Abstract
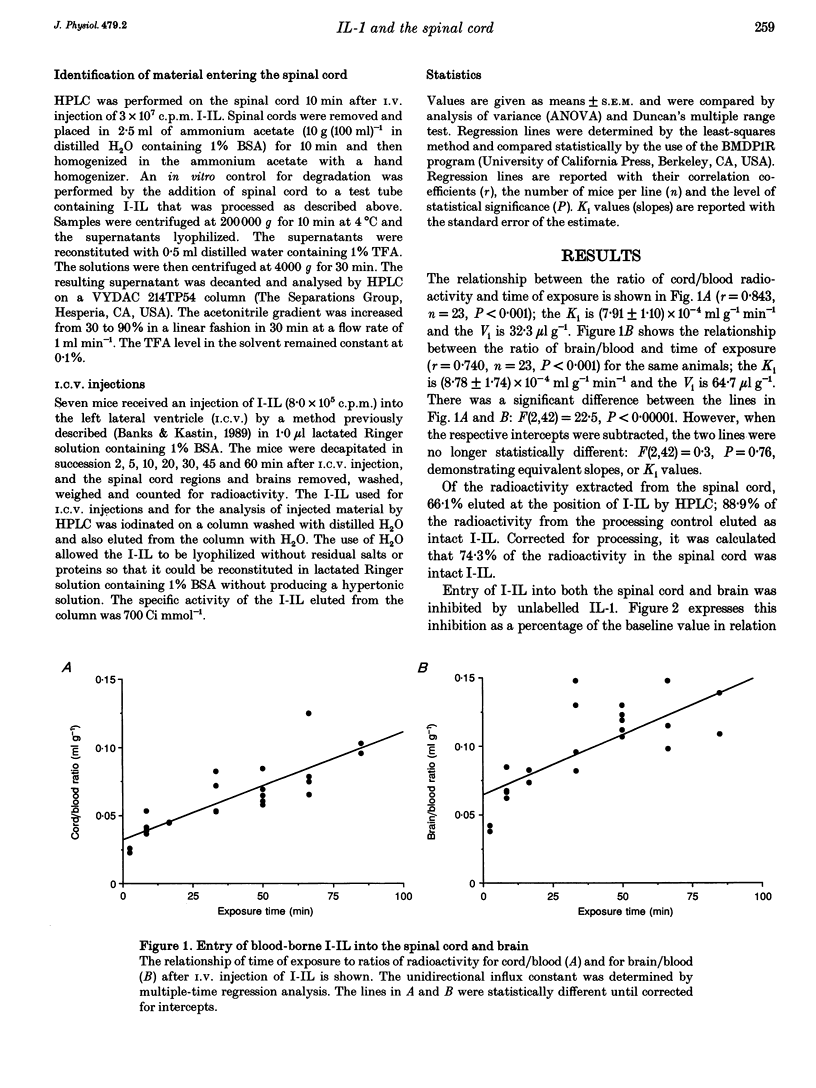
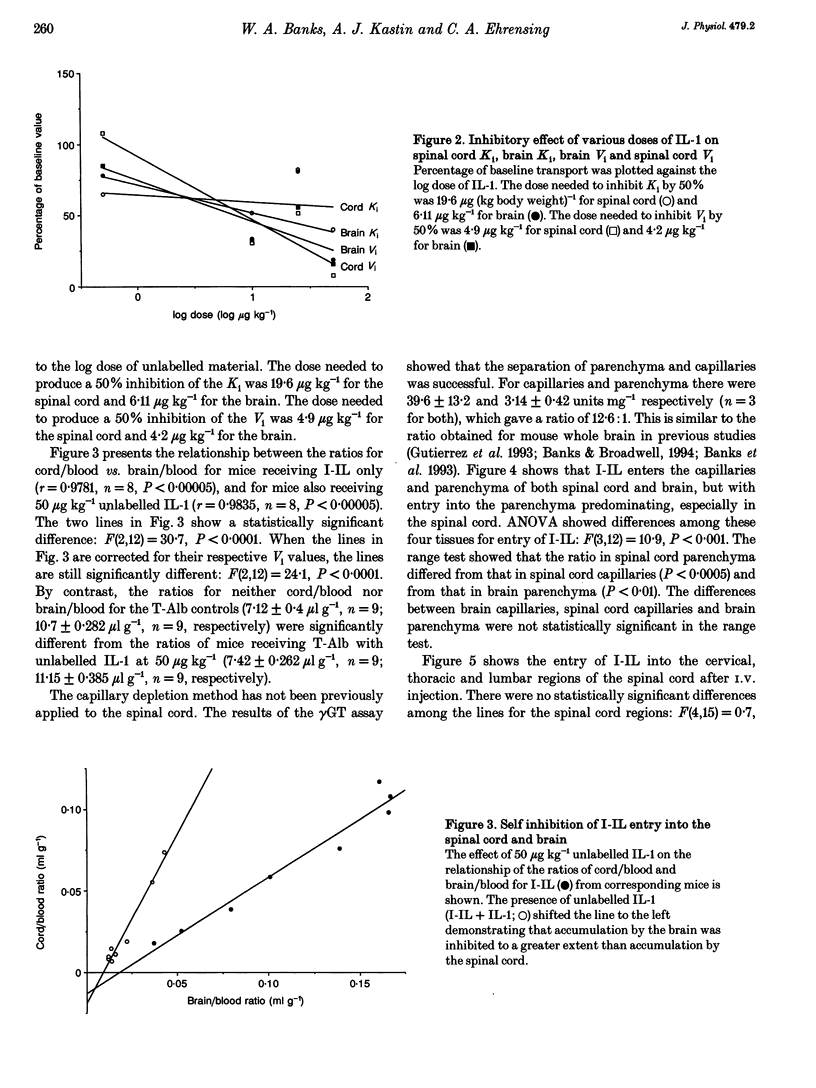
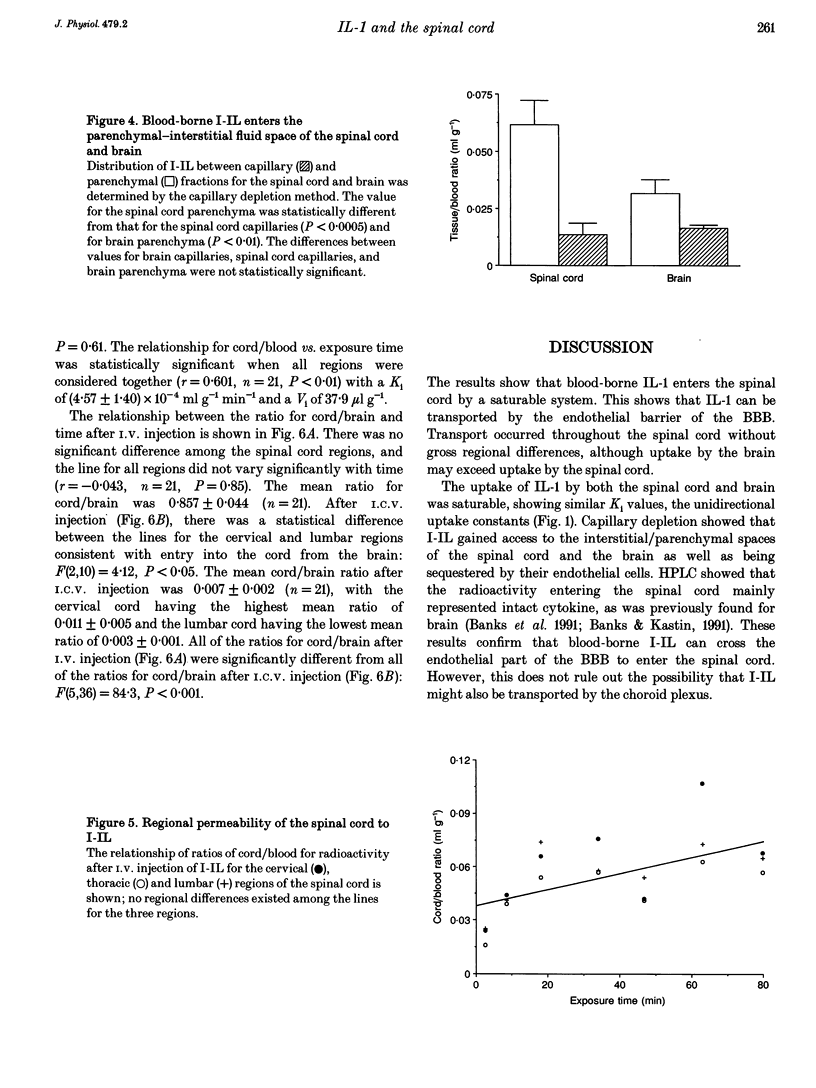
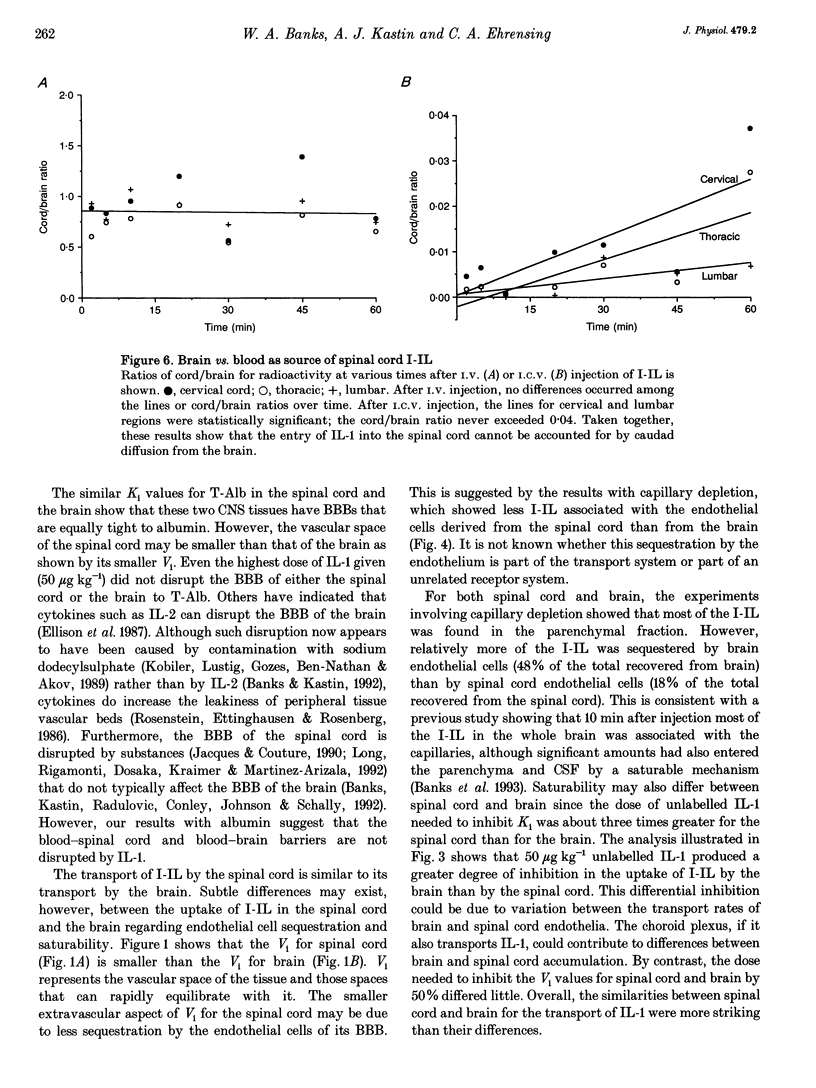
1. Previous work has shown that one mechanism by which blood-borne interleukin-1 alpha (IL-1) may be able to affect the central nervous system (CNS) is by direct transport into the brain across the blood-brain barrier (BBB). The BBB of the brain consists of endothelial (between blood and interstitial fluid) and ependymal (between blood and cerebrospinal fluid) barriers. Which of these barriers IL-1 can cross has not previously been investigated. At the spinal cord, which could be the site of action for some of the effects of IL-1 such as analgesia, the BBB consists only of the endothelial barrier. 2. We show here that IL-1 labelled with 125I (I-IL) is transported across the BBB of the spinal cord by a saturable system similar to the one previously described for the brain. High performance liquid chromatography (HPLC) showed that most of the material entering the spinal cord represented intact I-IL. The BBB of the spinal cord was no more leaky to radioactively labelled albumin than the BBB of the brain and was not disrupted by 50 micrograms kg-1 of IL-1. 3. Capillary depletion showed that most of the I-IL entered the parenchymal-interstitial fluid space of the spinal cord with only a modest amount being sequestered by the endothelial cells of its BBB. 4. I-IL entered the cervical, thoracic and lumbar regions of the spinal cord equally well. I-IL entering at the brain and diffusing caudally was estimated only to account for about 1% of the total radioactivity found in the spinal cord after i.v. injection.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks W. A., Broadwell R. D. Blood to brain and brain to blood passage of native horseradish peroxidase, wheat germ agglutinin, and albumin: pharmacokinetic and morphological assessments. J Neurochem. 1994 Jun;62(6):2404–2419. doi: 10.1046/j.1471-4159.1994.62062404.x. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J. Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci. 1991;48(25):PL117–PL121. doi: 10.1016/0024-3205(91)90385-o. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Durham D. A. Bidirectional transport of interleukin-1 alpha across the blood-brain barrier. Brain Res Bull. 1989 Dec;23(6):433–437. doi: 10.1016/0361-9230(89)90185-8. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Gutierrez E. G. Interleukin-1 alpha in blood has direct access to cortical brain cells. Neurosci Lett. 1993 Nov 26;163(1):41–44. doi: 10.1016/0304-3940(93)90224-9. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J. Quantifying carrier-mediated transport of peptides from the brain to the blood. Methods Enzymol. 1989;168:652–660. doi: 10.1016/0076-6879(89)68047-0. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J., Radulovic S., Conley F. K., Johnson D. L., Schally A. V. Selective uptake of the somatostatin analog RC-160 across the blood-brain tumor barrier of mice with KHT sarcomas. Anticancer Drugs. 1992 Oct;3(5):519–523. doi: 10.1097/00001813-199210000-00012. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Kastin A. J. The interleukins-1 alpha, -1 beta, and -2 do not acutely disrupt the murine blood-brain barrier. Int J Immunopharmacol. 1992 May;14(4):629–636. doi: 10.1016/0192-0561(92)90124-4. [DOI] [PubMed] [Google Scholar]
- Banks W. A., Ortiz L., Plotkin S. R., Kastin A. J. Human interleukin (IL) 1 alpha, murine IL-1 alpha and murine IL-1 beta are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther. 1991 Dec;259(3):988–996. [PubMed] [Google Scholar]
- Breder C. D., Dinarello C. A., Saper C. B. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988 Apr 15;240(4850):321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- Butter C., Baker D., O'Neill J. K., Turk J. L. Mononuclear cell trafficking and plasma protein extravasation into the CNS during chronic relapsing experimental allergic encephalomyelitis in Biozzi AB/H mice. J Neurol Sci. 1991 Jul;104(1):9–12. doi: 10.1016/0022-510x(91)90209-p. [DOI] [PubMed] [Google Scholar]
- Ellison M. D., Povlishock J. T., Merchant R. E. Blood-brain barrier dysfunction in cats following recombinant interleukin-2 infusion. Cancer Res. 1987 Nov 1;47(21):5765–5770. [PubMed] [Google Scholar]
- GRUNDY H. F. Circulation of cerebrospinal fluid in the spinal region of the cat. J Physiol. 1962 Oct;163:457–465. doi: 10.1113/jphysiol.1962.sp006989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E. G., Banks W. A., Kastin A. J. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993 Sep;47(2):169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- Hashimoto M., Ishikawa Y., Yokota S., Goto F., Bando T., Sakakibara Y., Iriki M. Action site of circulating interleukin-1 on the rabbit brain. Brain Res. 1991 Feb 1;540(1-2):217–223. doi: 10.1016/0006-8993(91)90510-3. [DOI] [PubMed] [Google Scholar]
- Jacobs C. A., Baker P. E., Roux E. R., Picha K. S., Toivola B., Waugh S., Kennedy M. K. Experimental autoimmune encephalomyelitis is exacerbated by IL-1 alpha and suppressed by soluble IL-1 receptor. J Immunol. 1991 May 1;146(9):2983–2989. [PubMed] [Google Scholar]
- Jacques L., Couture R. Studies on the vascular permeability induced by intrathecal substance P and bradykinin in the rat. Eur J Pharmacol. 1990 Aug 2;184(1):9–20. doi: 10.1016/0014-2999(90)90662-p. [DOI] [PubMed] [Google Scholar]
- Katsuura G., Arimura A., Koves K., Gottschall P. E. Involvement of organum vasculosum of lamina terminalis and preoptic area in interleukin 1 beta-induced ACTH release. Am J Physiol. 1990 Jan;258(1 Pt 1):E163–E171. doi: 10.1152/ajpendo.1990.258.1.E163. [DOI] [PubMed] [Google Scholar]
- Kennedy M. K., Torrance D. S., Picha K. S., Mohler K. M. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992 Oct 1;149(7):2496–2505. [PubMed] [Google Scholar]
- Kobiler D., Lustig S., Gozes Y., Ben-Nathan D., Akov Y. Sodium dodecylsulphate induces a breach in the blood-brain barrier and enables a West Nile virus variant to penetrate into mouse brain. Brain Res. 1989 Sep 4;496(1-2):314–316. doi: 10.1016/0006-8993(89)91079-2. [DOI] [PubMed] [Google Scholar]
- Long J. B., Rigamonti D. D., Dosaka K., Kraimer J. M., Martinez-Arizala A. Somatostatin causes vasoconstriction, reduces blood flow and increases vascular permeability in the rat central nervous system. J Pharmacol Exp Ther. 1992 Mar;260(3):1425–1432. [PubMed] [Google Scholar]
- Nakamura H., Nakanishi K., Kita A., Kadokawa T. Interleukin-1 induces analgesia in mice by a central action. Eur J Pharmacol. 1988 Apr 27;149(1-2):49–54. doi: 10.1016/0014-2999(88)90040-4. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Blasberg R. G., Fenstermacher J. D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983 Mar;3(1):1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán C. R. Immunoregulators in the nervous system. Neurosci Biobehav Rev. 1991 Summer;15(2):185–215. doi: 10.1016/s0149-7634(05)80001-6. [DOI] [PubMed] [Google Scholar]
- Rosenstein M., Ettinghausen S. E., Rosenberg S. A. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986 Sep 1;137(5):1735–1742. [PubMed] [Google Scholar]
- Sharief M. K., Noori M. A., Ciardi M., Cirelli A., Thompson E. J. Increased levels of circulating ICAM-1 in serum and cerebrospinal fluid of patients with active multiple sclerosis. Correlation with TNF-alpha and blood-brain barrier damage. J Neuroimmunol. 1993 Mar;43(1-2):15–21. doi: 10.1016/0165-5728(93)90070-f. [DOI] [PubMed] [Google Scholar]
- Sirko S., Bishai I., Coceani F. Prostaglandin formation in the hypothalamus in vivo: effect of pyrogens. Am J Physiol. 1989 Mar;256(3 Pt 2):R616–R624. doi: 10.1152/ajpregu.1989.256.3.R616. [DOI] [PubMed] [Google Scholar]
- Triguero D., Buciak J., Pardridge W. M. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem. 1990 Jun;54(6):1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]


