Abstract
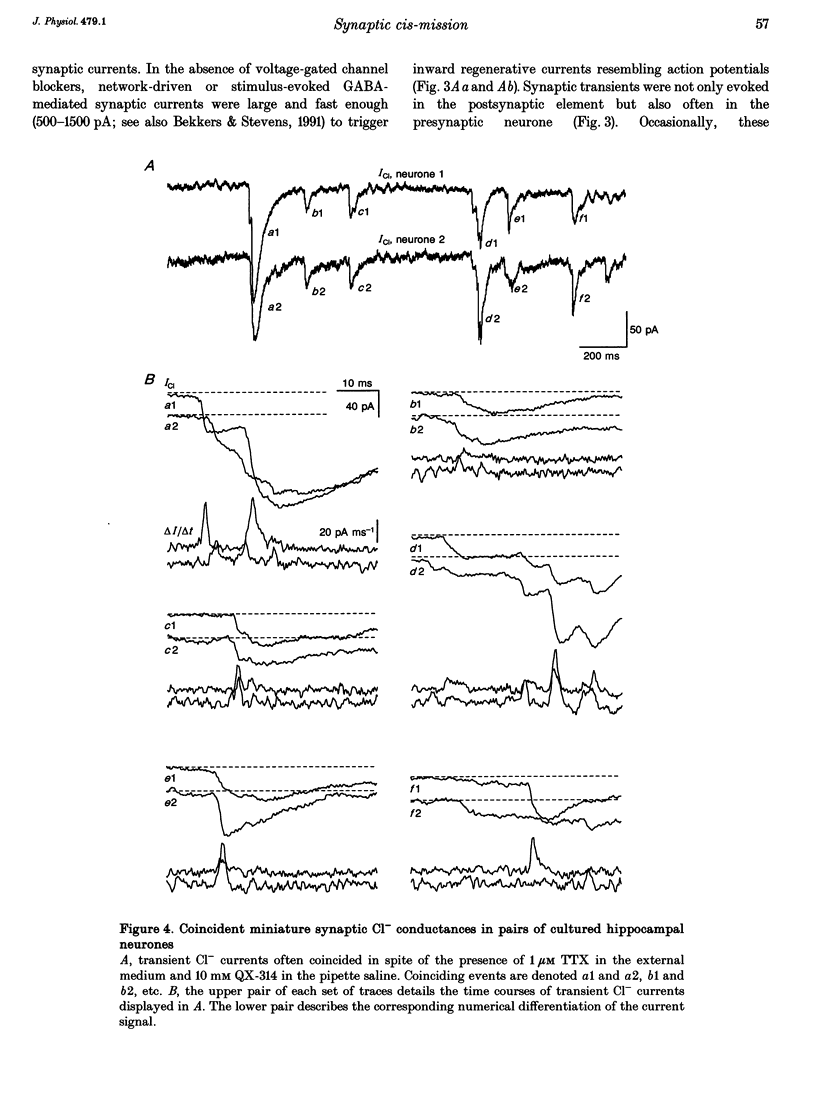
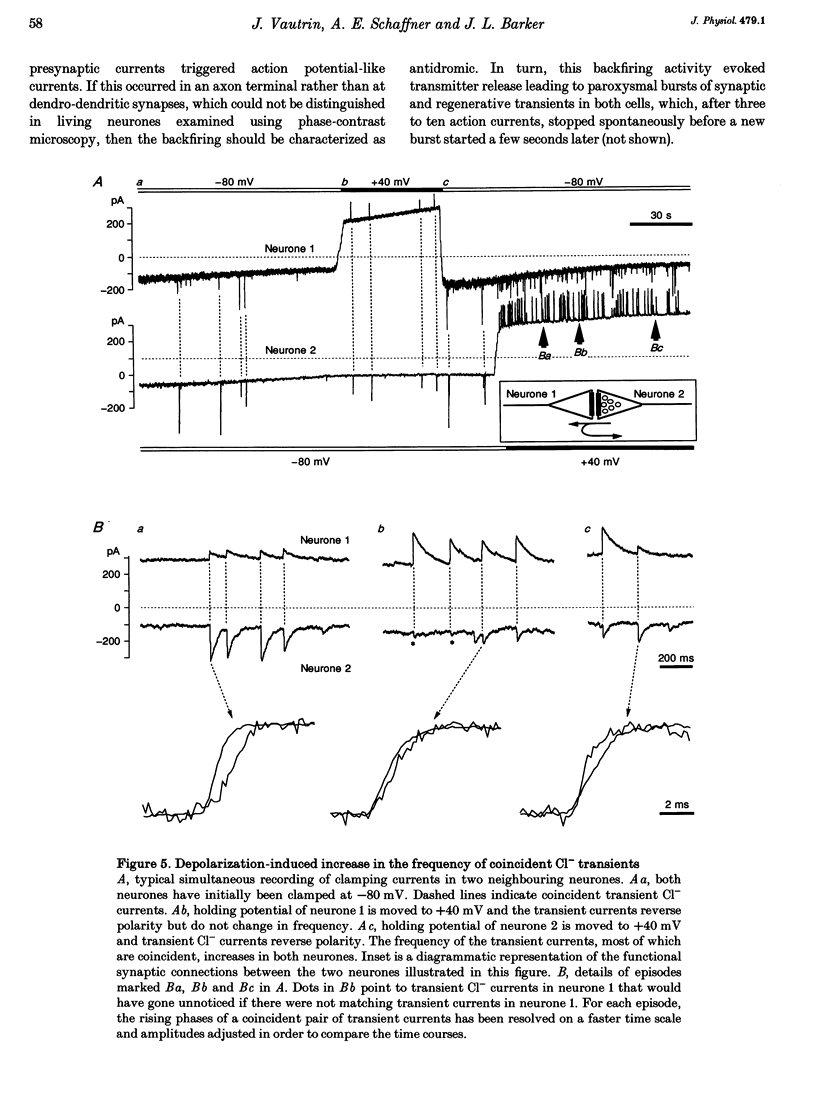
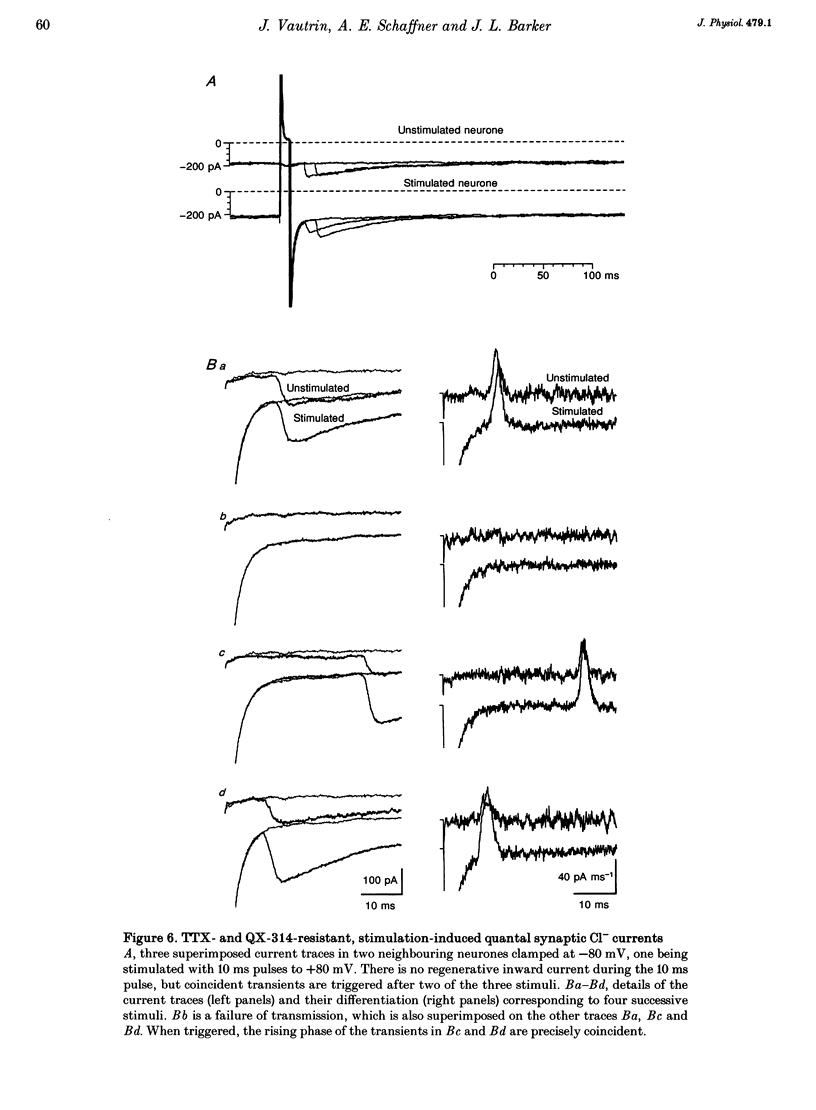
1. Hippocampal neurones cultured from the 18-day-old embryonic rat for 3 days to 3 weeks were recorded with Cl(-)-filled patch pipettes. Spontaneous synaptic currents, which reversed at the equilibrium potential for Cl- ions (ECl) and were blocked by the GABAA (gamma-aminobutyric acid) receptor antagonists bicuculline or picrotoxin, were recorded in every culture. At 25 degrees C and -80 mV they decayed with a time constant > or = 20 ms that invariably increased at positive potentials. After 2 weeks, 50-75% of all neurones were GABA immunoreactive. 2. In pairs-recordings, coincident synaptic currents in both cells were either spontaneous or evoked by stimulation of one cell. In the presence of tetrodotoxin and using pipettes containing lidocaine (lignocaine) N-ethyl bromide, coincident spontaneous Cl- transients still occurred in both neurones far more frequently than expected by chance. 3. Holding the potential of one neurone at a positive value reversed the synaptic transients in that cell and, in half of the cells, increased the frequency of coincident events in both cells. 4. In neurones where depolarization increased the frequency of coinciding events and all regenerative current apparent at the soma was abolished, short depolarizing pulses occasionally evoked all-or-none, pre- and postsynaptic currents with matching transmission failures and identical delays in transmission. 5. The results suggest that the same pulse of GABA simultaneously activates GABAA receptor-coupled Cl- channels on both sides of the same synaptic cleft, producing immediate auto-transmission in the absence of collaterals or interneurones.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. A., Grünert U. Three-dimensional structure of bidirectional, excitatory chemical synapses in the jellyfish Cyanea capillata. Synapse. 1988;2(6):606–613. doi: 10.1002/syn.890020605. [DOI] [PubMed] [Google Scholar]
- BIRKS R., HUXLEY H. E., KATZ B. The fine structure of the neuromuscular junction of the frog. J Physiol. 1960 Jan;150:134–144. doi: 10.1113/jphysiol.1960.sp006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Harrison N. L. Outward rectification of inhibitory postsynaptic currents in cultured rat hippocampal neurones. J Physiol. 1988 Sep;403:41–55. doi: 10.1113/jphysiol.1988.sp017237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7834–7838. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUTEAUX R. Morphological and cytochemical observations on the post-synaptic membrane at motor end-plates and ganglionic synapses. Exp Cell Res. 1958;14(Suppl 5):294–322. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Local activity at a depolarized nerve-muscle junction. J Physiol. 1955 May 27;128(2):396–411. doi: 10.1113/jphysiol.1955.sp005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y., Poo M. M. Quantal transmitter secretion from myocytes loaded with acetylcholine. Nature. 1992 Oct 22;359(6397):733–736. doi: 10.1038/359733a0. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erxleben C., Kriebel M. E. Subunit composition of the spontaneous miniature end-plate currents at the mouse neuromuscular junction. J Physiol. 1988 Jun;400:659–676. doi: 10.1113/jphysiol.1988.sp017142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Floran B., Silva I., Nava C., Aceves J. Presynaptic modulation of the release of GABA by GABAA receptors in pars compacta and by GABAB receptors in pars reticulata of the rat substantia nigra. Eur J Pharmacol. 1988 Jun 10;150(3):277–286. doi: 10.1016/0014-2999(88)90008-8. [DOI] [PubMed] [Google Scholar]
- HORRIDGE G. A., CHAPMAN D. M., MACKAY B. Naked axons and symmetrical synapses in an elementary nervous system. Nature. 1962 Mar 3;193:899–900. doi: 10.1038/193899a0. [DOI] [PubMed] [Google Scholar]
- Kono K. Symmetrical axo-axonic synapses in the axon cap of the goldfish Mauthner cell. Brain Res. 1970 Oct 13;23(2):255–258. doi: 10.1016/0006-8993(70)90045-4. [DOI] [PubMed] [Google Scholar]
- Llano I., Gerschenfeld H. M. Inhibitory synaptic currents in stellate cells of rat cerebellar slices. J Physiol. 1993 Aug;468:177–200. doi: 10.1113/jphysiol.1993.sp019766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y. Y., Frank E. Activation of GABAA receptors causes presynaptic and postsynaptic inhibition at synapses between muscle spindle afferents and motoneurons in the spinal cord of bullfrogs. J Neurosci. 1989 May;9(5):1516–1522. doi: 10.1523/JNEUROSCI.09-05-01516.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. L., Powell T. P. The morphology of the granule cells of the olfactory bulb. J Cell Sci. 1970 Jul;7(1):91–123. doi: 10.1242/jcs.7.1.91. [DOI] [PubMed] [Google Scholar]
- Richards J. G., Schoch P., Häring P., Takacs B., Möhler H. Resolving GABAA/benzodiazepine receptors: cellular and subcellular localization in the CNS with monoclonal antibodies. J Neurosci. 1987 Jun;7(6):1866–1886. doi: 10.1523/JNEUROSCI.07-06-01866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropert N., Miles R., Korn H. Characteristics of miniature inhibitory postsynaptic currents in CA1 pyramidal neurones of rat hippocampus. J Physiol. 1990 Sep;428:707–722. doi: 10.1113/jphysiol.1990.sp018236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A. E., Behar T., Nadi S., Smallwood V., Barker J. L. Quantitative analysis of transient GABA expression in embryonic and early postnatal rat spinal cord neurons. Brain Res Dev Brain Res. 1993 Apr 16;72(2):265–276. doi: 10.1016/0165-3806(93)90192-d. [DOI] [PubMed] [Google Scholar]
- Segal M. M. Epileptiform activity in microcultures containing one excitatory hippocampal neuron. J Neurophysiol. 1991 Apr;65(4):761–770. doi: 10.1152/jn.1991.65.4.761. [DOI] [PubMed] [Google Scholar]
- Stasheff S. F., Mott D. D., Wilson W. A. Axon terminal hyperexcitability associated with epileptogenesis in vitro. II. Pharmacological regulation by NMDA and GABAA receptors. J Neurophysiol. 1993 Sep;70(3):976–984. doi: 10.1152/jn.1993.70.3.976. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Capogna M., Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993 Jun;16(6):222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- Van der Loos H., Glaser E. M. Autapses in neocortex cerebri: synapses between a pyramidal cell's axon and its own dendrites. Brain Res. 1972 Dec 24;48:355–360. doi: 10.1016/0006-8993(72)90189-8. [DOI] [PubMed] [Google Scholar]
- Vautrin J., Kriebel M. E. Characteristics of slow-miniature endplate currents show a subunit composition. Neuroscience. 1991;41(1):71–88. doi: 10.1016/0306-4522(91)90201-x. [DOI] [PubMed] [Google Scholar]
- Vautrin J., Schaffner A. E., Baker J. L. Two classes of spontaneous GABA-mediated miniature synaptic currents in cultured rat hippocampal neurons. Neurosci Lett. 1992 Apr 13;138(1):67–71. doi: 10.1016/0304-3940(92)90474-l. [DOI] [PubMed] [Google Scholar]
- Vautrin J., Schaffner A. E., Barker J. L. Quantal and subquantal GABAergic transmissions in cultured rat hippocampal neurons. Hippocampus. 1993 Jan;3(1):93–101. doi: 10.1002/hipo.450030110. [DOI] [PubMed] [Google Scholar]
- Wilcox K. S., Dichter M. A. Paired pulse depression in cultured hippocampal neurons is due to a presynaptic mechanism independent of GABAB autoreceptor activation. J Neurosci. 1994 Mar;14(3 Pt 2):1775–1788. doi: 10.1523/JNEUROSCI.14-03-01775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. J., Jackson M. B. GABA-activated chloride channels in secretory nerve endings. Science. 1993 Jan 22;259(5094):531–534. doi: 10.1126/science.8380942. [DOI] [PubMed] [Google Scholar]