Abstract
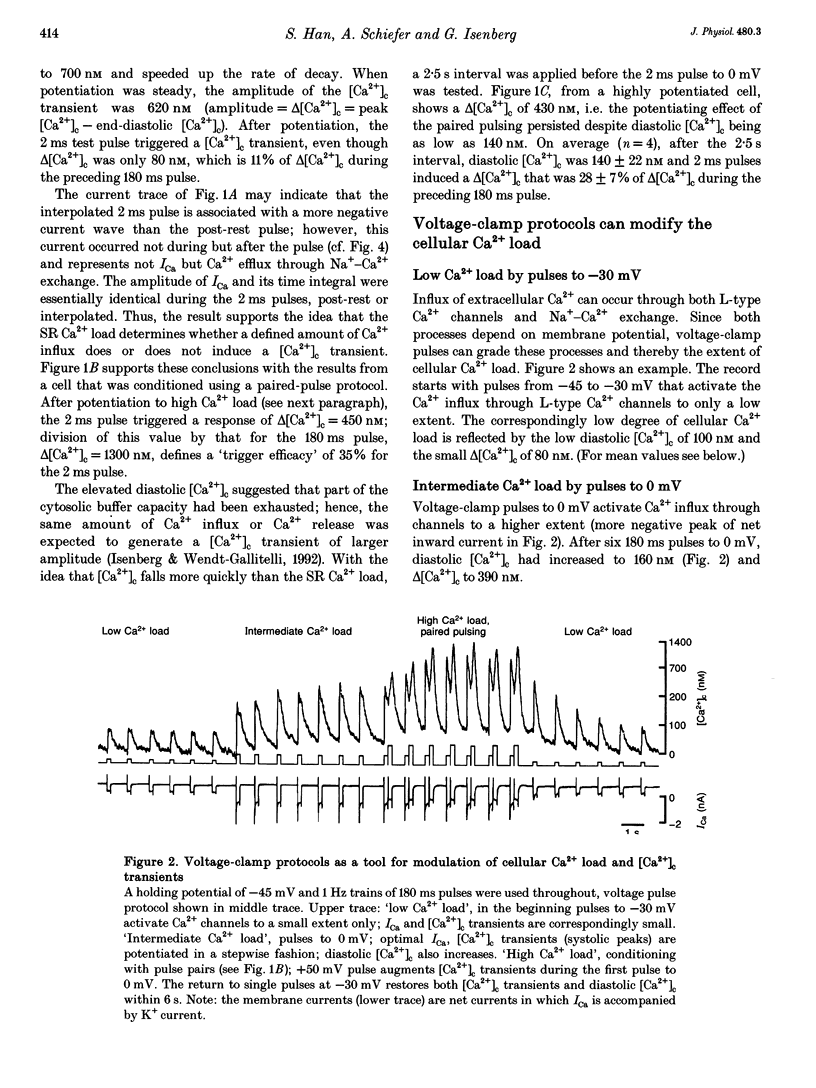
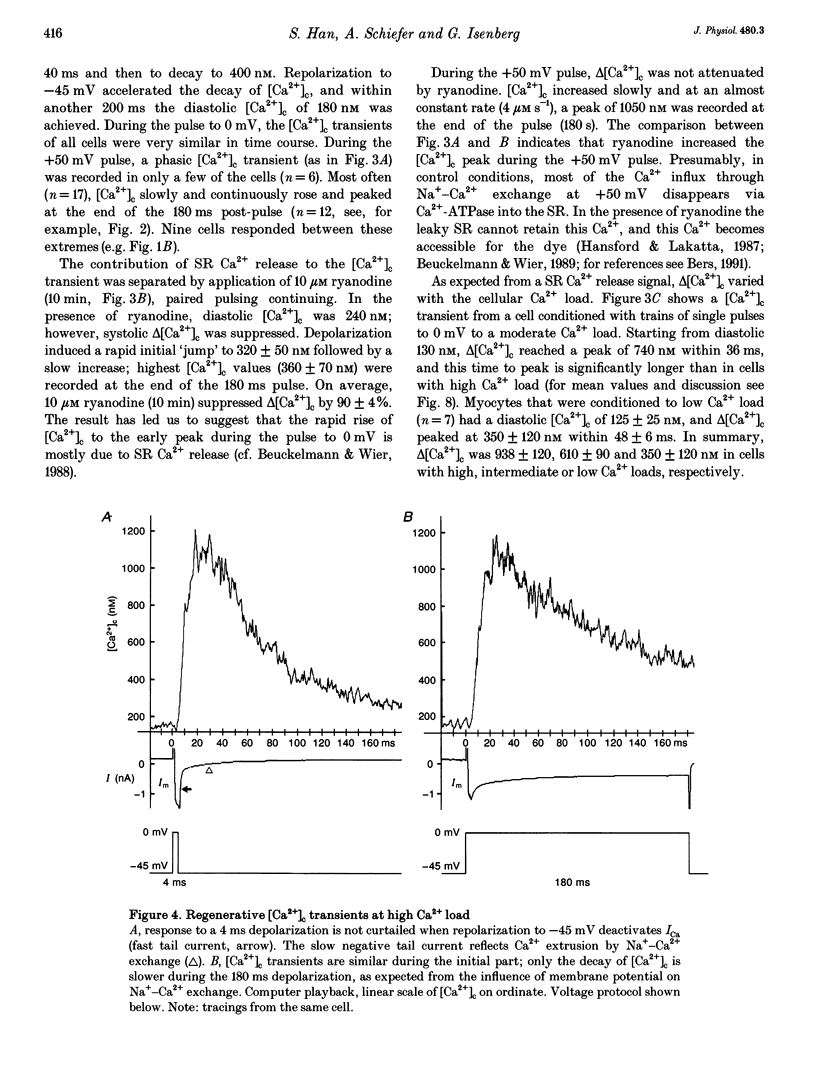
1. In guinea-pig ventricular cells, the concentration of ionized cytosolic calcium ([Ca2+]o) was estimated from the fluorescence of 100 microM K5-indo-1. At 36 degrees C and 2 mM [Ca2+]o, the Ca2+ load of the cells was varied by 1 Hz trains of conditioning clamp pulses to -30 mV (low Ca2+ load), 0 mV (intermediate Ca2+ load) and paired pulses (high Ca2+ load). After seven pulses potentiation was steady and short test pulses to 0 mV were tested for their efficacy in triggering [Ca2+]c transients. The influx of trigger Ca2+ was graded by varying the test-pulse duration between 1 and 180 ms. 2. After a 3 min rest period, [Ca2+]c was 100 +/- 20 nM (mean +/- S.E.M.) and 2 ms test pulses were unable to induce [Ca2+]c transients. Test pulses of 2 ms duration, however, induced [Ca2+]c transients after potentiation with single or paired pulses. 3. At high cellular Ca2+ load, the amplitude of the [Ca2+]c transients (delta[Ca2+]c) gradually increased with pulse durations up to 8 ms. Pulse durations between 8 and 160 ms, however, did not further increase delta[Ca2+]c as if the largest part of the [Ca2+]c transient was due to regenerative contribution of Ca(2+)-induced Ca2+ release. 4. Pulses of 160 ms duration induced 'saturating' responses whose amplitudes delta[Ca2+]c, t = infinity decreased from 938 +/- 120 nM at high Ca2+ load, to 610 +/- 90 and 350 +/- 120 nM at intermediate and low Ca2+ loads, respectively. 5. Delta[Ca2+]c was more sensitive to the duration of Ca2+ influx at low or intermediate Ca2+ loads than at high Ca2+ load. When delta[Ca2+]c was plotted against the test-pulse duration, 50% of delta[Ca2+]c, t = infinity was found to be at 9 +/- 2 ms (low), 4.6 +/- 1 ms (intermediate) or 1.8 +/- 0.5 ms pulses (high Ca2+ load). Correspondingly, the efficacy of 2 ms test pulses in triggering [Ca2+]c transients increased with the Ca2+ load. 6. At high Ca2+ load, [Ca2+]c peaked nearly independently of pulse duration at 19 +/- 3 ms. At intermediate or low Ca2+ load, time to peak increased with pulse duration. 7. The results confirm the theory that sarcoplasmic reticulum (SR) Ca2+ release contributes an amount to the [Ca2+]c transient that increases with the cellular Ca2+ load. The results are compatible with the hypothesis that SR Ca2+ release can be activated by both Ca2+ influx and by SR Ca2+ release and that the latter mechanism constitutes a positive feedback, the amplification of which increases with the amount of releasable Ca2+.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley R. H., Williams A. J. Divalent cation activation and inhibition of single calcium release channels from sheep cardiac sarcoplasmic reticulum. J Gen Physiol. 1990 May;95(5):981–1005. doi: 10.1085/jgp.95.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Lederer W. J., Berlin J. R. Intracellular Ca transients in rat cardiac myocytes: role of Na-Ca exchange in excitation-contraction coupling. Am J Physiol. 1990 May;258(5 Pt 1):C944–C954. doi: 10.1152/ajpcell.1990.258.5.C944. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in guinea-pig cardiac cells: exchange current and changes in intracellular Ca2+. J Physiol. 1989 Jul;414:499–520. doi: 10.1113/jphysiol.1989.sp017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich V Y. a., Isenberg G. Depolarization-mediated intracellular calcium transients in isolated smooth muscle cells of guinea-pig urinary bladder. J Physiol. 1991 Apr;435:187–205. doi: 10.1113/jphysiol.1991.sp018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hansford R. G., Lakatta E. G. Ryanodine releases calcium from sarcoplasmic reticulum in calcium-tolerant rat cardiac myocytes. J Physiol. 1987 Sep;390:453–467. doi: 10.1113/jphysiol.1987.sp016711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D. W., Collins A., Matsuoka S. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J Gen Physiol. 1992 Dec;100(6):933–961. doi: 10.1085/jgp.100.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Han S. Gradation of Ca(2+)-induced Ca2+ release by voltage-clamp pulse duration in potentiated guinea-pig ventricular myocytes. J Physiol. 1994 Nov 1;480(Pt 3):423–438. doi: 10.1113/jphysiol.1994.sp020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Wendt-Gallitelli M. F. Binding of calcium to myoplasmic buffers contributes to the frequency-dependent inotropy in heart ventricular cells. Basic Res Cardiol. 1992 Sep-Oct;87(5):411–417. doi: 10.1007/BF00795053. [DOI] [PubMed] [Google Scholar]
- Janczewski A. M., Lewartowski B. The effect of prolonged rest on calcium exchange and contractions in rat and guinea-pig ventricular myocardium. J Mol Cell Cardiol. 1986 Dec;18(12):1233–1242. doi: 10.1016/s0022-2828(86)80427-8. [DOI] [PubMed] [Google Scholar]
- Leblanc N., Hume J. R. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990 Apr 20;248(4953):372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Clusin W. T. Cytosolic calcium staircase in cultured myocardial cells. Circ Res. 1987 Dec;61(6):934–939. doi: 10.1161/01.res.61.6.934. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F. Ca-pools involved in the regulation of cardiac contraction under positive inotropy. X-ray microanalysis on rapidly-frozen ventricular muscles of guinea-pig. Basic Res Cardiol. 1986;81 (Suppl 1):25–32. doi: 10.1007/978-3-662-11374-5_3. [DOI] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F., Isenberg G. Total and free myoplasmic calcium during a contraction cycle: x-ray microanalysis in guinea-pig ventricular myocytes. J Physiol. 1991 Apr;435:349–372. doi: 10.1113/jphysiol.1991.sp018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F., Isenberg G. X-ray microanalysis of single cardiac myocytes frozen under voltage-clamp conditions. Am J Physiol. 1989 Feb;256(2 Pt 2):H574–H583. doi: 10.1152/ajpheart.1989.256.2.H574. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Egan T. M., López-López J. R., Balke C. W. Local control of excitation-contraction coupling in rat heart cells. J Physiol. 1994 Feb 1;474(3):463–471. doi: 10.1113/jphysiol.1994.sp020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- duBell W. H., Houser S. R. Voltage and beat dependence of Ca2+ transient in feline ventricular myocytes. Am J Physiol. 1989 Sep;257(3 Pt 2):H746–H759. doi: 10.1152/ajpheart.1989.257.3.H746. [DOI] [PubMed] [Google Scholar]


