Abstract
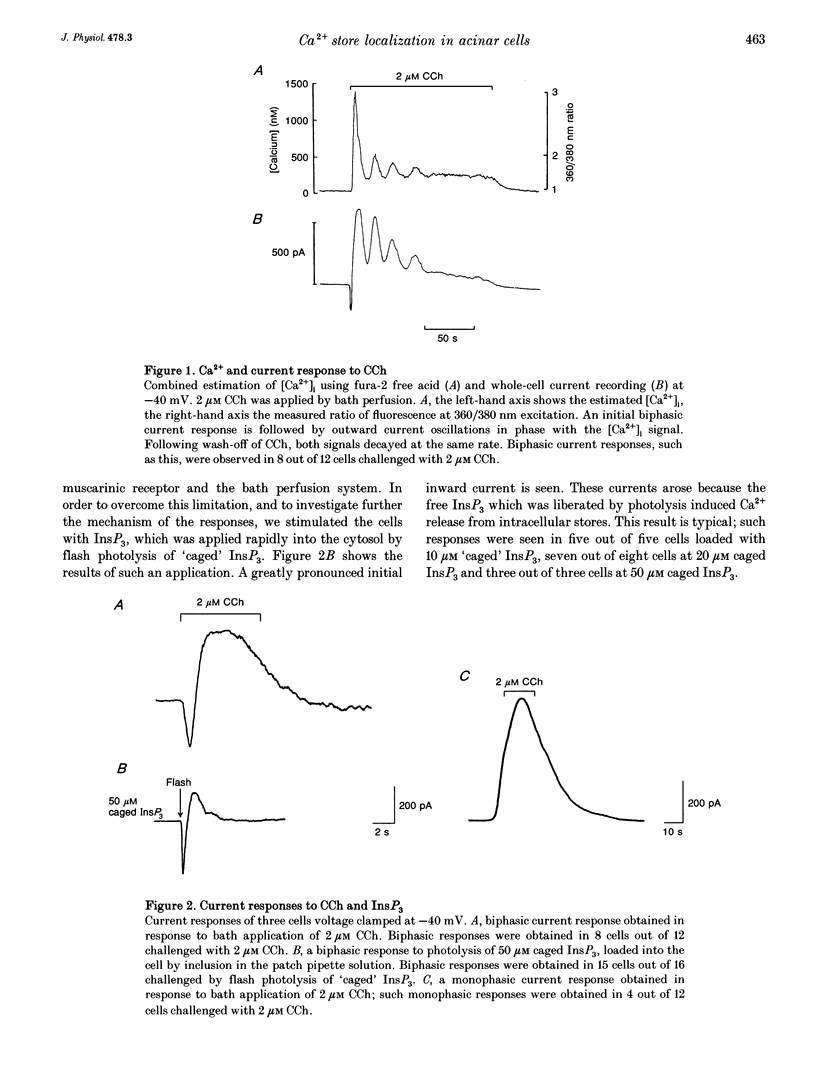
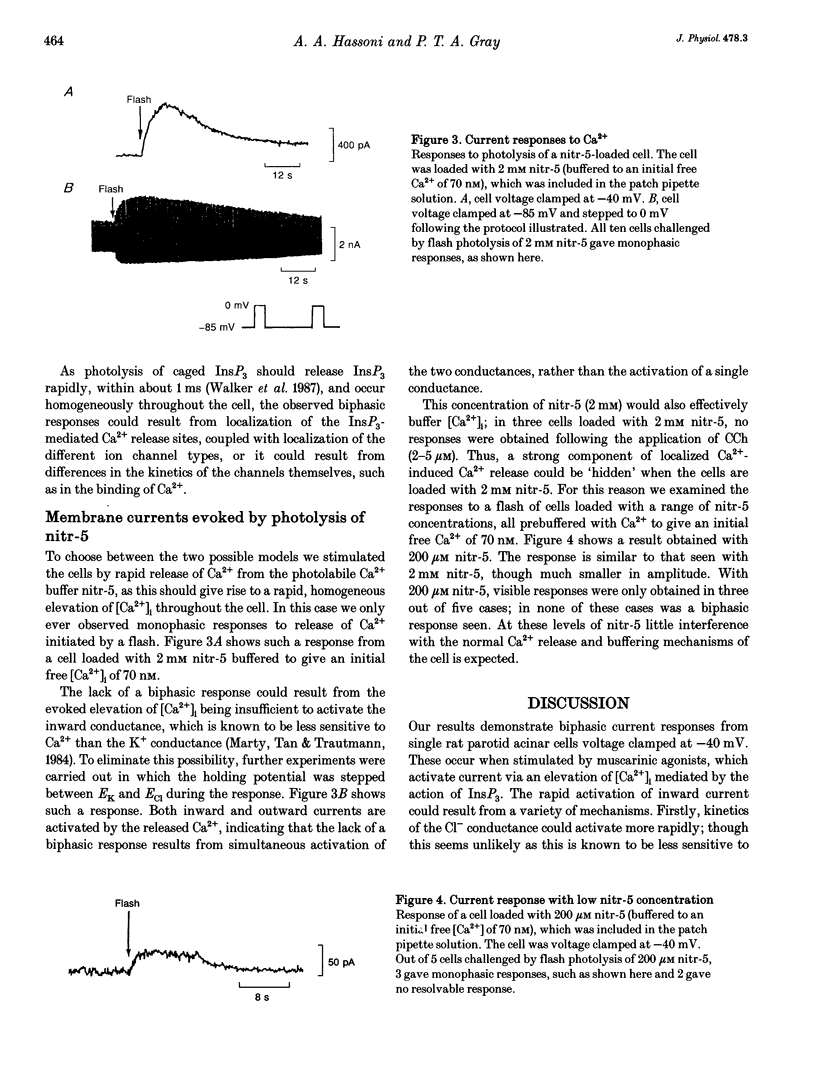
1. The temporal relationship between cytosolic free Ca2+ concentration ([Ca2+]i) and activation of membrane current responses in single rat parotid acinar cells has been examined. Activation of muscarinic receptors by carbachol (CCh) at -40 mV (midway between EK and ECl under our experimental conditions) frequently evoked biphasic current responses, application of 2 microM CCh leading to rapid activation of an inward current followed by a slower outward current. 2. Photochemical release of inositol 1,4,5-trisphosphate (InsP3), from 'caged' InsP3, by a brief near-UV flash, evoked similar biphasic current responses at -40 mV. In contrast, elevation of [Ca2+]i by photolysis of the caged calcium compound nitr-5 at -40 mV activated only monophasic current responses. 3. These results can be explained by a model in which the InsP3-sensitive Ca2+ release sites are localized at the luminal pole of the cell, combined with a relative preponderance of Ca(2+)-activated Cl- channels at that pole, and a relative preponderance of Ca(2+)-activated K+ channels at the basal end.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldys-Waligorska A., Pour A., Moriarty C. M., Dowd F. The effect of calcium and cyclic AMP on amylase release in digitonin-permeabilized parotid gland cells. Biochim Biophys Acta. 1987 Jul 6;929(2):190–196. doi: 10.1016/0167-4889(87)90175-3. [DOI] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett J. K., Gunter-Smith P. J., Melvin J. E., Turner R. J. Physiological localization of an agonist-sensitive pool of Ca2+ in parotid acinar cells. Proc Natl Acad Sci U S A. 1989 Jan;86(1):167–171. doi: 10.1073/pnas.86.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V., Petersen O. H. Electrophysiology of mouse parotid acini: effects of electrical field stimulation and ionophoresis of neurotransmitters. J Physiol. 1980 Aug;305:43–57. doi: 10.1113/jphysiol.1980.sp013348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. T. Oscillations of free cytosolic calcium evoked by cholinergic and catecholaminergic agonists in rat parotid acinar cells. J Physiol. 1988 Dec;406:35–53. doi: 10.1113/jphysiol.1988.sp017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gurney A. M., Tsien R. Y., Lester H. A. Activation of a potassium current by rapid photochemically generated step increases of intracellular calcium in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 May;84(10):3496–3500. doi: 10.1073/pnas.84.10.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Maruyama Y., Matsumoto O., Nishiyama A. Activation of Ca2+-dependent Cl- and K+ conductances in rat and mouse parotid acinar cells. Jpn J Physiol. 1985;35(6):933–944. doi: 10.2170/jjphysiol.35.933. [DOI] [PubMed] [Google Scholar]
- Kasai H., Augustine G. J. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990 Dec 20;348(6303):735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. An inexpensive digital tape recorder suitable for neurophysiological signals. J Neurosci Methods. 1985 Oct;15(1):1–13. doi: 10.1016/0165-0270(85)90057-3. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P. The initiation of calcium release following muscarinic stimulation in rat lacrimal glands. J Physiol. 1989 Dec;419:665–687. doi: 10.1113/jphysiol.1989.sp017892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Rink T. J. Rapid increases in cytosolic free calcium in response to muscarinic stimulation of rat parotid acinar cells. J Biol Chem. 1987 Apr 15;262(11):4958–4960. [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Stimulus-secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J Physiol. 1992 Mar;448:1–51. doi: 10.1113/jphysiol.1992.sp019028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp G., Güth K. A low cost high intensity flash device for photolysis experiments. Pflugers Arch. 1988 Feb;411(2):200–203. doi: 10.1007/BF00582315. [DOI] [PubMed] [Google Scholar]
- Streb H., Bayerdörffer E., Haase W., Irvine R. F., Schulz I. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81(3):241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Tan Y. P., Marty A., Trautmann A. High density of Ca(2+)-dependent K+ and Cl- channels on the luminal membrane of lacrimal acinar cells. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11229–11233. doi: 10.1073/pnas.89.23.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu E. C., Lawrie A. M., Petersen O. H., Gallacher D. V. Spatial and temporal distribution of agonist-evoked cytoplasmic Ca2+ signals in exocrine acinar cells analysed by digital image microscopy. EMBO J. 1992 Apr;11(4):1623–1629. doi: 10.1002/j.1460-2075.1992.tb05208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakui M., Osipchuk Y. V., Petersen O. H. Receptor-activated cytoplasmic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2(+)-induced Ca2+ release. Cell. 1990 Nov 30;63(5):1025–1032. doi: 10.1016/0092-8674(90)90505-9. [DOI] [PubMed] [Google Scholar]
- Walker J. W., Somlyo A. V., Goldman Y. E., Somlyo A. P., Trentham D. R. Kinetics of smooth and skeletal muscle activation by laser pulse photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1987 May 21;327(6119):249–252. doi: 10.1038/327249a0. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Intracellular calibration of the fluorescent calcium indicator Fura-2. Cell Calcium. 1990 Feb-Mar;11(2-3):75–83. doi: 10.1016/0143-4160(90)90061-x. [DOI] [PubMed] [Google Scholar]


