Abstract
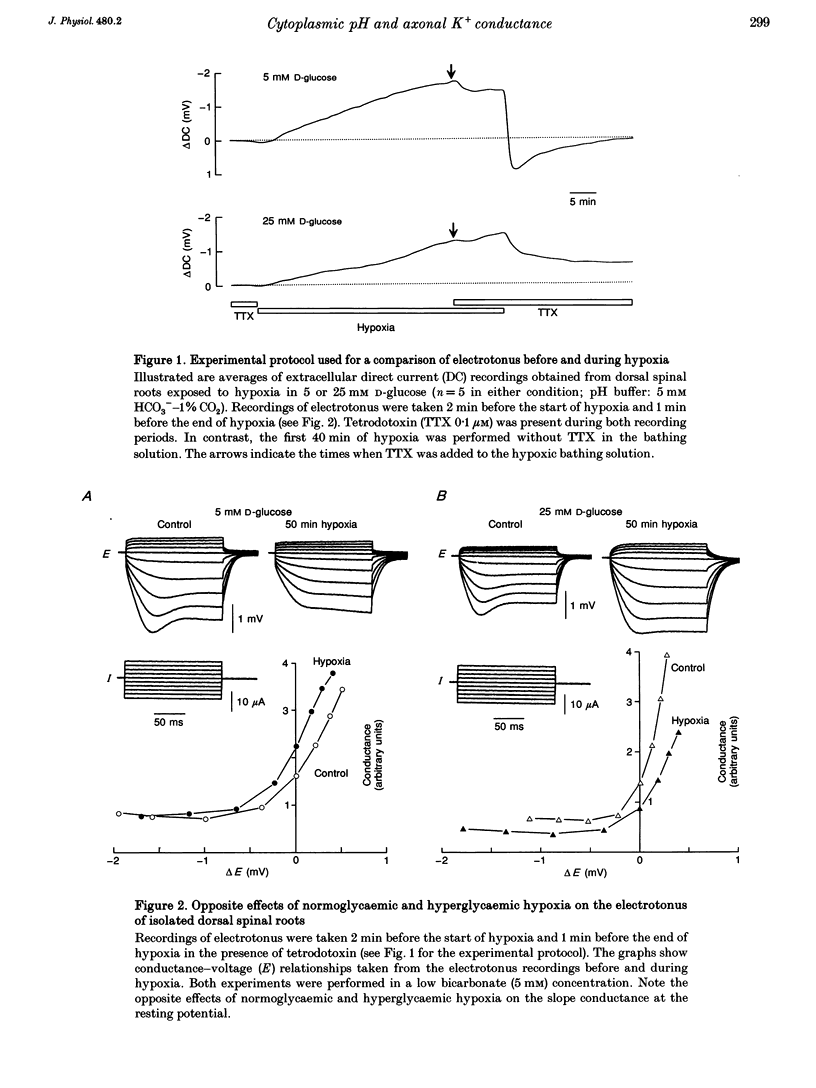
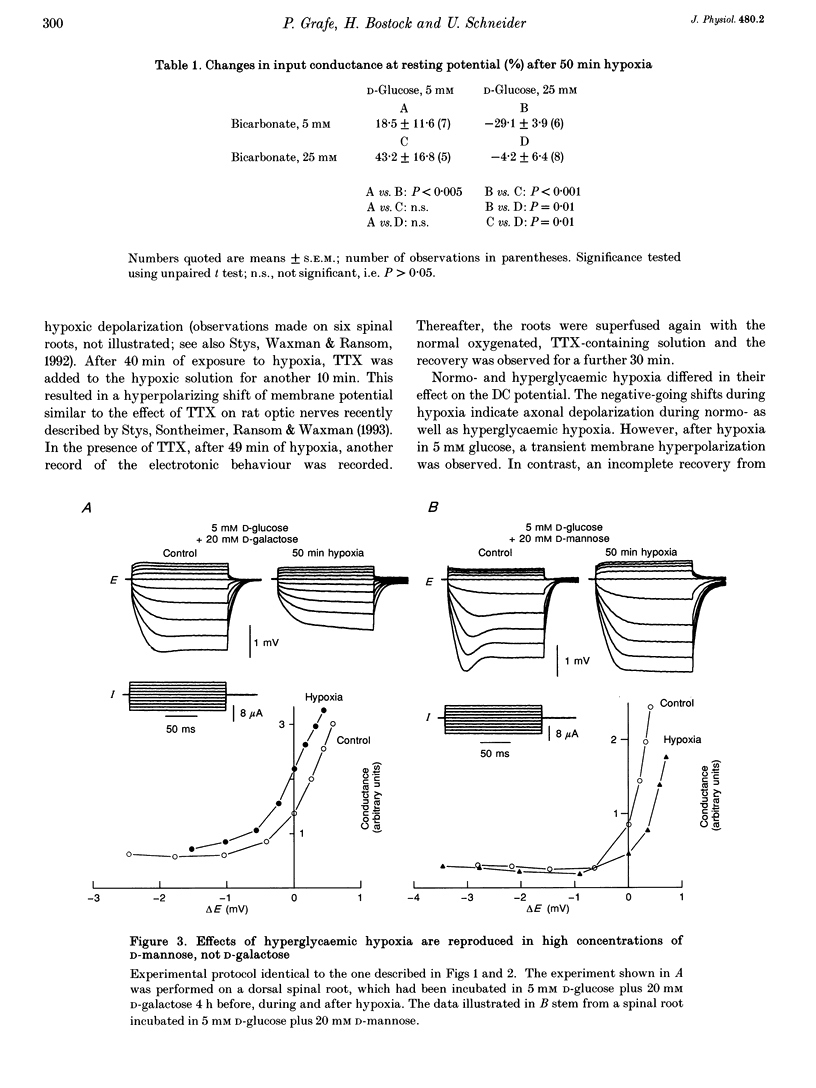
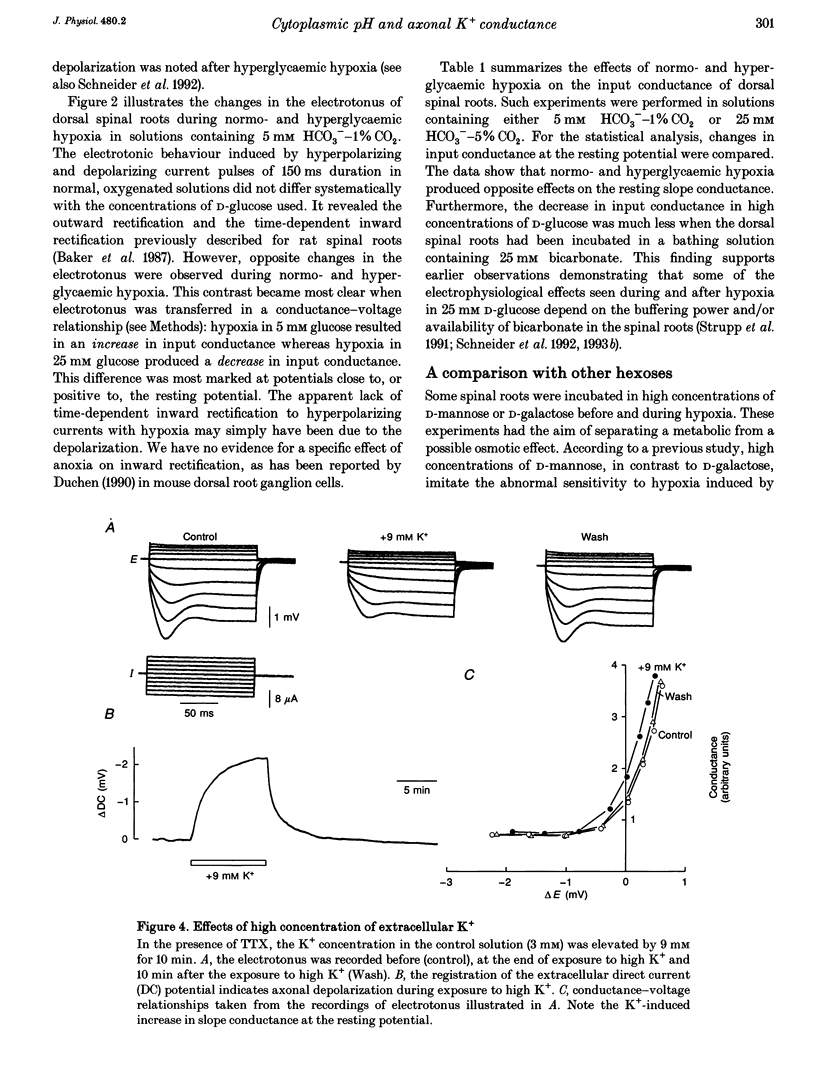
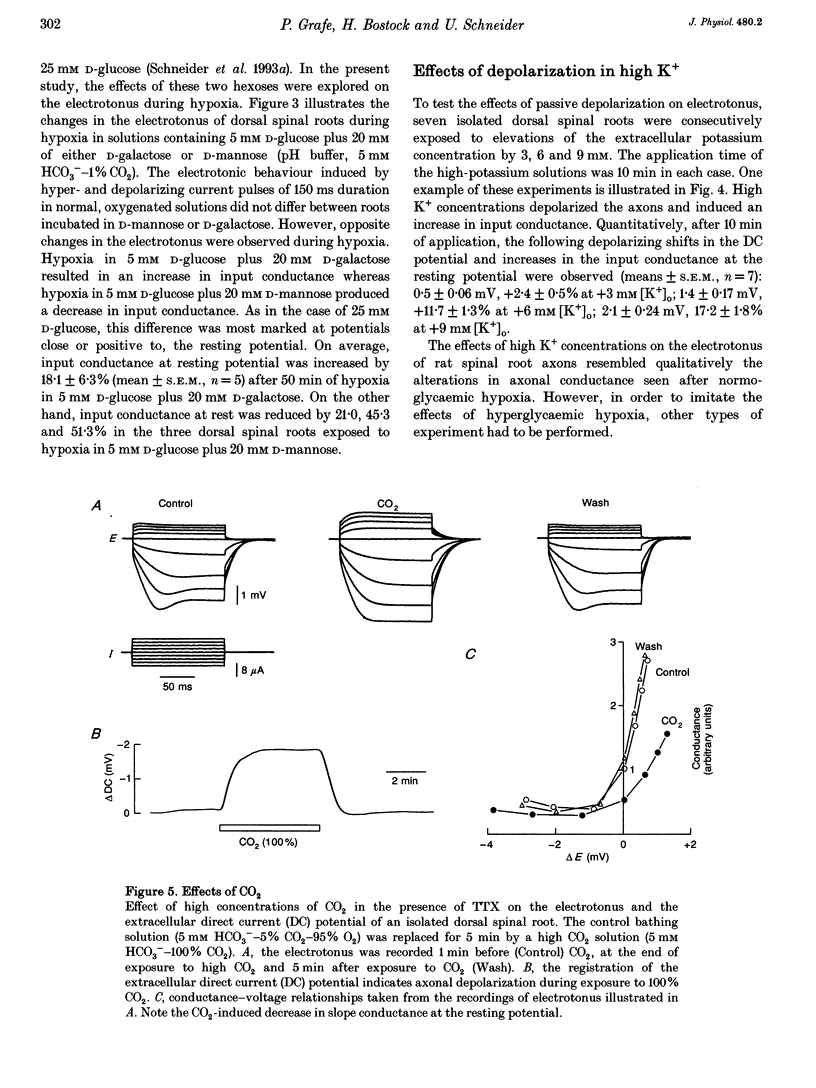
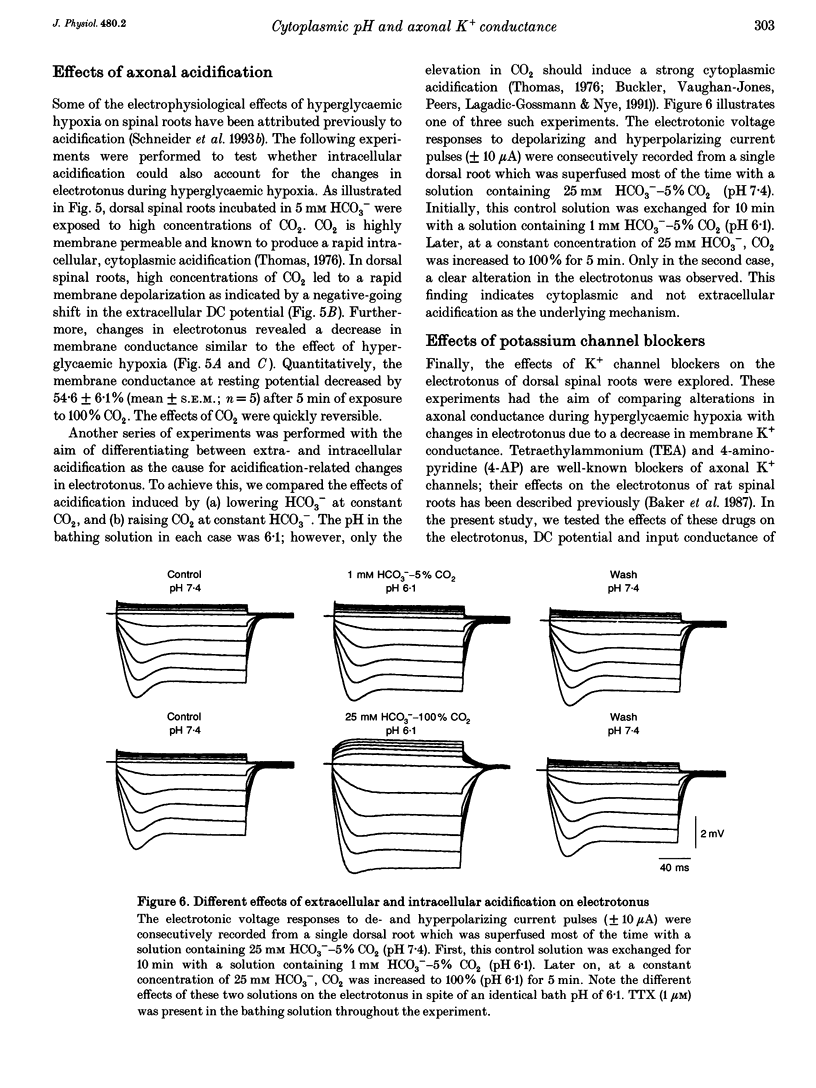
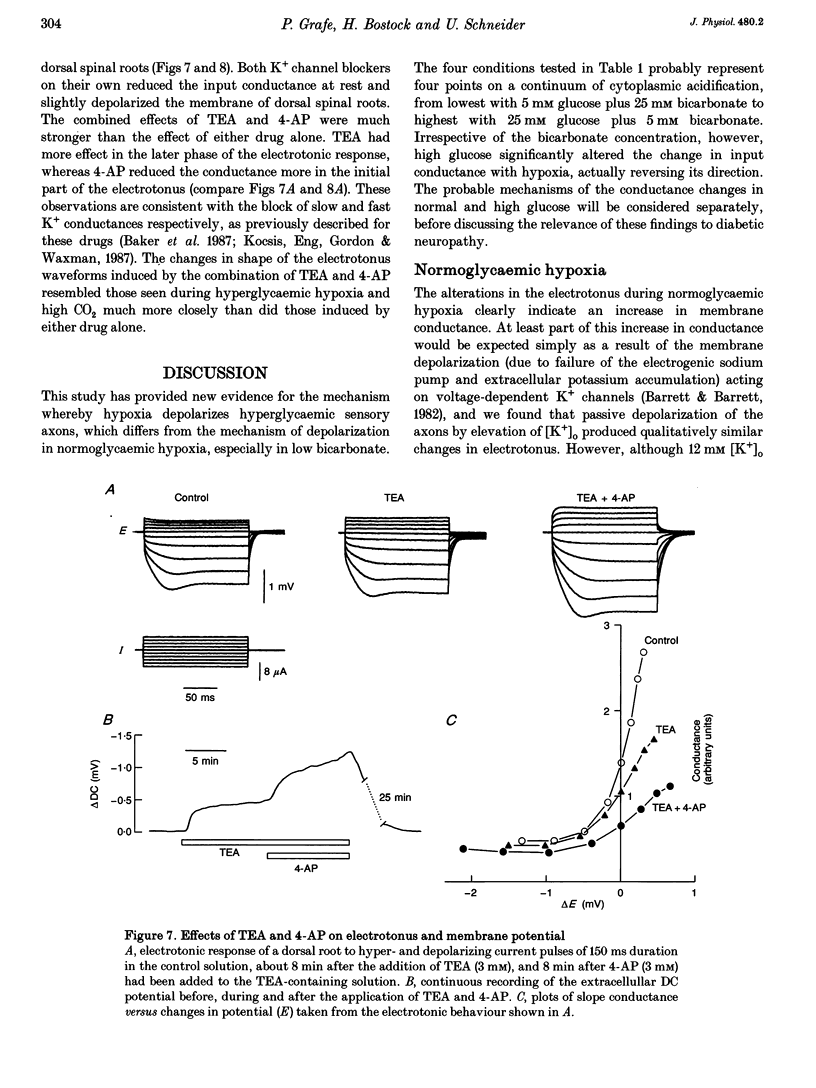
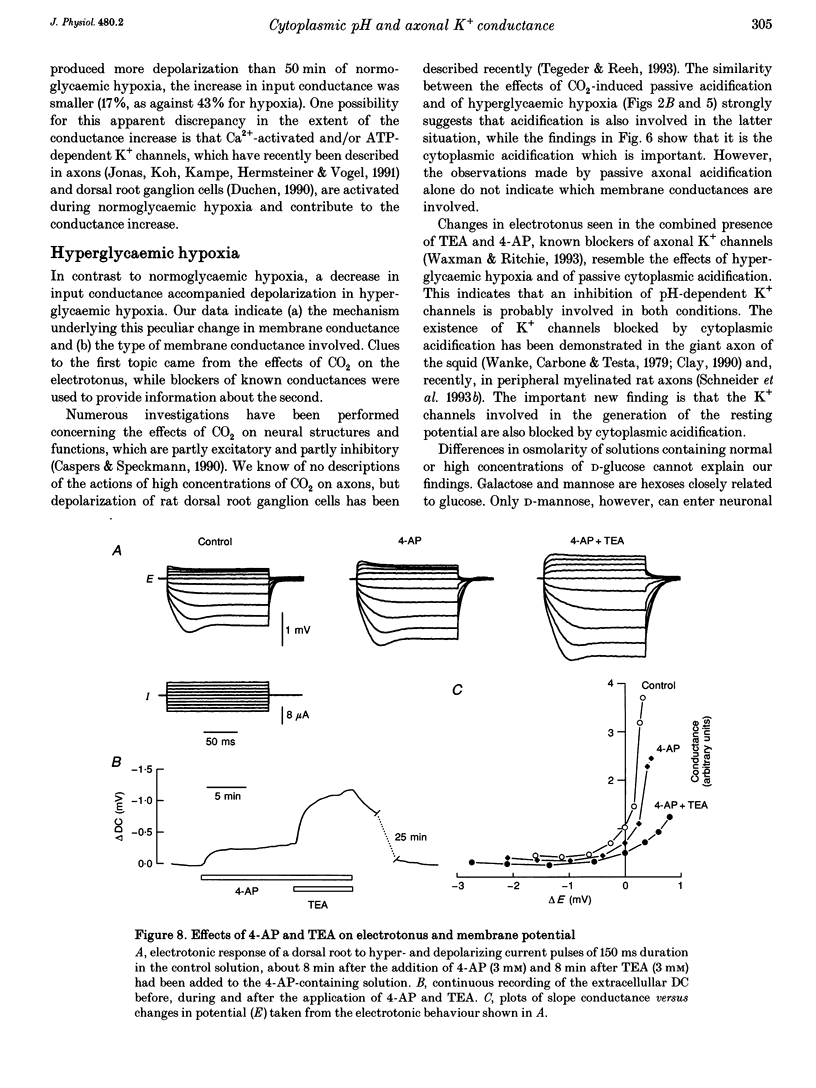
1. Electrotonic responses to 150 ms current pulses were recorded from isolated rat dorsal roots incubated for at least 3 h with either normal (5 mM) or high (25 mM) D-glucose solutions, and with either normal (25 mM) or low (5 mM) bicarbonate concentrations. 2. On replacement of O2 by N2 for 50 min, all the roots depolarized, but the changes in electrotonus differed systematically. With normal glucose, the depolarization was accompanied by an increase in input conductance. In contrast, for the hyperglycaemic roots the depolarization was slower and accompanied by a fall in input conductance which was exacerbated in low bicarbonate concentrations. 3. The changes induced by hyperglycaemic hypoxia in low bicarbonate could be mimicked by exposure of the roots either to 100% CO2 or to a combination of 3 mM tetraethylammonium chloride and 3 mM 4-aminopyridine, to block both fast and slow potassium channels. 4. These results indicate that the primary mechanism of hypoxic depolarization of these sensory axons is altered by hyperglycaemia. In normoglycaemia, the changes in electrotonus are consistent with an increase in axonal potassium conductance. The block of potassium channels seen in hyperglycaemic hypoxia is attributed to intra-axonal acidification by anaerobic glycolysis and may contribute to the pathogenesis of diabetic neuropathy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M., Bostock H., Grafe P., Martius P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol. 1987 Feb;383:45–67. doi: 10.1113/jphysiol.1987.sp016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol. 1982 Feb;323:117–144. doi: 10.1113/jphysiol.1982.sp014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D., Peers C., Lagadic-Gossmann D., Nye P. C. Effects of extracellular pH, PCO2 and HCO3- on intracellular pH in isolated type-I cells of the neonatal rat carotid body. J Physiol. 1991 Dec;444:703–721. doi: 10.1113/jphysiol.1991.sp018902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R. IK inactivation in squid axons is shifted along the voltage axis by changes in the intracellular pH. Biophys J. 1990 Sep;58(3):797–801. doi: 10.1016/S0006-3495(90)82423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R. Effects of metabolic inhibition on the membrane properties of isolated mouse primary sensory neurones. J Physiol. 1990 May;424:387–409. doi: 10.1113/jphysiol.1990.sp018073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Koh D. S., Kampe K., Hermsteiner M., Vogel W. ATP-sensitive and Ca-activated K channels in vertebrate axons: novel links between metabolism and excitability. Pflugers Arch. 1991 Mar;418(1-2):68–73. doi: 10.1007/BF00370453. [DOI] [PubMed] [Google Scholar]
- Kocsis J. D., Bowe C. M., Waxman S. G. Different effects of 4-aminopyridine on sensory and motor fibers: pathogenesis of paresthesias. Neurology. 1986 Jan;36(1):117–120. doi: 10.1212/wnl.36.1.117. [DOI] [PubMed] [Google Scholar]
- Kocsis J. D., Eng D. L., Gordon T. R., Waxman S. G. Functional differences between 4-aminopyridine and tetraethylammonium-sensitive potassium channels in myelinated axons. Neurosci Lett. 1987 Mar 31;75(2):193–198. doi: 10.1016/0304-3940(87)90296-5. [DOI] [PubMed] [Google Scholar]
- Kraig R. P., Chesler M. Astrocytic acidosis in hyperglycemic and complete ischemia. J Cereb Blood Flow Metab. 1990 Jan;10(1):104–114. doi: 10.1038/jcbfm.1990.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. A. Recent advances in the pathogenesis of diabetic neuropathy. Muscle Nerve. 1987 Feb;10(2):121–128. doi: 10.1002/mus.880100204. [DOI] [PubMed] [Google Scholar]
- Marsh S. J., Stansfeld C. E., Brown D. A., Davey R., McCarthy D. The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience. 1987 Oct;23(1):275–289. doi: 10.1016/0306-4522(87)90289-2. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M. A note on the mechanism of resistance to anoxia and ischaemia in pathophysiological mammalian myelinated nerve. J Neurol Neurosurg Psychiatry. 1985 Mar;48(3):274–277. doi: 10.1136/jnnp.48.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer W. W., Bunge R. P. Effects of calcium ion concentration on the degeneration of amputated axons in tissue culture. J Cell Biol. 1973 Nov;59(2 Pt 1):456–470. doi: 10.1083/jcb.59.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider U., Jund R., Nees S., Grafe P. Differences in sensitivity to hyperglycemic hypoxia of isolated rat sensory and motor nerve fibers. Ann Neurol. 1992 Jun;31(6):605–610. doi: 10.1002/ana.410310607. [DOI] [PubMed] [Google Scholar]
- Schneider U., Niedermeier W., Grafe P. The paradox between resistance to hypoxia and liability to hypoxic damage in hyperglycemic peripheral nerves. Evidence for glycolysis involvement. Diabetes. 1993 Jul;42(7):981–987. doi: 10.2337/diab.42.7.981. [DOI] [PubMed] [Google Scholar]
- Schneider U., Quasthoff S., Mitrović N., Grafe P. Hyperglycaemic hypoxia alters after-potential and fast K+ conductance of rat axons by cytoplasmic acidification. J Physiol. 1993 Jun;465:679–697. doi: 10.1113/jphysiol.1993.sp019700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. L., von Hanwehr R., Siesjö B. K. Changes in extra- and intracellular pH in the brain during and following ischemia in hyperglycemic and in moderately hypoglycemic rats. J Cereb Blood Flow Metab. 1986 Oct;6(5):574–583. doi: 10.1038/jcbfm.1986.104. [DOI] [PubMed] [Google Scholar]
- Strupp M., Jund R., Schneider U., Grafe P. Glucose availability and sensitivity to anoxia of isolated rat peroneal nerve. Am J Physiol. 1991 Sep;261(3 Pt 1):E389–E394. doi: 10.1152/ajpendo.1991.261.3.E389. [DOI] [PubMed] [Google Scholar]
- Stys P. K., Sontheimer H., Ransom B. R., Waxman S. G. Noninactivating, tetrodotoxin-sensitive Na+ conductance in rat optic nerve axons. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6976–6980. doi: 10.1073/pnas.90.15.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys P. K., Waxman S. G., Ransom B. R. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. J Neurosci. 1992 Feb;12(2):430–439. doi: 10.1523/JNEUROSCI.12-02-00430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson R., Peeling J., Sutherland G. Metabolic changes associated with altering blood glucose levels in short duration forebrain ischemia. Brain Res. 1993 Apr 16;608(2):288–298. doi: 10.1016/0006-8993(93)91470-d. [DOI] [PubMed] [Google Scholar]
- Wanke E., Carbone E., Testa P. L. K+ conductance modified by a titratable group accessible to protons from the intracellular side of the squid axon membrane. Biophys J. 1979 May;26(2):319–324. doi: 10.1016/S0006-3495(79)85251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. G., Ransom B. R., Stys P. K. Non-synaptic mechanisms of Ca(2+)-mediated injury in CNS white matter. Trends Neurosci. 1991 Oct;14(10):461–468. doi: 10.1016/0166-2236(91)90046-w. [DOI] [PubMed] [Google Scholar]
- Waxman S. G., Ritchie J. M. Molecular dissection of the myelinated axon. Ann Neurol. 1993 Feb;33(2):121–136. doi: 10.1002/ana.410330202. [DOI] [PubMed] [Google Scholar]


