Abstract
Background:
Medium vessel occlusion (MeVO) strokes, particularly affecting the M2 segment of the middle cerebral artery, represent a critical proportion of acute ischemic strokes, posing significant challenges in management and outcome prediction. The efficacy of mechanical thrombectomy (MT) in MeVO stroke may warrant reliable predictors of functional outcomes. This study aimed to investigate the prognostic value of follow-up infarct volume (FIV) for predicting 90-day functional outcomes in MeVO stroke patients undergoing MT.
Methods:
This multicenter, retrospective cohort study analyzed data from the Multicenter Analysis of primary Distal medium vessel occlusions: effect of Mechanical Thrombectomy (MAD-MT) registry, covering patients with acute ischemic stroke due to M2 segment occlusion treated with MT. We examined the relationship between 90-day functional outcomes, measured by the modified Rankin Scale (mRS), and follow-up infarct volume (FIV), assessed through CT or MRI within 12–36 h post-MT.
Results:
Among 130 participants, specific FIV thresholds were identified with high specificity and sensitivity for predicting outcomes. A FIV ⩽5 ml was highly specific for predicting favorable and excellent outcomes. The optimal cut-off for both prognostications was identified at ⩽15 ml by the Youden Index, with significant reductions in the likelihood of favorable outcomes observed above a 40 ml threshold. Receiver Operator Curve (ROC) analyses confirmed FIV as a superior predictor of functional outcomes compared to traditional recanalization scores, such as final modified thrombolysis in cerebral infarction score (mTICI). Multivariable analysis further highlighted the inverse relationship between FIV and positive functional outcomes.
Conclusions:
FIV within 36 h post-MT serves as a potent predictor of 90-day functional outcomes in patients with M2 segment MeVO strokes. Establishing FIV thresholds may aid in the prognostication of stroke outcomes, suggesting a role for FIV in guiding post intervention treatment decisions and informing clinical practice. Future research should focus on validating these findings across diverse patient populations and exploring the integration of FIV measurements with other clinical and imaging markers to enhance outcome prediction accuracy.
Keywords: Stroke, medium vessel occlusion, mechanical thrombectomy, follow-up infarct volume
Graphical abstract.
Introduction
Stroke is a multifaceted condition where clinical outcome is affected by an array of factors, including patient age, prior functional status, comorbidities, as well as elements related to post-stroke management and rehabilitation. Within the realm of stroke treatment, especially concerning endovascular interventions like mechanical thrombectomy (MT), the use of recanalization scores has emerged as a key surrogate marker for assessing the effectiveness of these therapies. Medium vessel occlusions (MeVOs), primarily occurring in the M2 and M3 segments of the middle cerebral artery (MCA), account for a substantial portion of acute ischemic strokes (AIS), with estimates ranging between 25% and 40%. MeVO strokes are clinically significant, given their potential to induce severe disabilities.1,2
The prevailing consensus in stroke management is the critical impact of vessel recanalization on clinical outcomes in AIS, 3 underpinning the rationale behind reperfusion therapies aimed at salvaging the ischemic penumbra to limit final infarct size. 4 This therapeutic goal addresses the mismatch between the ischemic core and penumbra, where the core expands at the penumbra’s expense due to collateral failure. 5 While experimental models of focal ischemia predominantly utilize infarct volume as an outcome measure, human studies have traditionally favored disability assessment scales, primarily due to the importance of targeting clinically meaningful endpoints as well as due to mixed results in correlating infarct volumes with clinical outcomes. These studies have faced challenges, including the heterogeneity of vascular occlusion sites and limited sample size, complicating the understanding of the infarct volume’s role in patient recovery.6–10
This study focuses on a homogeneous patient cohort with MCA-M2 segment occlusions undergoing MT, aiming to clarify the relationship between clinical outcomes, recanalization, and follow-up infarct volume (FIV). Advances in neuroimaging have enhanced the precision of FIV measurements,10–12 which are closely linked to neurological impairment and overall functional outcomes.13,14 FIV’s early evaluability and its reduced susceptibility to confounding factors position it as a potential indicator of therapeutic effect compared to traditional outcome measures.15–18 Previous research has underscored the significance of FIV in large vessel occlusion (LVO) strokes, including the identification of specific cut-off points correlating with outcomes. However, evidence remains limited on FIV’s predictive value in MeVO stroke, especially those treated with MT.19–24
Our investigation aims to assess whether FIV could serve as a more precise indicator of procedural success compared to recanalization scores and to identify FIV thresholds that predict favorable (mRS 0–2) and excellent (mRS 0–1) outcomes in patients with M2 segment stroke treated with MT. By focusing on this specific patient population, we aim to investigate the prognostic utility of FIV in the context of MeVO stroke and propose its potential as a surrogate marker for outcomes in ongoing MeVO reperfusion therapy trials.
Methods
This investigation is part of an analysis of the Multicenter Analysis of primary Distal medium vessel occlusions: effect of Mechanical Thrombectomy (MAD-MT) registry.25–35 The study received approval from the institutional review board or local ethical standards committee at each participating site, and informed consent from patients was waived given minimal patient risk. The de-identified data supporting this study’s findings are available from the corresponding author upon reasonable request. This study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline.36,37
Study population and setting
Inclusion criteria for this analysis were as follows: (1) middle cerebral artery acute ischemic stroke patients with DMVO in the M2 segment only; (2) MT with or without IVT; (3) availability of 90-day modified Rankin Scale (mRS) data; (3) Pre-stroke mRS of 0 or 1. Exclusion criteria encompassed with missing 90-day post-stroke mRS data; (2) Patients received intra-arterial urokinase (Supplemental Figure 1). Characteristics and outcomes of consecutive patients with acute ischemic stroke due to DMVO treated with MT or MT + IVT were collected at 37 centers in North America, Asia, and Europe.
Data collection and outcomes
Data were collected between September 2017 and July 2021. Data for this study were collected prospectively and reviewed retrospectively. The local neurointerventionalist reviewed all cases before sending their data to the MAD-MT consortium. They determined the angiographic treatment success before the data was sent to the consortium, which was self-reported by each center.
Baseline clinical and demographic characteristics were recorded for patients and included sex (male or female), age, hypertension, hypercholesterolemia, diabetes mellitus, atrial fibrillation, and smoking status. Pre-stroke modified Rankin Scale (mRS) score and occluded vessel were recorded. National Institutes of Health Stroke Scale (NIHSS) score was recorded at presentation. Baseline Alberta Stroke Program Early CT Score (ASPECTS) was assessed using non contrast head CT. 38 Hemorrhagic transformation was scored according to the Heidelberg Classification. 39
Other details of interest included antiplatelet and anticoagulation medication status, mothership versus drip-and-ship, time from onset to puncture and recanalization, vital sign readings (blood pressure, heart rate), glycemic readings, anesthesia type (general, sedation, or local), access site (femoral or radial), and imaging after MT (computed tomography (CT), magnetic resonance (MR), or none). Clinical outcome measures were favorable (mRS 0–2) and excellent outcome (mRS 0–1).
Procedural and technical details
Treatment consisted of MT alone or MT + IVT. MT access site, either femoral or radial artery, and endovascular strategy (aspiration, stent retriever, combined or rescue techniques) were left to the individual operator’s discretion. Similarly, the number of passes was left to the treating physician’s discretion and institutional guidelines. The final mTICI scores were site adjudicated.
Follow-up infarct volume (FIV) assessment
FIV was assessed on follow-up NCCT or MRI. If multiple follow-up scans were available, MRI was the preferred modality with a range of 12 h–36 h post MT. FIVs was calculated using either manual or semi-automated segmentation techniques and were reported per each center protocol. FIVs were calculated in milliliters (mL) by multiplying the number of voxels of the segmented ischemic lesions with its voxel size.
NCCT scans in our study were conducted using a helical scanning technique. The scans were performed with each slice having a thickness of 5 mm and a reconstruction resolution of 0.75 mm. The kilovoltage peak (kVp) was set at 120, and the milliampere-seconds (mAs) were set at 365. The rotation time of the CT scanner was maintained at 1 s, and the total acquisition time for each scan ranged between 6 and 8 s. The collimation of the scans was 128 mm × 0.6 mm, and a pitch value of 0.55 was used. All scans were performed in a craniocaudal direction.
Fluid-Attenuated Inversion Recovery (FLAIR) imaging was conducted using Siemens Aera or Skyra scanners (Erlangen, Germany). The FLAIR sequence parameters on 3T were: Repetition Time (TR) was set in the range of 9000 ms, Echo Time (TE) around 105 ms, and Inversion Time (TI) at 2500 ms. Flip Angle: 160; Field of View (FOV): 42.8 cm × 23 cm. The imaging was performed using either a 1.5 Tesla or 3 Tesla scanner. The slice thickness was maintained at 4 mm. At 1.5 T: Repetition Time (TR) was 7500 ms, Echo Time (TE) 78 ms, and Inversion Time (TI) at 2300 ms. Flip Angle: 180; FOV: 40.9 cm × 22 cm. The slice thickness was maintained at 5 mm. 35
The FLAIR images were reviewed and analyzed by experienced neuroradiologists, focusing on the presence, location, and volume of ischemic lesions. The volume of ischemic lesions on FLAIR was calculated using either manual or semi-automated segmentation techniques, with the FIV determined by measuring the hyperintense regions indicative of ischemic tissue.
These imaging protocols varied among centers; however, the parameters provided are representative of those used in the majority of centers.
Statistical analysis
Statistical analysis was conducted utilizing R software (version 4.2.2). 40 We presented categorical variables as frequencies with percentages, while continuous variables were summarized using medians and interquartile ranges (IQRs).
For inferential statistics, we employed logistic regression model to estimate odds ratios (ORs). To determine whether the FIV was independently associated with the functional outcome, we adjusted our model for age, admission NIHSS score, final mTICI score, administration of IVT, and the time from stroke onset to arterial puncture. A p-value of <0.05 was pre-specified as the threshold for statistical significance.
Separate receiver operating characteristic (ROC) curves were employed for a favorable and excellent outcome, and the area under the curve (AUC) calculated for each model. The optimal cutoff point for the predictive variables was determined using the Youden Index. We then calculated the positive predictive value (PPV), as well as the negative predictive value (NPV), sensitivity and specificity for each cut-off point, each with a corresponding 95% confidence interval.
Results
Baseline characteristics
The study included 130 patients with acute ischemic stroke in the MCA-M2 segment who underwent MT. The median age was 76 years (Interquartile Range (IQR): 66, 82), with 33% (43/130) being male. The prevalence of common stroke risk factors included hypertension (75%, 97/130), hypercholesterolemia (37%, 48/130), and diabetes mellitus (18%, 24/130). Atrial fibrillation was noted in 28% (37/130) of the patients, and 25% (33/130) were current smokers. Prior to the stroke, 85% (110/130) of the patients had a mRS score of 0, and 15% (20/130) had a score of 1. The median baseline NIHSS score was 11 (IQR: 5, 16; Table 1).
Table 1.
Baseline patient demographics and clinical characteristics.
| Characteristic | N = 130 |
|---|---|
| Male, n (%) | 43 (33) |
| Age, median (IQR) | 76 (66, 82) |
| Hypercholesterolemia, n (%) | 48 (37) |
| Hypertension, n (%) | 97 (75) |
| Diabetes, n (%) | 24 (18) |
| Atrial fibrillation, n (%) | 37 (28) |
| Current smokers, n (%) | 33 (25) |
| Previous use of antiplatelet drugs, n (%) | 36 (28) |
| Previous use of anticoagulant drugs, n (%) | 32 (25) |
| Pre-stroke mRS, n (%) | |
| 0 | 110 (85) |
| 1 | 20 (15) |
| ASPECTS, median (IQR) | 9.00 (8.00, 10.00) |
| Baseline NIHSS, median (IQR) | 11 (5, 16) |
| Stroke cause, n (%) | |
| Large artery atherosclerosis | 14 (11) |
| Cardioembolic | 91 (70) |
| Other etiology | 4 (3.1) |
| Unknown etiology despite work-up | 21 (16) |
Imaging, procedural, and clinical outcomes data
In terms of treatment, 62% (80/130) of patients received IVT. The median time from stroke onset to arterial puncture was 210 min (IQR: 148–313), and the median time from puncture to recanalization was 35 min (IQR: 20–60). Most patients (70%, 89/130) underwent general anesthesia during the procedure. Successful recanalization (TICI 2b-3) was achieved in 85% (107/130) of cases. At 90 days, 54% (70/130) of patients achieved an mRS score of 0–1, and 64% (83/130) achieved a mRS score of 0–2 (Table 2).
Table 2.
Imaging and procedural data with clinical outcomes.
| Characteristic | N = 130 |
|---|---|
| Given IVT, n (%) | 80 (62) |
| First line technique, n (%) | |
| Aspiration | 46 (38) |
| Both | 56 (46) |
| Stentretriever | 19 (16) |
| Side, n (%) | |
| Right | 53 (41) |
| Left | 77 (59) |
| Onset to arterial puncture (min), median (IQR) | 210 (148, 313) |
| Puncture to recanalization time (min), median (IQR) | 35 (20, 60) |
| Onset to recanalization (min), median (IQR) | 251 (189, 366) |
| Onset to IVT needle time (min), median (IQR) | 120 (85, 180) |
| Anesthesia, n (%) | |
| CS/LA | 39 (30) |
| GA | 89 (70) |
| Follow-up infarct volume (FIV; ml) | 3.6 (13.65, 27.34) |
| Imaging after MT, n (%) | |
| CT | 96 (75) |
| Both | 10 (7.8) |
| MRI | 22 (17) |
| Total number of passes, median (IQR) | 1.50 (1.00, 2.75) |
| Day one NIHSS, Median (IQR) | 4.0 (1.0, 8.8) |
| NIHSS shift, Median (IQR) | −4 (−9, −1) |
| TICI 2c-3, n (%) | 67 (53) |
| Successful Recanalization (TICI 2b-3), n (%) | 107 (85) |
| FPE, n (%) | 34 (27) |
| 90-day mRS 0–1, n (%) | 70 (54) |
| 90-day mRS 0–2, n (%) | 83 (64) |
| 90-day Mortality, n (%) | 14 (11) |
| sICH, n (%) | 8 (6.2) |
| Intracranial hemorrhage (any type), n (%) | 39 (30) |
Predictive values for different FIV thresholds
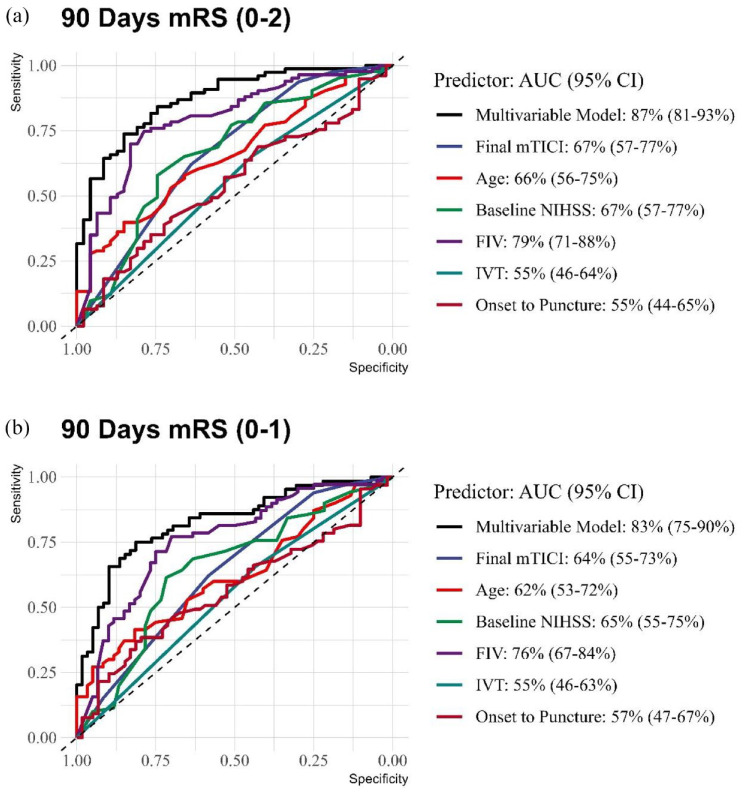
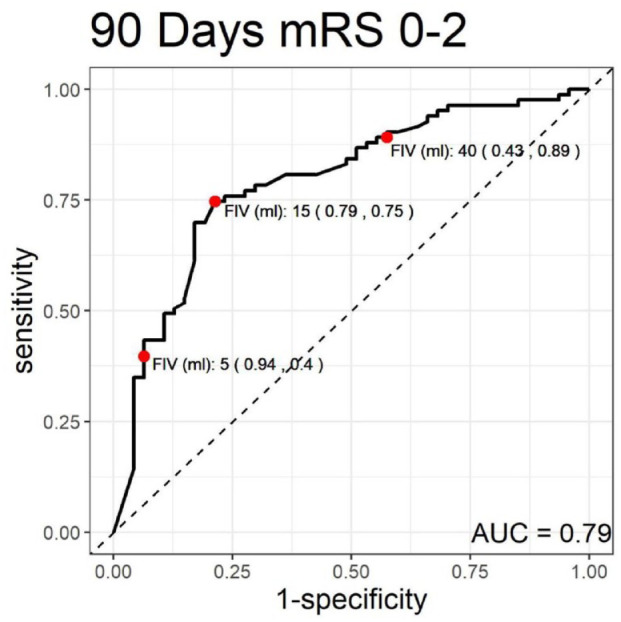
The analysis of predictive values for different FIV thresholds revealed that a FIV of ⩽5 ml had a high specificity of 94% (95% CI: 82%–99%) for predicting a favorable outcome (mRS 0–2), as illustrated in Figure 1, and 90% specificity (95% CI: 79%–96%) for predicting an excellent outcome (mRS 0–1), as illustrated in Figure 2. Notably, ⩽15 ml was determined to be the optimal cut-off point for both outcomes, with a sensitivity of 75% (95% CI: 64%–84%) and a specificity of 79% (95% CI: 64%–89%) for mRS 0–2, and a sensitivity of 77% (95% CI: 66%–86%) and a specificity of 70% (95% CI: 57%–81%) for mRS 0–1 (Table 3).
Figure 1.
Receiver operating characteristic (ROC) curve for 90-day modified Rankin scale (mRS) scores of 0–2.
Figure 2.
Receiver operating characteristic (ROC) curve for 90-day modified Rankin scale (mRS) scores of 0–1.
Table 3.
Predictive values for different follow-up infarct volumes (FIV) in stroke outcomes.
| FIV (ml) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| mRS 0–2 | ||||
| ⩽5 ml | 0.4 (0.29–0.51) | 0.94 (0.82–0.99) | 0.92 (0.78–0.98) | 0.47 (0.36–0.57) |
| ⩽15 ml a | 0.75 (0.64–0.84) | 0.79 (0.64–0.89) | 0.86 (0.76–0.93) | 0.64 (0.5–0.76) |
| ⩽40 ml | 0.89 (0.8–0.95) | 0.43 (0.28–0.58) | 0.73 (0.64–0.82) | 0.69 (0.49–0.85) |
| mRS 0–1 | ||||
| ⩽5 ml | 0.43 (0.31–0.55) | 0.9 (0.79–0.96) | 0.83 (0.67–0.94) | 0.57 (0.47–0.68) |
| ⩽15 ml a | 0.77 (0.66–0.86) | 0.7 (0.57–0.81) | 0.75 (0.63–0.84) | 0.72 (0.59–0.83) |
| ⩽40 ml | 0.9 (0.8–0.96) | 0.37 (0.25–0.5) | 0.62 (0.52–0.72) | 0.76 (0.56–0.9) |
PPV: Positive predictive value; NPV: Negative predictive value; FIV (ml): follow up infarct volume; CI: Confidence Interval.
Youden index cut-off point.
For the threshold of 40 ml, this cut-off point had a sensitivity of 89% (95% CI: 80%–95%) for predicting a favorable outcome (mRS 0–2) and 90% sensitivity (95% CI: 80%–96%) for predicting an excellent outcome (mRS 0–1).
Univariable and multivariable logistic regression models
In the univariable model, every 10 ml increase in FIV was associated with a 30% reduction in the odds of achieving mRS 0–2 (OR: 0.70, 95% CI: 0.58–0.82, p < 0.001) and a 28% reduction for mRS 0–1 (OR: 0.72, 95% CI: 0.60–0.85, p < 0.001). These associations persisted even after adjustment (mRS 0–2: adjusted OR: 0.69, 95% CI: 0.53–0.84, p = 0.002; mRS 0–1: adjusted OR: 0.73, 95% CI: 0.58–0.89, p = 0.006).
At the optimal (15 ml) cut-off points, the odds of achieving functional independence with an FIV of ⩽15 ml were 13 times higher (OR: 13.0, 95% CI: 4.50–43.4, p < 0.001) in the multivariable model, while the odds for an excellent outcome were increased by over seven times (OR: 7.09, 95% CI: 2.77–19.7, p < 0.001; Table 4).
Table 4.
Univariable and multivariable logistic regression models to predict mRS 0–1 and mRS 0–2.
| FIV (ml) | Univariable model |
Multivariable model
a
|
||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| mRS 0–2 | ||||
| FIV (per 10 ml) | 0.70 (0.58–0.82) | <0.001 | 0.69 (0.53–0.84) | 0.002 |
| ⩽5 ml | 9.68 (3.19–42.2) | <0.001 | 11.0 (2.93–60.9) | 0.001 |
| ⩽15 ml b | 10.9 (4.80–26.9) | <0.001 | 13.0 (4.50–43.4) | <0.001 |
| ⩽40 ml | 6.09 (2.54–15.6) | <0.001 | 7.93 (2.56–28.6) | <0.001 |
| mRS 0–1 | ||||
| FIV (per 10 ml) | 0.72 (0.60–0.85) | <0.001 | 0.73 (0.58–0.89) | 0.006 |
| ⩽5 ml | 6.75 (2.72–19.4) | <0.001 | 7.70 (2.64–26.1) | <0.00 |
| ⩽15 ml b | 7.88 (3.67–17.7) | <0.001 | 7.09 (2.77–19.7) | <0.001 |
| ⩽40 ml | 5.21 (2.12–14.3) | <0.001 | 5.09 (1.70–17.6) | 0.006 |
All estimates are adjusted for age, admission NIHSS score, final mTICI, administration of IVT and stroke onset to arterial puncture time.
Youden Index cut-off point.
Receiver operating characteristic (ROC) analysis for predictive models
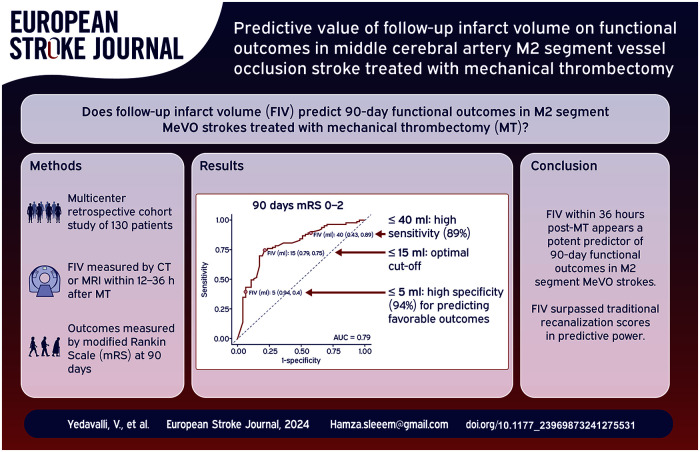
The multivariable model for predicting functional independence (mRS 0–2) achieved an AUC of 87% (95% CI: 81%–93%), reflecting a high level of discriminative ability (Figure 3(a)). Within this model, the mTICI score, patient age, baseline NIHSS score, and FIV yielded AUCs of 67%, 66%, 67%, and 79%, respectively. For the prediction of excellent outcomes (mRS 0–1), the multivariable model exhibited an AUC of 83% (95% CI: 75%–90%), and the individual AUCs for final mTICI, age, baseline NIHSS, and FIV were 64%, 62%, 65%, and 76%, respectively (Figure 3(b)).
Figure 3.
Comparative receiver operating characteristic (ROC) curves for 90-day mRS outcomes. (a) 90-day mRS (0–2). (b) 90-day mRS (0–1).
Discussion
In this multicenter cohort analysis, we have explored the role of FIV as a prognostic imaging biomarker for 90 days outcomes prediction. In addition, we have sought to identify optimal thresholds of FIV for predicting favorable and excellent outcomes after stroke, focusing on M2 stroke patients that underwent MT.
The analysis revealed that a threshold of ⩽5 ml FIV was highly specific for predicting both favorable and excellent outcomes, with ⩽15 ml as the optimal cut-off for these prognostications. Notably, a FIV of 40 ml emerged as a critical marker, above which the likelihood of achieving these desired outcomes diminishes significantly. Our regression models, both univariable and multivariable, consistently demonstrated that each increment of 10 ml in FIV substantially reduced the odds of 90-days mRS of 0–2 or 0–1. Furthermore, our study underscored FIV as the primary determinant of functional outcomes with AUC of 0.79 for predicting mRS 0–2 by FIV alone, surpassing the predictive value of recanalization status.
To the best of our knowledge, this study is the first to examine the association between FIV in MCA-M2 segment strokes and 90-day functional outcomes, and to compare its predictive power with the mTICI score. Prior research, including the HERMES dataset, has incorporated M2 occlusions within a larger, more heterogeneous array of occlusion sites, identifying a 96 ml threshold for high specificity in predicting poor outcomes. In contrast, our focused analysis on the M2 segment has identified a lower threshold of 40 ml that carries similar prognostic specificity. This discrepancy can be attributed to differences in the occlusion sites being studied. The HERMES meta-analysis focused on LVOs, whereas our study concentrated on the M2 segment, suggesting a lesser infarct volume is required to influence cortical functionality and outcomes adversely in these cases as a smaller volume of brain tissue is also initially affected. Moreover, our inclusion of FIV measurements within a narrower time window post-MT (12–36 h) aimed to minimize the confounding influence of lesion growth, in contrast to HERMES, which accounted for imaging up to 1 week post-stroke, potentially overestimating FIV due to cerebral edema.41–49
Other studies were not able to establish a robust association between FIV and patient outcomes, a discrepancy we attribute to the examination of a diverse patient cohort with varying vascular occlusion sites and different treatments.9,50 To mitigate these confounding factors, our study focused exclusively on patients with a homogeneous vascular occlusion site—specifically M2 segment occlusion, and those who had undergone MT, which further enhances the reliability of our findings. Moreover, we advocate for further research into how FIV may act within different vascular territories, suggesting that the impact of FIV on stroke outcomes may vary across different regions of the brain, underscoring the need for a territory-specific approach in future studies. 42
In this study, we used FLAIR and NCCT for assessing FIV rather than diffusion-weighted imaging (DWI), as FLAIR imaging is considered the gold standard for identifying subacute infarcts, and it provides superior contrast in the subacute phase of ischemia compared to DWI.16–18 Most cases in our cohort had MRIs performed after 24 h of stroke onset, aligning with the subacute phase where FLAIR is particularly effective.15,35 NCCT was only used for patients who did not undergo follow-up MRI.
Our study is subject to several limitations that warrant consideration. Primarily, its retrospective design introduces inherent selection bias. Although infarct volumes were measured using both CT and MRI, and FLAIR imaging is recognized for its accuracy, existing literature suggests that both modalities offer comparable precision in FIV estimation. 51 A notable limitation arises from the decentralization of FIV adjudication, performed by individual participating centers rather than a centralized imaging facility. This approach, while potentially enhancing the generalizability of our findings, may introduce variability due to differing institutional protocols and readers. However, it’s important to note that previous studies have demonstrated high intra- and inter-rater reliability for infarct size delineation on MRI and CT, especially for infarcts larger than 10 ml.6,12,52–54
Our analysis acknowledges that the proposed infarct volume thresholds for guiding treatment decisions necessitate further validation, particularly using pre-treatment infarct volumes. Moreover, although FIV emerged as a potent predictor of functional outcomes post-MT, the integration of additional imaging metrics with FIV is likely to refine outcome predictions. 55 Such enhancements, however, demand further investigation, including both clinical and imaging validations, before lesion topography can be reliably utilized for outcome prediction in clinical settings. 56
Furthermore, our analysis did not fully incorporate the potential impact of edema-related lesion expansion, although this factor could improve clinical predictions by identifying patients prone to malignant edema patterns. Consequently, future research is imperative to establish reliable edema measurement techniques and to explore the comprehensive utility of FIV as a surrogate marker by rigorously testing the treatment-FIV-functional outcome causal pathway.6,57 Despite these limitations, our study is strengthened by its incorporation of large scale, multinational, multicenter, and real-world data, thereby improving generalizability.
Conclusion
In conclusion, this study underscores the significance of FIV as a pivotal predictor of functional outcomes in MeVO stroke patients undergoing MT. By demonstrating the potential superiority of FIV over traditional recanalization scores and establishing precise thresholds for favorable and excellent prognoses, our findings advocate for the integration of neuroimaging in the upcoming endovascular stroke trial as surrogate for the intervention effect. This study paves the way for future research to validate these thresholds across broader populations and investigate the synergy between FIV and other prognostic markers with long-term stroke outcomes.
Supplemental Material
Supplemental material, sj-jpg-1-eso-10.1177_23969873241275531 for Predictive value of follow-up infarct volume on functional outcomes in middle cerebral artery M2 segment vessel occlusion stroke treated with mechanical thrombectomy by Vivek Yedavalli, Hamza Adel Salim, Basel Musmar, Nimer Adeeb, Kareem El Naamani, Nils Henninger, Sri Hari Sundararajan, Anna Luisa Kühn, Jane Khalife, Sherief Ghozy, Luca Scarcia, Benjamin YQ Tan, Robert W Regenhardt, Jeremy J Heit, Nicole M Cancelliere, Joshua D Bernstock, Aymeric Rouchaud, Jens Fiehler, Sunil Sheth, Ajit S Puri, Christian Dyzmann, Marco Colasurdo, Xavier Barreau, Leonardo Renieri, João Pedro Filipe, Pablo Harker, Răzvan Alexandru Radu, Mohamad Abdalkader, Piers Klein, Thomas R Marotta, Julian Spears, Takahiro Ota, Ashkan Mowla, Pascal Jabbour, Arundhati Biswas, Frédéric Clarençon, James E Siegler, Thanh N Nguyen, Ricardo Varela, Amanda Baker, Muhammed Amir Essibayi, David Altschul, Nestor R Gonzalez, Markus A Möhlenbruch, Vincent Costalat, Benjamin Gory, Christian Paul Stracke, Mohammad Ali Aziz-Sultan, Constantin Hecker, Hamza Shaikh, David S Liebeskind, Alessandro Pedicelli, Andrea M Alexandre, Illario Tancredi, Tobias D Faizy, Erwah Kalsoum, Boris Lubicz, Aman B Patel, Vitor Mendes Pereira, Adrien Guenego and Adam A Dmytriw in European Stroke Journal
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Regenhardt serves on a DSMB for a trial sponsored by Rapid Medical, serves as site PI for studies sponsored by Penumbra and Microvention, and receives stroke research grant funding from the National Institutes of Health, Society of Vascular and Interventional Neurology, and Heitman Stroke Foundation.
Dr. Guenego reports consultancy for Rapid Medical and Phenox, not directly related to the present work.
Dr. Clarençon reports conflicts of interest with Medtronic, Balt Extrusion (consultant), ClinSearch (core lab), Penumbra, Stryker (payment for reading) and Artedrone (Board); all not directly related to the present work.
Dr. Henninger received support from W81XWH-19-PRARP-RPA form the CDMRP/DoD, NS131756 and U24NS113844 from the NINDS, and NR020231 from the NINR and received compensation from Myrobalan, Inc. and General Dynamics during the conduct of this study unrelated to this work.
Dr. Liebeskind is consultant as Imaging Core Lab to Cerenovus, Genentech, Medtronic, Stryker, Rapid Medical.
Dr. Yeo reports Advisory work for AstraZeneca, Substantial support from NMRC Singapore and is a medical advisor for See-mode, Cortiro and Sunbird Bio, with equity in Ceroflo. All unrelated to the present work.
Dr. Griessenauer reports a proctoring agreement with Medtronic and research funding by Penumbra.
Dr. Marnat reports conflicts of interest with Microvention Europe, Stryker Neurovascular, Balt (consulting), Medtronic, Johnson & Johnson and Phenox (paid lectures), all not directly related to the present work.
Dr. Puri is a consultant for Medtronic Neurovascular, Stryker NeurovascularBalt, Q’Apel Medical, Cerenovus, Microvention, Imperative Care, Agile, Merit, CereVasc and Arsenal Medical, he received research grants from NIH, Microvention, Cerenovus, Medtronic Neurovascular and Stryker Neurovascular, and holds stocks in InNeuroCo, Agile, Perfuze, Galaxy and NTI.
Dr. Tjoumakaris is a consultant for Medtronic and Microvention (funds paid to institution, not personally).
Dr. Jabbour is a consultant for Medtronic, Microvention and Cerus.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent and ethical approval: The study received approval from the local ethical standards committee at each participating site, and informed consent from patients was waived. The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Guarantor: Vivek Yedavalli, MD
Contributorship: V.Y, H.S, B.M, N.A, K.N, N.H, S.S, A.K, J.K, S.G, L.S, B.T, R.R, J.H, N.C, J.B, A.R, J.F, S.S, A.P, C.D, M.C, X.B, L.R, J.F, P.H, R.R, M.A, P.K, T.M, J.S, T.O, A.M, P.J, A.B, F.C, J.S, T.N, R.V, A.B, M.E, D.A, N.G, M.M, V.C, B.G, C.S, M.A, C.H, H.S, D.L, A.P, A.A, I.T, T.F, E.K, B.L, A.P, V.P, A.G, A.D. contributed to the conception and design of the work.
V.Y, H.S, B.M, N.A, K.N, N.H, S.S, A.K, J.K, S.G, L.S, B.T, R.R, J.H, N.C, J.B, A.R, J.F, S.S, A.P, C.D, M.C, X.B, L.R, J.F, P.H, R.R, M.A, P.K, T.M, J.S, T.O, A.M, P.J, A.B, F.C, J.S, T.N, R.V, A.B, M.E, D.A, N.G, M.M, V.C, B.G, C.S, M.A, C.H, H.S, D.L, A.P, A.A, I.T, T.F, E.K, B.L, A.P, V.P, A.G, A.D, . were involved in the acquisition of data, and data analysis and interpretation.
V.Y, H.S, B.M, N.A, K.N, N.H, S.S, A.K, J.K, S.G, L.S, B.T, R.R, J.H, N.C, J.B, A.R, J.F, S.S, A.P, C.D, M.C, X.B, L.R, J.F, P.H, R.R, M.A, P.K, T.M, J.S, T.O, A.M, P.J, A.B, F.C, J.S, T.N, R.V, A.B, M.E, D.A, N.G, M.M, V.C, B.G, C.S, M.A, C.H, H.S, D.L, A.P, A.A, I.T, T.F, E.K, B.L, A.P, V.P, A.G, A.D. drafted the work and revised it critically for important intellectual content.
ORCID iDs: Hamza Adel Salim  https://orcid.org/0000-0002-5208-8425
https://orcid.org/0000-0002-5208-8425
Basel Musmar  https://orcid.org/0009-0000-4910-6090
https://orcid.org/0009-0000-4910-6090
Luca Scarcia  https://orcid.org/0000-0002-1316-0383
https://orcid.org/0000-0002-1316-0383
Robert W. Regenhardt  https://orcid.org/0000-0003-2958-3484
https://orcid.org/0000-0003-2958-3484
Jens Fiehler  https://orcid.org/0000-0001-8533-7478
https://orcid.org/0000-0001-8533-7478
Răzvan Alexandru Radu  https://orcid.org/0000-0001-6375-8466
https://orcid.org/0000-0001-6375-8466
Piers Klein  https://orcid.org/0000-0001-7468-137X
https://orcid.org/0000-0001-7468-137X
Takahiro Ota  https://orcid.org/0000-0002-5108-6719
https://orcid.org/0000-0002-5108-6719
Pascal Jabbour  https://orcid.org/0000-0002-8965-2413
https://orcid.org/0000-0002-8965-2413
Thanh N. Nguyen  https://orcid.org/0000-0002-2810-1685
https://orcid.org/0000-0002-2810-1685
Muhammed Amir Essibayi  https://orcid.org/0000-0001-8325-2382
https://orcid.org/0000-0001-8325-2382
Nestor R. Gonzalez  https://orcid.org/0000-0002-8277-6317
https://orcid.org/0000-0002-8277-6317
Markus A. Möhlenbruch  https://orcid.org/0000-0002-5075-704X
https://orcid.org/0000-0002-5075-704X
David S. Liebeskind  https://orcid.org/0000-0002-5109-8736
https://orcid.org/0000-0002-5109-8736
Andrea M. Alexandre  https://orcid.org/0000-0002-8080-3916
https://orcid.org/0000-0002-8080-3916
Supplemental material: Supplemental material for this article is available online.
References
- 1. Ospel JM, Goyal M. A review of endovascular treatment for medium vessel occlusion stroke. J Neurointerv Surg 2021; 13: 623–630. [DOI] [PubMed] [Google Scholar]
- 2. Saver JL, Chapot R, Agid R, et al.; Distal Thrombectomy Summit Group. Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke 2020; 51: 2872–2884. [DOI] [PubMed] [Google Scholar]
- 3. The impact of recanalization on ischemic stroke outcome. Stroke. https://www.ahajournals.org/doi/10.1161/01.str.0000258112.14918.24 (accessed March 2, 2024). [DOI] [PubMed]
- 4. Pathophysiology of acute ischemic stroke : continuum: lifelong learning in neurology. https://journals.lww.com/continuum/abstract/2008/12000/pathophysiology_of_acute_ischemic_stroke.4.aspx (accessed March 2, 2024).
- 5. Macrae IM. Preclinical stroke research–advantages and disadvantages of the most common rodent models of focal ischaemia. Br J Pharmacol 2011; 164: 1062–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoo AJ, Chaudhry ZA, Nogueira RG, et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke 2012; 43: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 7. Serial changes in ischemic lesion volume and neurological recovery after t-PA therapy. PubMed. https://pubmed.ncbi.nlm.nih.gov/21397255/ (accessed March 2, 2024). [DOI] [PubMed]
- 8. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. Stroke. https://www.ahajournals.org/doi/10.1161/01.STR.30.2.293 (accessed March 2, 2024). [DOI] [PubMed]
- 9. Wardlaw JM, Keir SL, Bastin ME, et al. Is diffusion imaging appearance an independent predictor of outcome after ischemic stroke? Neurology 2002; 59: 1381–1387. [DOI] [PubMed] [Google Scholar]
- 10. Thijs VN, Lansberg MG, Beaulieu C, et al. Is early ischemic lesion volume on diffusion-weighted imaging an independent predictor of stroke outcome? A multivariable analysis. Stroke 2000; 31: 2597–2602. [DOI] [PubMed] [Google Scholar]
- 11. van Everdingen KJ, van der Grond J, Kappelle LJ, et al. Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke 1998; 29: 1783–1790. [DOI] [PubMed] [Google Scholar]
- 12. Reproducibility of measurements of cerebral infarct volume on CT scans. Stroke. https://pubmed.ncbi.nlm.nih.gov/11157177/ (accessed January 15, 2024). [DOI] [PubMed]
- 13. Saunders DE, Clifton AG, Brown MM. Measurement of infarct size using MRI predicts prognosis in middle cerebral artery infarction. Stroke 1995; 26: 2272–2276. [DOI] [PubMed] [Google Scholar]
- 14. Correlation of perfusion- and diffusion-weighted MRI with NIHSS score in acute (<<6.5 hour) ischemic stroke. PubMed. https://pubmed.ncbi.nlm.nih.gov/9566364/ (accessed January 15, 2024). [DOI] [PubMed]
- 15. Artzi M, Aizenstein O, Jonas-Kimchi T, et al. FLAIR lesion segmentation: application in patients with brain tumors and acute ischemic stroke. Eur J Radiol 2013; 82: 1512–1518. [DOI] [PubMed] [Google Scholar]
- 16. Farr TD, Wegener S. Use of magnetic resonance imaging to predict outcome after stroke: a review of experimental and clinical evidence. J Cereb Blood Flow Metab 2010; 30: 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schiemanck SK, Kwakkel G, Post MW, et al. Predictive value of ischemic lesion volume assessed with magnetic resonance imaging for neurological deficits and functional outcome poststroke: a critical review of the literature. Neurorehabil Neural Repair 2006; 20: 492–502. [DOI] [PubMed] [Google Scholar]
- 18. Ricci PE, Burdette JH, Elster AD, et al. A comparison of fast spin-echo, fluid-attenuated inversion-recovery, and diffusion-weighted MR imaging in the first 10 days after cerebral infarction. AJNR Am J Neuroradiol 1999; 20: 1535–1542. [PMC free article] [PubMed] [Google Scholar]
- 19. Pretreatment diffusion-weighted imaging lesion volume predicts favorable outcome after intravenous thrombolysis with tissue-type plasminogen activator in acute ischemic stroke. Stroke. https://www.ahajournals.org/doi/10.1161/strokeaha.110.600148 (accessed February 26, 2024). [DOI] [PubMed]
- 20. Parsons MW, Christensen S, McElduff P, et al.; Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) Investigators. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab 2010; 30: 1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kruetzelmann A, Köhrmann M, Sobesky J, et al. Pretreatment diffusion-weighted imaging lesion volume predicts favorable outcome after intravenous thrombolysis with tissue-type plasminogen activator in acute ischemic stroke. Stroke 2011; 42: 1251–1254. [DOI] [PubMed] [Google Scholar]
- 22. MRI-based selection for intra-arterial stroke therapy. Stroke. https://www.ahajournals.org/doi/full/10.1161/STROKEAHA.108.541656 (accessed February 26, 2024).
- 23. Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006; 60: 508–517. [DOI] [PubMed] [Google Scholar]
- 24. The cortical ischemic core and not the consistently present penumbra is a determinant of clinical outcome in acute middle cerebral artery occlusion. Stroke. https://www.ahajournals.org/doi/full/10.1161/01.STR.0000091232.81947.C9 (accessed February 26, 2024). [DOI] [PubMed]
- 25. Radu RA, Costalat V, Fahed R, et al. First pass effect as an independent predictor of functional outcomes in medium vessel occlusions: an analysis of an international multicenter study. Eur Stroke J 2024; 9: 123. DOI: 10.1177/23969873231208276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siegler J, Shaikh H, Khalife J, et al. Aspiration versus stent-retriever as first-line endovascular therapy technique for primary medium and distal intracranial occlusions: a propensity-score matched multicenter analysis. Stroke 2023; 3. https://www.ahajournals.org/doi/10.1161/SVIN.123.000931 [Google Scholar]
- 27. Dmytriw AA, Musmar B, Salim H, et al. Incidence and clinical outcomes of perforations during mechanical thrombectomy for medium vessel occlusion in acute ischemic stroke: A retrospective, multicenter, and multinational study. Eur Stroke J 2024; 9(2): 328–337. 10.1177/23969873231219412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salim H, Musmar B, Adeeb N, et al. Outcomes of mechanical thrombectomy in anticoagulated patients with acute distal and medium vessel stroke. Eur Stroke J Published online May 10, 2024. DOI: 10.1177/23969873241249295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aspiration versus stent-retriever as first-line endovascular therapy technique for primary medium and distal intracranial occlusions: a propensity-score matched multicenter analysis. Stroke Vasc Interv Neurol. https://www.ahajournals.org/doi/10.1161/SVIN.123.000931 (accessed January 27, 2024).
- 30. Salim HA, Yedavalli V, Musmar B, et al. Endovascular therapy versus best medical management in distal medium middle cerebral artery acute ischaemic stroke: a multinational multicentre propensity score-matched study. J Neurol Neurosurg Psychiatry 2024. DOI: 10.1136/jnnp-2024-333669 [DOI] [PubMed] [Google Scholar]
- 31. Yedavalli V, Salim HA, Musmar B, et al. Pretreatment predictors of very poor clinical outcomes in medium vessel occlusion stroke patients treated with mechanical thrombectomy. Int J Stroke 2024. Published online July 29, 2024. DOI: 10.1177/17474930241270524 [DOI] [PubMed] [Google Scholar]
- 32. Symptomatic intracerebral hemorrhage in proximal and distal medium middle cerebral artery occlusion patients treated with mechanical thrombectomy. J NeuroInterv Surg. https://jnis.bmj.com/content/early/2024/07/08/jnis-2024-021879 (accessed July 12, 2024). [DOI] [PubMed]
- 33. Outcomes with general anesthesia compared to conscious sedation for endovascular treatment of medium vessel occlusions: results of an international multicentric study. Clin Neuroradiol. https://link.springer.com/article/10.1007/s00062-024-01415-1 (accessed May 16, 2024). [DOI] [PubMed]
- 34. Kühn A, Puri A, Salim H, et al. Multicenter evaluation of mechanical thrombectomy for distal medium vessel occlusions with National Institute of Health Stroke Scale Scores ⩾ 6 and ⩽ 6. J Neurol. Published online July 5, 2024. DOI: 10.1007/s00415-024-12537-4 [DOI] [PubMed] [Google Scholar]
- 35. Salim H, Lakhani DA, Balar A, et al. Follow-up infarct volume on fluid attenuated inversion recovery (FLAIR) imaging in distal medium vessel occlusions: the role of cerebral blood volume index. J Neurol 2024; 271: 3389–3397. Published online March 20, 2024. DOI: 10.1007/s00415-024-12279-3 [DOI] [PubMed] [Google Scholar]
- 36. Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019; 13: S31–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. von Elm E, Altman DG, Egger M, et al.; STROBE Initiative. The strengthening the reporting of observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program early CT score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 2001; 22: 1534–1542. [PMC free article] [PubMed] [Google Scholar]
- 39. The Heidelberg bleeding classification . Stroke. https://www.ahajournals.org/doi/10.1161/STROKEAHA.115.010049 (accessed January 18, 2024).
- 40. Citing RStudio. Posit Support. Published December 16, 2023. http://www.rstudio.com/ (accessed December 25, 2023).
- 41. Bucker A, Boers AM, Bot JCJ, et al.; MR CLEAN Trial Investigators (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands). Associations of ischemic lesion volume with functional outcome in patients with acute ischemic stroke: 24-Hour versus 1-Week imaging. Stroke 2017; 48: 1233–1240. [DOI] [PubMed] [Google Scholar]
- 42. Zaidi SF, Aghaebrahim A, Urra X, et al. Final infarct volume is a stronger predictor of outcome than recanalization in patients with proximal middle cerebral artery occlusion treated with endovascular therapy. Stroke 2012; 43: 3238–3244. [DOI] [PubMed] [Google Scholar]
- 43. Aslan A, Abuzahra S, Adeeb N, et al. The feasibility of mechanical thrombectomy versus medical management for acute stroke with a large ischemic territory. J Neurointerv Surg 2024. Published online March 12, 2024. pii: jnis-2023-021368. DOI: 10.1136/jnis-2023-021368 [DOI] [PubMed] [Google Scholar]
- 44. Dmytriw A, Salim H, Musmar B, et al. Dual layer vs single layer woven endobridge device in the treatment of intracranial aneurysms: a propensity score-matched analysis. Neurosurg Rev 2024; 47: 116. [DOI] [PubMed] [Google Scholar]
- 45. Dmytriw A, Musmar B, Salim H, et al. The impact of postoperative aspirin in patients undergoing Woven EndoBridge: a multicenter, institutional, propensity score-matched analysis. J Neurointerv Surg. Published online January 19, 2024. DOI: 10.1136/jnis-2023-021082 [DOI] [PubMed] [Google Scholar]
- 46. Lakhani DA, Balar AB, Salim H, et al. CT perfusion derived rCBV << 42% lesion volume is independently associated with followup FLAIR infarct volume in anterior circulation large vessel occlusion. Diagnostics 2024; 14: 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mei J, Salim HA, Lakhani DA, et al. Lower admission stroke severity is associated with good collateral status in distal medium vessel occlusion stroke. J Neuroimaging 2024; 34: 424–429 (accessed June 30, 2024). [DOI] [PubMed] [Google Scholar]
- 48. Dmytriw AA, Salim HA, Musmar B, et al.; Flow diversion Multinational Observational cohort Device comparsion (FlowMOD) Investigators. Comparative efficacy of flow diverter devices in the treatment of carotid sidewall intracranial aneurysms: a Retrospective, Multicenter Study. Clin Neuroradiol 2024. Published online July 18, 2024. DOI: 10.1007/s00062-024-01435-x [DOI] [PubMed] [Google Scholar]
- 49. Musmar B, Spellicy S, Salim H, et al. Comparative outcomes of middle meningeal artery embolization with statins versus embolization alone in the treatment of chronic subdural hematoma: a systematic review and meta-analysis. Neurosurg Rev 2023; 46: 262. [DOI] [PubMed] [Google Scholar]
- 50. Hand PJ, Wardlaw JM, Rivers CS, et al. MR diffusion-weighted imaging and outcome prediction after ischemic stroke. Neurology 2006; 66: 1159–1163. [DOI] [PubMed] [Google Scholar]
- 51. Boers AMM, Jansen IGH, Beenen LFM, et al. Association of follow-up infarct volume with functional outcome in acute ischemic stroke: a pooled analysis of seven randomized trials. J Neurointerv Surg 2018; 10: 1137–1142. [DOI] [PubMed] [Google Scholar]
- 52. Ay H, Arsava EM, Vangel M, et al. Interexaminer difference in infarct volume measurements on MRI: a source of variance in stroke research. Stroke 2008; 39: 1171–1176. [DOI] [PubMed] [Google Scholar]
- 53. Luby M, Bykowski JL, Schellinger PD, et al. Intra- and interrater reliability of ischemic lesion volume measurements on Diffusion-Weighted, mean transit time and fluid-attenuated inversion recovery MRI. Stroke 2006; 37: 2951–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ebinger M, Christensen S, De Silva DA, et al.; Echoplanar Imaging Thrombolytic Evaluation Trial Investigators. Expediting MRI-based proof-of-concept stroke trials using an earlier imaging end point. Stroke 2009; 40: 1353–1358. [DOI] [PubMed] [Google Scholar]
- 55. Puig J, Pedraza S, Blasco G, et al. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol 2011; 32: 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. The real estate factor. Stroke. https://www.ahajournals.org/doi/10.1161/01.str.0000251792.76080.45 (accessed March 2, 2024).
- 57. Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke. Stroke. https://www.ahajournals.org/doi/10.1161/01.STR.27.4.599 (accessed March 2, 2024). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-eso-10.1177_23969873241275531 for Predictive value of follow-up infarct volume on functional outcomes in middle cerebral artery M2 segment vessel occlusion stroke treated with mechanical thrombectomy by Vivek Yedavalli, Hamza Adel Salim, Basel Musmar, Nimer Adeeb, Kareem El Naamani, Nils Henninger, Sri Hari Sundararajan, Anna Luisa Kühn, Jane Khalife, Sherief Ghozy, Luca Scarcia, Benjamin YQ Tan, Robert W Regenhardt, Jeremy J Heit, Nicole M Cancelliere, Joshua D Bernstock, Aymeric Rouchaud, Jens Fiehler, Sunil Sheth, Ajit S Puri, Christian Dyzmann, Marco Colasurdo, Xavier Barreau, Leonardo Renieri, João Pedro Filipe, Pablo Harker, Răzvan Alexandru Radu, Mohamad Abdalkader, Piers Klein, Thomas R Marotta, Julian Spears, Takahiro Ota, Ashkan Mowla, Pascal Jabbour, Arundhati Biswas, Frédéric Clarençon, James E Siegler, Thanh N Nguyen, Ricardo Varela, Amanda Baker, Muhammed Amir Essibayi, David Altschul, Nestor R Gonzalez, Markus A Möhlenbruch, Vincent Costalat, Benjamin Gory, Christian Paul Stracke, Mohammad Ali Aziz-Sultan, Constantin Hecker, Hamza Shaikh, David S Liebeskind, Alessandro Pedicelli, Andrea M Alexandre, Illario Tancredi, Tobias D Faizy, Erwah Kalsoum, Boris Lubicz, Aman B Patel, Vitor Mendes Pereira, Adrien Guenego and Adam A Dmytriw in European Stroke Journal