6% and a specificity of 85.7% 55 .
More recently, several studies showed that p-tau217 is the phosphorylated tau isoform with the earliest changes 45,46,47 . Evidence indicated that p-tau217 concentrations in CSF and plasma increase at the same time amyloid PET becomes positive and before tau PET changes 45,46,47,56 . Compared to p-tau181, p-tau217 exhibited a better correlation with amyloid PET and better differentiated A+ individuals from A- individuals 57 . Additionally, p-tau217 proved to be a better marker of cognitive progression to dementia in patients with MCI than p-tau181 58 . On the other hand, p-tau205 changed later and was better associated with tau PET than with amyloid PET 57 .
Although the p-tau dosage in CSF serves as a biomarker for tau pathology, the soluble tau isoforms do not correlate with the load of insoluble p-tau aggregates (represented by NFTs). Recently, the measurement of MTBR-tau243 residues was investigated as a new CSF biomarker specific for insoluble tau aggregates 59 . Changes in MTBR-tau243 in CSF occur simultaneously with changes in tau PET 59 .
Total p-tau (t-tau) measures all isoforms of p-tau, regardless of phosphorylation state; therefore, is not specific to AD. In fact, elevated t-tau levels can be observed in other conditions involving neuronal damage, both degenerative (e.g., Creutzfeldt-Jakob disease) and acute non-degenerative (e.g., cerebrovascular accident). Thus, t-tau is considered a nonspecific biomarker of neurodegeneration. Recently, the measurement of t-tau in CSF has been increasingly replaced by other neurodegeneration markers, especially neurofilament light chain (NfL). Similar to t-tau, increased NfL concentrations are not specific to AD and can rise in other neurodegenerative disorders, such as amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). NfL is promising as it may predict disease progression and potentially response to disease-modifying treatments 60 .
Recent CSF AD biomarkers time course study involving Chinese participants during the 20 years preceding clinical diagnosis of sporadic Alzheimer’s disease, showed changes in CSF biomarkers concentration, as follows: Aβ42, 18 years; Aβ42/Aβ40 ratio, 14 years; p-tau 181, 11 years; t-tau, 10 years; and NfL, 9 years. As cognitive impairment progressed, the changes in CSF biomarker levels in the AD group initially accelerated and then slowed 61 .
Despite numerous studies demonstrating the good accuracy of CSF biomarkers for AD, one of the major challenges has been the significant variability between laboratories 51,62 . This variability often leads clinicians to disregard CSF results when they conflict with the diagnostic hypothesis and to seek molecular neuroimaging methods as biomarkers. This variability primarily stems from errors and failures in the pre-analytical (sample collection, storage, and transportation) and analytical (actual execution of the test) phases. It is estimated that pre-analytical problems account for up to 70% of errors in clinical laboratories 63 . To address this issue, the AA, in partnership with the Neurochemistry Laboratory at the University of Gothenburg (Sweden), established an external quality control (QC) program in 2009. The program aims to monitor variations in biomarker tests across different locations and batches and to help the participating laboratories synchronize their procedures. This external QC program is free for participating laboratories, whether they are devoted to research or clinical activities 62 .
Among the core biomarkers for AD, the measurement of Aβ42 is the most affected by the pre-analytical phase. Falsely reduced results, or potential false positives, can occur due to the adsorption of the peptide onto the walls of the tubes used for CSF collection. The Aβ42/Aβ40 ratio shows less variation than the concentration of Aβ42 alone 62 . The recommendation is to use low-binding polypropylene tubes for the collection and storage of CSF, avoiding glass tubes. Other interfering factors include sample handling, tube transfers (aliquoting), freeze-thaw cycles, and storage temperature.
Recently, a working group led by the AA, consisting of experts from academia and industry, proposed a new simplified and standardized pre-analytical protocol for CSF collection, handling, and analysis for clinical routine use. This proposal came up after a comprehensive analysis of the impact of pre-analytical factors, including the type of tube, sample handling procedures, and storage/transport conditions 62 .
The most used methods for measuring biomarkers are manual immunoassays, specifically enzyme-linked immunosorbent assay (ELISA). Even when following the recommendations suggested by the AA, the coefficient of variation (CV) for this technique between laboratories can reach 15–25% (medium to high). Recently, single molecule array (SIMOA™) technology, which involves encapsulating individual molecules in femtoliter reaction chambers using paramagnetic markers, has also been used for biomarker measurement due to its ability to detect very small concentrations of substances in CSF and plasma.
Fully automated platforms have been developed in recent years. Bittner et al. published a 100% automated method based on electrochemiluminescence immunoassay 64 . Automated assays offer significant advantages over the ELISA methodology as they eliminate manual steps, reducing the possibility of human interference in the analytical process, and they exhibit superior performance (low CVs: Aβ42 ≤ 6%; p-tau and t-tau: ≤ 2.5%) with a shorter test execution time (~20 minutes). A recent study demonstrated that the ratios of different automated platforms (p-tau/Aβ42 and Aβ42/Aβ40) in CSF show excellent concordance (positive and negative) with amyloid PET classification 65 . These data suggest that the p-tau/Aβ42 and Aβ42/Aβ40 ratios provide similar clinical information in evaluating amyloid pathology, thereby bringing greater reliability to clinical practice results. However, these automated platforms are still costly. Two automated electrochemiluminescence platforms have received regulatory approval in the United States (through FDA, Food and Drug Administration) and in the European Union (EMA, European Medicines Agency) to date 66,67 . The approval of these two platforms was conditioned on achieving accuracy close to 90% compared to amyloid PET. The sensitivity of these platforms ranges from 88–97%, while specificity ranges from 84–89% 66,67 .
We strongly recommend that clinicians observe the methodology used in these measures and, whenever possible, choose laboratories that use fully automated platforms to minimize analytic bias.
Finally, it is important to emphasize that CSF analysis is recommended in cases of rapidly progressive dementia, early-onset dementia, or atypical dementias, to exclude infectious, inflammatory autoimmune, or neoplastic diseases 38 . In these cases, the CSF examination must include a red blood cell and nucleated cell count, differential leukocyte analysis, total protein measurement, and glucose levels. If an infection is suspected, the analysis should include venereal disease reaction level, Gram stain, fungal and acid-fast bacilli tests, and culture with antibiogram. At the physician’s discretion, other analyses may be included (e.g., neoplastic cell search, specific immunological reactions, or oligoclonal band search).
BLOOD-BASED BIOMARKERS
In recent years, there has been significant improvement in the technologies that enable the measurement of Aβ peptide and different epitopes of p-tau in plasma. The latest availability of plasma assays for these biomarkers was made possible by developing more precise techniques, as the plasma concentration of these proteins is much lower than in CSF. The use of assays based on mass spectrometry or more sensitive immunoassays (e.g., SIMOA™) has made it a reality to measure these biomarkers in plasma 42,68,69 .
Compared to well-established CSF and PET methods, plasma biomarkers offer a less invasive, more feasible, and potentially more cost-effective option in clinical practice. Additionally, they have the potential to significantly reduce screening time and costs in clinical trials and can be used as surrogate outcomes to evaluate the efficacy of therapies. However, the validation of these plasma biomarkers is still in development, and currently, they cannot be used alone for diagnosis. While a recent study suggests the possible superiority of plasma over CSF biomarkers 45 , another study using machine learning to predict AD-associated cognitive decline suggests the superiority of CSF biomarkers 70 .
Besides the mixed results regarding the superiority of plasma biomarkers for AD diagnosis, the limitation of real-world clinical validation, lack of studies in ethnically diverse populations and local norms (e.g., for the Brazilian population), and some methodological issues (pre-analytical care protocols and QC programs like those existing for CSF biomarkers) hinder the applicability of plasma biomarker 68 . For all these reasons, the systematic and routine use of plasma biomarkers in clinical practice is not currently recommended. Additionally, none of the plasma biomarker assays have been approved by the FDA as of June 2024 4 .
Plasma Aβ42, Aβ40 and Aβ42/Aβ40 ratio
The development of various immunoprecipitation methods based on mass spectrometry has greatly improved the Aβ42/Aβ40 ratio accuracy. However, accuracy varies among the different methods used. While mass spectrometry-based assays have an area under the curve (AUC) ranging from 0.76–0.87, immunoassay methods have lower performance, with AUC ranging from 0.64–0.78 48 . This significant variability in accuracy between assay types is one of the main limitations to the clinical use of plasma Aβ peptide biomarkers 48,71 . The measurement of Aβ42 alone has not shown good accuracy and is therefore not performed; the Aβ42/Aβ40 ratio is always recommended.
An inherent problem with the plasma Aβ42/Aβ40 ratio, regardless of the method used, is that AD patients show only a small reduction in this parameter compared to controls. For reference, in the plasma of AD patients, the Aβ42/Aβ40 ratio is reduced by 8–15% compared to controls, while in CSF, the reduction is 40–60% 42,68,71 . This makes the plasma test less robust than the CSF Aβ42/Aβ40 ratio. One possible explanation for this difference is that the reduction in the Aβ42/Aβ40 ratio quantified in plasma reflects only a fraction of the Aβ detected in the blood, as the test does not distinguish between Aβ derived from the brain and that of extracerebral origin, the latter presumably not affected by AD pathology 42,68 . Developing plasma biomarkers that reflect the brain-derived fraction of Aβ remains a challenge.
Other important issues include the need for “real-world” studies to verify the robustness of the test, studies that validate it in diverse populations, and an understanding of intra-individual variability and disease-associated variability, as well as the potential impact of comorbidities and medications 42,68 . For these reasons, it is not recommended to use the Aβ42/Aβ40 ratio alone as the sole amyloid biomarker, for example, to indicate anti-amyloid therapy 42,68 . Plasma biomarkers are not recommended as unique diagnostic criteria for anti-amyloid therapy.
Combining the Aβ42/Aβ40 ratio with other biomarkers can improve its accuracy. West et al. designed a mass spectrometry platform to quantify plasma levels of Aβ42 and Aβ40 and identify specific peptides of the apolipoprotein E (ApoE) isoform — proteotyping, an indirect method of determining ApoE genotype 72 . In this study, the accuracy of Aβ42/Aβ40 increased from 81 to 86% when combined with ApoE proteotyping. The authors also developed an amyloid probability score (APS) by combining the Aβ42/Aβ40 ratio, ApoE proteotyping, and the patient’s age. The scores are categorized as low (0–35), intermediate (36–57), and high (58–100), corresponding to the likelihood of amyloid PET positivity, with a sensitivity of 84.9% and a specificity of 96.0% 73 . Although this platform is already being commercialized, it is emphasized that the measurement methods for Aβ42 and Aβ40 peptides still require better validation, particularly in the Brazilian population. Another aspect to consider in a blood matrix is identifying confounding factors that affect levels and their clinical utility before routine implementation. Recent evidence suggests that reduced renal function may be associated with increased plasma biomarker concentrations 74 . No platform for measuring Aβ42 and Aβ40 in plasma has received regulatory approval in the United States or the European Union 4 .
Plasma phosphorylated tau
Similar to advancements in detecting plasma Aβ peptide through mass spectrometry, in recent years, several assays using this method have been developed for detecting p-tau in the blood. However, these assays are not yet validated and standardized for routine use (real-world studies) outside the context of clinical research. An inherent problem with p-tau is its extremely low plasma concentration. This consideration is necessary for interpreting study results and, obviously, for considering its application in clinical practice. Significant progress was achieved in p-tau measurement techniques, and in some cases, accuracy values equivalent to tau PET and even superior to CSF have been achieved 45 .
The referenced assays use antibodies to detect tau epitopes, as the intact protein is even less available in peripheral blood due to proteolytic processes. These epitopes are named according to the position of the amino acid at which they are phosphorylated. The most widely used epitopes in assays are p-tau181, p-tau217, and p-tau231. Tests with each of these variants have shown that they can differentiate patients with and without AD pathology 56,75,76,77 . Studies with neuropathological information have shown that plasma levels of p-tau217 reflect both the density of NFTs and amyloid plaques 78 . Notably, the increase in levels of these p-tau forms seems specific to AD and does not occur in other tauopathies such as corticobasal degeneration (CBD), progressive supranuclear palsy (PSP), or frontotemporal lobar degeneration (FTLD) 68,75 . Both p-tau181 and p-tau217 have also shown excellent accuracy in predicting the progression of patients with MCI to a stage of dementia due to AD 56,75 . On the other hand, p-tau231 is the first to change on the AD continuum, indicating a closer association with amyloid pathology than with tau pathology itself 79 .
Of all the isoforms, p-tau217 has shown the highest sensitivity and specificity for the diagnosis of AD 80 . Unlike the Aβ42/Aβ40 ratio, which decreases by less than 15% in AD patients, p-tau217 increases by 300 to 700% 42 . This makes p-tau217 a much more robust test for detecting AD compared to the Aβ42/Aβ40 ratio. In a study comparing ten different plasma p-tau assays, the measurement of p-tau217 using a mass spectrometry-based assay presented an AUC of 0.947 and a correlation of 0.891 with CSF p-tau concentration 56 . Interestingly, p-tau217 levels also showed an excellent correlation with amyloid pathology 42 . Brum et al. developed an algorithm using p-tau217, ApoE genotyping, and age as a screening test to determine Aβ pathology positivity in patients with MCI 81 . This algorithm could predict amyloid pathology in 80% of cases (in the remaining 20%, the Aβ42/Aβ40 ratio in CSF was needed). In another study with 786 participants from three cohorts using an immunoassay, plasma p-tau217 concentration had high accuracy in predicting Aβ pathology (AUC=0.92–0.96; 95%CI 0.89–0.99) and tau pathology (AUC=0.93–0.97; 95%CI 0.84–0.99) 76 . Despite the need for more real-world validation studies, plasma p-tau217 (especially when using mass spectrometry-based assays) emerges as a promising biomarker for detecting proteinopathies (amyloid and tau) 45 . The ratio of plasma p-tau217 concentrations to phosphorylated p-tau at different sites (np-tau217) has been used in some studies to further increase the diagnostic accuracy of p-tau217 82,83 . Most recently, an algorithm combining the plasma Aβ42/Aβ40 ratio and the percentage of p-tau217 relative to np-tau217 to produce an amyloid probability score 2 (APS2) had accuracy of 88% (AUC=0.94; 95%CI 0.92–0.96), with 88% agreement with amyloid PET 84 .
As with plasma Aβ, the presence of certain comorbidities can significantly affect the plasma concentration of tau epitopes. Understandably, chronic kidney disease — once it affects protein clearance—is associated with higher levels of p-tau181 and p-tau217. Additionally, conditions such as hypertension, stroke, and myocardial infarction also elevate these levels 85 . These conditions may presumably modify the safe cutoff points for the test, but this relationship has not yet been established.
NEUROIMAGING BIOMARKERS
Among the neuroimaging biomarkers for evaluating AD, structural neuroimaging methods such as computed tomography (CT) and magnetic resonance imaging (MRI) stand out, along with nuclear medicine techniques.
Molecular neuroimaging and nuclear medicine methods include conventional brain scintigraphy, acquired in gamma cameras in three-dimensional form (single-photon emission computed tomography — SPECT), and the PET. In these exams, the radioactive source emanates from the patient, who receives an intravenous injection of radiotracers. These procedures are safe, with no significant adverse events or reported allergies. PET has a higher spatial resolution than SPECT. These exams are currently performed on hybrid multimodal machines (SPECT/CT, PET/CT, and, more recently, PET/MRI) to compensate for their lack of spatial resolution. Today, almost all PET machines have a PET/CT configuration 86 .
These methods are highly sensitive for selecting patients for anti-amyloid therapies and for prognosis evaluation/stratification. They are commonly used for outcome assessments in most recent AD studies, mainly due to their ability to detect specific pathological or physiological changes. However, their use is limited in monitoring the safety of new anti-amyloid therapies compared to MRI, the gold standard in neuroradiology. MRI has recognized superiority in detecting general conditions such as bleeding, stroke, edema, and amyloid-related imaging abnormalities (ARIA) 87 .
MRI is very useful in the initial evaluation of individuals with dementia due to its ability to detect structural causes (e.g., cerebrovascular disease) or potentially reversible causes (e.g., benign neoplasms) of cognitive decline 88 . In some cases, especially when MRI is not accessible, CT can be used as a substitute. However, RM use is not limited to initial evaluation; it can also be employed as a biomarker of neurodegeneration, as highlighted hereinafter.
Imaging exams can function as biomarkers for AD throughout the entire AT(N) staging 44 . Using PET scans with amyloid or tau tracers, it is possible to detect the cerebral deposition of Aβ peptide plaques (“A” staging) or intracellular tangles of phosphorylated p-tau (“T” staging), respectively, as well as their removal after treatments. While fluid biomarkers (CSF and plasma) reflect the production or clearance of the soluble forms of proteinopathies, PET tracers mark the insoluble aggregates, measuring the cumulative effects of the proteinopathies and providing information on the neuroanatomical distribution of the pathology 89,90 .
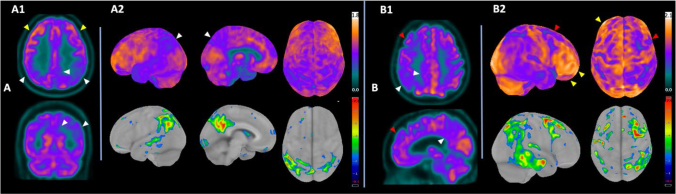
PET scans assessing glucose metabolism [18F]-Fluorodeoxyglucose (FDG-PET), cerebral perfusion by SPECT, and MRI can identify signs of neurodegeneration (“N” staging) 91 . FDG-PET, besides being an N biomarker for investigating AD, can aid in differential diagnosis by demonstrating neurodegeneration patterns suggestive of AD or other non-AD degenerative diseases, as outlined by the NIA-AA Research Framework 43 and confirmed in a Brazilian study 92 . Furthermore, FDG-PET is particularly useful for characterizing atypical forms of AD (logopenic variant, posterior cortical atrophy, and dysexecutive-behavior variant).
In Brazil, the main limitations of molecular neuroimaging assessment are the unavailability of certain tracers (especially tau PET), the high cost combined with the limited availability of others (amyloid PET) in routine clinical practice, and, most notably, the high cost of combining multiple tests, as detailed thereafter.
Amyloid plaque marker positron emission tomography
The PET scan with an amyloid plaque marker (amyloid PET) represents a significant technical advancement in AD imaging assessment, being included as an “A” staging marker in the latest diagnostic criteria 4,44,49 .
This method detects extracellular cerebral deposits of Aβ peptide in moderate/frequent neuritic plaques with high sensitivity and specificity (96% and 100%, respectively) 2,93 . A normal amyloid PET scan excludes AD as a diagnosis in patients with cognitive impairment. Therefore, it is indicated as a prerequisite to confirm or exclude a clinical syndrome related to AD, with high specificity and reproducibility, making it an excellent tool for selecting patients for clinical trials and approved treatments.
There are four most common and commercially available amyloid PET tracers. One of them, labeled with carbon-11, 11C-Pittsburgh compound-B ([11C]-PiB), is considered open-access; however, it requires the presence of a cyclotron at the exam location due to the very short half-life of carbon-11. The other three tracers, labeled with fluorine-18, have commercial use due to the longer half-life of fluorine-18 and were approved by European and United States regulatory agencies for clinical use: [18F]-Florbetaben, [18F]-Florbetapir, and [18F]-Flutemetamol. Currently, the tracers available in Brazil are [11C]-PiB and [18F]-Florbetaben.
Amyloid PET scans are generally dichotomous and classified as positive or negative (A+ or A-), facilitating the selection of individuals for clinical studies (Figure 1). Negative tests (A-) rule out the possibility of AD. The incidence of A+ in cognitively normal individuals can range from 2.7% in people aged 50 to 59 years to 41.3% in people aged 80 to 89 years 94 , typically varying around 10–20% in individuals aged between 60 and 70 years. Conversely, about 25–30% of individuals with a clinical diagnosis of AD have negative (A-) scans in most studies, indicating a high percentage of patients with clinically suggestive AD but with another underlying pathology 95 . There are minimal variations in the percentage of A+ between cohorts and concordant results with international studies using [11C]-PiB. The Brazilian setting demonstrates 18% positivity among controls (mean age ± standard deviation [SD] = 71.19±6.1 years) and 76% in individuals with clinically suggestive AD (mean age ± SD = 73.7±7.3 years) 92 , highlighting the limitation of solely clinical diagnosis of the disease.
Figure 1. Axial images of amyloid PET of two individuals with [11C]-PiB showing significant cortical uptake of the tracer (A - upper row - a “positive” scan, or A+) and absence of significant uptake of the tracer (B - lower row - a “negative” scan, or A-). In the A+ individual, a diffuse uptake is seen in the frontal, parietal, and temporal neocortices, as well as in the medial parietal and frontal lobes (precuneus, posterior and anterior cingulate gyrus), with an intensity similar to or more intense than the physiological uptake in the white matter. A negative scan (B) shows an uptake restricted to white matter tracts. Red localizers show the correct way to visualize the medial parietal and medial frontal cortices in the medial views (at the cortex level as localized in the coronal or axial views). Note the absence of uptake in the cerebellar cortex (a reference for negativity) and the physiological uptake in the brain stem (a reference for positivity).
Source: Images from the personal archive of one of the authors (A.M.C.) and acquired at the Nuclear Medicine Center of the Institute of Radiology, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP), São Paulo, SP, Brazil.
In response to the initial results of studies with amyloid PET, joint recommendations from the Society of Nuclear Medicine and the AA for the use of amyloid PET were published in 2013 96 . This consensus recommended the method in the following cases:
Persistent or progressive MCI of unexplained cause;
Patients with clinical criteria for possible AD, but with atypical clinical presentation or possible mixed etiology; and
Patients with progressive dementia and atypical age of onset (pre-senile), defined as symptom onset at 65 years or younger.
The same recommendations suggested not using amyloid PET in the following situations:
Patients who meet clinical criteria for probable AD with typical age of onset;
To determine the severity of dementia;
Based solely on a family history of dementia or the presence of the ApoE ε4 allele;
Patients with SCD;
As a substitute for genotyping in carriers of autosomal dominant mutations;
Asymptomatic individuals; and
Non-medical use (e.g., legal purposes, insurance coverage evaluation, employment, or occupational assessment).
It is important to note that these recommendations were published in 2013, before the adoption of biological diagnostic criteria for AD research based on biomarkers.
Amyloid PET scans have been included as biomarkers used as biological outcome variables in all clinical trials of anti-amyloid drugs approved so far on the United States market to monitor the biological success of anti-amyloid therapy. Based on their high specificity, the reduction of amyloid load was considered one of the aspects that contributed to the approval of these drugs 97,98,99,100,101 . In October 2023, the Centers for Medicare & Medicaid Services in the United States approved coverage for one amyloid PET scan during the lifetime of patients with AD, primarily to appropriately select patients for anti-amyloid treatment 102 . Regarding clinical use for early detection or differential diagnosis of neurodegenerative diseases, some authors have suggested the ideal timing for using this method, including its optimal timing relative to other molecular imaging methods 2,86 .
The interpretation of typical images obtained with amyloid PET depends on the knowledge of the progression of amyloid deposition and its topography during the course of AD. Initially, plaques form in the neocortex (frontal, parietal, temporal, occipital) (Thal phase 1); next, in the allocortex (entorhinal cortex, subiculum, hippocampus [CA1]), cingulate cortex, and amygdala (Thal phase 2); then the basal ganglia, thalamus, and hypothalamus are affected (Thal phase 3); and finally, the midbrain, pons, and cerebellum (Thal phases 4 and 5) 25,103,104 .
Tau protein positron emission tomography
PET scans with p-tau deposit tracers are not yet part of routine clinical practice, even in centers in developed countries. However, research results indicate that this exam may eventually be included in clinical practice due to its potential to replace older exams by combining the detection of pathological processes with disease staging and differential diagnosis 86,105,106,107 .
In the revised criteria for diagnosis and staging of AD, published by AA workgroup, it is clearly stated that tau PET can discriminate between biological stages, categorized from A to D, whereas fluid biomarkers can only establish that an individual is in stage A or higher. Stage A corresponds to positive amyloid PET and negative tau PET (A+T2); tau PET with uptake only in the medial temporal region (A+T2MTL+) is equivalent to stage B; tau PET with moderate neocortical uptake (A+T2MOD+) is stage C; and high tau PET high neocortical uptake (A+T2HIGH+) represents stage D. So, for staging AD, tau PET is currently the only available biomarker 4 .
P-tau tracers have been validated by post-mortem pathological studies, showing a strong association with NFTs 47,89,107 . However, studies comparing p-tau levels in fluids with tau PET have shown that p-tau increases in both CSF and plasma before tau PET becomes positive 42 . Despite this, tau PET is highly associated with the neuroanatomical distribution of NFTs, thus reflecting the clinical manifestations, including the phenotypic variants of AD (i.e., non-amnestic presentations) 107 .
The interpretation of typical images obtained with tau PET depends on the topographical knowledge and progressive distribution or staging of NFT appearance in the course of AD. Initially, the transentorhinal stage is identified (Braak & Braak stages I–II), followed by the limbic stage (Braak & Braak stages III–IV), and finally the isocortical stage (Braak & Braak stages V–VI) 25,108 .
The first generation of tau tracers, most notably the radiopharmaceutical [18F]-FAV1451 (formerly [18F]-T807 and commercially known as [18F]-Flortaucipir), has affinity only for 3R/4R helical tau filaments, which are exclusive to AD. These methods are excellent for selecting T+ or T- individuals in AD and for staging the disease, as their deposition follows the Braak & Braak staging observed in pathological studies 109 , which can help assess disease progression. However, this marker has not been able to identify deposits of 3R or 4R tau related to primary tauopathies, which are mainly represented by FTLD, CBD, and PSP 110 . Currently, [18F]-Flortaucipir is the only p-tau tracer approved for clinical use in the United Sates 111 .
Second-generation tau tracers appear to overcome the limitations related to primary tauopathies and are mainly represented by [18F]-MK6240 and [18F]-PI2620. Their use allows for the identification of 3R/4R tau deposits in AD with high affinity, staging/stratifying AD, and differentiating it from other tauopathies both by the spatial distribution of tracer deposition and by lower affinity and intensity of uptake in 4R tauopathies 105,112,113 . This method could also evaluate the evolutionary stage as a staging marker in these conditions.
Once clinically tested and approved, these second-generation radiopharmaceuticals could be used to select individuals based on their pathological status (strongly T+ individuals are generally A+), perform differential diagnoses with other tauopathies, and simultaneously stratify and classify diseases with 3R/4R or 4R tau deposition. They could also help differentiate AD from both tauopathies and alpha-synucleinopathies, the latter presenting negative scans (absence of tracer deposition). Thus, this method has enormous potential for routine clinical use in the future. These tracers represent a significant advancement not only in selecting individuals for AD trials but also for anti-tau treatments in diseases such as PSP.
Tau PET methods are not currently available in Brazil, and there are no perspective for their clinical use in the short term. Additionally, the costs of these procedures are expected to be high. There are also no well-defined criteria for their visual analysis in clinical practice. This is a hindrance to the use of the newly proposed revised criteria for staging AD in clinical practice in Brazil and in many other countries.
[18F]- Fluorodeoxyglucose positron emission tomography
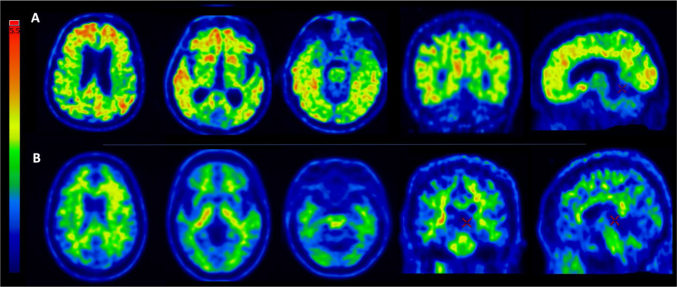
FDG-PET remains the primary molecular neuroimaging method available in clinical practice. The pattern of regional hypometabolism in the temporoparietal association areas, medial temporal, precuneus, and posterior cingulate shows high sensitivity and specificity in indirectly detecting AD-related pathology (Figure 2). The accuracy is around 90% in AD or slightly higher in various recent cohorts, including in the prediction of in vivo amyloid in national series, both in individuals with a broad spectrum of amnestic syndrome 92 and in non-amnestic variants such as corticobasal syndrome 114 . It is important to note that the test shows higher accuracy in the dementia phase or in cases of MCI due to AD in more advanced stages of neurodegeneration, and lower accuracy in the early stages of MCI due to AD.
Figure 2. Typical pattern of neurodegeneration in Alzheimer’s Disease as seen on FDG-PET at different stages of progression in two individuals with predominantly amnestic presentation and confirmed cortical deposition of beta-amyloid plaques by [11C]-PiB PET. (A) Hypometabolism in the posterior temporal neocortex, temporoparietal associative cortex, posterior cingulate, and precuneus, slightly more evident on the left side, highlighted by white arrows. (A1) Images in axial and coronal planes, showing preserved frontal metabolism (yellow arrows). (A2) 3D-SSP* quantification images demonstrating in the top row the projection of metabolism on the cortical surface in left lateral, medial, and superior projections; in the bottom row, the projection of the Z-score of hypometabolic areas in relation to a normal control database by the same method is shown. (B) Patient in the moderate phase of the neurodegeneration process, with prefrontal hypometabolism (red arrows) in addition to hypometabolism in posterior temporoparietal regions (white arrows). Notable is the preservation of metabolism in polar and supraorbital frontal regions (yellow arrows), an important differentiation from other pathological processes (e.g., frontotemporal dementia). (B2) 3D-SSP* quantification images demonstrating in the top row the projection of metabolism on the cortical surface in right lateral and superior projections; in the bottom row, the projection of the Z-score of hypometabolic areas in relation to a normal control database is shown.
Source: Images from the personal archive of one of the authors (A.M.C.) and acquired at the Nuclear Medicine Center of Institute of Radiology, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP), São Paulo, SP, Brazil.
Note: *3D-SSP images obtained by Cortex ID Suite, GE Healthcare. Color scales to the right – upper bars in standardized uptake value ratio (SUVr) with the global cortex mean as the normalization reference; lower bars in Z-score values relative to an age-matched normal control database.
On the other hand, while a normal FDG-PET scan does not exclude the diagnosis of a degenerative disease, it suggests a favorable prognosis of potential cognitive stability over an average follow-up period of three years 115 . Additionally, the combination of PET with CT in PET/CT equipment provides the advantage of simultaneously evaluating cerebral metabolism, structural changes, and vascular load. Although this is less refined than MRI, it is often sufficient for clinical purposes.
Ossenkoppele et al. indicated that the neuroanatomical pattern of hypometabolism in FDG-PET showed a strong negative correlation with areas of tracer hyper-uptake for p-tau. In other words, the hypometabolism pattern (neurodegeneration) reflects the distribution of tau pathology in AD 106 . This overlap between p-tau accumulation (tau PET) and areas of hypometabolism (FDG-PET) has a significant implication: FDG-PET can be a good substitute for tau PET in locations where it is unavailable 43,92 . However, it is important to note that, according to the amyloid cascade hypothesis, tau PET changes precede those observed in FDG-PET, a marker of neurodegeneration. In post-mortem analyses in which FDG-PET was evaluated by physicians who were not molecular imaging specialists, the exam showed a sensitivity of 80% and a specificity of 84% 40 . Previous studies, however, demonstrated wide variability in these values, with sensitivity ranging from 88–98% and specificity from 80–95% 40 . Generally, older studies, in which exams were not reviewed by specialists or did not use quantitative methods in clinical practice, presented more modest results.
Motara et al. evaluated the clinical impact of using FDG-PET according to its indications: diagnostic difficulty after formal clinical procedures and inconclusive structural analysis, pre-senile dementia, differentiation between AD and FTD, atypical cases of AD and FTD, psychiatric comorbidities associated with cognitive decline, and inconclusive neuropsychological evaluation 116 . In this evaluation, FDG-PET impacted clinical management in 81% of individuals (79/98), changing the pre-test diagnosis in 35% of cases, reducing the need for further investigations by 42%, and altering therapy in 32%. These results are similar to those of Laforce Jr et al., who showed a clinical management impact of 56%, with a diagnosis change in 29% of cases 117 .
A consensus article based on expert opinions from the European Associations of Nuclear Medicine (four individuals) and Neurology (three individuals) determined in 2018 several clinical situations in the context of neurodegenerative diseases where FDG-PET had sufficient evidence to recommend its clinical use, in addition to clinical/neuropsychological examination. Naturally, there were differences in the degree of evidence and recommendation of indication among the evaluators, but ultimately, FDG-PET was recommended in several circunstances:
To aid in the diagnosis of atypical AD;
To differentiate AD from DLB, FTD, and vascular dementia;
To differentiate DLB from FTD, and Parkinson’s disease from PSP; and
To suggest pathophysiology in corticobasal syndrome, primary progressive aphasia, and assess cortical dysfunction in Parkinson’s disease.
In all indications, the routine use of semi-automated quantification to assist visual analysis was recommended, which has been internalized in the European guidelines for performing the exam 118 . The indications of FDG-PET related to AD were those where in general, there was the greatest consensus among specialists 119 .
Cerebral perfusion assessment by SPECT can be a more cost-effective alternative to FDG-PET but has several disadvantages, including lower spatial resolution and accuracy 120 , fewer commercially available semiquantitative programs for the method, and the lack of integrated CT in most available machines. However, SPECT can be useful for differential diagnosis between AD and FTD, provided that the exam is interpreted by an experienced nuclear medicine physician 121 . Additionally, the accuracy of SPECT is not affected by glycemic control or diabetes, which can potentially influence FDG-PET results 122,123 .
Magnetic resonance imaging as a marker of neurodegeneration
In addition to its role in excluding reversible and irreversible structural causes of cognitive decline, MRI can also be used as a biomarker of neurodegeneration. However, its accuracy in this evaluation is generally inferior to FDG-PET when only visual analysis of structural images is used. Structural changes occur later than those detected by other biomarkers, except for cognitive markers, which may appear even later or coincide with the structural changes observed on MRI.
However, MRI shows a significant increase in accuracy when its analysis is optimized using quantitative analysis programs, such as those for measuring gray matter volume or cortical thickness reduction. Initially available in research settings, these programs have recently been adapted for commercial use. More specifically, the most commonly used neurodegeneration marker of atrophy of the medial temporal lobe is preferably characterized by the medial temporal lobe atrophy (MTA) score 124 . The interpretation of the MTA score is based on age and degree of atrophy, with a score above 1 considered abnormal for patients up to 74 years and a score above 2 considered abnormal for patients aged 75 years or older. Other scales used for measuring the atrophy commonly found in AD patients are the Koedam and entorhinal cortical atrophy scale (ERICA), respectively quantifying parietal and entorhinal regions.
BIOMARKERS IN ETHNICALLY DIVERSE POPULATIONS
Despite the recent increased availability of biomarkers, many of the studies validating cutoff points have been conducted in populations of white people living in high-income countries, like the United States and European countries. Biomarkers seem to behave differently in black individuals 42,125,126 . Recent clinical trials of anti-amyloid therapies that used AD biomarkers as inclusion criteria had a large disproportion of black participants who did not meet the inclusion criteria based on these biomarkers, suggesting that the cutoff points in this population may differ 99,101 . In the lecanemab clinical trial, only 2% of the participants were black 99 .
PERSPECTIVES
The field of biomarker research has grown significantly. Certainly, the development of plasma biomarkers will make AD diagnosis more accessible and less expensive. However, the quality of studies remains variable. Recently, the Global CEO Initiative on Alzheimer’s Disease convened a BBM Workgroup to consider the minimum acceptable performance of blood-based biomarkers (BBM) tests for clinical use 127 . A BBM test should have a sensitivity ≥90% with a specificity ≥85% in primary care and ≥75–85% in secondary care in case of screening test prior to subsequent confirmatory testing (PET or CSF). If it is used as a confirmatory test, a plasma biomarker should have performance equivalent to that of CSF tests (with sensitivity and specificity of at least ~90%) 127 .
The BBM Workgroup also suggests that plasma biomarkers could have two cutoffs to define three categories of result: positive, intermediate, and negative 127 . Patients with a result below the lower cutoff (negative) are highly likely not to have AD, and those with a result above the upper cutoff (positive) are highly likely to be diagnosed with AD. In cases where the result is between the two cutoffs (intermediate), a confirmatory test (PET or CSF) or a repeat plasma biomarker should be considered in one year. However, the BBM Workgroup does not yet endorse any specific test 127 .
In addition to the improvement of Aβ and p-tau pathology biomarkers, there is a search for biomarkers related to other aspects of pathology. For example, biomarkers of neuroinflammation associated with AD, such as markers of astrocytic and microglial activation. Among these, the plasma measurement of glial fibrillary acidic protein (GFAP), expressed in astrocytes, stands out 42,128,129 . Similar to fluid-based biomarkers of neuroinflammation, molecular neuroimaging for neuroinflammation has shown promise with several studies in the last decades 130,131,132,133,134,135 . Radiopharmaceuticals have been developed, some that demonstrate both microglial activation (e.g., [11C]-PK11195) 131,133 , and others that show astrocytic activity (e.g., [11C]-Acetate) 134,135 . However, PET for neuroinflammation is still restricted to research, with no applicability in clinical practice to date.
Another area of intense study is the search for biomarkers of other proteinopathies and cerebrovascular diseases that frequently occur as co-pathologies in patients with AD. Among these, alpha-synuclein is one of the most relevant 136 .
PRACTICAL RECOMMENDATIONS FOR REQUESTING BIOMARKERS IN CLINICAL PRACTICE
Biomarkers for AD are not yet perfect but play an important role in aiding the clinical diagnosis in symptomatic patients, particularly those with atypical presentations, and have implications for indicating disease-modifying therapy. The request and interpretation of biomarkers are not trivial and should be performed by physicians well-trained in the field, usually from the medical specialties of Geriatrics, Neurology, Psychiatry (Old-Age Psychiatry). Biomarkers should only be requested for cognitively symptomatic patients with objectively demonstrated deficits in cognitive testing, i.e., with a clinical dementia rating (CDR) score of 0.5 or above. They may be also requested in FTD patients, with significant behavioral changes for differential diagnosis. Biomarkers can be indicated when it is necessary to make a precise etiological diagnosis of cognitive impairment.
Thus, biomarkers for AD are not indicated in asymptomatic individuals, patients with SCD, or in cases of advanced dementia (where etiological diagnosis will not change clinical management).
Biomarkers should not be requested to predict the risk of developing cognitive decline in asymptomatic individuals for several reasons:
There is no disease-modifying treatment for the preclinical phase; and
Not all individuals with positive biomarkers will progress to MCI or dementia stages in AD.
However, biomarkers can be recommended in the following clinical contexts:
Early-onset dementias (symptoms onset before 65 years of age);
Rapidly progressive dementias;
Atypical dementia;
Available disease-modifying treatment (e.g. anti-amyloid therapy); and
Differential diagnosis with other neurodegenerative dementias 41,137 .
Biomarkers can also be recommended when patients want to know if their cognitive decline is caused by AD 138 . In all situations mentioned above, the interpretation of biomarkers should be done with caution, in the context of the clinical picture, and by a clinician with solid experience in the area.
Therefore, requesting biomarkers for AD is not a trivial procedure and should not be performed in the absence of clinical symptoms. It should also not be done by medical professionals who are not trained to correctly interpret the results and manage the consequences of the diagnosis for the patient and family.
The current objective of biomarkers for AD is to assist in the diagnosis of symptomatic patients. A future objective may be the monitoring of therapeutic response and disease progression. The diagnosis of AD based on biomarkers has important implications for treatment with new disease-modifying therapies, being fundamental in the indication or contraindication of these therapies. Table 3 summarizes the commercially accessible biomarkers for clinical use (some of which are not yet available in Brazil). We recommend that laboratories provide the ratios between analytes in CSF (Aβ42/Aβ40, p-tau181/Aβ42) as they have better accuracy than the isolated value of the analyte (especially in the case of Aβ42).
Table 3. Currently available biomarkers for clinical use.
| Category | CSF biomarkers | Plasma biomarkers | Neuroimaging biomarkers |
|---|---|---|---|
| Proteinopathy Aβ (A) | ↓ Aβ42/Aβ40
↑ p-tau/Aβ42 |
↓ Aβ42/Aβ40 | Positive amyloid PET [11C]-PiB, [18F]-Florbetaben, [18F]-Florbetapir*, [18F]-Flutemetamol* |
| Proteinopathy phosphorylated tau (T) | ↑ p-tau181 ↑ p-tau181/Aβ42 |
↑ p-tau 217*
↑ p-tau217/np-tau217 |
Positive tau PET ([18F]-Flortaucipir*) |
| Neurodegeneration (N) | ↑ NfL ↑ t-tau |
↑ NfL*
↑ t-tau |
FDG-PET with temporoparietal, posterior cingulate and precuneus hypometabolism Brain MRI with medial temporal lobe atrophy (particularly the entorhinal cortex), precuneus and temporoparietal cortex. |
Abbreviations: CSF, cerebrospinal fluid; Aβ, amyloid beta; p-tau: phosphorylated tau; PET, positron emission tomography; NfL, light chain neurofilaments; FDG, [18F]-Fluorodeoxyglucose; MRI, magnetic resonance imaging.
Note: *Biomarkers not commercially available in Brazil.
The validation of some biomarkers, especially plasma analytes, is in the process of development, particularly in populations underrepresented in clinical research, which is generally conducted in high-income countries with a predominance of white participants.
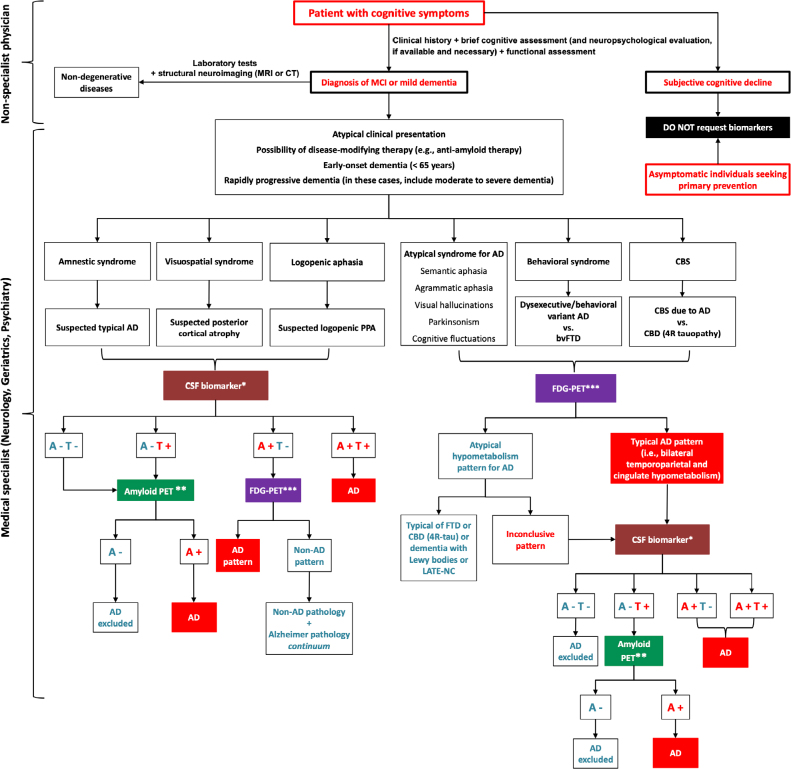
We have elaborated a flowchart to assist physicians in requesting biomarkers, as shown in Figure 3. Plasma biomarkers were not included in this algorithm because they still need to be validated and have a quality control certificate. These tests are promising, such as p-tau217, and once they are adequately validated, the algorithm will certainly be updated to include these plasma biomarkers. For a plasma biomarker to be indicated, it must demonstrate an accuracy of at least ~90% compared to an amyloid PET or an already approved CSF biomarker platform by regulatory agencies.
Figure 3. Suggestion for a rational use algorithm of biomarkers based on typical variant, and atypical cognitive syndromes of Alzheimer’s disease. At present, plasma biomarkers require better validation and, therefore, have not been included in this flowchart.
Abbreviations: AD, Alzheimer’s disease; MCI, mild cognitive impairment; PPA, primary progressive aphasias; MRI, magnetic resonance imaging; CT, computed tomography; PET, positron emission tomography; FDG, fluorodeoxyglucose; FTD, frontotemporal dementia; CBD, corticobasal degeneration; LATE, Limbic-predominant age-related TDP-43 encephalopathy.
Notes: *CSF biomarker should be performed on automated platforms (e.g., electrochemiluminescence immunoassay or mass spectrometry); **Caution should be exercised when interpreting amyloid PET in patients aged >80 years, as they may be positive in cognitively normal individuals in this age group; ***In the absence of FDG-PET, a SPECT with quantification can be considered.
Regarding CSF biomarkers, we suggest verifying if the laboratory is compliant with pre-analytical and analytical procedures and if there is quality control as required by the Alzheimer’s Association. We also recommend that laboratories adopt automated platforms, such as electrochemiluminescence immunoassay, instead of the ELISA technique.
Regarding PET scans, we propose that they be conducted in clinics with experienced nuclear medicine physicians qualified at evaluating brain PET scans, and whose analysis includes semiquantitative methods, not just visual. We recommend avoiding amyloid PET and other amyloid research tests isolated in patients aged 80 or older due to the high positivity rate in asymptomatic individuals in this age group, except when the goal is to rule out AD diagnosis (i.e., negative result). We also consider that measuring biomarkers in CSF is more available and less costly than amyloid PET (though more invasive due to lumbar puncture), and therefore recommend CSF examination as the first option over amyloid PET. Finally, the request for biomarkers should be made after a structured clinical evaluation, including detailed history, physical examination, and cognitive and functional assessments, in addition to neuropsychological evaluation if necessary 137,139 .
Laboratory tests and structural neuroimaging (ideally cranial MRI, with CT as a second option) should always be requested for all patients to exclude non-degenerative etiologies. Much has been discussed about diagnosing AD exclusively through biomarkers (biological diagnosis), regardless of clinical manifestations 44,49 . However, we recommend that the search for an AD diagnosis should begin with the presence of symptoms and that the characterization of the cognitive-behavioral syndrome should be the starting point for selecting which biomarkers to request 7 . It should also be noted that in many cases of patients diagnosed with AD in vivo, post-mortem evaluation reveals multiple pathologies (e.g., alpha-synuclein, TDP-43, and cerebrovascular disease) 14,140,141 . This means that the positivity of an AD biomarker does not necessarily indicate that the patient’s symptoms are solely due to AD. Therefore, the interpretation of biomarkers should be made in the context of the clinical syndrome to avoid the risk of false diagnoses.
Finally, we recommend that validated CSF and PET biomarkers for clinical use be submitted to the Brazilian Health Regulatory Agency (ANVISA, Agência Nacional de Vigilância Sanitária) for regulatory approval in Brazil. This would enable their implementation in both the Brazilian Unified Health System (SUS, Sistema Único de Saúde) and the supplementary health system. Box 1 provides a summary of the main recommendations.
Box 1. Recommendations from the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology for the use and interpretation of Alzheimer’s disease biomarkers in clinical practice in Brazil.
Before requesting AD biomarkers, a structured clinical evaluation should be performed (detailed history, physical and neurological examination, and cognitive/functional assessment).
Requests should only be made by well-trained physicians with solid experience in the field, usually from the following medical specialties: Geriatrics, Neurology, and Psychiatry (Old-Age Psychiatry).
Requests should not be made by medical professionals who are not qualified to interpret the results and handle the consequences of the diagnosis for the patient and their family.
Biomarkers should only be requested for cognitively and/or behaviorally symptomatic patients (objectively demonstrated in cognitive testing and clinical evaluation).
Biomarkers for AD are not indicated in asymptomatic individuals and patients with subjective cognitive decline (cognitive complaints without test abnormalities)
Biomarkers for AD are not indicated in cases of advanced dementia (except when the goal is to rule out AD diagnosis, which may change treatment; e.g., discontinuation of cholinesterase inhibitors or memantine).
Biomarkers can be recommended in the following clinical contexts: early-onset dementias (symptoms onset before 65 years), rapidly progressive dementias, atypical AD manifestations (e.g., non-amnestic presentations), and other atypical clinical manifestations (e.g., when there is a suspected overlap of symptoms of two or more neurodegenerative dementias).
Biomarkers can be recommended when there is suspicion of mild cognitive impairment or mild dementia due to AD and there is a possibility of indicating anti-amyloid therapy (or other disease-modifying therapies that may be approved in the future).
Laboratory tests and structural neuroimaging (ideally cranial MRI, with CT as a second option) should always be requested for all patients to exclude non-degenerative etiologies.
CSF biomarkers should be conducted in laboratories with the required pre-analytical and analytical procedures and quality control as required by the Alzheimer’s Association.
Laboratories should adopt automated platforms (e.g., electrochemiluminescence immunoassay or mass spectrometry) instead of the ELISA technique.
Laboratories should provide ratios between analytes in CSF (Aβ42/Aβ40, p-tau181/Aβ42) as they have better accuracy than the isolated value of the analyte (especially in the case of Aβ42).
Plasma biomarkers still need to be validated and have a quality control certificate (an accuracy of at least close to 90% is recommended compared to an amyloid PET or a CSF biomarker platform already approved by regulatory agencies).
We recommend caution in performing amyloid PET and other amyloid research tests in patients aged 80 or older due to the high positivity rate in asymptomatic individuals in this age group.
We recommend CSF examination as the first option over amyloid PET (see flowchart in Figure 3), as measuring biomarkers in CSF is more available and less costly than amyloid PET.
Regarding PET scans, we suggest to be performed in clinics with experienced nuclear medicine physicians who are experienced in evaluating brain PET scans, and whose analysis includes semiquantitative methods, not just visual.
In the absence of FDG-PET, a SPECT with quantification can be considered.
We recommend that validated CSF and PET biomarkers for clinical use be submitted to ANVISA for regulatory approval in Brazil.
We recommend that ANVISA-approved biomarkers be implemented in both the Brazilian Unified Health System (SUS) and the supplementary health system.
SUPPLEMENTARY MATERIAL
Diretrizes para o uso e interpretação dos biomarcadores de Doença de Alzheimer no Brasil: Recomendações do Departamento Científico de Neurologia Cognitiva e do Envelhecimento da Academia Brasileira de Neurologia, available at: https://www.demneuropsy.com.br/wp-content/uploads/2024/10/DN_2024C01_PT_15-10-aprovado.pdf.
Funding Statement
ASN has received honoraria for participation in advisory board for Roche. BJAP has participated as a speaker in symposiums promoted by the laboratories Sandoz, Roche, Knight Therapeutics, Novo Nordisk, and Libbs, participated in the advisory board of Roche and received financial support for participation in events from Novo Nordisk. OVF is a principal investigator in a clinical trial for Biogen (ENVISION). EPFR received support from Proneuro for the preparation of educational materials, from Novo Nordisk for conference attendance, and from Roche for participation in educational activities. PHFB has received honoraria from Neuroimmun, RMC received Alzheimer’s Association Grant (AARGD-21-846545) and is funded by CNPq, Brazil (research productivity grant). GBPF has received participation as speaker in symposium sponsored by Lilly and Roche. MLFB participates in research sponsored by Roche. NAFF has received honoraria for participation in advisory boards for Roche. LPS has received honoraria for participation in advisory boards for Biogen, Knight, Lilly, Novo Nordisk e Roche and for the development of continuing medical education material by Aché, Apsen, Biogen, Knight, Libbs, Novo Nordisk e Roche. LCS has received honoraria for participation in advisory boards for Biogen and Lilly, for the development of continuing medical education material, and participation as a speaker in a symposium sponsored by Abbott, Biogen, Knight, and Novo Nordisk; he is a principal investigator in a clinical trial for PassageBio. SMDB has received honoraria for participation in the advisory board for Biogen, Novo Nordisk, Lilly, Roche, and Adium. PC has participated in a clinical trial sponsored by Novo Nordisk, has participated in consulting activities for Aché, Danone, Eurofarma, Knight Therapeutics and Roche and has prepared continuing medical education materials and participation as speaker in symposia sponsored by Aché, Danone, Grupo Fleury, Novo Nordisk and Roche. RN and SMDB are funded by CNPq, Brazil (research productivity grant). AMC, WVB, MNMS, HRG, MTB, JS, EE have no conflicts of interest to declare.
Footnotes
Funding: ASN has received honoraria for participation in advisory board for Roche. BJAP has participated as a speaker in symposiums promoted by the laboratories Sandoz, Roche, Knight Therapeutics, Novo Nordisk, and Libbs, participated in the advisory board of Roche and received financial support for participation in events from Novo Nordisk. OVF is a principal investigator in a clinical trial for Biogen (ENVISION). EPFR received support from Proneuro for the preparation of educational materials, from Novo Nordisk for conference attendance, and from Roche for participation in educational activities. PHFB has received honoraria from Neuroimmun, RMC received Alzheimer’s Association Grant (AARGD-21-846545) and is funded by CNPq, Brazil (research productivity grant). GBPF has received participation as speaker in symposium sponsored by Lilly and Roche. MLFB participates in research sponsored by Roche. NAFF has received honoraria for participation in advisory boards for Roche. LPS has received honoraria for participation in advisory boards for Biogen, Knight, Lilly, Novo Nordisk e Roche and for the development of continuing medical education material by Aché, Apsen, Biogen, Knight, Libbs, Novo Nordisk e Roche. LCS has received honoraria for participation in advisory boards for Biogen and Lilly, for the development of continuing medical education material, and participation as a speaker in a symposium sponsored by Abbott, Biogen, Knight, and Novo Nordisk; he is a principal investigator in a clinical trial for PassageBio. SMDB has received honoraria for participation in the advisory board for Biogen, Novo Nordisk, Lilly, Roche, and Adium. PC has participated in a clinical trial sponsored by Novo Nordisk, has participated in consulting activities for Aché, Danone, Eurofarma, Knight Therapeutics and Roche and has prepared continuing medical education materials and participation as speaker in symposia sponsored by Aché, Danone, Grupo Fleury, Novo Nordisk and Roche. RN and SMDB are funded by CNPq, Brazil (research productivity grant). AMC, WVB, MNMS, HRG, MTB, JS, EE have no conflicts of interest to declare.
REFERENCES
- 1.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6(3):131–44. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 2.Chételat G, Arbizu J, Barthel H, Garibotto V, Law I, Morbelli S, et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020;19(11):951–62. doi: 10.1016/S1474-4422(20)30314-8. [DOI] [PubMed] [Google Scholar]
- 3.Bouwman FH, Frisoni GB, Johnson SC, Chen X, Engelborghs S, Ikeuchi T, et al. Clinical application of CSF biomarkers for Alzheimer’s disease: from rationale to ratios. Alzheimers Dement (Amst) 2022;14(1):e12314. doi: 10.1002/dad2.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Andrews JS, Beach TG, Buracchio T, Dunn B, Graf A, et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024;20(8):5143–69. doi: 10.1002/alz.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattke S, Santos OCS, Filho, Hanson M, Mateus EF, Reis JP, Neto, Souza LC, et al. Preparedness of the Brazilian health-care system to provide access to a disease-modifying Alzheimer’s disease treatment. Alzheimers Dement. 2023;19(1):375–81. doi: 10.1002/alz.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.arra MA, Orellana P, Leon T, Victoria CG, Henriquez F, Gomez R, et al. Biomarkers for dementia in Latin American countries: gaps and opportunities. Alzheimers Dement. 2023;19(2):721–35. doi: 10.1002/alz.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frisoni GB, Festari C, Massa F, Ramusino MC, Orini S, Aarsland D, et al. European intersocietal recommendations for the biomarker-based diagnosis of neurocognitive disorders. Lancet Neurol. 2024;23(3):302–12. doi: 10.1016/S1474-4422(23)00447-7. [DOI] [PubMed] [Google Scholar]
- 8.Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood) 2018;243(3):213–21. doi: 10.1177/1535370217750088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klunk WE. Biological markers of Alzheimer’s disease. Neurobiol Aging. 1998;19(2):145–7. doi: 10.1016/s0197-4580(98)00013-x. [DOI] [PubMed] [Google Scholar]
- 10.Khoury R, Ghossoub E. Diagnostic biomarkers of Alzheimer’s disease: a state-of-the-art review. Biomark Neuropsychiatry. 2019;1:100005. doi: 10.1016/j.bionps.2019.100005. [DOI] [Google Scholar]
- 11.Thal DR, Capetillo-Zarate E, Del Tredici K, Braak H. The development of amyloid beta protein deposits in the aged brain. Sci Aging Knowledge Environ. 2006;2006(6):re1. doi: 10.1126/sageke.2006.6.re1. [DOI] [PubMed] [Google Scholar]
- 12.Bayer TA, Schäfer S, Breyhan H, Wirths O, Treiber C, Multhaup G. A vicious circle: role of oxidative stress, intraneuronal Abeta and Cu in Alzheimer’s disease. Clin Neuropathol. 2006;25(4):163–71. [PubMed] [Google Scholar]
- 13.D’Andrea MR, Nagele RG. Morphologically distinct types of amyloid plaques point the way to a better understanding of Alzheimer’s disease pathogenesis. Biotech Histochem. 2010;85(2):133–47. doi: 10.3109/10520290903389445. [DOI] [PubMed] [Google Scholar]
- 14.DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):32. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 16.Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359(6393):322–5. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 17.Gabriele RMC, Abel E, Fox NC, Wray S, Arber C. Knockdown of amyloid precursor protein: biological consequences and clinical opportunities. Front Neurosci. 2022;16:835645. doi: 10.3389/fnins.2022.835645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3(1):75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- 19.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118(1):5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann S, Dumurgier J, Ayrignac X, Marelli C, Alcolea D, Ormaechea JF, et al. Cerebrospinal fluid A beta 1-40 peptides increase in Alzheimer’s disease and are highly correlated with phospho-tau in control individuals. Alzheimers Res Ther. 2020;12(1):123. doi: 10.1186/s13195-020-00696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Goyal R. Amyloid beta plaque: a culprit for neurodegeneration. Acta Neurol Belg. 2016;116(4):445–50. doi: 10.1007/s13760-016-0639-9. [DOI] [PubMed] [Google Scholar]
- 22.Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, Melcher K, et al. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin. 2017;38(9):1205–35. doi: 10.1038/aps.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–81. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer’s disease. Trends Neurosci. 1993;16(11):460–5. doi: 10.1016/0166-2236(93)90078-z. [DOI] [PubMed] [Google Scholar]
- 25.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 26.Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994;33(32):9511–22. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- 27.Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol. 2017;133(5):665–704. doi: 10.1007/s00401-017-1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goedert M, Jakes R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 1990;9(13):4225–30. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282(5395):1914–7. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- 30.Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179(2):312–39. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tapia-Rojas C, Cabezas-Opazo F, Deaton CA, Vergara EH, Johnson GVW, Quintanilla RA. It’s all about tau. Prog Neurobiol. 2019;175:54–76. doi: 10.1016/j.pneurobio.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawat P, Sehar U, Bisht J, Selman A, Culberson J, Reddy PH. Phosphorylated Tau in Alzheimer’s disease and other tauopathies. Int J Mol Sci. 2022;23(21):12841. doi: 10.3390/ijms232112841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karikari TK, Ashton NJ, Brinkmalm G, Brum WS, Benedet AL, Montoliu-Gaya L, et al. Blood phospho-tau in Alzheimer disease: analysis, interpretation, and clinical utility. Nat Rev Neurol. 2022;18(7):400–18. doi: 10.1038/s41582-022-00665-2. [DOI] [PubMed] [Google Scholar]
- 34.Ishida K, Yamada K, Nishiyama R, Hashimoto T, Nishida I, Abe Y, et al. Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration. J Exp Med. 2022;219(3):e20211275. doi: 10.1084/jem.20211275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alzheimer A. Uber eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeitschrift fur Psychiatrie und Psychisch-gerichtliche Medizin. 1907;64:146–8. [Google Scholar]
- 36.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 37.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lesman-Segev OH, La Joie R, Iaccarino L, Lobach I, Rosen HJ, Seo SW, et al. Diagnostic accuracy of amyloid versus 18F-fluorodeoxyglucose positron emission tomography in autopsy-confirmed dementia. Ann Neurol. 2021;89(2):389–401. doi: 10.1002/ana.25968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindler SE. Continuum. 3. Vol. 28. Minneap Minn: 2022. Fluid biomarkers in dementia diagnosis; pp. 822–33. [DOI] [PubMed] [Google Scholar]
- 42.Hansson O, Blennow K, Zetterberg H, Dage J. Blood biomarkers for Alzheimer’s disease in clinical practice and trials. Nat Aging. 2023;3(5):506–19. doi: 10.1038/s43587-023-00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knopman DS, Haeberlein SB, Carrillo MC, Hendrix JA, Kerchner G, Margolin R, et al. The National Institute on Aging and the Alzheimer’s Association Research Framework for Alzheimer’s disease: perspectives from the research Roundtable. Alzheimers Dement. 2018;14(4):563–75. doi: 10.1016/j.jalz.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barthélemy NR, Salvadó G, Schindler SE, He Y, Janelidze S, Collij LE, et al. Highly accurate blood test for Alzheimer’s disease is similar or superior to clinical cerebrospinal fluid tests. Nat Med. 2024;30(4):1085–95. doi: 10.1038/s41591-024-02869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groot C, Smith R, Stomrud E, Binette AP, Leuzy A, Wuestefeld A, et al. Phospho-tau with subthreshold tau-PET predicts increased tau accumulation rates in amyloid-positive individuals. Brain. 2023;146(4):1580–91. doi: 10.1093/brain/awac329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leuzy A, Smith R, Cullen NC, Strandberg O, Vogel JW, Binette AP, et al. Biomarker-based prediction of longitudinal tau positron emission tomography in Alzheimer disease. JAMA Neurol. 2022;79(2):149–58. doi: 10.1001/jamaneurol.2021.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janelidze S, Teunissen CE, Zetterberg H, Allué JA, Sarasa L, Eichenlaub U, et al. Head-to-Head Comparison of 8 Plasma Amyloid-β 42/40 Assays in Alzheimer Disease. JAMA Neurol. 2021;78(11):1375–82. doi: 10.1001/jamaneurol.2021.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubois B, Villain N, Frisoni GB, Rabinovici GD, Sabbagh M, Cappa S, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol. 2021;20(6):484–96. doi: 10.1016/S1474-4422(21)00066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blennow K, Zetterberg H. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med. 2018;284(6):643–63. doi: 10.1111/joim.12816. [DOI] [PubMed] [Google Scholar]
- 52.Lewczuk P, Matzen A, Blennow K, Parnetti L, Molinuevo JL, Eusebi P, et al. Cerebrospinal Fluid Aβ42/40 Corresponds Better than Aβ42 to Amyloid PET in Alzheimer’s Disease. J Alzheimers Dis. 2017;55(2):813–22. doi: 10.3233/JAD-160722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caroli A, Frisoni GB. Alzheimer’s Disease Neuroimaging Initiative.The dynamics of Alzheimer’s disease biomarkers in the Alzheimer’s Disease Neuroimaging Initiative cohort. Neurobiol Aging. 2010;31(8):1263–74. doi: 10.1016/j.neurobiolaging.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmqvist S, Mattsson N, Hansson O. Alzheimer’s Disease Neuroimaging Initiative.Cerebrospinal fluid analysis detects cerebral amyloid-β accumulation earlier than positron emission tomography. Brain. 2016;139(Pt 4):1226–36. doi: 10.1093/brain/aww015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66(3):382–9. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 56.Janelidze S, Berron D, Smith R, Strandberg O, Proctor NK, Dage JL, et al. Associations of Plasma Phospho-Tau217 levels with tau positron emission tomography in early Alzheimer Disease. JAMA Neurol. 2021;78(2):149–56. doi: 10.1001/jamaneurol.2020.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barthélemy NR, Li Y, Joseph-Mathurin N, Gordon BA, Hassenstab J, Benzinger TLS, et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat Med. 2020;26(3):398–407. doi: 10.1038/s41591-020-0781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mielke MM, Aakre JA, Algeciras-Schimnich A, Proctor NK, Machulda MM, Eichenlaub U, et al. Comparison of CSF phosphorylated tau 181 and 217 for cognitive decline. Alzheimers Dement. 2022;18(4):602–11. doi: 10.1002/alz.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horie K, Salvadó G, Barthélemy NR, Janelidze S, Li Y, He Y, et al. CSF MTBR-tau243 is a specific biomarker of tau tangle pathology in Alzheimer’s disease. Nat Med. 2023;29(8):1954–63. doi: 10.1038/s41591-023-02443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hampel H, Cummings J, Blennow K, Gao P, Jack CR, Jr, Vergallo A. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat Rev Neurol. 2021;17(9):580–9. doi: 10.1038/s41582-021-00520-w. [DOI] [PubMed] [Google Scholar]
- 61.Jia J, Ning Y, Chen M, Wang S, Yang H, Li F, et al. Biomarker changes during 20 years preceding Alzheimer’s disease. N Engl J Med. 2024;390(8):712–22. doi: 10.1056/NEJMoa2310168. [DOI] [PubMed] [Google Scholar]
- 62.Hansson O, Batrla R, Brix B, Carrillo MC, Corradini V, Edelmayer RM, et al. The Alzheimer’s Association international guidelines for handling of cerebrospinal fluid for routine clinical measurements of amyloid β and tau. Alzheimers Dement. 2021;17(9):1575–82. doi: 10.1002/alz.12316. [DOI] [PubMed] [Google Scholar]
- 63.Lippi G, Chance JJ, Church S, Dazzi P, Fontana R, Giavarina D, et al. Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med. 2011;49(7):1113–26. doi: 10.1515/CCLM.2011.600. [DOI] [PubMed] [Google Scholar]
- 64.Bittner T, Zetterberg H, Teunissen CE, Ostlund RE, Jr, Militello M, Andreasson U, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12(5):517–26. doi: 10.1016/j.jalz.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 65.Campbell MR, Ashrafzadeh-Kian S, Petersen RC, Mielke MM, Syrjanen JA, van Harten AC, et al. P-tau/Aβ42 and Aβ42/40 ratios in CSF are equally predictive of amyloid PET status. Alzheimer’s Dement (Amst) 2021;13(1):e12190. doi: 10.1002/dad2.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Food and Drug Administration Evaluation of automatic class III designation for Lumipulse G β-amyloid ratio (1-42/1-40) Decision Summary [Internet] [[cited on Dec 5, 2024]]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf20/DEN200072.pdf .
- 67.Food and Drug Administration Center for Devices and Radiological Health. Elecsys β-Amyloid (1-42) CSF II, Elecsys Phospho-Tau (181P) CSF: 510(k) substantial equivalence determination decision summary [Internet] 2023. [[cited on Dec 5, 2024]]. Available from: https://www.accessdata.fda.gov/cdrh_docs/reviews/K221842.pdf .
- 68.Hansson O, Edelmayer RM, Boxer AL, Carrillo MC, Mielke MM, Rabinovici GD, et al. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dement. 2022;18(12):2669–86. doi: 10.1002/alz.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teunissen CE, Verberk IMW, Thijssen EH, Vermunt L, Hansson O, Zetterberg H, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 2022;21(1):66–77. doi: 10.1016/S1474-4422(21)00361-6. [DOI] [PubMed] [Google Scholar]
- 70.Meeker KL, Luckett PH, Barthélemy NR, Hobbs DA, Chen C, Bollinger J, et al. Comparison of cerebrospinal fluid, plasma and neuroimaging biomarker utility in Alzheimer’s disease. Brain Commun. 2024;6(2):fcae081. doi: 10.1093/braincomms/fcae081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rabe C, Bittner T, Jethwa A, Suridjan I, Manuilova E, Friesenhahn M, et al. Clinical performance and robustness evaluation of plasma amyloid-β42/40 prescreening. Alzheimers Dement. 2023;19(4):1393–402. doi: 10.1002/alz.12801. [DOI] [PubMed] [Google Scholar]
- 72.West T, Kirmess KM, Meyer MR, Holubasch MS, Knapik SS, Hu Y, et al. A blood-based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol Neurodegener. 2021;16(1):30. doi: 10.1186/s13024-021-00451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fogelman I, West T, Braunstein JB, Verghese PB, Kirmess KM, Meyer MR, et al. Independent study demonstrates amyloid probability score accurately indicates amyloid pathology. Ann Clin Transl Neurol. 2023;10(5):765–78. doi: 10.1002/acn3.51763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Syrjanen JA, Campbell MR, Algeciras-Schimnich A, Vemuri P, Graff-Radford J, Machulda MM, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 2022;18(6):1128–40. doi: 10.1002/alz.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer Disease vs other Neurodegenerative disorders. JAMA. 2020;324(8):772–81. doi: 10.1001/jama.2020.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ashton NJ, Brum WS, Di Molfetta G, Benedet AL, Arslan B, Jonaitis E, et al. Diagnostic accuracy of a plasma phosphorylated tau 217 immunoassay for Alzheimer disease pathology. JAMA Neurol. 2024;81(3):255–63. doi: 10.1001/jamaneurol.2023.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moscoso A, Grothe MJ, Ashton NJ, Karikari TK, Rodriguez JL, Snellman A, et al. Time course of phosphorylated-tau181 in blood across the Alzheimer’s disease spectrum. Brain. 2021;144(1):325–39. doi: 10.1093/brain/awaa399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mattsson-Carlgren N, Janelidze S, Bateman RJ, Smith R, Stomrud E, Serrano GE, et al. Soluble P-tau217 reflects amyloid and tau pathology and mediates the association of amyloid with tau. EMBO Mol Med. 2021;13(6):e14022. doi: 10.15252/emmm.202114022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milà-Alomà M, Ashton NJ, Shekari M, Salvadó G, Ortiz-Romero P, Montoliu-Gaya L, et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nat Med. 2022;28(9):1797–801. doi: 10.1038/s41591-022-01925-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bayoumy S, Verberk IMW, den Dulk B, Hussainali Z, Zwan M, van der Flier WM, et al. Clinical and analytical comparison of six Simoa assays for plasma P-tau isoforms P-tau181, P-tau217, and P-tau231. Alzheimers Res Ther. 2021;13(1):198. doi: 10.1186/s13195-021-00939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brum WS, Cullen NC, Janelidze S, Ashton NJ, Zimmer ER, Therriault J, et al. A two-step workflow based on plasma p-tau217 to screen for amyloid β positivity with further confirmatory testing only in uncertain cases. Nat Aging. 2023;3(9):1079–90. doi: 10.1038/s43587-023-00471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barthélemy NR, Saef B, Li Y, Gordon BA, He Y, Horie K, et al. CSF tau phosphorylation occupancies at T217 and T205 represent improved biomarkers of amyloid and tau pathology in Alzheimer’s disease. Nat Aging. 2023;3(4):391–401. doi: 10.1038/s43587-023-00380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rissman RA, Langford O, Raman R, Donohue MC, Abdel-Latif S, Meyer MR, et al. Plasma Aβ42/Aβ40 and phospho-tau217 concentration ratios increase the accuracy of amyloid PET classification in preclinical Alzheimer’s disease. Alzheimers Dement. 2024;20(2):1214–24. doi: 10.1002/alz.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyer MR, Kirmess KM, Eastwood S, Wente-Roth TL, Irvin F, Holubasch MS, et al. Clinical validation of the PrecivityAD2 blood test: A mass spectrometry-based test with algorithm combining %p-tau217 and Aβ42/40 ratio to identify presence of brain amyloid. Alzheimers Dement. 2024;20(5):3179–92. doi: 10.1002/alz.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mielke MM, Dage JL, Frank RD, Algeciras-Schimnich A, Knopman DS, Lowe VJ, et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med. 2022;28(7):1398–405. doi: 10.1038/s41591-022-01822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Studart A, Neto, Coutinho AM. From clinical phenotype to proteinopathy: molecular neuroimaging in neurodegenerative dementias. Arq Neuropsiquiatr. 2022;80(5 Suppl 1):24–35. doi: 10.1590/0004-282X-ANP-2022-S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Filippi M, Cecchetti G, Spinelli EG, Vezzulli P, Falini A, Agosta F. Amyloid-related imaging abnormalities and β-amyloid-targeting antibodies: a systematic review. JAMA Neurol. 2022;79(3):291–304. doi: 10.1001/jamaneurol.2021.5205. [DOI] [PubMed] [Google Scholar]
- 88.Barbosa BJAP, Siqueira JI, Alves GS, Sudo FK, Suemoto CK, Tovar-Moll F, et al. Diagnosis of vascular cognitive impairment: recommendations of the scientific department of cognitive neurology and aging of the Brazilian Academy of Neurology. Dement Neuropsychol. 2022;16(3 Suppl 1):53–72. doi: 10.1590/1980-5764-DN-2022-S104PT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fleisher AS, Pontecorvo MJ, Devous MD, Lu M, Arora AK, Truocchio SP, et al. Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 2020;77(7):829–39. doi: 10.1001/jamaneurol.2020.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lowe VJ, Lundt ES, Albertson SM, Przybelski SA, Senjem ML, Parisi JE, et al. Neuroimaging correlates with neuropathologic schemes in neurodegenerative disease. Alzheimers Dement. 2019;15(7):927–39. doi: 10.1016/j.jalz.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Therriault J, Pascoal TA, Lussier FZ, Tissot C, Chamoun M, Bezgin G, et al. Biomarker modeling of Alzheimer’s disease using PET-based Braak staging. Nat Aging. 2022;2(6):526–35. doi: 10.1038/s43587-022-00204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coutinho AM, Busatto GF, Porto FHG, Faria DP, Ono CR, Garcez AT, et al. Brain PET amyloid and neurodegeneration biomarkers in the context of the 2018 NIA-AA research framework: an individual approach exploring clinical-biomarker mismatches and sociodemographic parameters. Eur J Nucl Med Mol Imaging. 2020;47(11):2666–80. doi: 10.1007/s00259-020-04714-0. [DOI] [PubMed] [Google Scholar]
- 93.Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11(8):669–78. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 94.Roberts RO, Aakre JA, Kremers WK, Vassilaki M, Knopman DS, Mielke MM, et al. Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol. 2018;75(8):970–9. doi: 10.1001/jamaneurol.2018.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rabinovici GD, Rosen HJ, Alkalay A, Kornak J, Furst AJ, Agarwal N, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology. 2011;77(23):2034–42. doi: 10.1212/WNL.0b013e31823b9c5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. J Nucl Med. 2013;54(3):476–90. doi: 10.2967/jnumed.113.120618. [DOI] [PubMed] [Google Scholar]
- 97.Cummings J, Lee G, Zhong K, Fonseca J, Taghva K. Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement (NY) 2021;7(1):e12179. doi: 10.1002/trc2.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Budd Haeberlein S, Aisen PS, Barkhof F, Chalkias S, Chen T, Cohen S, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimer’s Dis. 2022;9(2):197–210. doi: 10.14283/jpad.2022.30. [DOI] [PubMed] [Google Scholar]
- 99.van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 100.Rafii MS, Sperling RA, Donohue MC, Zhou J, Roberts C, Irizarry MC, et al. The AHEAD 3-45 study: design of a prevention trial for Alzheimer’s disease. Alzheimers Dement. 2023;19(4):1227–33. doi: 10.1002/alz.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, et al. Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512–27. doi: 10.1001/jama.2023.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beta amyloid positron emission tomography in dementia and neurodegenerative disease [Internet] 2023. [[cited on Dec 5, 2023]]. Available from: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=308 .
- 103.Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 104.Thal DR, Walter J, Saido TC, Fändrich M. Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer’s disease. Acta Neuropathol. 2015;129(2):167–82. doi: 10.1007/s00401-014-1375-y. [DOI] [PubMed] [Google Scholar]
- 105.Tagai K, Ono M, Kubota M, Kitamura S, Takahata K, Seki C, et al. High-contrast in vivo imaging of tau pathologies in Alzheimer’s and non-Alzheimer’s disease tauopathies. Neuron. 2021;109(1):42–58.e8. doi: 10.1016/j.neuron.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 106.Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(Pt 5):1551–67. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ossenkoppele R, Reimand J, Smith R, Leuzy A, Strandberg O, Palmqvist S, et al. Tau PET correlates with different Alzheimer’s disease-related features compared to CSF and plasma p-tau biomarkers. EMBO Mol Med. 2021;13(8):e14398. doi: 10.15252/emmm.202114398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vogel JW, Iturria-Medina Y, Strandberg OT, Smith R, Levitis E, Evans AC, et al. Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nat Commun. 2020;11(1):2612. doi: 10.1038/s41467-020-15701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lagarde J, Olivieri P, Caillé F, Gervais P, Baron J-C, Bottlaender M, et al. [18F]-AV-1451 tau PET imaging in Alzheimer’s disease and suspected non-AD tauopathies using a late acquisition time window. J Neurol. 2019;266(12):3087–97. doi: 10.1007/s00415-019-09530-7. [DOI] [PubMed] [Google Scholar]
- 111.Food and Drug Administration FDA approves first drug to image tau pathology in patients being evaluated for Alzheimer’s disease [Internet] 2020. [[cited on Dec 5, 2024]]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-image-tau-pathology-patients-being-evaluated-alzheimers-disease .
- 112.Aguero C, Dhaynaut M, Normandin MD, Amaral AC, Guehl NJ, Neelamegam R, et al. Autoradiography validation of novel tau PET tracer [F-18]-MK-6240 on human postmortem brain tissue. Acta Neuropathol Commun. 2019;7(1):37. doi: 10.1186/s40478-019-0686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brendel M, Barthel H, van Eimeren T, Marek K, Beyer L, Song M, et al. Assessment of 18F-PI-2620 as a biomarker in progressive supranuclear palsy. JAMA Neurol. 2020;77(11):1408–19. doi: 10.1001/jamaneurol.2020.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Parmera JB, Coutinho AM, Aranha MR, Studart A, Neto, Carneiro CG, Almeida IJ, et al. FDG-PET patterns predict amyloid deposition and clinical profile in corticobasal syndrome. Mov Disord. 2021;36(3):651–61. doi: 10.1002/mds.28373. [DOI] [PubMed] [Google Scholar]
- 115.Silverman DH, Small GW, Chang CY, Lu CS, Aburto MAK, Chen W, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA. 2001;286(17):2120–7. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 116.Motara H, Olusoga T, Russell G, Jamieson S, Ahmed S, Brindle N, et al. Clinical impact and diagnostic accuracy of 2-[18F]-fluoro-2-deoxy-d-glucose positron-emission tomography/computed tomography (PET/CT) brain imaging in patients with cognitive impairment: a tertiary centre experience in the UK. Clin Radiol. 2017;72(1):63–73. doi: 10.1016/j.crad.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 117.Laforce R, Jr, Soucy JP, Sellami L, Dallaire-Théroux C, Brunet F, Bergeron D, et al. Molecular imaging in dementia: past, present, and future. Alzheimers Dement. 2018;14(11):1522–52. doi: 10.1016/j.jalz.2018.06.2855. [DOI] [PubMed] [Google Scholar]
- 118.Guedj E, Varrone A, Boellaard R, Albert NL, Barthel H, van Berckel B, et al. EANM procedure guidelines for brain PET imaging using [18F]FDG, version 3. Eur J Nucl Med Mol Imaging. 2022;49(2):632–51. doi: 10.1007/s00259-021-05603-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nobili F, Arbizu J, Bouwman F, Drzezga A, Agosta F, Nestor P, et al. European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18 F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur J Neurol. 2018;25(10):1201–17. doi: 10.1111/ene.13728. [DOI] [PubMed] [Google Scholar]
- 120.O’Brien JT, Firbank MJ, Davison C, Barnett N, Bamford C, Donaldson C, et al. 18F-FDG PET and perfusion SPECT in the diagnosis of Alzheimer and Lewy body dementias. J Nucl Med. 2014;55(12):1959–65. doi: 10.2967/jnumed.114.143347. [DOI] [PubMed] [Google Scholar]
- 121.Athanasio BS, Oliveira ACS, Pedrosa AL, Borges RS, Neto AOM, Oliveira RA, et al. The role of brain perfusion SPECT in the diagnosis of frontotemporal dementia: a systematic review. J Neuroimaging. 2024;34(3):308–19. doi: 10.1111/jon.13189. [DOI] [PubMed] [Google Scholar]
- 122.Valotassiou V, Angelidis G, Psimadas D, Tsougos I, Georgoulias P. In the era of FDG PET, is it time for brain perfusion SPECT to gain a place in Alzheimer’s disease imaging biomarkers? Eur J Nucl Med Mol Imaging. 2021;48(4):969–71. doi: 10.1007/s00259-020-05077-2. [DOI] [PubMed] [Google Scholar]
- 123.Nitrini R, Buchpiguel CA, Caramelli P, Bahia VS, Mathias SC, Nascimento CM, et al. SPECT in Alzheimer’s disease: features associated with bilateral parietotemporal hypoperfusion. Acta Neurol Scand. 2000;101(3):172–6. doi: 10.1034/j.1600-0404.2000.101003172.x. [DOI] [PubMed] [Google Scholar]
- 124.Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55(10):967–72. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Groechel RC, Tripodis Y, Alosco ML, Mez J, Qiu WQ, Goldstein L, et al. Biomarkers of Alzheimer’s disease in black and/or African American Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants. Neurobiol Aging. 2023;131:144–52. doi: 10.1016/j.neurobiolaging.2023.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gleason CE, Zuelsdorff M, Gooding DC, Kind AJH, Johnson AL, James TT, et al. Alzheimer’s disease biomarkers in black and non-Hispanic white cohorts: a contextualized review of the evidence. Alzheimers Dement. 2022;18(8):1545–64. doi: 10.1002/alz.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schindler SE, Galasko D, Pereira AC, Rabinovici GD, Salloway S, Suárez-Calvet M, et al. Acceptable performance of blood biomarker tests of amyloid pathology – recommendations from the Global CEO Initiative on Alzheimer’s Disease. Nat Rev Neurol. 2024;20(7):426–39. doi: 10.1038/s41582-024-00977-5. [DOI] [PubMed] [Google Scholar]
- 128.Benedet AL, Milà-Alomà M, Vrillon A, Ashton NJ, Pascoal TA, Lussier F, et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 2021;78(12):1471–83. doi: 10.1001/jamaneurol.2021.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cicognola C, Janelidze S, Hertze J, Zetterberg H, Blennow K, Mattsson-Carlgren N, et al. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res Ther. 2021;13(1):68. doi: 10.1186/s13195-021-00804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou R, Ji B, Kong Y, Qin L, Ren W, Guan Y, et al. PET imaging of neuroinflammation in Alzheimer’s disease. Front Immunol. 2021;12:739130. doi: 10.3389/fimmu.2021.739130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Q, Chen G, Schindler SE, Christensen J, McKay NS, Liu J, et al. Baseline microglial activation correlates with brain amyloidosis and longitudinal cognitive decline in Alzheimer disease. Neurol Neuroimmunol Neuroinflamm. 2022;9(3):e1152. doi: 10.1212/NXI.0000000000001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schilling LP, Zimmer ER, Shin M, Leuzy A, Pascoal TA, Benedet AL, et al. Imaging Alzheimer’s disease pathophysiology with PET. Dement Neuropsychol. 2016;10(2):79–90. doi: 10.1590/S1980-5764-2016DN1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parbo P, Ismail R, Hansen KV, Amidi A, Mårup FH, Gottrup H, et al. Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer’s disease. Brain. 2017;140(7):2002–11. doi: 10.1093/brain/awx120. [DOI] [PubMed] [Google Scholar]
- 134.Nam MH, Ko HY, Kim D, Lee S, Park YM, Hyeon SJ, et al. Visualizing reactive astrocyte-neuron interaction in Alzheimer’s disease using 11C-acetate and 18F-FDG. Brain. 2023;146(7):2957–74. doi: 10.1093/brain/awad03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Duong MT, Chen YJ, Doot RK, Young AJ, Lee H, Cai J, et al. Astrocyte activation imaging with 11C-acetate and amyloid PET in mild cognitive impairment due to Alzheimer pathology. Nucl Med Commun. 2021;42(11):1261–9. doi: 10.1097/MNM.0000000000001460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Simuni T, Chahine LM, Poston K, Brumm M, Buracchio T, Campbell M, et al. A biological definition of neuronal α-synuclein disease: towards an integrated staging system for research. Lancet Neurol. 2024;23(2):178–90. doi: 10.1016/S1474-4422(23)00405-2. [DOI] [PubMed] [Google Scholar]
- 137.Smid J, Studart A, Neto, César-Freitas KG, Dourado MCN, Kochhann R, Barbosa BJAP, et al. Subjective cognitive decline, mild cognitive impairment, and dementia - syndromic approach: recommendations of the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology. Dement Neuropsychol. 2022;16(3 Suppl 1):1–24. doi: 10.1590/1980-5764-DN-2022-S101P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Molinuevo JL, Blennow K, Dubois B, Engelborghs S, Lewczuk P, Perret-Liaudet A, et al. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement. 2014;10(6):808–17. doi: 10.1016/j.jalz.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 139.Schilling LP, Balthazar MLF, Radanovic M, Forlenza OV, Silagi ML, Smid J, et al. Diagnosis of Alzheimer’s disease: recommendations of the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology. Dement Neuropsychol. 2022;16(3 Suppl 1):25–39. doi: 10.1590/1980-5764-DN-2022-S102PT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Karanth S, Nelson PT, Katsumata Y, Kryscio RJ, Schmitt FA, Fardo DW, et al. Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol. 2020;77(10):1299–307. doi: 10.1001/jamaneurol.2020.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Suemoto CK, Ferretti-Rebustini REL, Rodriguez RD, Leite REP, Soterio L, Brucki SMD, et al. Neuropathological diagnoses and clinical correlates in older adults in Brazil: a cross-sectional study. PLoS Med. 2017;14(3):e1002267. doi: 10.1371/journal.pmed.1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]