Abstract
1. The role of the giant neuropile glial cells in the buffering of activity-related extracellular pH changes was studied in segmental ganglia of the leech Hirudo medicinalis L. using pH-sensitive microelectrodes and a slow, two-electrode voltage-clamp system. Neuronal activity was induced by electrical stimulation of a ganglionic side nerve (20 Hz, 1 min). 2. In CO2-HCO3(-)-buffered saline the glial cells were depolarized by 6.5 +/- 2.3 mV and alkalinized by 0.024 +/- 0.006 pH units (mean +/- SD) during the stimulation. The stimulation induced an acidification of 0.032 +/- 0.006 pH units in the extracellular spaces (ECS). 3. Voltage clamping the glial cells suppressed the stimulus-induced glial depolarization and turned the intraglial alkalinization into an acidification of 0.045 +/- 0.021 pH units (n = 6) that closely resembled the acidification observed in the presence of the anion transport blocker DIDS (4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid, 0.5 mM), and in CO2-HCO(3-)-free saline. 4. Voltage clamping the glial cell resulted in the appearance of a distinct stimulus-induced extracellular alkalinization of 0.024 +/- 0.013 pH units at the onset of the stimulation, as also observed during DIDS application and in the absence of CO2-HCO3-. 5. The results suggest that glial uptake of bicarbonate is mediated by depolarization-induced activation of the electrogenic Na(+)-HCO3- cotransport, which suppresses the profound alkalinization of the ECS during neuronal activity. This is the first direct evidence the glial cells actively modulate extracellular pH changes in a voltage-dependent manner.
Full text
PDF

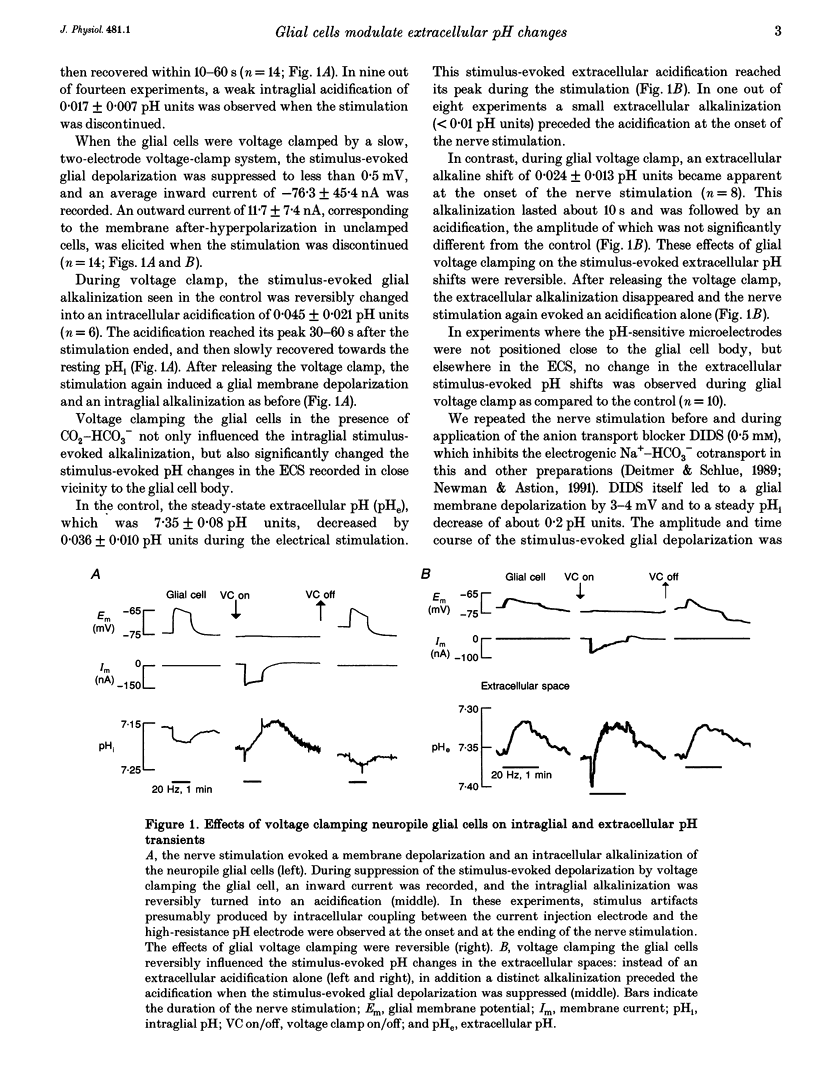

Selected References
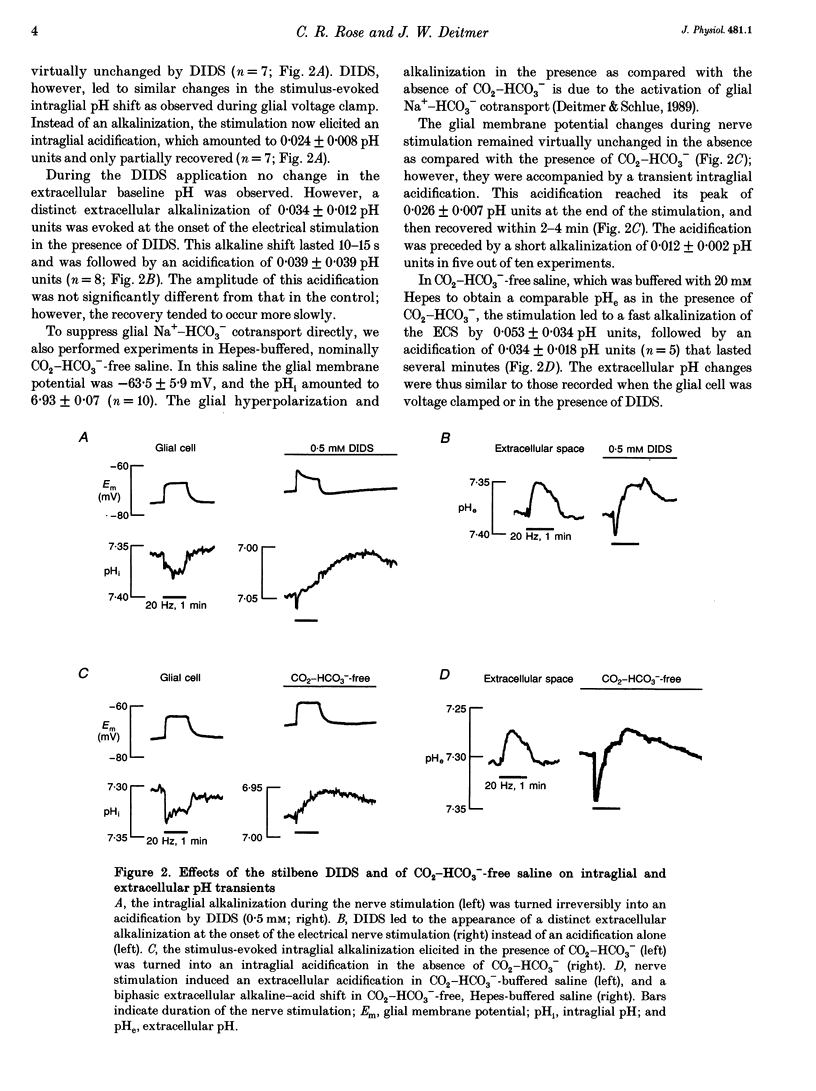
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Intracellular pH changes induced by calcium influx during electrical activity in molluscan neurons. J Gen Physiol. 1980 Apr;75(4):403–426. doi: 10.1085/jgp.75.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aram J. A., Lodge D. Epileptiform activity induced by alkalosis in rat neocortical slices: block by antagonists of N-methyl-D-aspartate. Neurosci Lett. 1987 Dec 29;83(3):345–350. doi: 10.1016/0304-3940(87)90112-1. [DOI] [PubMed] [Google Scholar]
- Balestrino M., Somjen G. G. Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. J Physiol. 1988 Feb;396:247–266. doi: 10.1113/jphysiol.1988.sp016961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M., Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992 Oct;15(10):396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Chesler M., Kraig R. P. Intracellular pH transients of mammalian astrocytes. J Neurosci. 1989 Jun;9(6):2011–2019. doi: 10.1523/JNEUROSCI.09-06-02011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34(5):401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Coles J. A. Functions of glial cells in the retina of the honeybee drone. Glia. 1989;2(1):1–9. doi: 10.1002/glia.440020102. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W. Electrogenic sodium-dependent bicarbonate secretion by glial cells of the leech central nervous system. J Gen Physiol. 1991 Sep;98(3):637–655. doi: 10.1085/jgp.98.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W. Evidence for glial control of extracellular pH in the leech central nervous system. Glia. 1992;5(1):43–47. doi: 10.1002/glia.440050107. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Schlue W. R. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol. 1989 Apr;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Szatkowski M. Membrane potential dependence of intracellular pH regulation by identified glial cells in the leech central nervous system. J Physiol. 1990 Feb;421:617–631. doi: 10.1113/jphysiol.1990.sp017965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolimek W., Misgeld U., Lux H. D. Activity dependent alkaline and acid transients in guinea pig hippocampal slices. Brain Res. 1989 Dec 29;505(2):225–232. doi: 10.1016/0006-8993(89)91447-9. [DOI] [PubMed] [Google Scholar]
- Munsch T., Deitmer J. W. Sodium-bicarbonate cotransport current in identified leech glial cells. J Physiol. 1994 Jan 1;474(1):43–53. doi: 10.1113/jphysiol.1994.sp020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. A., Astion M. L. Localization and stoichiometry of electrogenic sodium bicarbonate cotransport in retinal glial cells. Glia. 1991;4(4):424–428. doi: 10.1002/glia.440040411. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Carlini W. G., Connors B. W. Brain extracellular space: developmental studies in rat optic nerve. Ann N Y Acad Sci. 1986;481:87–105. doi: 10.1111/j.1749-6632.1986.tb27141.x. [DOI] [PubMed] [Google Scholar]
- Ransom B. R. Glial modulation of neural excitability mediated by extracellular pH: a hypothesis. Prog Brain Res. 1992;94:37–46. doi: 10.1016/s0079-6123(08)61737-9. [DOI] [PubMed] [Google Scholar]
- Syková E., Jendelová P., Simonová Z., Chvátal A. K+ and pH homeostasis in the developing rat spinal cord is impaired by early postnatal X-irradiation. Brain Res. 1992 Oct 23;594(1):19–30. doi: 10.1016/0006-8993(92)91025-a. [DOI] [PubMed] [Google Scholar]
- Voipio J., Kaila K. Interstitial PCO2 and pH in rat hippocampal slices measured by means of a novel fast CO2/H(+)-sensitive microelectrode based on a PVC-gelled membrane. Pflugers Arch. 1993 May;423(3-4):193–201. doi: 10.1007/BF00374394. [DOI] [PubMed] [Google Scholar]


