Abstract
1. Single-channel studies were performed to clarify how tonic changes in intracellular Ca2+ concentrations ([Ca2+]i) modulate cardiac L-type Ca2+ channels. Currents were recorded from fura-2-loaded guinea-pig ventricular myocytes in the cell-attached configuration. Fura-2 fluorescence signals were recorded simultaneously during pulses to elicit channel activity. 2. The myocyte [Ca2+]i was altered through changes in bath Ca2+ concentration during K+ depolarization. When [Ca2+]i exceeded approximately 2 times the resting level (estimated [Ca2+]i around 180-400 nM), the activity of Ca2+ channels was reversibly potentiated without changes in unitary current amplitudes. 3. Increased channel open probability during Ca(2+)-dependent potentiation resulted from increased availability and increased open probability during non-blank sweeps. Closed time analysis revealed a distribution best fitted with two exponentials. Increased [Ca2+]i reduced the longer time constant, but had no effect on the shorter time constant. The open time constant was unchanged in most cases. Current records occasionally included sweeps with long openings (approximately 10 ms or more), whose appearance increased during potentiation. 4. When [Ca2+]i was increased after cAMP-dependent upregulation of Ca2+ channels, the change in channel activity was diminished. Similar results were observed when Ca(2+)-dependent potentiation was examined in myocytes exposed to a membrane-permeant protein kinase inhibitor, H-89. This suggests that channel phosphorylation may be responsible for Ca(2+)-dependent potentiation. 5. When [Ca2+]i was further increased, but remained below the threshold for contraction (estimated [Ca2+]i above 600 nM), Ca2+ channel activity was suppressed. 6. Our results demonstrate directly at the single-channel level that [Ca2+]i modulates the activity of cardiac L-type Ca2+ channels, enhancing it with modest [Ca2+]i increases and decreasing it with greater [Ca2+]i increases.
Full text
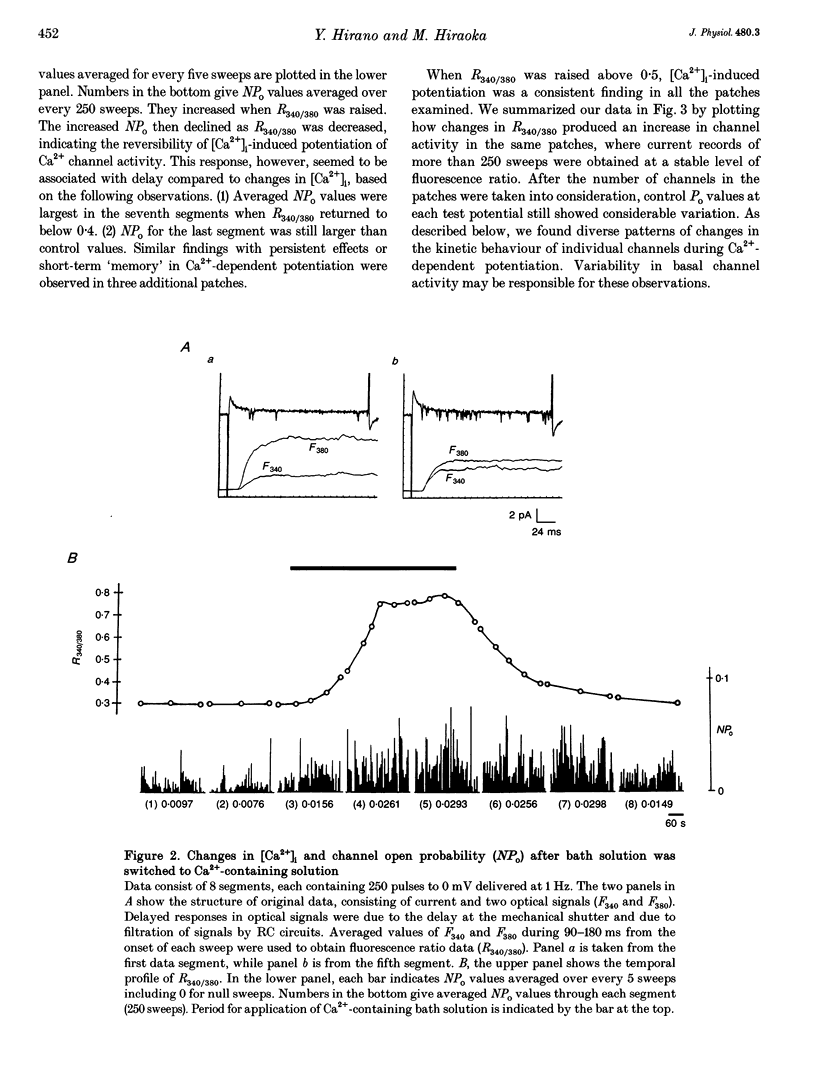
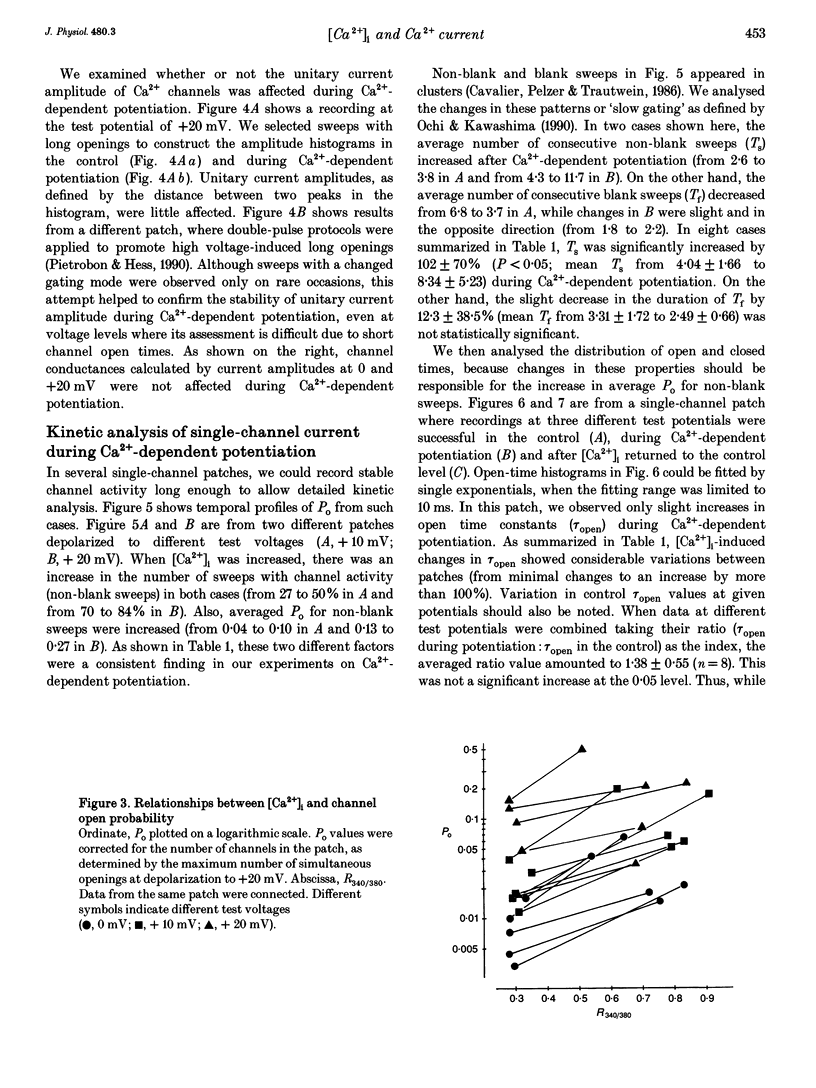
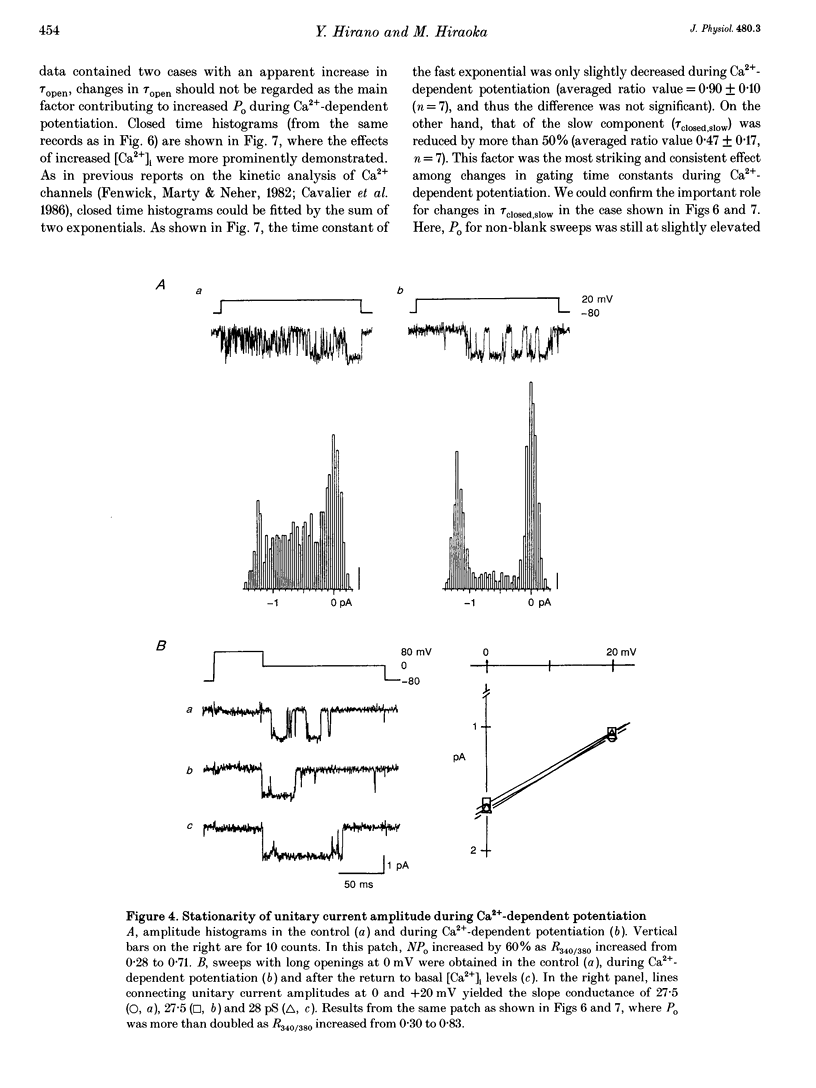
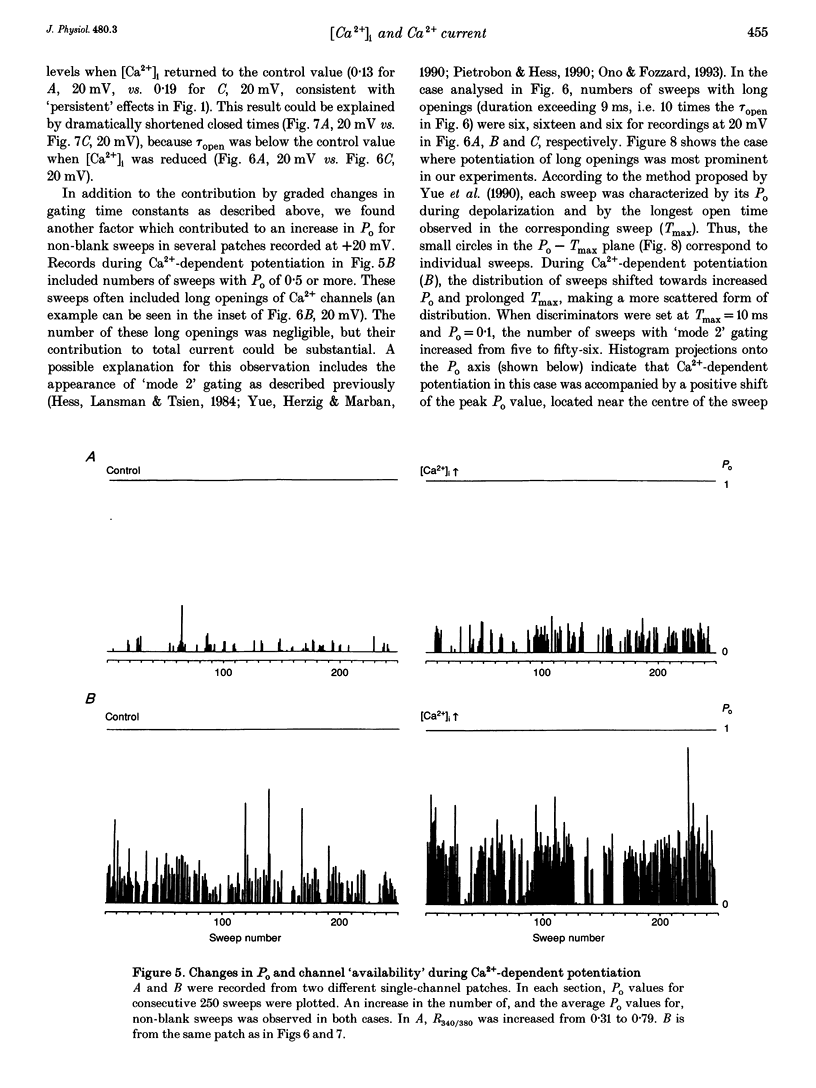
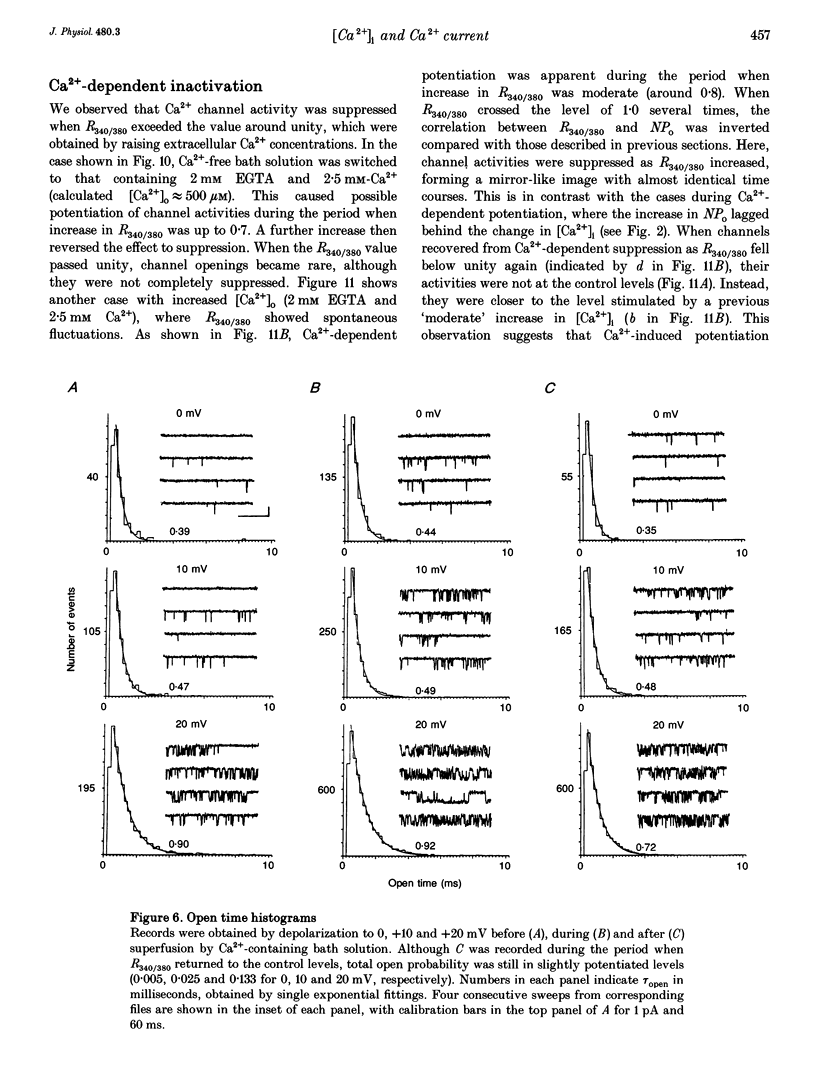
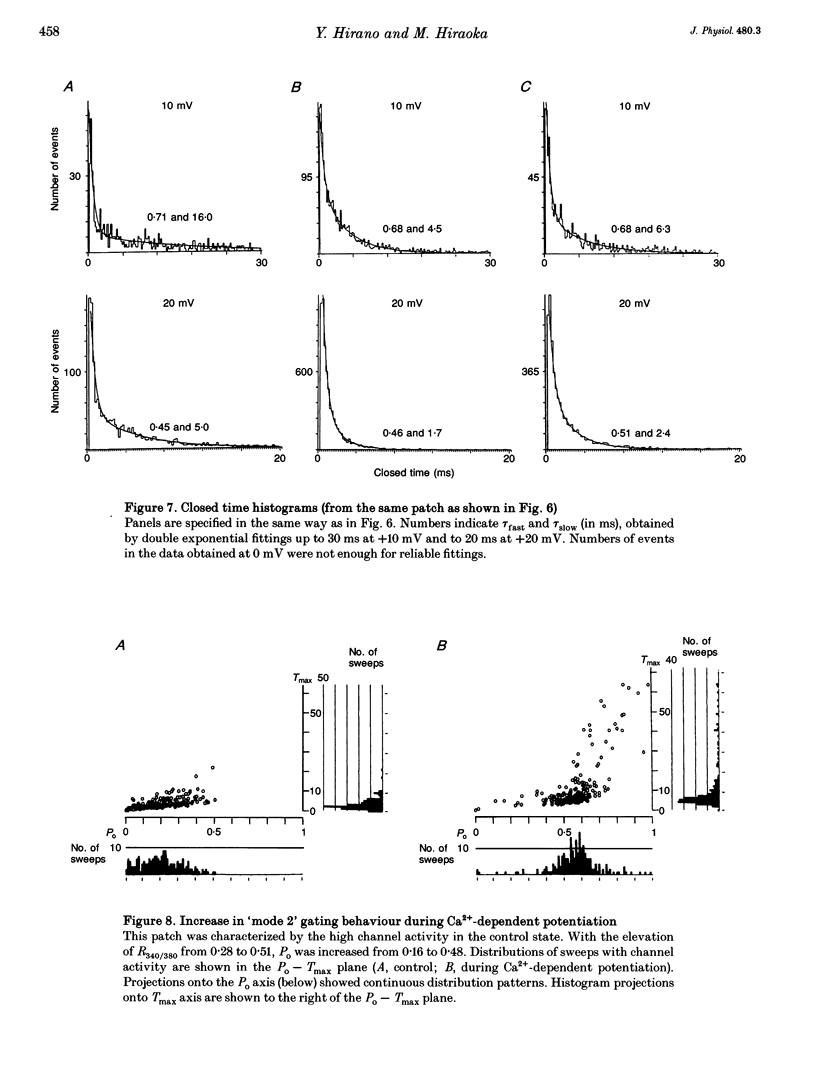
PDF

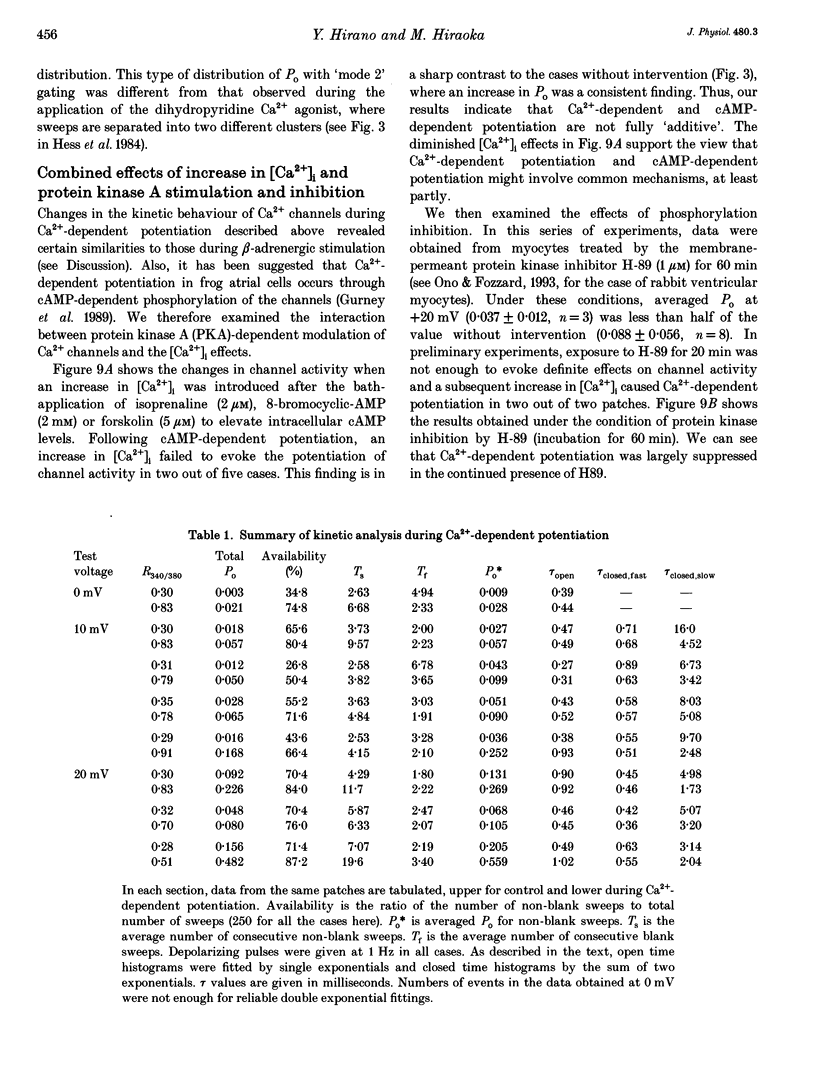
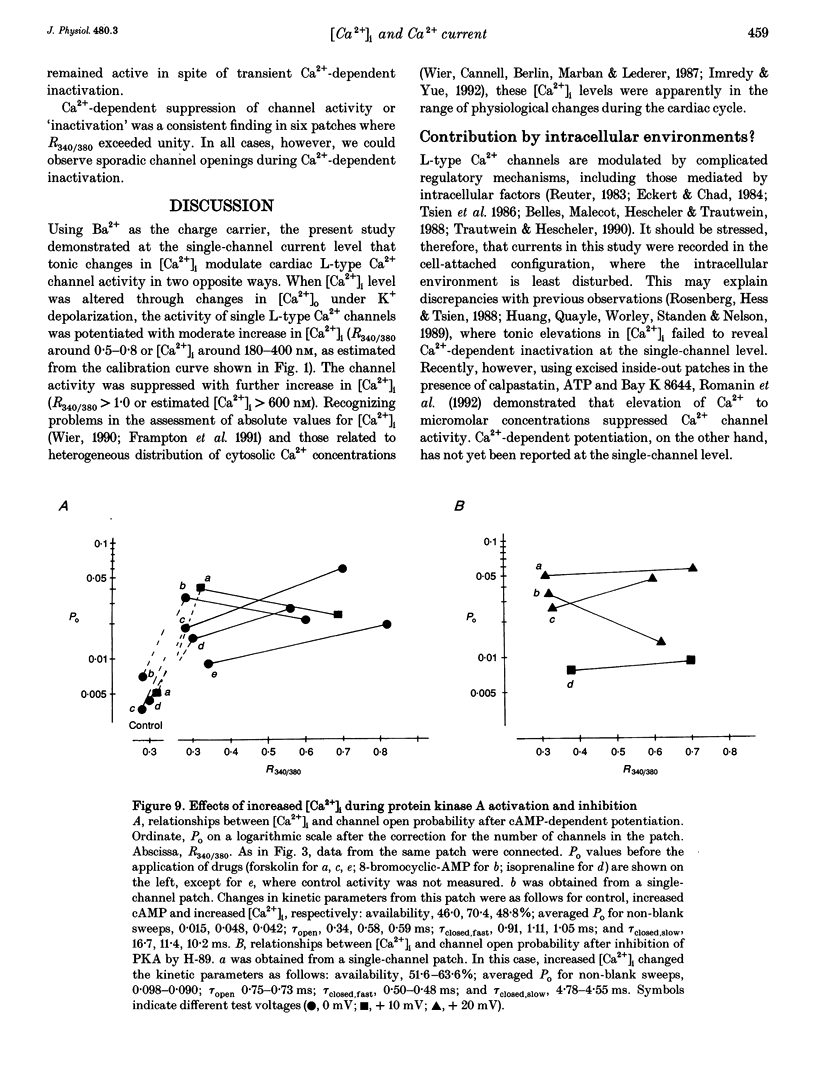
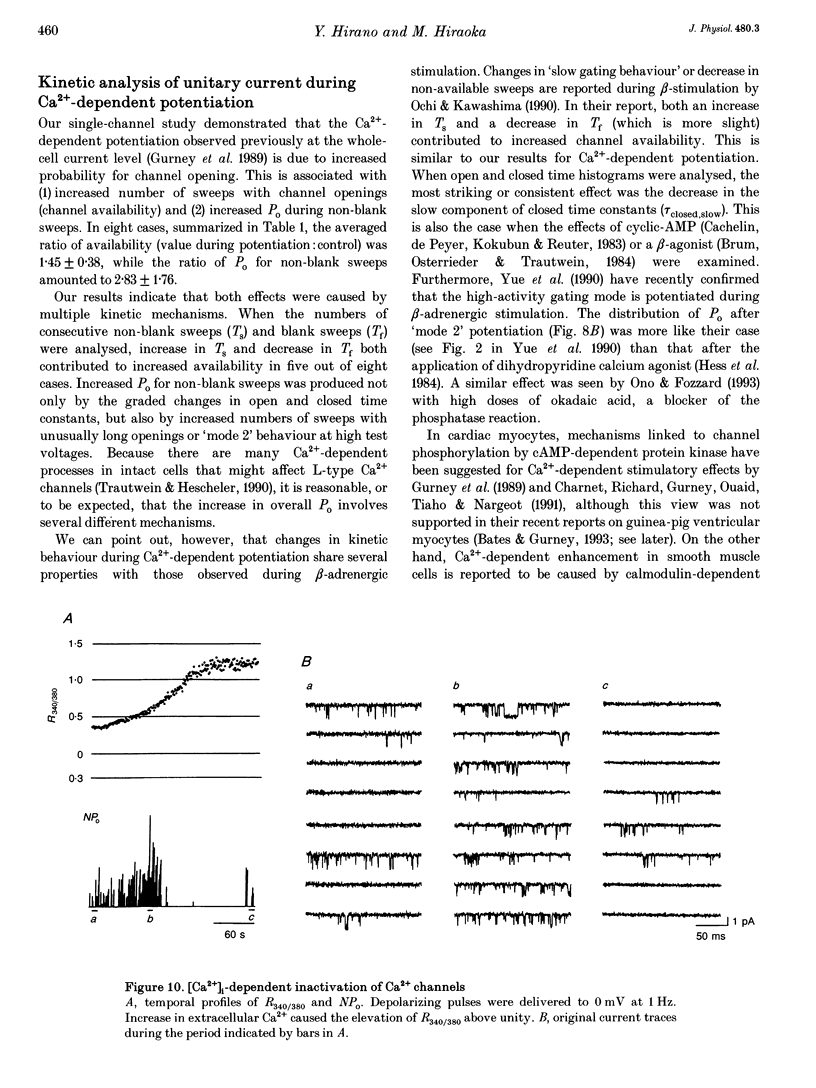
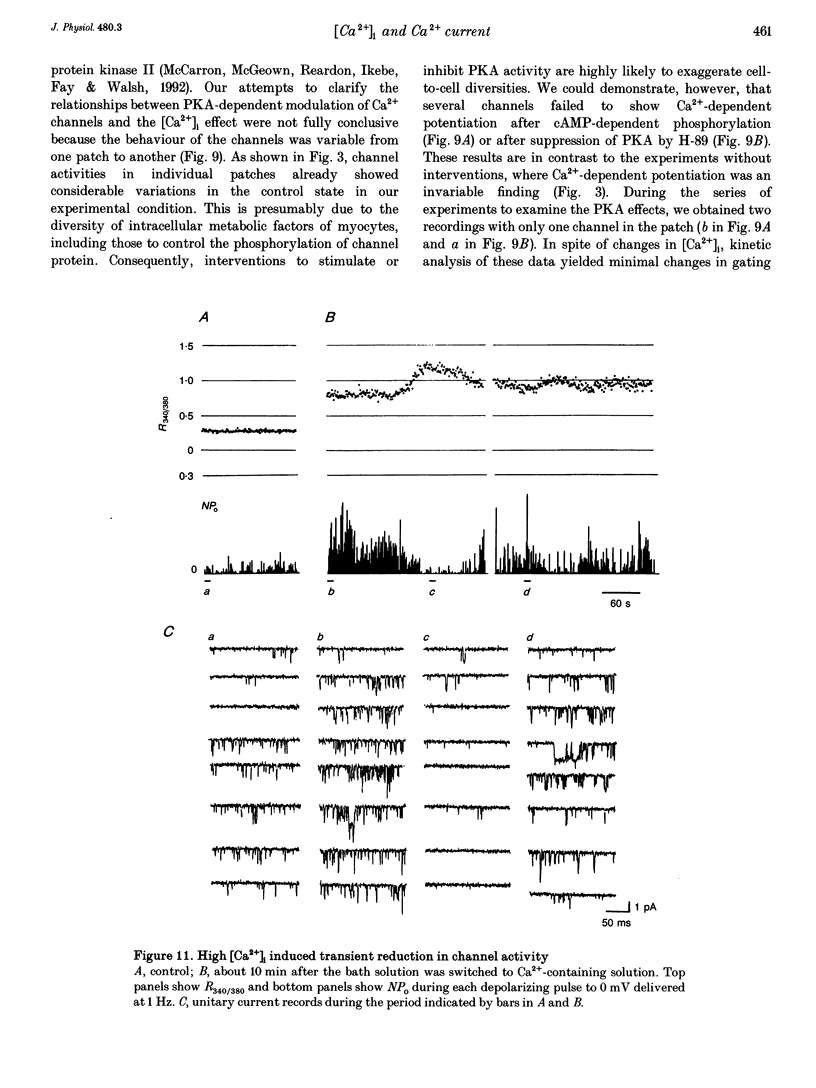


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates S. E., Gurney A. M. Ca(2+)-dependent block and potentiation of L-type calcium current in guinea-pig ventricular myocytes. J Physiol. 1993 Jul;466:345–365. [PMC free article] [PubMed] [Google Scholar]
- Belles B., Malécot C. O., Hescheler J., Trautwein W. "Run-down" of the Ca current during long whole-cell recordings in guinea pig heart cells: role of phosphorylation and intracellular calcium. Pflugers Arch. 1988 Apr;411(4):353–360. doi: 10.1007/BF00587713. [DOI] [PubMed] [Google Scholar]
- Brum G., Osterrieder W., Trautwein W. Beta-adrenergic increase in the calcium conductance of cardiac myocytes studied with the patch clamp. Pflugers Arch. 1984 Jun;401(2):111–118. doi: 10.1007/BF00583870. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., de Peyer J. E., Kokubun S., Reuter H. Ca2+ channel modulation by 8-bromocyclic AMP in cultured heart cells. Nature. 1983 Aug 4;304(5925):462–464. doi: 10.1038/304462a0. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Pelzer D., Trautwein W. Fast and slow gating behaviour of single calcium channels in cardiac cells. Relation to activation and inactivation of calcium-channel current. Pflugers Arch. 1986 Mar;406(3):241–258. doi: 10.1007/BF00640910. [DOI] [PubMed] [Google Scholar]
- Charnet P., Richard S., Gurney A. M., Ouadid H., Tiaho F., Nargeot J. Modulation of Ca currents in isolated frog atrial cells studied with photosensitive probes. Regulation by cAMP and Ca2+: a common pathway? J Mol Cell Cardiol. 1991 Mar;23(3):343–356. doi: 10.1016/0022-2828(91)90070-3. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Fedida D., Noble D., Spindler A. J. Mechanism of the use dependence of Ca2+ current in guinea-pig myocytes. J Physiol. 1988 Nov;405:461–475. doi: 10.1113/jphysiol.1988.sp017342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton J. E., Orchard C. H., Boyett M. R. Diastolic, systolic and sarcoplasmic reticulum [Ca2+] during inotropic interventions in isolated rat myocytes. J Physiol. 1991 Jun;437:351–375. doi: 10.1113/jphysiol.1991.sp018600. [DOI] [PMC free article] [PubMed] [Google Scholar]
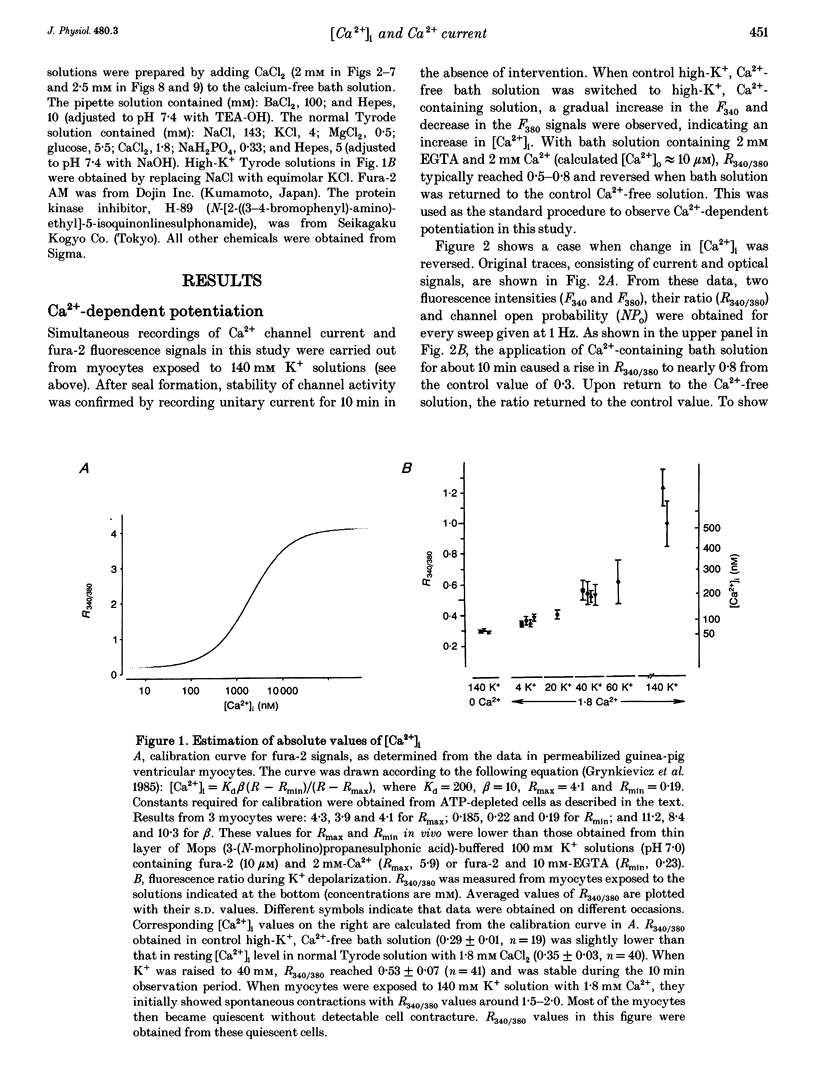
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gurney A. M., Charnet P., Pye J. M., Nargeot J. Augmentation of cardiac calcium current by flash photolysis of intracellular caged-Ca2+ molecules. Nature. 1989 Sep 7;341(6237):65–68. doi: 10.1038/341065a0. [DOI] [PubMed] [Google Scholar]
- Hadley R. W., Lederer W. J. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J Physiol. 1991 Dec;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Abe S., Sawanobori T., Hiraoka M. External ATP-induced changes in [Ca2+]i and membrane currents in mammalian atrial myocytes. Am J Physiol. 1991 Apr;260(4 Pt 1):C673–C680. doi: 10.1152/ajpcell.1991.260.4.C673. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Fozzard H. A., January C. T. Characteristics of L- and T-type Ca2+ currents in canine cardiac Purkinje cells. Am J Physiol. 1989 May;256(5 Pt 2):H1478–H1492. doi: 10.1152/ajpheart.1989.256.5.H1478. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Hiraoka M. Barium-induced automatic activity in isolated ventricular myocytes from guinea-pig hearts. J Physiol. 1988 Jan;395:455–472. doi: 10.1113/jphysiol.1988.sp016929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Quayle J. M., Worley J. F., Standen N. B., Nelson M. T. External cadmium and internal calcium block of single calcium channels in smooth muscle cells from rabbit mesenteric artery. Biophys J. 1989 Nov;56(5):1023–1028. doi: 10.1016/S0006-3495(89)82747-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imredy J. P., Yue D. T. Submicroscopic Ca2+ diffusion mediates inhibitory coupling between individual Ca2+ channels. Neuron. 1992 Aug;9(2):197–207. doi: 10.1016/0896-6273(92)90159-b. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Altschuld R. A., Stokes B. T. Quantitation of intracellular free calcium in single adult cardiomyocytes by fura-2 fluorescence microscopy: calibration of fura-2 ratios. Biochem Biophys Res Commun. 1987 Aug 31;147(1):120–126. doi: 10.1016/s0006-291x(87)80095-5. [DOI] [PubMed] [Google Scholar]
- Marban E., Tsien R. W. Enhancement of calcium current during digitalis inotropy in mammalian heart: positive feed-back regulation by intracellular calcium? J Physiol. 1982 Aug;329:589–614. doi: 10.1113/jphysiol.1982.sp014321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J., Liu Y. M. Gating of L-type Ca2+ channels in embryonic chick ventricle cells: dependence on voltage, current and channel density. J Physiol. 1991 Nov;443:307–334. doi: 10.1113/jphysiol.1991.sp018835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron J. G., McGeown J. G., Reardon S., Ikebe M., Fay F. S., Walsh J. V., Jr Calcium-dependent enhancement of calcium current in smooth muscle by calmodulin-dependent protein kinase II. Nature. 1992 May 7;357(6373):74–77. doi: 10.1038/357074a0. [DOI] [PubMed] [Google Scholar]
- O'Rourke B., Backx P. H., Marban E. Phosphorylation-independent modulation of L-type calcium channels by magnesium-nucleotide complexes. Science. 1992 Jul 10;257(5067):245–248. doi: 10.1126/science.1321495. [DOI] [PubMed] [Google Scholar]
- Ochi R., Kawashima Y. Modulation of slow gating process of calcium channels by isoprenaline in guinea-pig ventricular cells. J Physiol. 1990 May;424:187–204. doi: 10.1113/jphysiol.1990.sp018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Fozzard H. A. Two phosphatase sites on the Ca2+ channel affecting different kinetic functions. J Physiol. 1993 Oct;470:73–84. doi: 10.1113/jphysiol.1993.sp019848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D., Hess P. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 1990 Aug 16;346(6285):651–655. doi: 10.1038/346651a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Romanin C., Karlsson J. O., Schindler H. Activity of cardiac L-type Ca2+ channels is sensitive to cytoplasmic calcium. Pflugers Arch. 1992 Aug;421(5):516–518. doi: 10.1007/BF00370266. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. L., Hess P., Tsien R. W. Cardiac calcium channels in planar lipid bilayers. L-type channels and calcium-permeable channels open at negative membrane potentials. J Gen Physiol. 1988 Jul;92(1):27–54. doi: 10.1085/jgp.92.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein W., Hescheler J. Regulation of cardiac L-type calcium current by phosphorylation and G proteins. Annu Rev Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]
- Tseng G. N. Calcium current restitution in mammalian ventricular myocytes is modulated by intracellular calcium. Circ Res. 1988 Aug;63(2):468–482. doi: 10.1161/01.res.63.2.468. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Cannell M. B., Berlin J. R., Marban E., Lederer W. J. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 1987 Jan 16;235(4786):325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]
- Wier W. G. Cytoplasmic [Ca2+] in mammalian ventricle: dynamic control by cellular processes. Annu Rev Physiol. 1990;52:467–485. doi: 10.1146/annurev.ph.52.030190.002343. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Herzig S., Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990 Jan;87(2):753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt A. C., Maylie J. Stimulation-dependent facilitation of the high threshold calcium current in guinea-pig ventricular myocytes. J Physiol. 1990 Sep;428:653–671. doi: 10.1113/jphysiol.1990.sp018233. [DOI] [PMC free article] [PubMed] [Google Scholar]


