Abstract
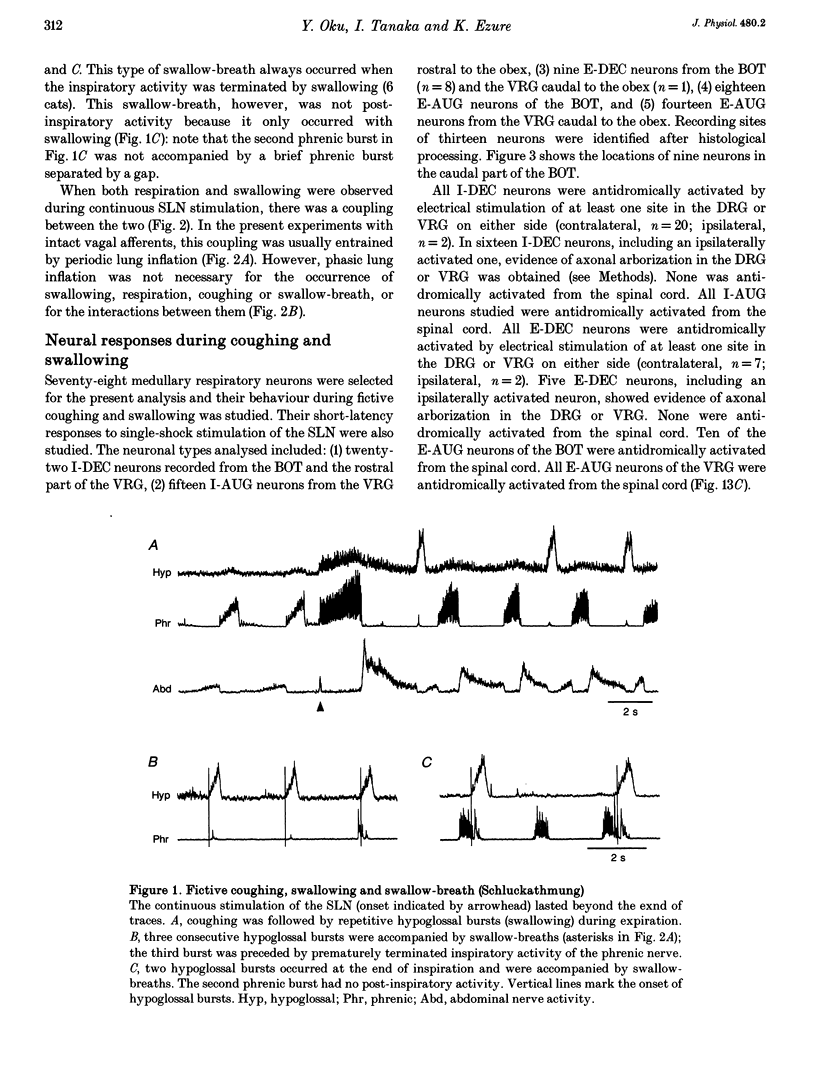
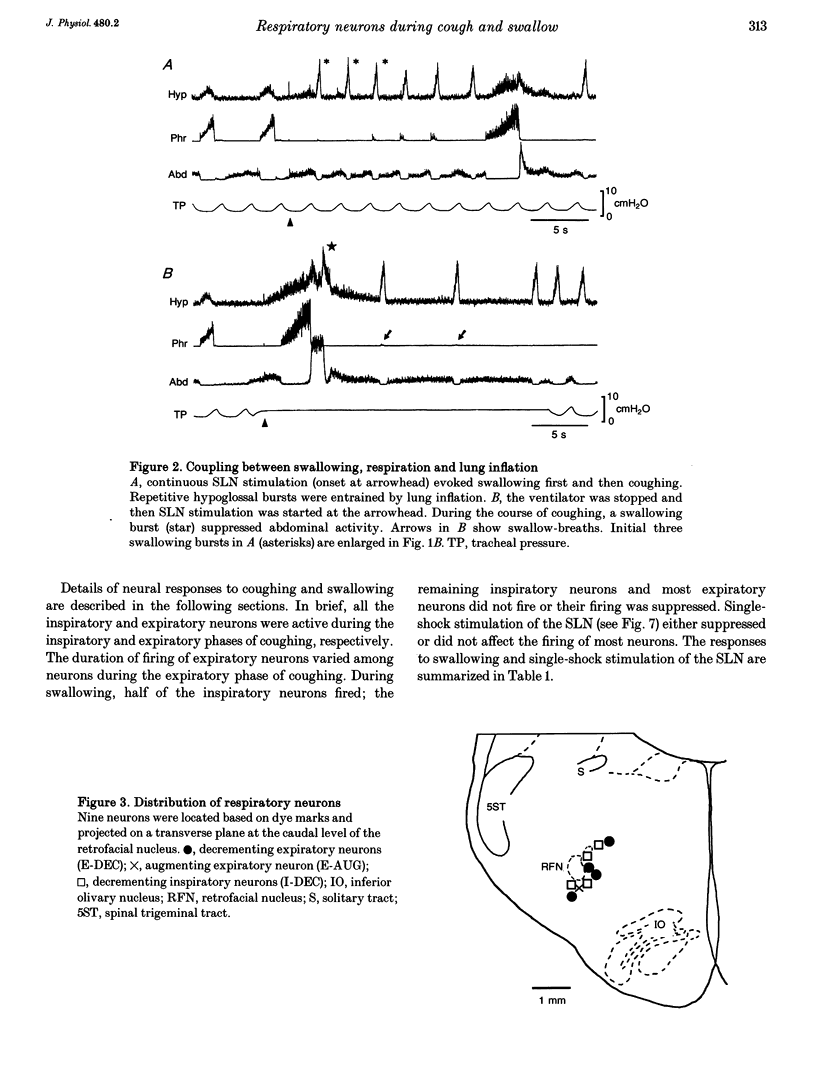
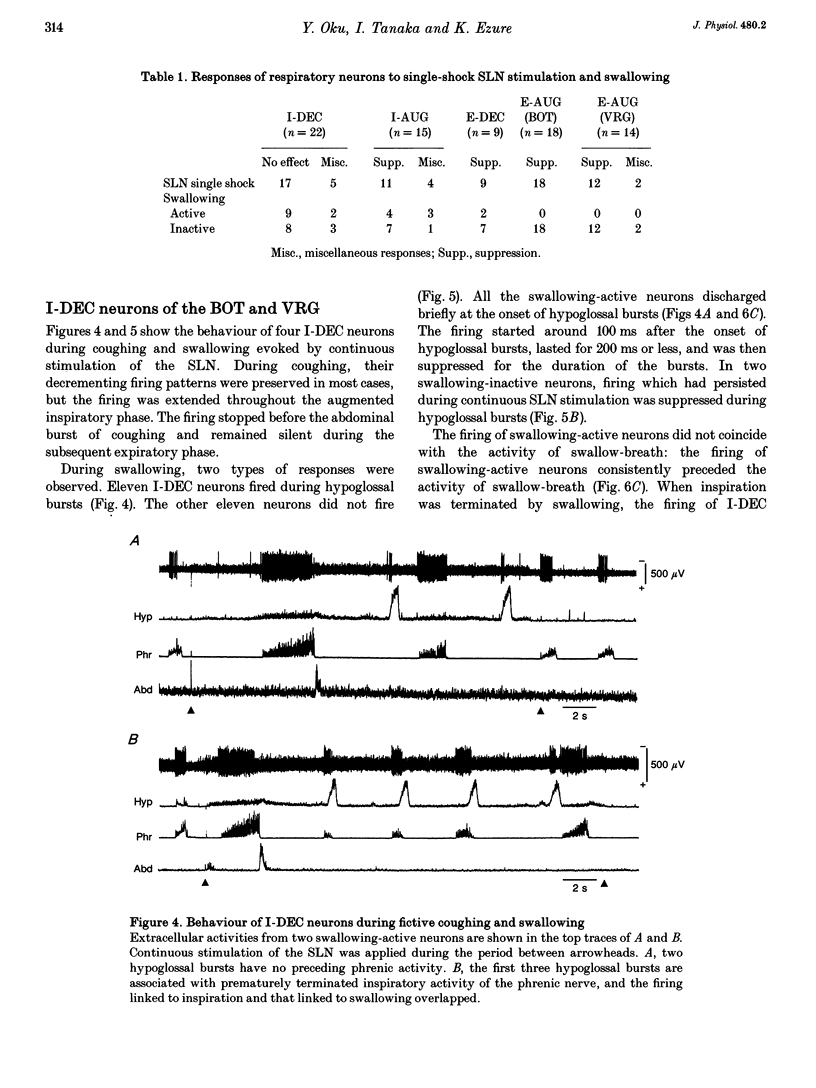
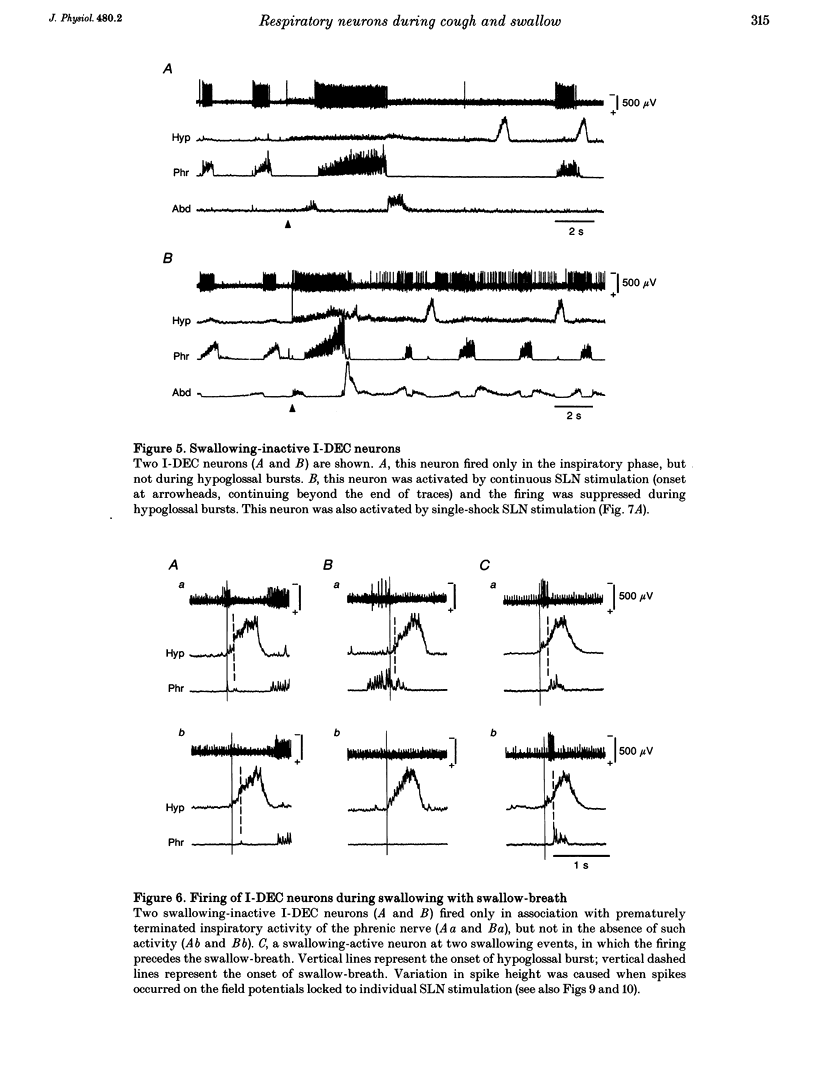
1. The behaviour of medullary respiratory neurons was studied during fictive coughing and swallowing evoked by electrical stimulation of the superior laryngeal nerve (SLN) in decerebrate, paralysed and artificially ventilated cats. Fictive coughing, swallowing and respiration were monitored by recording activities of the phrenic, hypoglossal and abdominal nerves. 2. Extracellular recordings were made from respiratory neurons in the ventral respiratory group (VRG) and in the Bötzinger complex (BOT). The neuronal types analysed included decrementing inspiratory neurons (I-DEC), augmenting expiratory neurons (E-AUG) and decrementing expiratory neurons (E-DEC) from the BOT area, and augmenting inspiratory neurons (I-AUG) and augmenting expiratory neurons (E-AUG) from the VRG area. 3. During fictive coughing, all the inspiratory and expiratory neurons were active during the inspiratory and expiratory phases of coughing, respectively. The firing of both I-DEC and I-AUG neurons was increased and prolonged in association with the augmented inspiratory activity of the phrenic nerve. The activity of E-AUG neurons of the VRG did not parallel the abdominal nerve activity, suggesting the existence of additional neurons which participate in the generation of abdominal nerve activity during fictive coughing. 4. During fictive swallowing, half of I-DEC neurons fired transiently at the onset of hypoglossal bursts associated with swallowing; the firing was suppressed during the rest of the hypoglossal bursts. Other I-DEC neurons were silent during hypoglossal bursts. Some I-AUG neurons fired during the initial half of hypoglossal bursts, and others were silent. The brief phrenic activity accompanying the swallowing might have originated from this activity in I-AUG neurons. The discharges of all E-AUG neurons (BOT and VRG) and the majority of E-DEC BOT neurons were suppressed during swallowing. 5. We conclude that these five types of respiratory neurons of the BOT and VRG are involved in the generation of the spatiotemporally organized activity of coughing and swallowing, and that at least a part of the neuronal network for respiration is shared by networks for these non-respiratory activities.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amri M., Car A., Jean A. Medullary control of the pontine swallowing neurones in sheep. Exp Brain Res. 1984;55(1):105–110. doi: 10.1007/BF00240503. [DOI] [PubMed] [Google Scholar]
- Bolser D. C. Fictive cough in the cat. J Appl Physiol (1985) 1991 Dec;71(6):2325–2331. doi: 10.1152/jappl.1991.71.6.2325. [DOI] [PubMed] [Google Scholar]
- Cohen M. I. Neurogenesis of respiratory rhythm in the mammal. Physiol Rev. 1979 Oct;59(4):1105–1173. doi: 10.1152/physrev.1979.59.4.1105. [DOI] [PubMed] [Google Scholar]
- DOTY R. W. Influence of stimulus pattern on reflex deglutition. Am J Physiol. 1951 Jul;166(1):142–158. doi: 10.1152/ajplegacy.1951.166.1.142. [DOI] [PubMed] [Google Scholar]
- Davis J. N., Plum F. Separation of descending spinal pathways to respiratory motoneurons. Exp Neurol. 1972 Jan;34(1):78–94. doi: 10.1016/0014-4886(72)90189-6. [DOI] [PubMed] [Google Scholar]
- Dick T. E., Oku Y., Romaniuk J. R., Cherniack N. S. Interaction between central pattern generators for breathing and swallowing in the cat. J Physiol. 1993 Jun;465:715–730. doi: 10.1113/jphysiol.1993.sp019702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K., Manabe M. Decrementing expiratory neurons of the Bötzinger complex. II. Direct inhibitory synaptic linkage with ventral respiratory group neurons. Exp Brain Res. 1988;72(1):159–166. doi: 10.1007/BF00248511. [DOI] [PubMed] [Google Scholar]
- Ezure K., Manabe M., Otake K. Excitation and inhibition of medullary inspiratory neurons by two types of burst inspiratory neurons in the cat. Neurosci Lett. 1989 Oct 9;104(3):303–308. doi: 10.1016/0304-3940(89)90593-4. [DOI] [PubMed] [Google Scholar]
- Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol. 1990;35(6):429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- Fedorko L., Duffin J., England S. Inhibition of inspiratory neurons of the nucleus retroambigualis by expiratory neurons of the Botzinger complex in the cat. Exp Neurol. 1989 Oct;106(1):74–77. doi: 10.1016/0014-4886(89)90146-5. [DOI] [PubMed] [Google Scholar]
- Grélot L., Milano S. Diaphragmatic and abdominal muscle activity during coughing in the decerebrate cat. Neuroreport. 1991 Apr;2(4):165–168. doi: 10.1097/00001756-199104000-00001. [DOI] [PubMed] [Google Scholar]
- Grélot L., Milano S., Portillo F., Miller A. D., Bianchi A. L. Membrane potential changes of phrenic motoneurons during fictive vomiting, coughing, and swallowing in the decerebrate cat. J Neurophysiol. 1992 Dec;68(6):2110–2119. doi: 10.1152/jn.1992.68.6.2110. [DOI] [PubMed] [Google Scholar]
- HUKUHARA T., OKADA H. Effects of deglutition upon the spike discharges of neurones in the respiratory center. Jpn J Physiol. 1956 Jun 15;6(2):162–166. doi: 10.2170/jjphysiol.6.162. [DOI] [PubMed] [Google Scholar]
- Iscoe S., Feldman J. L., Cohen M. I. Properties of inspiratory termination by superior laryngeal and vagal stimulation. Respir Physiol. 1979 Apr;36(3):353–366. doi: 10.1016/0034-5687(79)90047-1. [DOI] [PubMed] [Google Scholar]
- Iscoe S., Grélot L. Regional intercostal activity during coughing and vomiting in decerebrate cats. Can J Physiol Pharmacol. 1992 Aug;70(8):1195–1199. doi: 10.1139/y92-166. [DOI] [PubMed] [Google Scholar]
- Jakus J., Tomori Z., Stránsky A. Activity of bulbar respiratory neurones during cough and other respiratory tract reflexes in cats. Physiol Bohemoslov. 1985;34(2):127–136. [PubMed] [Google Scholar]
- Jiang C., Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Bötzinger complex in the cat. Exp Brain Res. 1990;81(3):639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- Jiang C., Lipski J. Synaptic inputs to medullary respiratory neurons from superior laryngeal afferents in the cat. Brain Res. 1992 Jul 3;584(1-2):197–206. doi: 10.1016/0006-8993(92)90895-g. [DOI] [PubMed] [Google Scholar]
- Jodkowski J. S., Berger A. J. Influences from laryngeal afferents on expiratory bulbospinal neurons and motoneurons. J Appl Physiol (1985) 1988 Apr;64(4):1337–1345. doi: 10.1152/jappl.1988.64.4.1337. [DOI] [PubMed] [Google Scholar]
- KAWASAKI M., OGURA J. H., TAKENOUCHI S. NEUROPHYSIOLOGIC OBSERVATIONS OF NORMAL DEGLUTITION. I. ITS RELATIONSHIP TO THE RESPIRATORY CYCLE. Laryngoscope. 1964 Dec;74:1747–1765. doi: 10.1288/00005537-196412000-00004. [DOI] [PubMed] [Google Scholar]
- KAWASAKI M., OGURA J. H., TAKENOUCHI S. NEUROPHYSIOLOGIC OBSERVATIONS OF NORMAL DEGLUTITION. II. ITS RELATIONSHIP TO ALLIED PHENOMENA. Laryngoscope. 1964 Dec;74:1766–1780. doi: 10.1288/00005537-196412000-00005. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Proceedings: Monosynaptic excitation of thoracic expiratory motoneurones from lateral respiratory neurones in the medulla of the cat. J Physiol. 1973 Oct;234(2):87P–89P. [PubMed] [Google Scholar]
- Long S., Duffin J. The neuronal determinants of respiratory rhythm. Prog Neurobiol. 1986;27(2):101–182. doi: 10.1016/0301-0082(86)90007-9. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Fedorko L. Monosynaptic inhibition of phrenic motoneurons: a long descending projection from Bötzinger neurons. J Neurosci. 1984 Sep;4(9):2350–2353. doi: 10.1523/JNEUROSCI.04-09-02350.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill E. G., Lipski J., Kubin L., Fedorko L. Origin of the expiratory inhibition of nucleus tractus solitarius inspiratory neurones. Brain Res. 1983 Mar 14;263(1):43–50. doi: 10.1016/0006-8993(83)91198-8. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Ezure K. Behavior of inhibitory and excitatory propriobulbar respiratory neurons during fictive vomiting. Brain Res. 1992 Apr 24;578(1-2):168–176. doi: 10.1016/0006-8993(92)90245-5. [DOI] [PubMed] [Google Scholar]
- Miller A. J. Characteristics of the swallowing reflex induced by peripheral nerve and brain stem stimulation. Exp Neurol. 1972 Feb;34(2):210–222. doi: 10.1016/0014-4886(72)90168-9. [DOI] [PubMed] [Google Scholar]
- Miller A. J., Loizzi R. F. Anatomical and functional differentiation of superior laryngeal nerve fibers affecting swallowing and respiration. Exp Neurol. 1974 Feb;42(2):369–387. doi: 10.1016/0014-4886(74)90033-8. [DOI] [PubMed] [Google Scholar]
- Mori M., Sakai Y. Re-examination of centrally-induced cough in cats using a micro-stimulation technique. Jpn J Pharmacol. 1972 Oct;22(5):635–643. doi: 10.1254/jjp.22.635. [DOI] [PubMed] [Google Scholar]
- Nishino T., Honda Y., Kohchi T., Shirahata M., Yonezawa T. Effects of increasing depth of anaesthesia on phrenic nerve and hypoglossal nerve activity during the swallowing reflex in cats. Br J Anaesth. 1985 Feb;57(2):208–213. doi: 10.1093/bja/57.2.208. [DOI] [PubMed] [Google Scholar]
- Oku Y., Tanaka I., Ezure K. Possible inspiratory off-switch neurones in the ventrolateral medulla of the cat. Neuroreport. 1992 Oct;3(10):933–936. doi: 10.1097/00001756-199210000-00029. [DOI] [PubMed] [Google Scholar]
- Orem J., Brooks E. G. The activity of retrofacial expiratory cells during behavioral respiratory responses and active expiration. Brain Res. 1986 May 28;374(2):409–412. doi: 10.1016/0006-8993(86)90440-3. [DOI] [PubMed] [Google Scholar]
- Orem J., Trotter R. H. Medullary respiratory neuronal activity during augmented breaths in intact unanesthetized cats. J Appl Physiol (1985) 1993 Feb;74(2):761–769. doi: 10.1152/jappl.1993.74.2.761. [DOI] [PubMed] [Google Scholar]
- Price W. M., Batsel H. L. Respiratory neurons participating in sneeze and in response to resistance to expiration. Exp Neurol. 1970 Dec;29(3):554–570. doi: 10.1016/0014-4886(70)90080-4. [DOI] [PubMed] [Google Scholar]
- Remmers J. E., Richter D. W., Ballantyne D., Bainton C. R., Klein J. P. Reflex prolongation of stage I of expiration. Pflugers Arch. 1986 Aug;407(2):190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- SUMI T. The activity of brain-stem respiratory neurons and spinal respiratory motoneurons during swallowing. J Neurophysiol. 1963 May;26:466–477. doi: 10.1152/jn.1963.26.3.466. [DOI] [PubMed] [Google Scholar]
- Tomori Z., Widdicombe J. G. Muscular, bronchomotor and cardiovascular reflexes elicited by mechanical stimulation of the respiratory tract. J Physiol. 1969 Jan;200(1):25–49. doi: 10.1113/jphysiol.1969.sp008680. [DOI] [PMC free article] [PubMed] [Google Scholar]


