Abstract
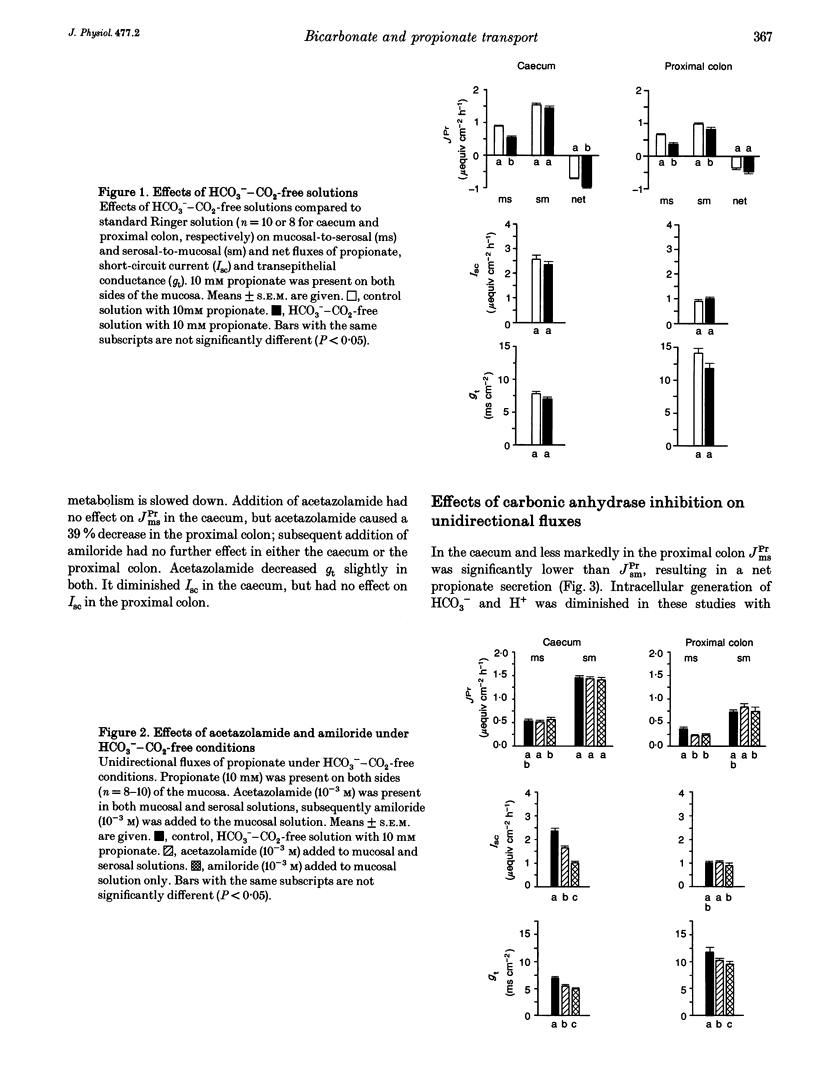
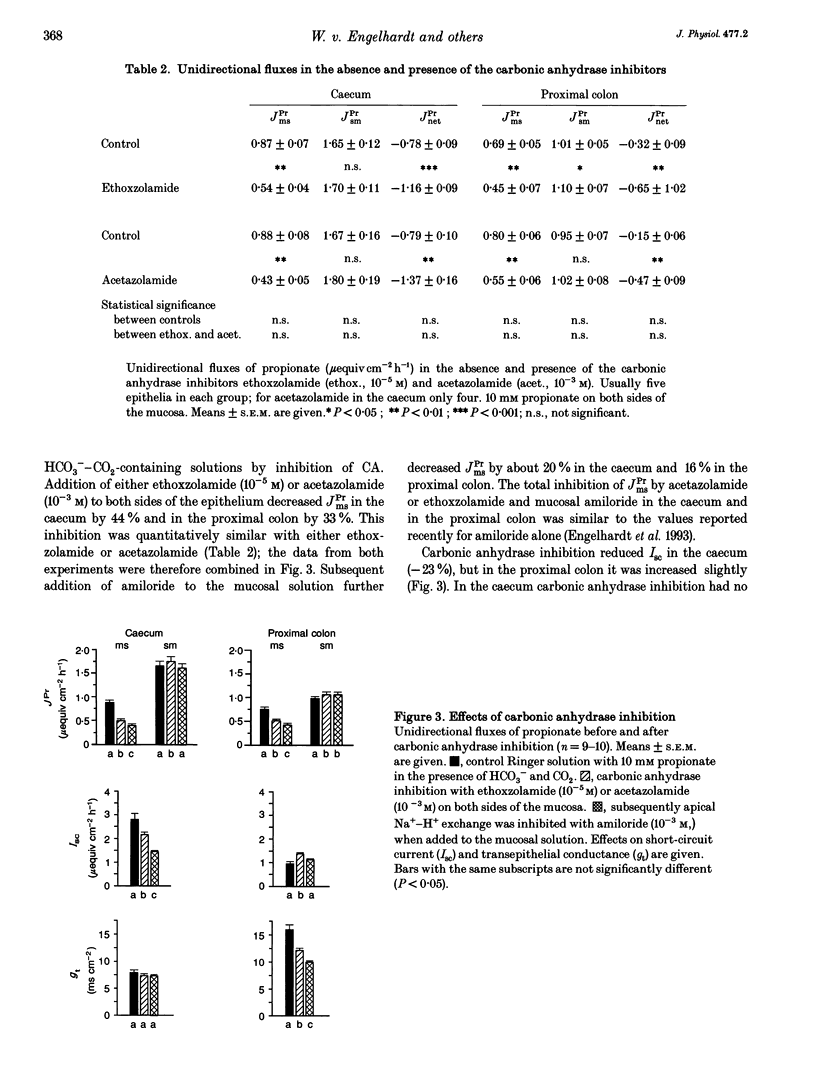
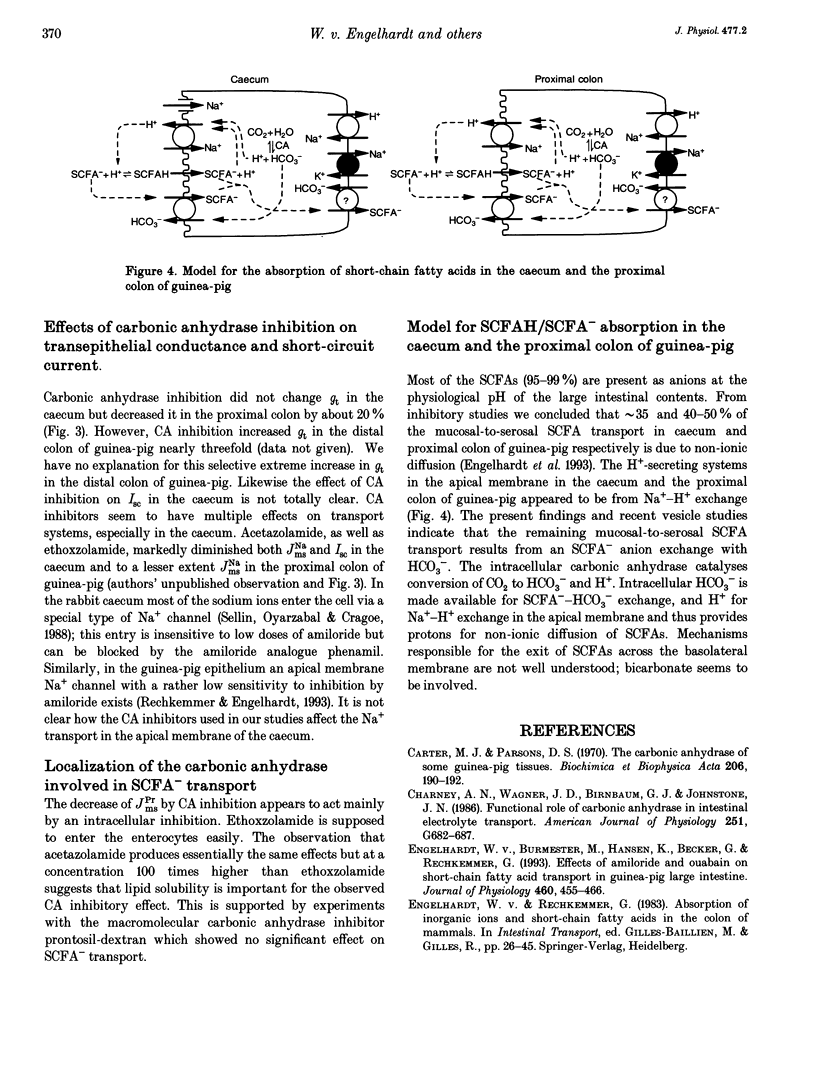
1. Unidirectional fluxes of propionate across isolated epithelia from the guinea-pig caecum and proximal colon were measured under short-circuit current conditions. In the caecum and proximal colon the serosal-to-mucosal propionate flux (JPrsm) was higher than mucosal-to-serosal flux (JPrms), resulting in a net secretory flux of propionate. 2. HCO3(-)-CO2-free solution reduced JPrms in the caecum and proximal colon markedly; JPrsm was not (caecum) or little (proximal colon) affected. The subsequent addition of acetazolamide caused a further decrease in JPrms in the proximal colon, but not in the caecum. 3. In HCO3(-)-containing solutions acetazolamide or ethoxzolamide inhibited JPrms; JPrsm was not affected. A macromolecular carbonic anhydrase inhibitor, prontosil-dextran, had no effect on propionate fluxes, indicating that the intracellular carbonic anhydrase is of importance for short-chain fatty acid transport. 4. Subsequent to carbonic anhydrase inhibition, mucosal addition of amiloride caused a slight further decrease of JPrms in the caecum and proximal colon; JPrsm was not affected. 5. Results support the view that a considerable proportion of short-chain fatty acids (SCFAs) is absorbed via a SCFA(-)-HCO3- exchange.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter M. J., Parsons D. S. The carbonic anhydrases of some guinea-pig tissues. Biochim Biophys Acta. 1970 Apr 22;206(1):190–192. doi: 10.1016/0005-2744(70)90099-9. [DOI] [PubMed] [Google Scholar]
- Charney A. N., Wagner J. D., Birnbaum G. J., Johnstone J. N. Functional role of carbonic anhydrase in intestinal electrolyte transport. Am J Physiol. 1986 Nov;251(5 Pt 1):G682–G687. doi: 10.1152/ajpgi.1986.251.5.G682. [DOI] [PubMed] [Google Scholar]
- Geers C., Gros G., Gärtner A. Extracellular carbonic anhydrase of skeletal muscle associated with the sarcolemma. J Appl Physiol (1985) 1985 Aug;59(2):548–558. doi: 10.1152/jappl.1985.59.2.548. [DOI] [PubMed] [Google Scholar]
- Halm D. R., Frizzell R. A. Active K transport across rabbit distal colon: relation to Na absorption and Cl secretion. Am J Physiol. 1986 Aug;251(2 Pt 1):C252–C267. doi: 10.1152/ajpcell.1986.251.2.C252. [DOI] [PubMed] [Google Scholar]
- Lacy E. R., Colony P. C. Localization of carbonic anhydrase activity in the developing rat colon. Gastroenterology. 1985 Jul;89(1):138–150. doi: 10.1016/0016-5085(85)90754-1. [DOI] [PubMed] [Google Scholar]
- Luciano L., Reale E., Rechkemmer G., von Engelhardt W. Structure of zonulae occludentes and the permeability of the epithelium to short-chain fatty acids in the proximal and the distal colon of guinea pig. J Membr Biol. 1984;82(2):145–156. doi: 10.1007/BF01868939. [DOI] [PubMed] [Google Scholar]
- Lönnerholm G. Carbonic anhydrase in the intestinal tract of the guinea-pig. Acta Physiol Scand. 1977 Jan;99(1):53–61. doi: 10.1111/j.1748-1716.1977.tb10352.x. [DOI] [PubMed] [Google Scholar]
- Mascolo N., Rajendran V. M., Binder H. J. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology. 1991 Aug;101(2):331–338. doi: 10.1016/0016-5085(91)90008-9. [DOI] [PubMed] [Google Scholar]
- Rechkemmer G., Rönnau K., von Engelhardt W. Fermentation of polysaccharides and absorption of short chain fatty acids in the mammalian hindgut. Comp Biochem Physiol A Comp Physiol. 1988;90(4):563–568. doi: 10.1016/0300-9629(88)90668-8. [DOI] [PubMed] [Google Scholar]
- Sellin J. H., DeSoignie R. Short-chain fatty acid absorption in rabbit colon in vitro. Gastroenterology. 1990 Sep;99(3):676–683. doi: 10.1016/0016-5085(90)90954-y. [DOI] [PubMed] [Google Scholar]
- Sellin J. H., Oyarzabal H., Cragoe E. J. Electrogenic sodium absorption in rabbit cecum in vitro. J Clin Invest. 1988 Apr;81(4):1275–1283. doi: 10.1172/JCI113445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus E., Ahearn G. A. Short-chain fatty acid transport in the intestine of a herbivorous teleost. J Exp Biol. 1988 Mar;135:77–94. doi: 10.1242/jeb.135.1.77. [DOI] [PubMed] [Google Scholar]
- von Engelhardt W., Burmester M., Hansen K., Becker G., Rechkemmer G. Effects of amiloride and ouabain on short-chain fatty acid transport in guinea-pig large intestine. J Physiol. 1993 Jan;460:455–466. doi: 10.1113/jphysiol.1993.sp019481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt W., Rechkemmer G. Segmental differences of short-chain fatty acid transport across guinea-pig large intestine. Exp Physiol. 1992 May;77(3):491–499. doi: 10.1113/expphysiol.1992.sp003609. [DOI] [PubMed] [Google Scholar]


