Abstract
1. Contractile properties were investigated on single skinned-fibre preparations from rat leg muscles. Following the mechanical measurements, the myosin heavy chain (HC) composition of the same fibre was analysed by gradient gel electrophoresis. 2. Fibres were typed according to their myosin HC isoform composition (HCI, type I; HCIIA, type IIA; HCIID, type IID; HCIIB, type IIB). Many fibres showed the co-existence of two myosin HC isoforms (hybrid fibres). 3. A strong correlation was found between fibre type and time characteristics of stretch-induced delayed force increase (stretch activation) of fully Ca(2+)-activated fibres. 4. The maximal unloaded shortening velocity (Vmax), as measured with the slack test, was lowest in type I fibres. Within the type II group, a continuum of Vmax values was found, with large overlaps of the different fibre types. 5. The results suggest that the kinetics of stretch activation is determined by the myosin HCs whereas unloaded fibre shortening seems to be determined by other myofibrillar proteins in addition to the myosin HCs. Assuming that stretch activation represents certain steps of the cross-bridge turnover under isometric conditions and Vmax reflects cross-bridge detachment under unloaded conditions it can be deduced that different myofibrillar proteins are responsible for different steps within the cross-bridge turnover.
Full text
PDF


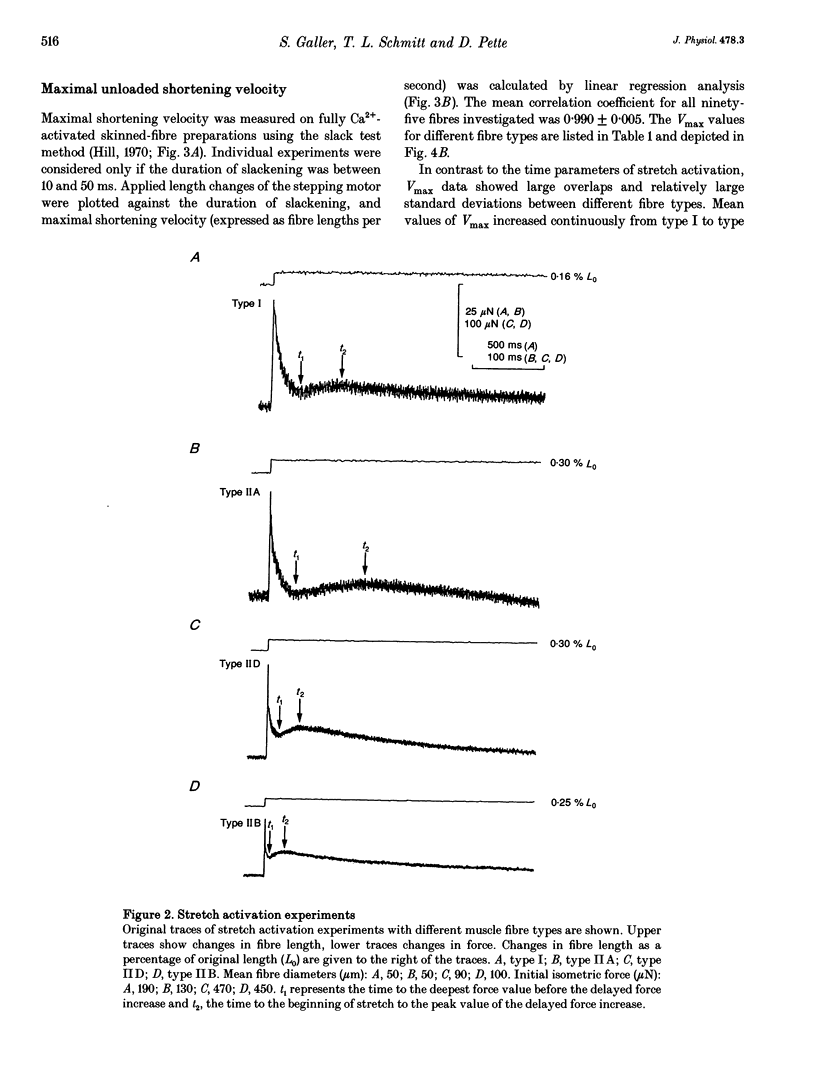
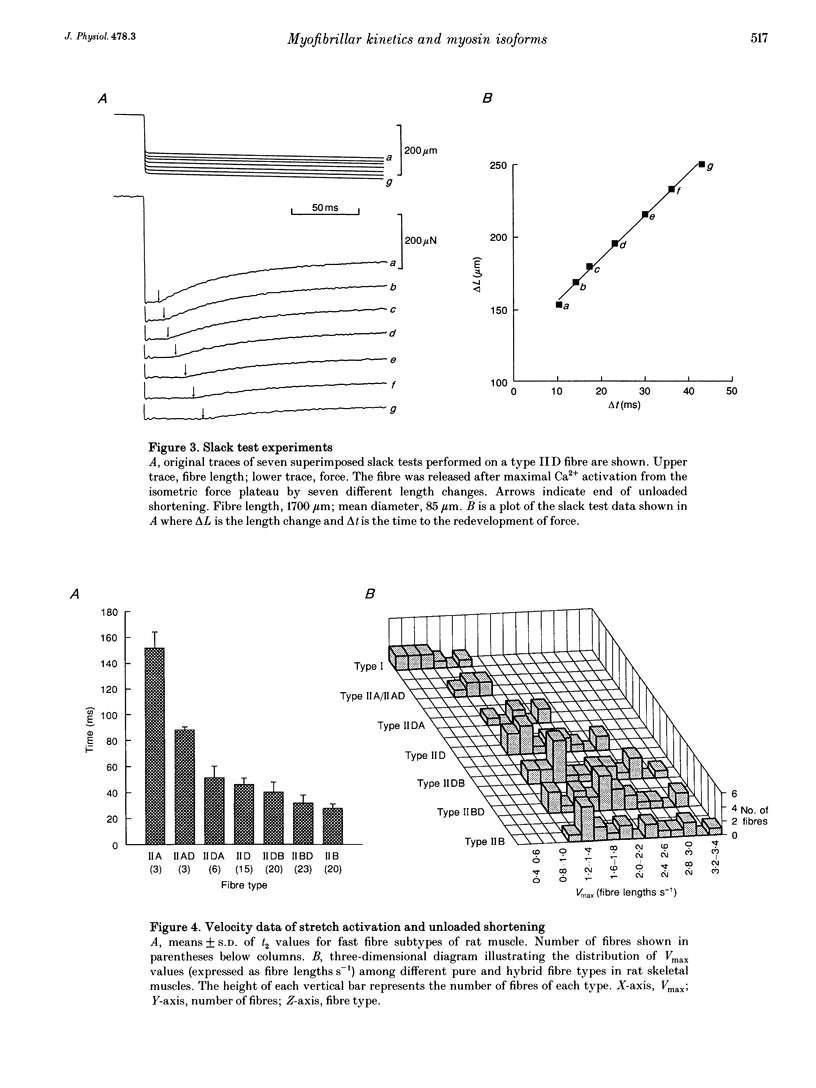




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottinelli R., Schiaffino S., Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991 Jun;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär A., Pette D. Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett. 1988 Aug 1;235(1-2):153–155. doi: 10.1016/0014-5793(88)81253-5. [DOI] [PubMed] [Google Scholar]
- Eddinger T. J., Moss R. L. Mechanical properties of skinned single fibers of identified types from rat diaphragm. Am J Physiol. 1987 Aug;253(2 Pt 1):C210–C218. doi: 10.1152/ajpcell.1987.253.2.C210. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C., Schiaffino S., te Kronnie G. Maximum velocity of shortening related to myosin isoform composition in frog skeletal muscle fibres. J Physiol. 1988 Jan;395:679–694. doi: 10.1113/jphysiol.1988.sp016941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L., Chen Y. Cross-bridge model of muscle contraction. Quantitative analysis. Biophys J. 1980 Feb;29(2):195–227. doi: 10.1016/S0006-3495(80)85126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler S., Hutzler C., Haller T. Effects of taurine on Ca2(+)-dependent force development of skinned muscle fibre preparations. J Exp Biol. 1990 Sep;152:255–264. doi: 10.1242/jeb.152.1.255. [DOI] [PubMed] [Google Scholar]
- Galler S., Rathmayer W. Shortening velocity and force/pCa relationship in skinned crab muscle fibres of different types. Pflugers Arch. 1992 Feb;420(2):187–193. doi: 10.1007/BF00374989. [DOI] [PubMed] [Google Scholar]
- Galler S. Stretch activation of skeletal muscle fibre types. Pflugers Arch. 1994 Jun;427(3-4):384–386. doi: 10.1007/BF00374550. [DOI] [PubMed] [Google Scholar]
- Gorza L. Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem. 1990 Feb;38(2):257–265. doi: 10.1177/38.2.2137154. [DOI] [PubMed] [Google Scholar]
- Greaser M. L., Moss R. L., Reiser P. J. Variations in contractile properties of rabbit single muscle fibres in relation to troponin T isoforms and myosin light chains. J Physiol. 1988 Dec;406:85–98. doi: 10.1113/jphysiol.1988.sp017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Heinl P., Kuhn H. J., Rüegg J. C. Tension responses to quick length changes of glycerinated skeletal muscle fibres from the frog and tortoise. J Physiol. 1974 Mar;237(2):243–258. doi: 10.1113/jphysiol.1974.sp010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen N., Pette D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem. 1993 May;41(5):733–743. doi: 10.1177/41.5.8468455. [DOI] [PubMed] [Google Scholar]
- Larsson L., Edström L., Lindegren B., Gorza L., Schiaffino S. MHC composition and enzyme-histochemical and physiological properties of a novel fast-twitch motor unit type. Am J Physiol. 1991 Jul;261(1 Pt 1):C93–101. doi: 10.1152/ajpcell.1991.261.1.C93. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G., Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol. 1978 Feb;275:241–262. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pette D., Staron R. S. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Greaser M. L., Moss R. L. Myosin heavy chain composition of single cells from avian slow skeletal muscle is strongly correlated with velocity of shortening during development. Dev Biol. 1988 Oct;129(2):400–407. doi: 10.1016/0012-1606(88)90387-9. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985 Aug 5;260(16):9077–9080. [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Ausoni S., Vianello M., Gundersen K., Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989 Jun;10(3):197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Squire J. M. Muscle filament lattices and stretch-activation: the match-mismatch model reassessed. J Muscle Res Cell Motil. 1992 Apr;13(2):183–189. doi: 10.1007/BF01874155. [DOI] [PubMed] [Google Scholar]
- Staron R. S., Pette D. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in rabbit muscle fibers. Histochemistry. 1986;86(1):19–23. doi: 10.1007/BF00492341. [DOI] [PubMed] [Google Scholar]
- Steiger J. G., Abbott R. H. Biochemical interpretation of tension transients produced by a four-state mechanical model. J Muscle Res Cell Motil. 1981 Sep;2(3):245–260. doi: 10.1007/BF00713264. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Sréter F. A., Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem. 1988 Jun 25;263(18):9034–9039. [PubMed] [Google Scholar]
- Termin A., Staron R. S., Pette D. Myosin heavy chain isoforms in histochemically defined fiber types of rat muscle. Histochemistry. 1989;92(6):453–457. doi: 10.1007/BF00524756. [DOI] [PubMed] [Google Scholar]
- Wada M., Pette D. Relationships between alkali light-chain complement and myosin heavy-chain isoforms in single fast-twitch fibers of rat and rabbit. Eur J Biochem. 1993 May 15;214(1):157–161. doi: 10.1111/j.1432-1033.1993.tb17908.x. [DOI] [PubMed] [Google Scholar]



