Abstract
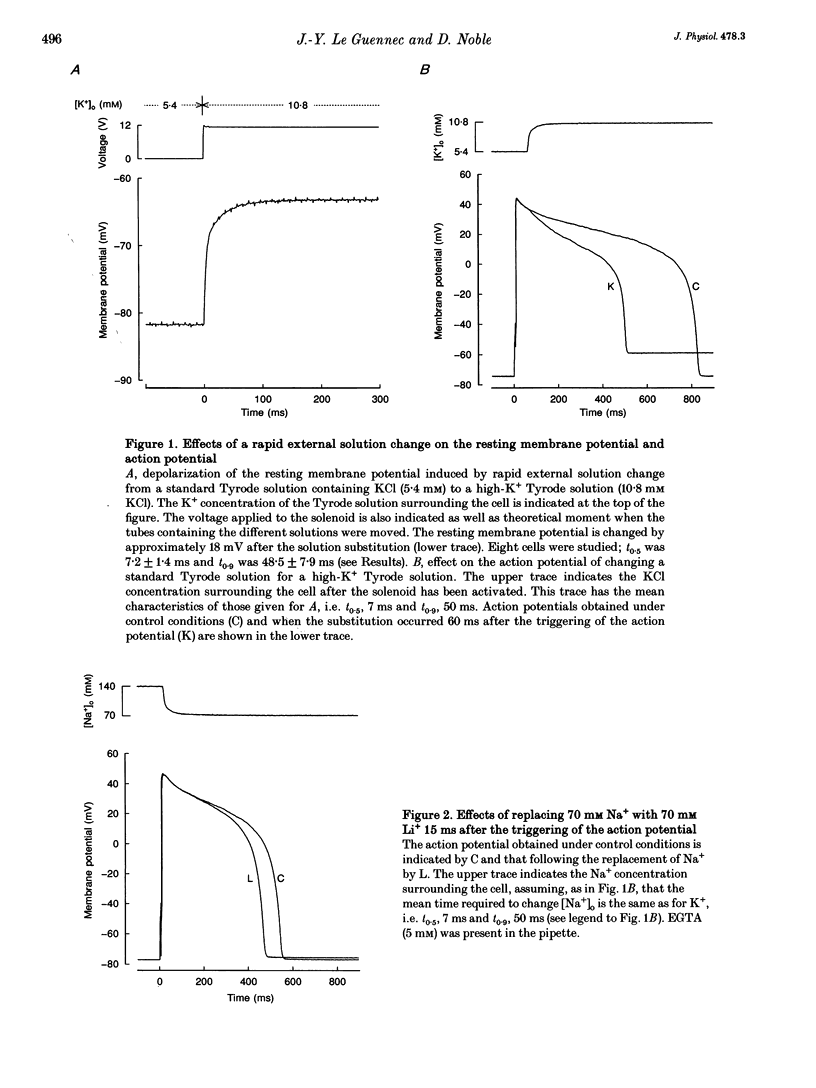
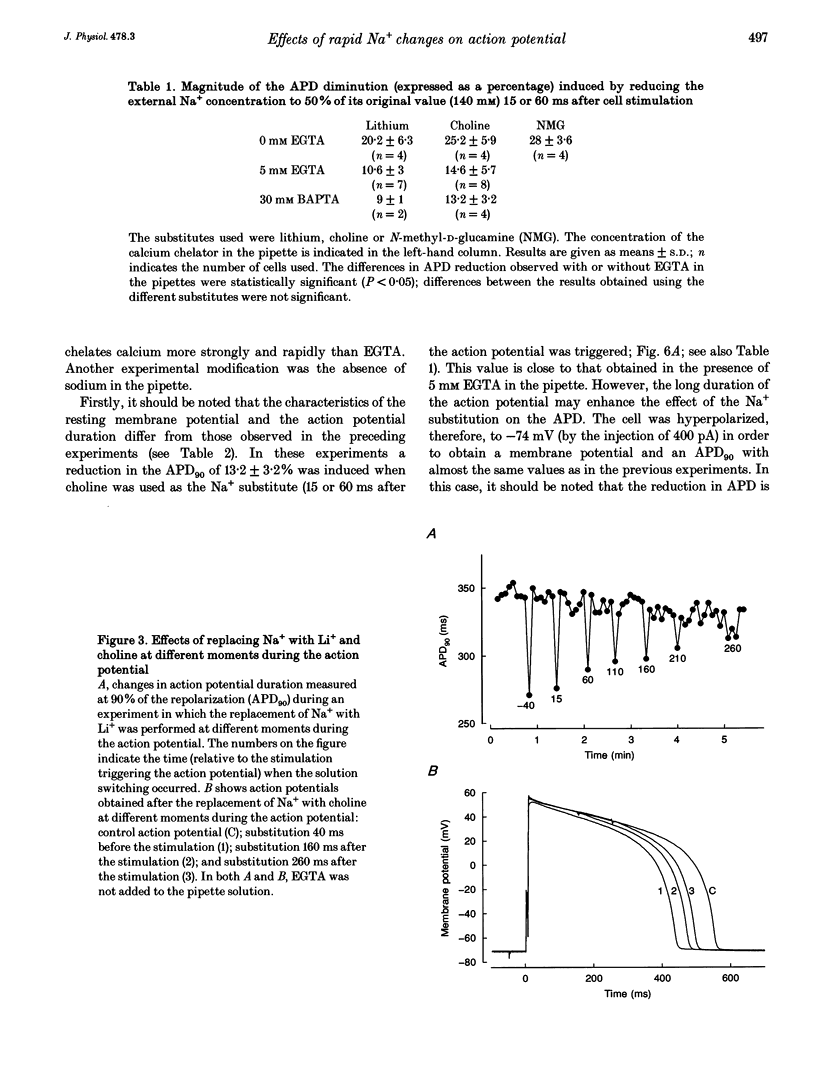
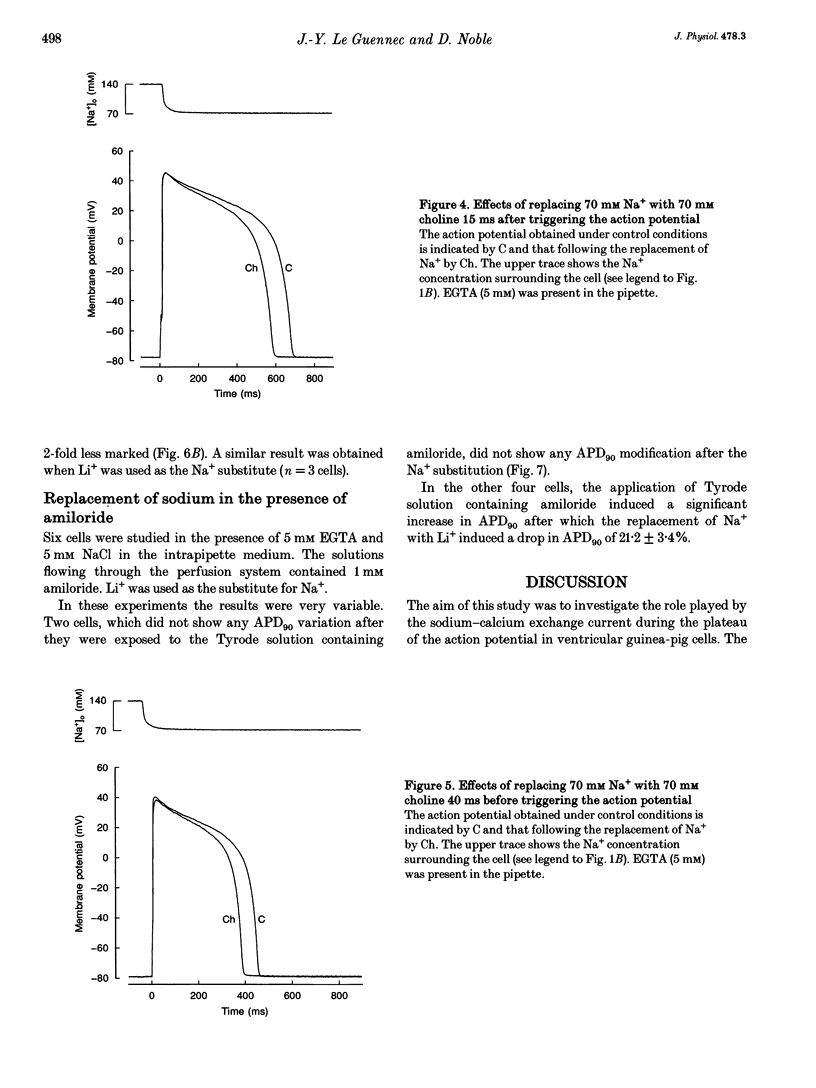
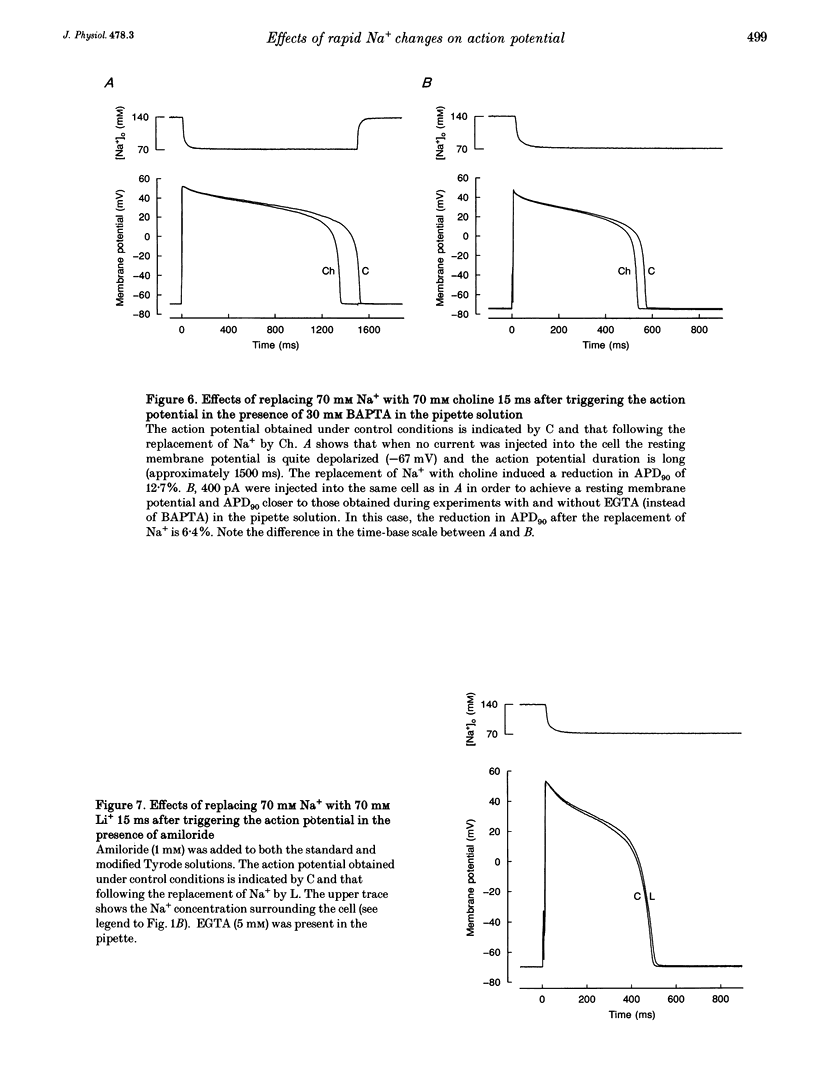
1. A rapid solution-changing system using a solenoid was set up. The half-time for changing the external solution surrounding a ventricular cardiac cell was 7.2 +/- 1.4 ms, whereas the time needed to change 90% of this solution was 48.5 +/- 7.9 ms. This rapid switching system was used to reduce the external sodium concentration at different moments during the action potential (recorded using the whole-cell method) to 50% of its original value. This was performed in order to investigate the effect on the shape and duration of the action potential of modifying the activity of the sodium-calcium exchanger. 2. A diminution of the action potential duration was seen irrespective of the substitute used for reducing the NaCl concentration from 140 to 70 mM. The magnitude of this diminution depended on the presence or absence of EGTA (5 mM) in the pipette solution and also on the moment during the action potential at which the NaCl substitution occurred. 3. Some differences were observed depending on whether the NaCl substitute used was lithium chloride or choline chloride. When choline chloride or N-methyl-D-glucamine was used as the NaCl substitute, the amplitude of the action potential was slightly reduced (by 2-5 mV) when the solution was changed 40 ms before the action potential was triggered. This reduction was never observed when LiCl was used as the NaCl substitute. 4. The effects on the shape of the action potential of changing from a solution containing 140 mM NaCl to one containing 70 mM NaCl and 70 mM LiCl were much more rapid when these changes occurred at a later stage during the action potential. The rate of repolarization was more than doubled when the change occurred at a late stage of the action potential but was hardly changed at the beginning of the plateau. 5. These experiments confirm the role of the sodium-calcium exchange current in determining the duration of the mammalian ventricular action potential. However, it is also possible that the sodium background current plays a significant role in determining the shape of the action potential.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Cohen I., Eisner D., Ohba M., Ojeda C. The steady state TTX-sensitive ("window") sodium current in cardiac Purkinje fibres. Pflugers Arch. 1979 Mar 16;379(2):137–142. doi: 10.1007/BF00586939. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. E. INFLUENCE OF LITHIUM IONS ON THE TRANSMEMBRANE POTENTIAL AND CATION CONTENT OF CARDIAC CELLS. J Gen Physiol. 1964 Jan;47:501–530. doi: 10.1085/jgp.47.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. A fuzzy subsarcolemmal space for intracellular Na+ in cardiac cells? Cardiovasc Res. 1992 May;26(5):433–442. doi: 10.1093/cvr/26.5.433. [DOI] [PubMed] [Google Scholar]
- Doerr T., Denger R., Doerr A., Trautwein W. Ionic currents contributing to the action potential in single ventricular myocytes of the guinea pig studied with action potential clamp. Pflugers Arch. 1990 May;416(3):230–237. doi: 10.1007/BF00392058. [DOI] [PubMed] [Google Scholar]
- Earm Y. E., Ho W. K., So I. S. Inward current generated by Na-Ca exchange during the action potential in single atrial cells of the rabbit. Proc R Soc Lond B Biol Sci. 1990 May 22;240(1297):61–81. doi: 10.1098/rspb.1990.0027. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Spindler A. J., Twist V. W. Sodium-calcium exchange during the action potential in guinea-pig ventricular cells. J Physiol. 1989 Apr;411:639–661. doi: 10.1113/jphysiol.1989.sp017596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre J. F., Findlay I. Action potential duration and activation of ATP-sensitive potassium current in isolated guinea-pig ventricular myocytes. Biochim Biophys Acta. 1990 Nov 2;1029(1):167–172. doi: 10.1016/0005-2736(90)90450-3. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horackova M., Vassort G. Sodium-calcium exchange in regulation of cardiac contractility. Evidence for an electrogenic, voltage-dependent mechanism. J Gen Physiol. 1979 Apr;73(4):403–424. doi: 10.1085/jgp.73.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Uehara A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. J Physiol. 1985 Nov;368:525–544. doi: 10.1113/jphysiol.1985.sp015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosue T., Arita M., Muramatsu H., Spindler A. J., Noble D. Ionic mechanisms of action potential prolongation at low temperature in guinea-pig ventricular myocytes. J Physiol. 1993 Aug;468:85–106. doi: 10.1113/jphysiol.1993.sp019761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosue T., Spindler A. J., Noble S. J., Noble D. Background inward current in ventricular and atrial cells of the guinea-pig. Proc Biol Sci. 1993 Apr 22;252(1333):65–74. doi: 10.1098/rspb.1993.0047. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Calcium-activated inward current and contraction in rat and guinea-pig ventricular myocytes. J Physiol. 1987 Oct;391:545–560. doi: 10.1113/jphysiol.1987.sp016755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. The effects of ryanodine, EGTA and low-sodium on action potentials in rat and guinea-pig ventricular myocytes: evidence for two inward currents during the plateau. Br J Pharmacol. 1984 Mar;81(3):543–550. doi: 10.1111/j.1476-5381.1984.tb10107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y., Kimura J. Sodium-calcium exchange current. Dependence on internal Ca and Na and competitive binding of external Na and Ca. J Gen Physiol. 1989 Jun;93(6):1129–1145. doi: 10.1085/jgp.93.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Niedergerke R., Orkand R. K. The dependence of the action potential of the frog's heart on the external and intracellular sodium concentration. J Physiol. 1966 May;184(2):312–334. doi: 10.1113/jphysiol.1966.sp007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Noble S. J., Bett G. C., Earm Y. E., Ho W. K., So I. K. The role of sodium-calcium exchange during the cardiac action potential. Ann N Y Acad Sci. 1991;639:334–353. doi: 10.1111/j.1749-6632.1991.tb17323.x. [DOI] [PubMed] [Google Scholar]
- Powell T., Twist V. W. A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun. 1976 Sep 7;72(1):327–333. doi: 10.1016/0006-291x(76)90997-9. [DOI] [PubMed] [Google Scholar]
- Schouten V. J., ter Keurs H. E. The slow repolarization phase of the action potential in rat heart. J Physiol. 1985 Mar;360:13–25. doi: 10.1113/jphysiol.1985.sp015601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd N., Kavaler F. Direct control of contraction force of single frog atrial cells by extracellular ions. Am J Physiol. 1986 Nov;251(5 Pt 1):C653–C661. doi: 10.1152/ajpcell.1986.251.5.C653. [DOI] [PubMed] [Google Scholar]
- Spitzer K. W., Bridge J. H. A simple device for rapidly exchanging solution surrounding a single cardiac cell. Am J Physiol. 1989 Feb;256(2 Pt 1):C441–C447. doi: 10.1152/ajpcell.1989.256.2.C441. [DOI] [PubMed] [Google Scholar]
- Terrar D. A., White E. Changes in cytosolic calcium monitored by inward currents during action potentials in guinea-pig ventricular cells. Proc R Soc Lond B Biol Sci. 1989 Nov 22;238(1291):171–188. doi: 10.1098/rspb.1989.0074. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Shortening of the cardiac action potential due to a brief injection of KCl following the onset of activity. J Physiol. 1956 Apr 27;132(1):157–163. doi: 10.1113/jphysiol.1956.sp005510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettwer E., Himmel H., Ravens U. Amiloride derivatives as blockers of Na+/Ca2+ exchange: effects on mechanical and electrical function of guinea-pig myocardium. Pharmacol Toxicol. 1992 Aug;71(2):95–102. doi: 10.1111/j.1600-0773.1992.tb00526.x. [DOI] [PubMed] [Google Scholar]


