Abstract
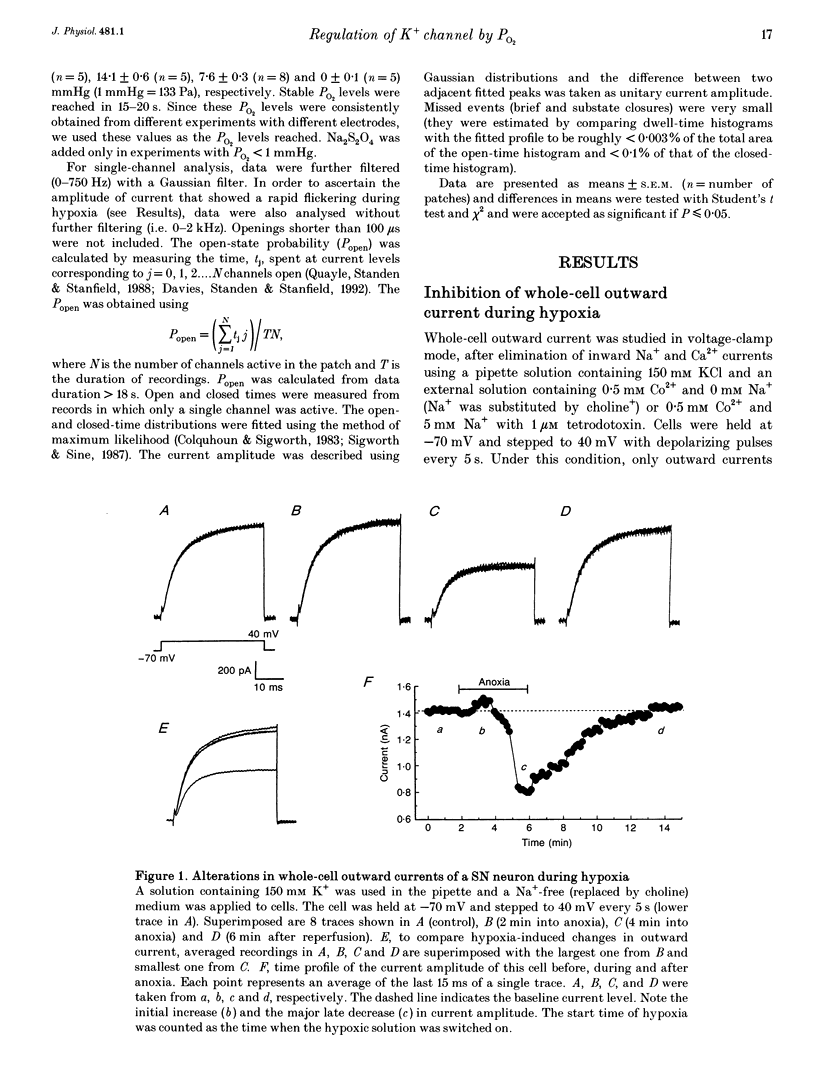
1. K+ channel modulation has been shown to be an integral and important cellular response to O2 deprivation. Although part of this modulation occurs as a result of changes in concentrations of several cytosolic factors such as ATP and Ca2+, it is unknown whether there are mechanisms other than those originating from the cytosol. To test the hypothesis that membrane-delimited mechanisms participate in the O2-sensing process and are involved in the modulation of K+ channel activity in central neurons, we performed experiments using patch-clamp techniques and dissociated cells from the rat neocortex and substantia nigra. 2. Whole-cell outward currents were studied in voltage-clamp mode using Na(+)-free or low-Na+ (5 mM, with 1 microM tetrodotoxin) extracellular medium plus 0.5 mM Co2+. O2 deprivation produced a biphasic response in current amplitude, i.e. an initial transient increase followed by a pronounced decrease in outward currents. The reduction in outward currents was a reversible process since perfusion with a medium of PO2 > 100 mmHg (1 mmHg = 133 Pa) led to a complete recovery. 3. In cell-free excised membrane patches, we found that a specific K+ current (large conductance, inhibited by micromolar concentrations of ATP and activated by Ca2+) was reversibly inhibited by lack of O2. This was characterized by a marked decrease in channel open-state probability and a slight reduction in unitary conductance. The magnitude of channel inhibition by O2 deprivation was closely dependent on O2 tension. The PO2 level for 50% channel inhibition was about 10 mmHg with little or no inhibition at PO2 > or = 20 mmHg. 4. Single-channel kinetic analysis showed that channel open times consisted of two components and closed times were composed of three. The hypoxia-induced inhibition of K+ channel activity was mediated by selective suppression of the longer time constant channel openings without significantly affecting closed time constants. This led to an increase in frequency of opening and closing and rapid channel flickerings. 5. Our data showed that O2 deprivation had no effect on another K+ current characterized by a much smaller conductance and Ca2+ independence. This provides evidence for the selective nature of the hypoxia-induced inhibition of some species of K+ channels. 6. These results therefore provide the first evidence for regulation of K+ channel activity by O2 deprivation in cell-free excised patches from central neurons.
Full text
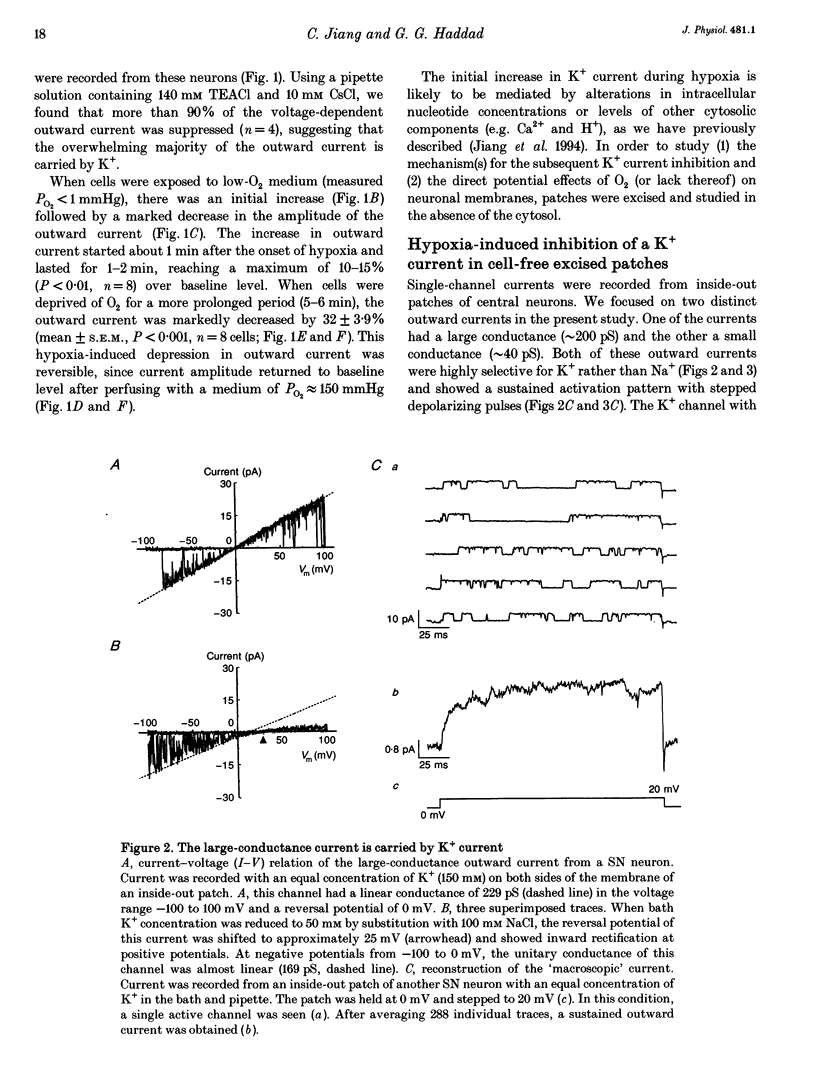
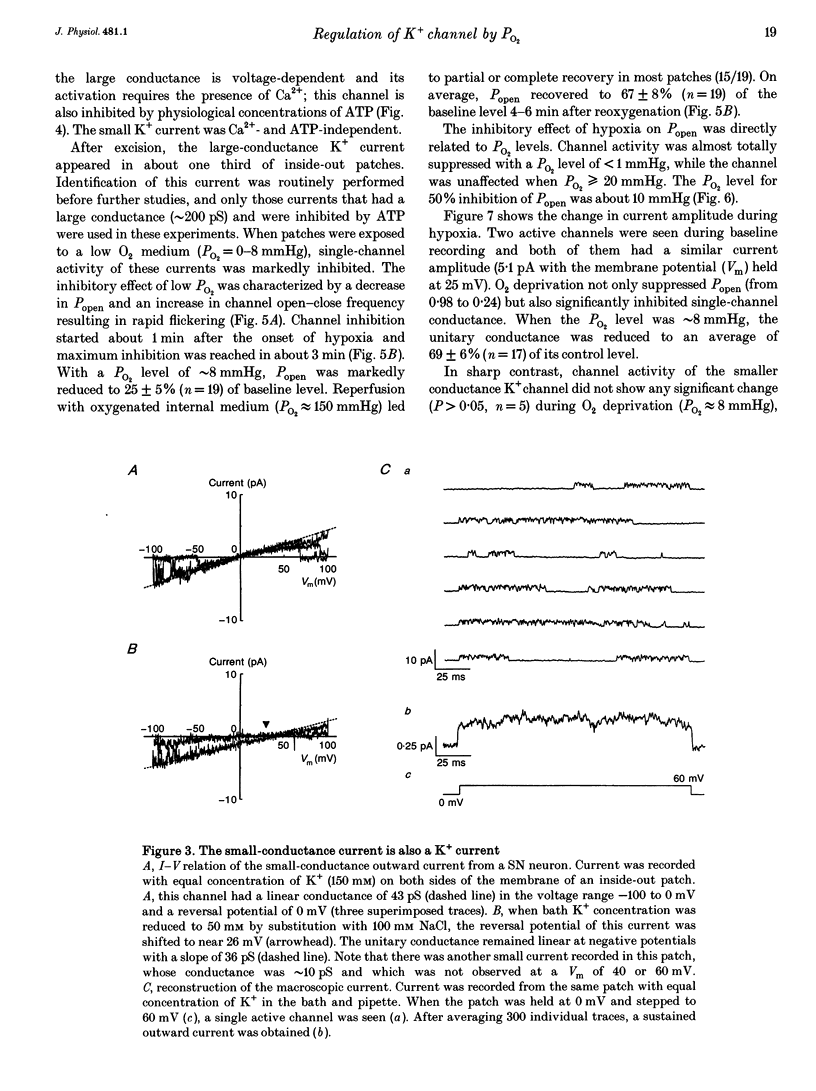
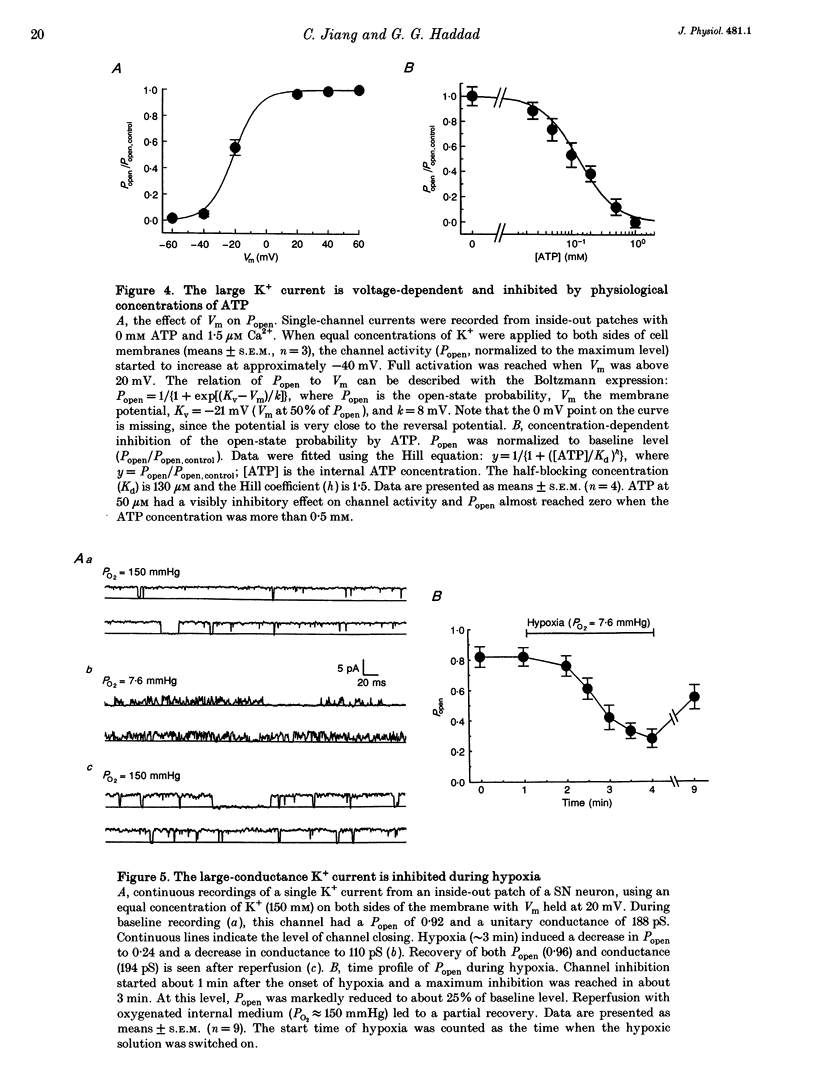
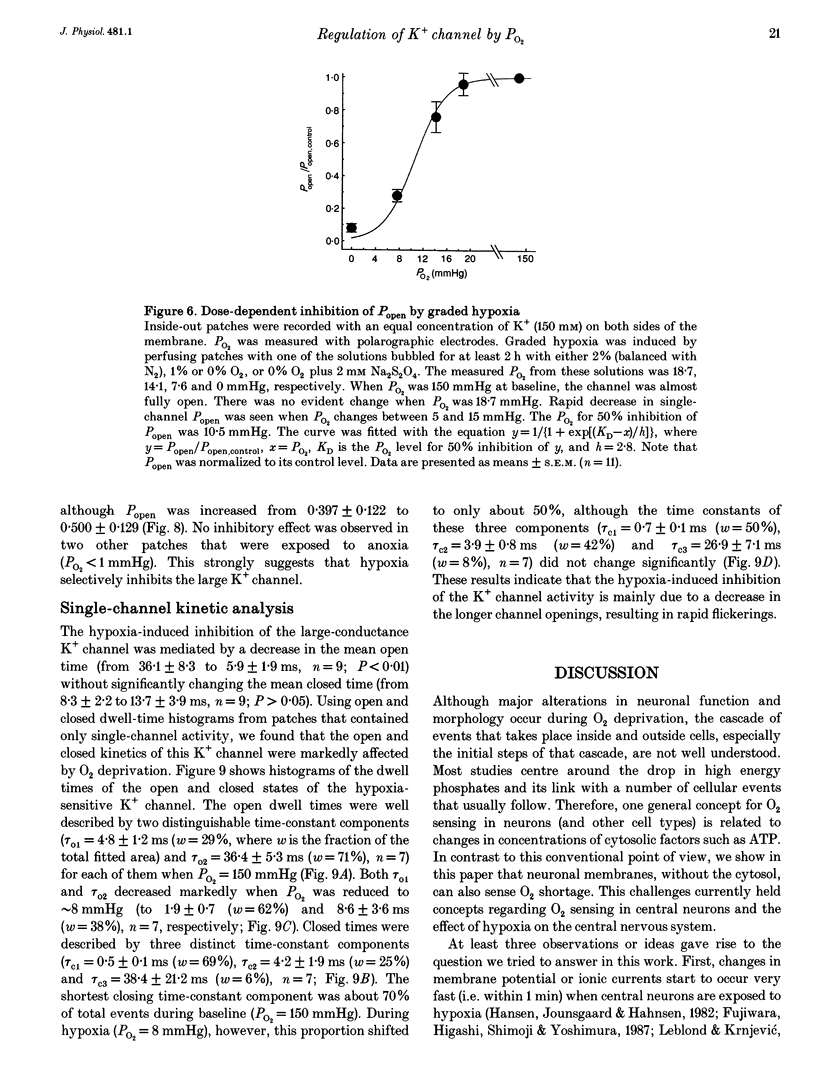
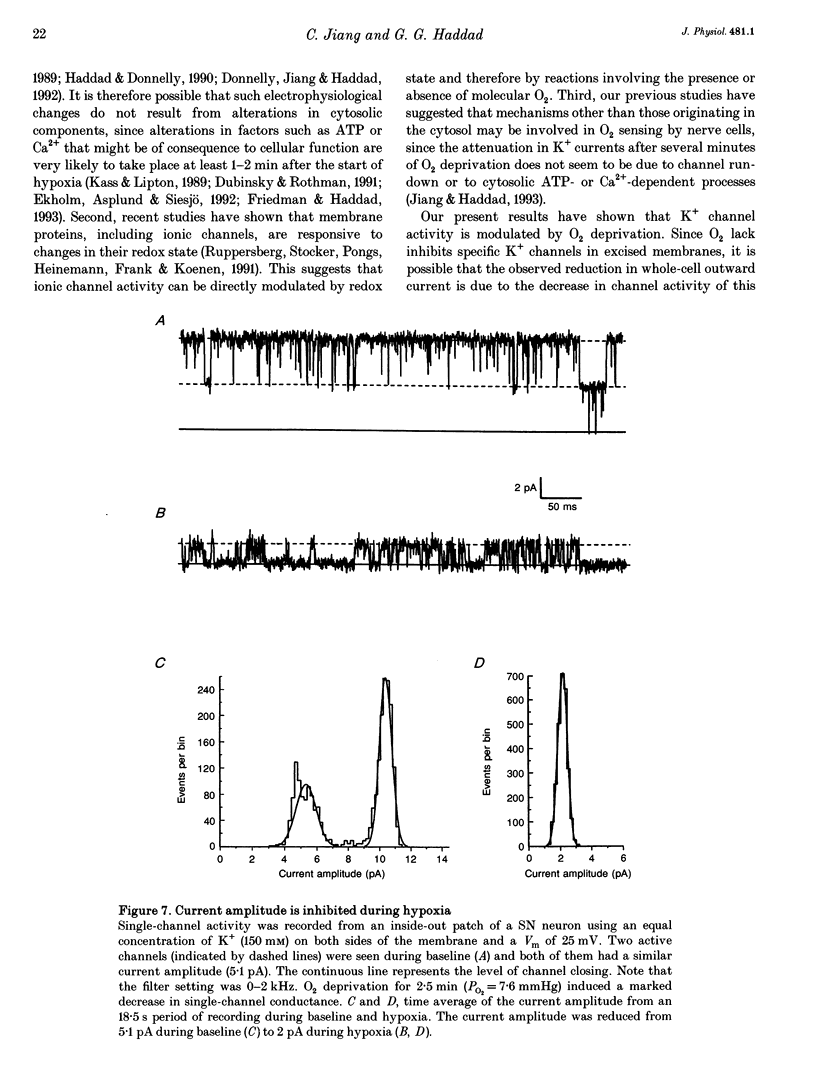
PDF


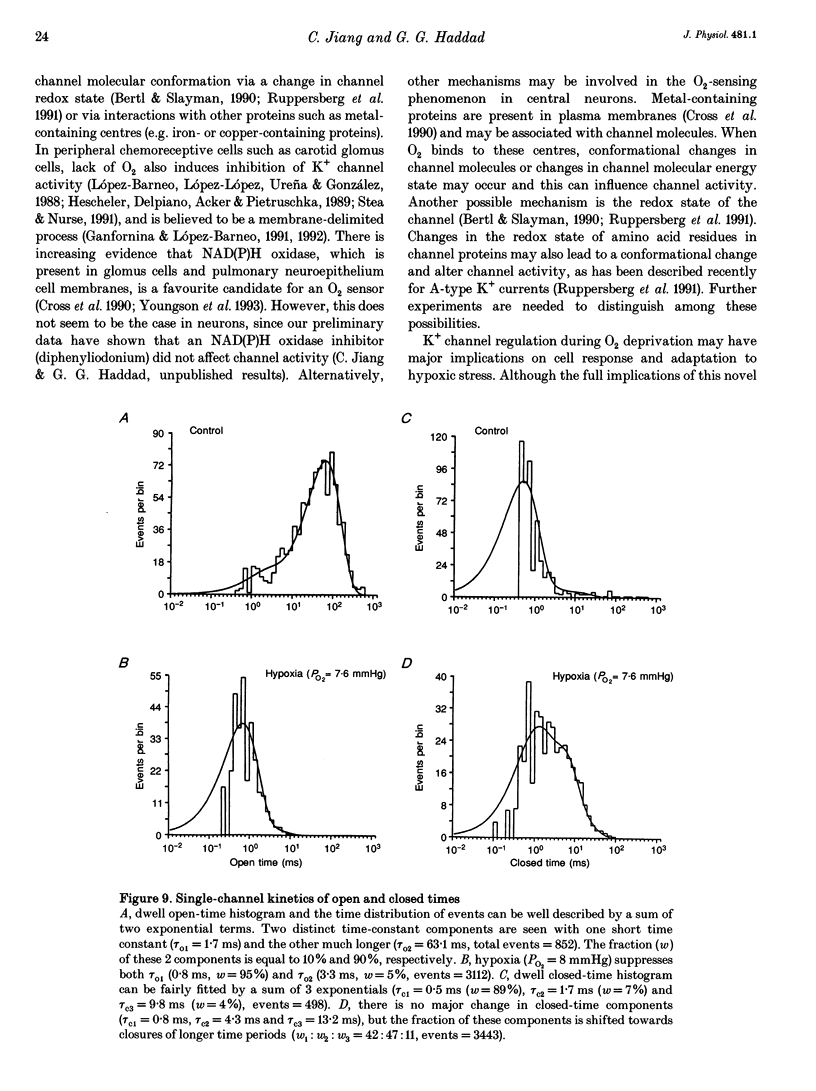


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertl A., Slayman C. L. Cation-selective channels in the vacuolar membrane of Saccharomyces: dependence on calcium, redox state, and voltage. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7824–7828. doi: 10.1073/pnas.87.20.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Henderson L., Jones O. T., Delpiano M. A., Hentschel J., Acker H. Involvement of an NAD(P)H oxidase as a pO2 sensor protein in the rat carotid body. Biochem J. 1990 Dec 15;272(3):743–747. doi: 10.1042/bj2720743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins T. R., Jiang C., Haddad G. G. Human neocortical excitability is decreased during anoxia via sodium channel modulation. J Clin Invest. 1993 Feb;91(2):608–615. doi: 10.1172/JCI116241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. W., Standen N. B., Stanfield P. R. The effect of intracellular pH on ATP-dependent potassium channels of frog skeletal muscle. J Physiol. 1992 Jan;445:549–568. doi: 10.1113/jphysiol.1992.sp018939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domesick V. B., Stinus L., Paskevich P. A. The cytology of dopaminergic and nondopaminergic neurons in the substantia nigra and ventral tegmental area of the rat: a light- and electron-microscopic study. Neuroscience. 1983 Apr;8(4):743–765. doi: 10.1016/0306-4522(83)90007-6. [DOI] [PubMed] [Google Scholar]
- Donnelly D. F., Jiang C., Haddad G. G. Comparative responses of brain stem and hippocampal neurons to O2 deprivation: in vitro intracellular studies. Am J Physiol. 1992 May;262(5 Pt 1):L549–L554. doi: 10.1152/ajplung.1992.262.5.L549. [DOI] [PubMed] [Google Scholar]
- Dubinsky J. M., Rothman S. M. Intracellular calcium concentrations during "chemical hypoxia" and excitotoxic neuronal injury. J Neurosci. 1991 Aug;11(8):2545–2551. doi: 10.1523/JNEUROSCI.11-08-02545.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm A., Asplund B., Siesjö B. K. Perturbation of cellular energy state in complete ischemia: relationship to dissipative ion fluxes. Exp Brain Res. 1992;90(1):47–53. doi: 10.1007/BF00229255. [DOI] [PubMed] [Google Scholar]
- Friedman J. E., Haddad G. G. Major differences in Ca2+i response to anoxia between neonatal and adult rat CA1 neurons: role of Ca2+o and Na+o. J Neurosci. 1993 Jan;13(1):63–72. doi: 10.1523/JNEUROSCI.13-01-00063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N., Higashi H., Shimoji K., Yoshimura M. Effects of hypoxia on rat hippocampal neurones in vitro. J Physiol. 1987 Mar;384:131–151. doi: 10.1113/jphysiol.1987.sp016447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina M. D., López-Barneo J. Potassium channel types in arterial chemoreceptor cells and their selective modulation by oxygen. J Gen Physiol. 1992 Sep;100(3):401–426. doi: 10.1085/jgp.100.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina M. D., López-Barneo J. Single K+ channels in membrane patches of arterial chemoreceptor cells are modulated by O2 tension. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2927–2930. doi: 10.1073/pnas.88.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. F., Lowenstein D., Fernyak S., Hisanaga K., Simon R., Sharp F. R. Induction of heat shock protein 72-like immunoreactivity in the hippocampal formation following transient global ischemia. Brain Res Bull. 1991 Feb;26(2):241–250. doi: 10.1016/0361-9230(91)90234-b. [DOI] [PubMed] [Google Scholar]
- Grace A. A., Onn S. P. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989 Oct;9(10):3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad G. G., Donnelly D. F. O2 deprivation induces a major depolarization in brain stem neurons in the adult but not in the neonatal rat. J Physiol. 1990 Oct;429:411–428. doi: 10.1113/jphysiol.1990.sp018265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad G. G., Jiang C. Mechanisms of anoxia-induced depolarization in brainstem neurons: in vitro current and voltage clamp studies in the adult rat. Brain Res. 1993 Oct 22;625(2):261–268. doi: 10.1016/0006-8993(93)91067-3. [DOI] [PubMed] [Google Scholar]
- Haddad G. G., Jiang C. O2 deprivation in the central nervous system: on mechanisms of neuronal response, differential sensitivity and injury. Prog Neurobiol. 1993 Mar;40(3):277–318. doi: 10.1016/0301-0082(93)90014-j. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hansen A. J. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985 Jan;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Hansen A. J., Hounsgaard J., Jahnsen H. Anoxia increases potassium conductance in hippocampal nerve cells. Acta Physiol Scand. 1982 Jul;115(3):301–310. doi: 10.1111/j.1748-1716.1982.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Delpiano M. A., Acker H., Pietruschka F. Ionic currents on type-I cells of the rabbit carotid body measured by voltage-clamp experiments and the effect of hypoxia. Brain Res. 1989 May 1;486(1):79–88. doi: 10.1016/0006-8993(89)91280-8. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W. Defense strategies against hypoxia and hypothermia. Science. 1986 Jan 17;231(4735):234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Jiang C., Agulian S., Haddad G. G. O2 tension in adult and neonatal brain slices under several experimental conditions. Brain Res. 1991 Dec 24;568(1-2):159–164. doi: 10.1016/0006-8993(91)91392-e. [DOI] [PubMed] [Google Scholar]
- Jiang C., Haddad G. G. Differential responses of neocortical neurons to glucose and/or O2 deprivation in the human and rat. J Neurophysiol. 1992 Dec;68(6):2165–2173. doi: 10.1152/jn.1992.68.6.2165. [DOI] [PubMed] [Google Scholar]
- Jiang C., Haddad G. G. Effect of anoxia on intracellular and extracellular potassium activity in hypoglossal neurons in vitro. J Neurophysiol. 1991 Jul;66(1):103–111. doi: 10.1152/jn.1991.66.1.103. [DOI] [PubMed] [Google Scholar]
- Jiang C., Haddad G. G. Short periods of hypoxia activate a K+ current in central neurons. Brain Res. 1993 Jun 18;614(1-2):352–356. doi: 10.1016/0006-8993(93)91055-w. [DOI] [PubMed] [Google Scholar]
- Jiang C., Sigworth F. J., Haddad G. G. Oxygen deprivation activates an ATP-inhibitable K+ channel in substantia nigra neurons. J Neurosci. 1994 Sep;14(9):5590–5602. doi: 10.1523/JNEUROSCI.14-09-05590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Xia Y., Haddad G. G. Role of ATP-sensitive K+ channels during anoxia: major differences between rat (newborn and adult) and turtle neurons. J Physiol. 1992 Mar;448:599–612. doi: 10.1113/jphysiol.1992.sp019060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku D. A., Giffard R. G., Choi D. W. Neuroprotective effects of glutamate antagonists and extracellular acidity. Science. 1993 Jun 4;260(5113):1516–1518. doi: 10.1126/science.8389056. [DOI] [PubMed] [Google Scholar]
- Kass I. S., Lipton P. Protection of hippocampal slices from young rats against anoxic transmission damage is due to better maintenance of ATP. J Physiol. 1989 Jun;413:1–11. doi: 10.1113/jphysiol.1989.sp017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods. 1986 May;16(3):227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Leblond J. Changes in membrane currents of hippocampal neurons evoked by brief anoxia. J Neurophysiol. 1989 Jul;62(1):15–30. doi: 10.1152/jn.1989.62.1.15. [DOI] [PubMed] [Google Scholar]
- Kuo S. S., Saad A. H., Koong A. C., Hahn G. M., Giaccia A. J. Potassium-channel activation in response to low doses of gamma-irradiation involves reactive oxygen intermediates in nonexcitatory cells. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):908–912. doi: 10.1073/pnas.90.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond J., Krnjevic K. Hypoxic changes in hippocampal neurons. J Neurophysiol. 1989 Jul;62(1):1–14. doi: 10.1152/jn.1989.62.1.1. [DOI] [PubMed] [Google Scholar]
- Levitan E. S., Kramer R. H. Neuropeptide modulation of single calcium and potassium channels detected with a new patch clamp configuration. Nature. 1990 Dec 6;348(6301):545–547. doi: 10.1038/348545a0. [DOI] [PubMed] [Google Scholar]
- López-Barneo J., López-López J. R., Ureña J., González C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988 Jul 29;241(4865):580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- Murphy K. P., Greenfield S. A. Neuronal selectivity of ATP-sensitive potassium channels in guinea-pig substantia nigra revealed by responses to anoxia. J Physiol. 1992;453:167–183. doi: 10.1113/jphysiol.1992.sp019222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post J. M., Hume J. R., Archer S. L., Weir E. K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992 Apr;262(4 Pt 1):C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., Standen N. B., Stanfield P. R. The voltage-dependent block of ATP-sensitive potassium channels of frog skeletal muscle by caesium and barium ions. J Physiol. 1988 Nov;405:677–697. doi: 10.1113/jphysiol.1988.sp017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rordorf G., Koroshetz W. J., Bonventre J. V. Heat shock protects cultured neurons from glutamate toxicity. Neuron. 1991 Dec;7(6):1043–1051. doi: 10.1016/0896-6273(91)90348-4. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A., Nurse C. A. Whole-cell and perforated-patch recordings from O2-sensitive rat carotid body cells grown in short- and long-term culture. Pflugers Arch. 1991 Mar;418(1-2):93–101. doi: 10.1007/BF00370457. [DOI] [PubMed] [Google Scholar]
- Xia Y., Jiang C., Haddad G. G. Oxidative and glycolytic pathways in rat (newborn and adult) and turtle brain: role during anoxia. Am J Physiol. 1992 Apr;262(4 Pt 2):R595–R603. doi: 10.1152/ajpregu.1992.262.4.R595. [DOI] [PubMed] [Google Scholar]
- Youngson C., Nurse C., Yeger H., Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993 Sep 9;365(6442):153–155. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]
- Yuan X. J., Goldman W. F., Tod M. L., Rubin L. J., Blaustein M. P. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol. 1993 Feb;264(2 Pt 1):L116–L123. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]


