Abstract
1. A single dose of tetanus toxin, injected under anaesthesia into one dorsal hippocampus of the rat, produces chronic epileptic foci involving both hippocampi. Generalized seizures occurred 1-6 weeks after injection and epileptic discharges were found in hippocampal slices in vitro. Here we measured the time course of decay of epileptic activity and the level of GABAA receptor-mediated inhibition in hippocampal slices 1-16 weeks after toxin injection in vivo. 2. Epileptic activity peaked in the dentate granule cell and CA3 pyramidal cell layers 2 weeks after toxin injection and at 4 weeks in CA1. Thresholds for evoking epileptic activity were lowest in the suprapyramidal blade of the dentate gyrus and area CA3c. Recovery from epileptic activity occurred more rapidly in the contralateral hippocampus. Polyspike activity ceased by 8 weeks and interictal activity by 16 weeks. Epileptic discharges could still be evoked from CA1 16 weeks after toxin injection. 3. The maximal monosynaptic fast inhibitory postsynaptic current (IPSC) conductance changes (gIPSC) decreased to < 10% of control values at the time of peak epileptic activity and remained lower than controls for 4 weeks ipsilaterally. In the contralateral hippocampus, gIPSC fell to ca 50% of control values for the first 2 weeks. Responses to exogenous GABA remained unchanged. 4. After 8 weeks dentate granule cells had gIPSC significantly larger than controls. No increase in gIPSC occurred in CA3. Epileptic activity persisted 8-10 weeks after recovery from disinhibition ipsilaterally and 4 weeks contralaterally. 5. Epileptic activity was seen when monosynaptic GABAA receptor-mediated IPSCs were normal or supranormal. At these times polysynaptic inhibition was still profoundly reduced. These observations provide strong evidence for long-term changes in the pattern of synaptic excitation contributing to a chronic epileptic syndrome syndrome following disinhibitory insult, and are consistent with weakened excitation of inhibitory neurones.
Full text
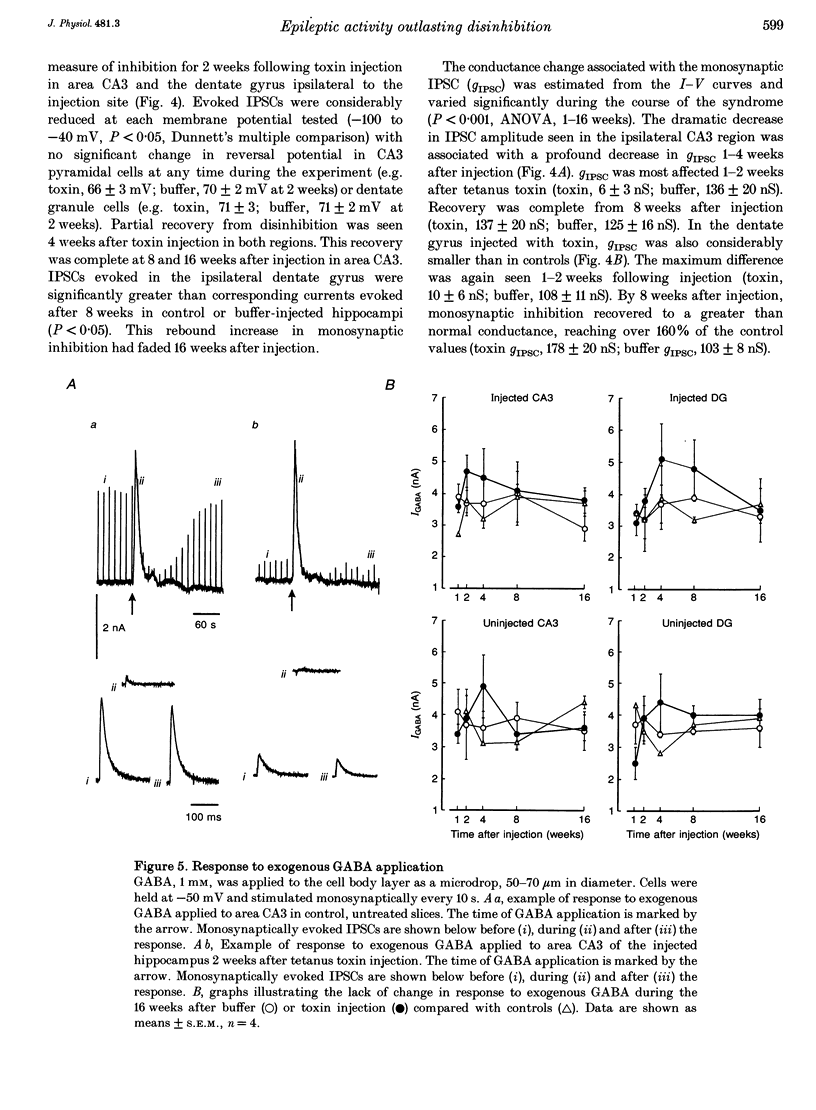
PDF

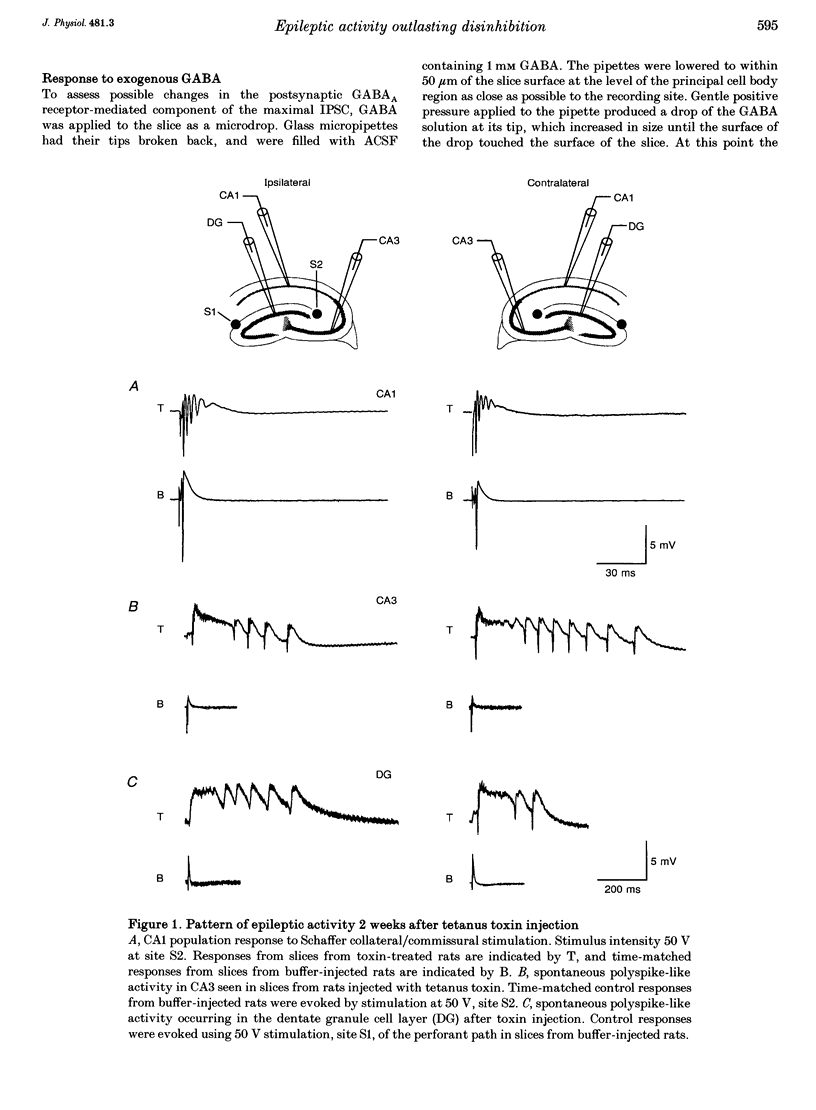
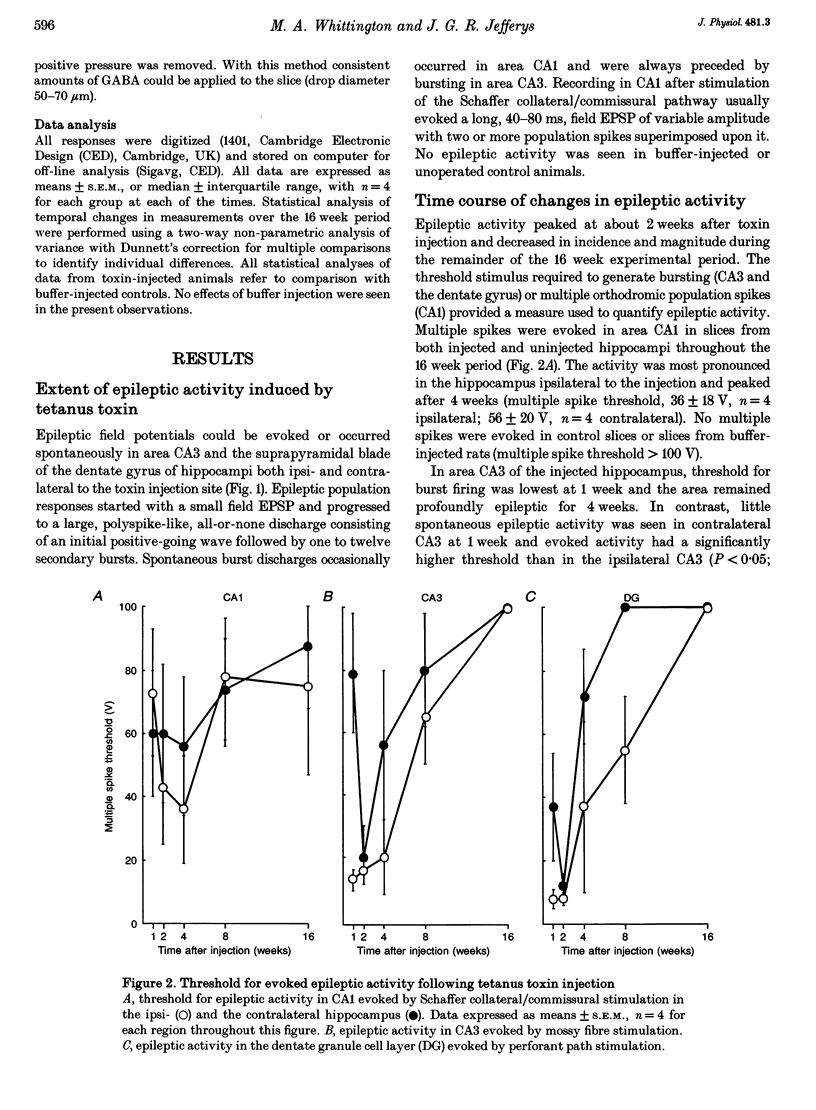
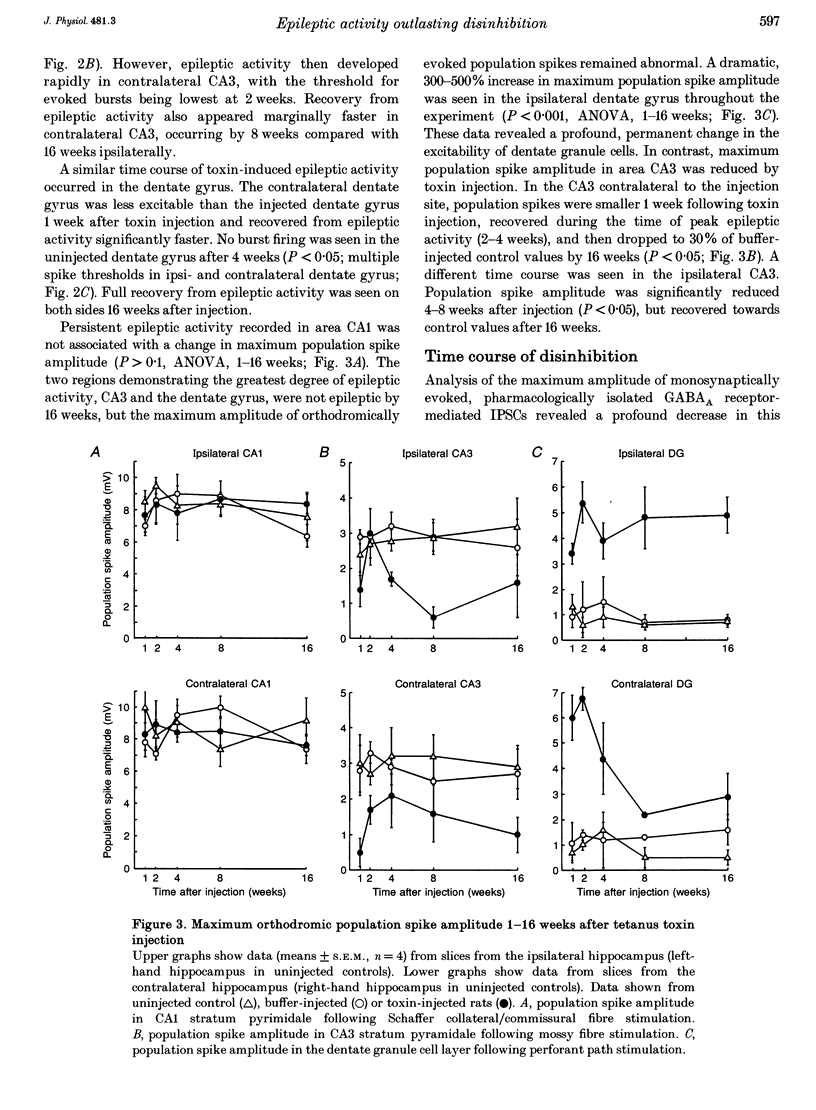
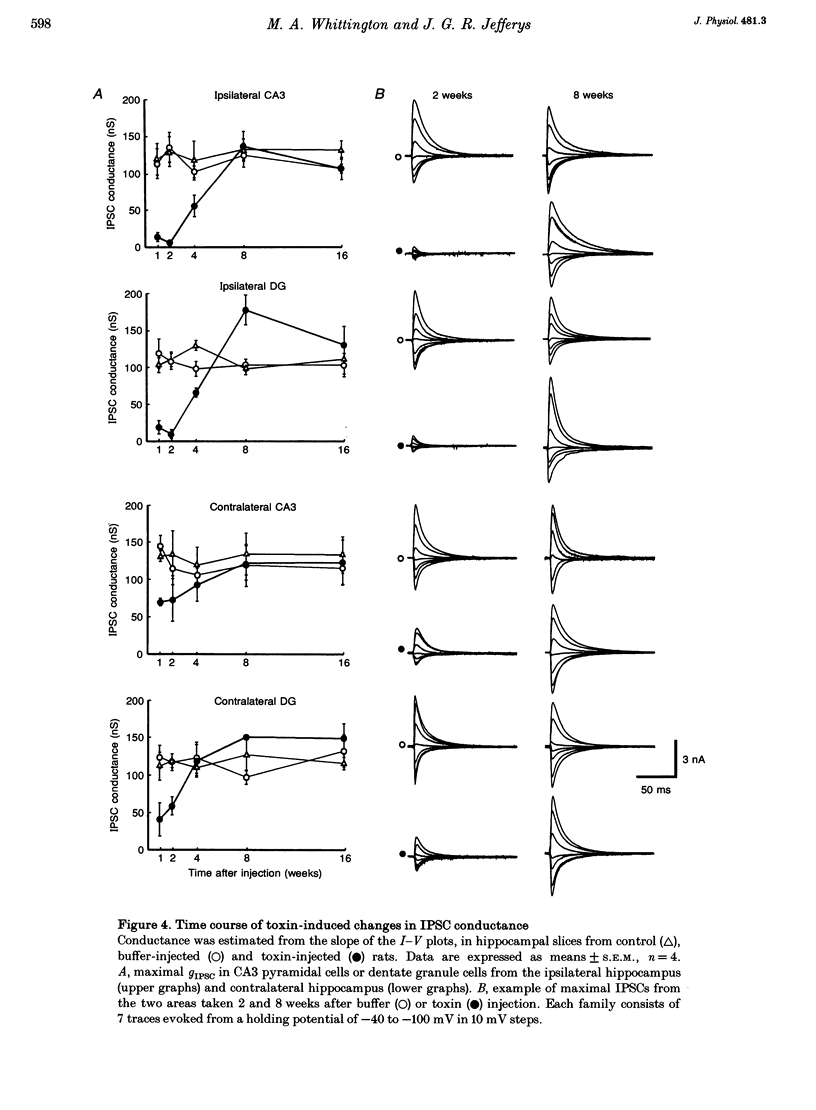



Images in this article
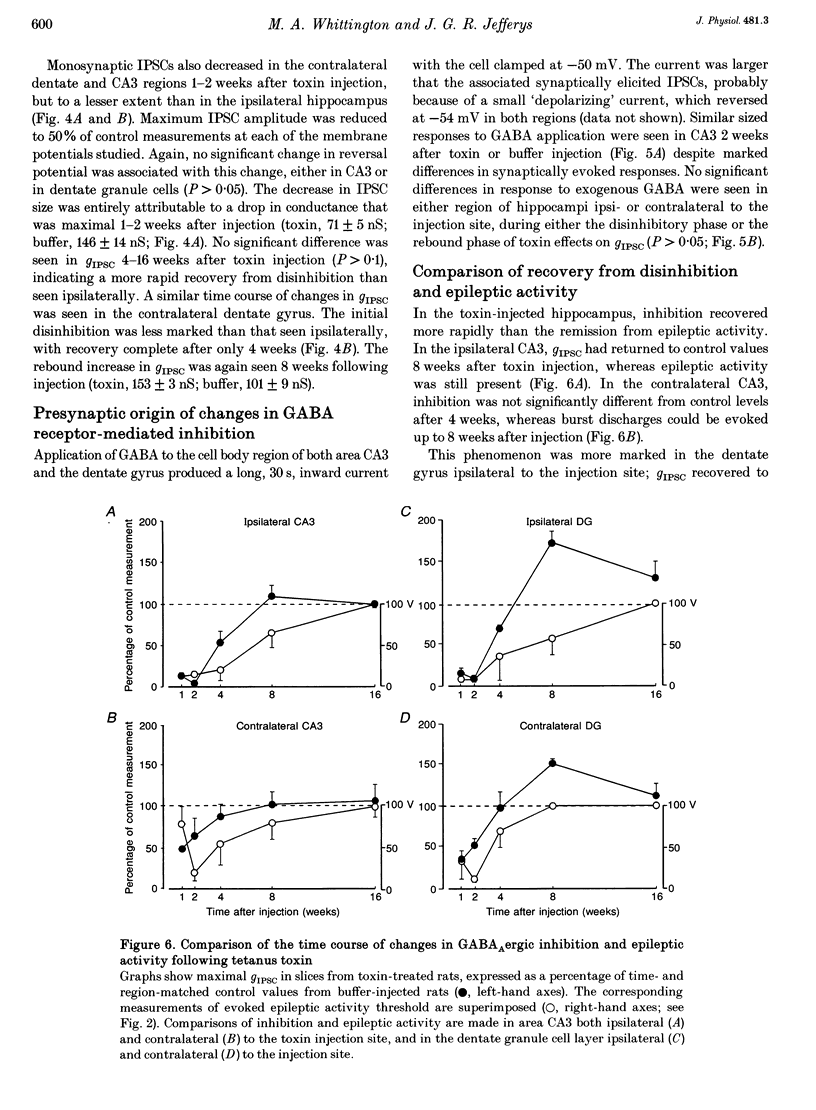
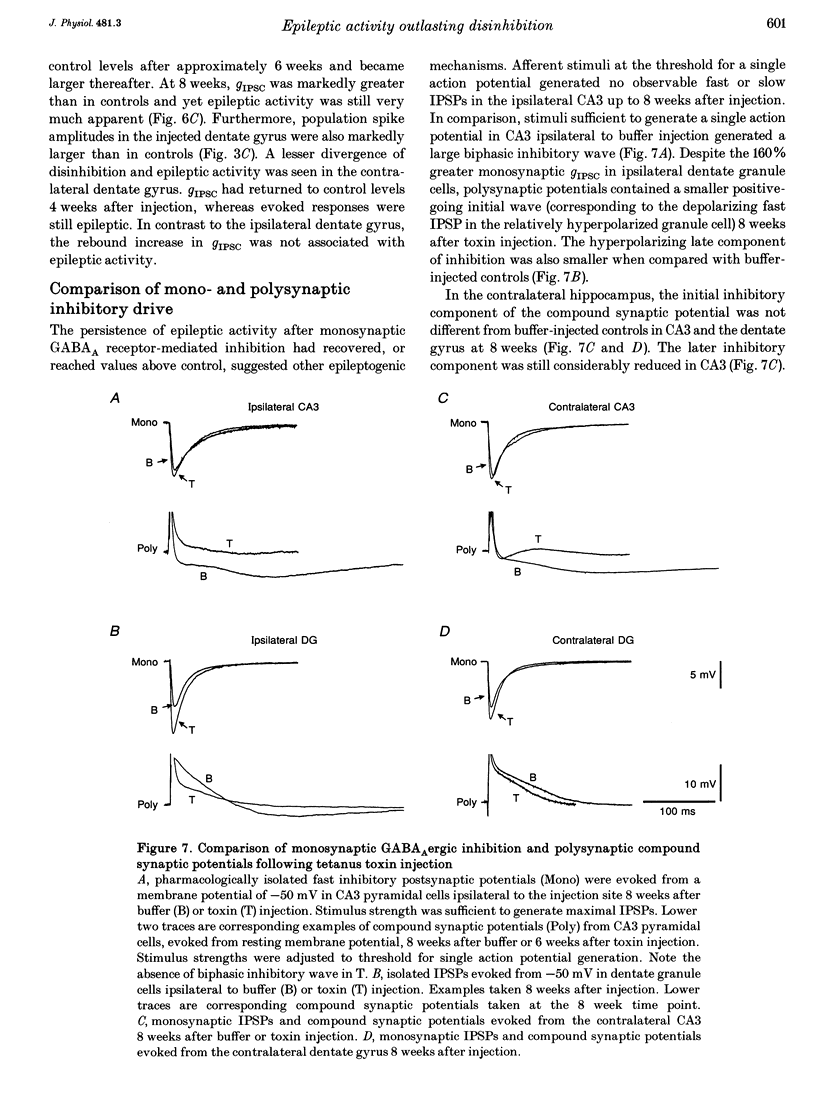
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekenstein J. W., Lothman E. W. Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science. 1993 Jan 1;259(5091):97–100. doi: 10.1126/science.8093417. [DOI] [PubMed] [Google Scholar]
- Bergey G. K., MacDonald R. L., Habig W. H., Hardegree M. C., Nelson P. G. Tetanus toxin: convulsant action on mouse spinal cord neurons in culture. J Neurosci. 1983 Nov;3(11):2310–2323. doi: 10.1523/JNEUROSCI.03-11-02310.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Wendon L. M. A study of the action of tetanus toxin at rat soleus neuromuscular junctions. J Physiol. 1984 Mar;348:1–17. doi: 10.1113/jphysiol.1984.sp015095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner M. A., Holz R. W. Effects of tetanus toxin on catecholamine release from intact and digitonin-permeabilized chromaffin cells. J Neurochem. 1988 Aug;51(2):451–456. doi: 10.1111/j.1471-4159.1988.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Brace H. M., Jefferys J. G., Mellanby J. Long-term changes in hippocampal physiology and learning ability of rats after intrahippocampal tetanus toxin. J Physiol. 1985 Nov;368:343–357. doi: 10.1113/jphysiol.1985.sp015861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener K., Amitai Y., Jefferys J. G., Gutnick M. J. Chronic epileptic foci in neocortex: in vivo and in vitro effects of tetanus toxin. Eur J Neurosci. 1991;3(1):47–54. doi: 10.1111/j.1460-9568.1991.tb00810.x. [DOI] [PubMed] [Google Scholar]
- Cavazos J. E., Golarai G., Sutula T. P. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci. 1991 Sep;11(9):2795–2803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Thompson P. A., Davies J., Mellanby J. In vitro effect of tetanus toxin on GABA release form rat hippocampal slices. J Neurochem. 1981 Oct;37(4):1039–1041. doi: 10.1111/j.1471-4159.1981.tb04492.x. [DOI] [PubMed] [Google Scholar]
- Cornish S. M., Wheal H. V. Long-term loss of paired pulse inhibition in the kainic acid-lesioned hippocampus of the rat. Neuroscience. 1989;28(3):563–571. doi: 10.1016/0306-4522(89)90005-5. [DOI] [PubMed] [Google Scholar]
- Darcey T. M., Williamson P. D. Chronic/semichronic limbic epilepsy produced by microinjection of tetanus toxin in cat hippocampus. Epilepsia. 1992 May-Jun;33(3):402–419. doi: 10.1111/j.1528-1157.1992.tb01684.x. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. N., Collingridge G. L. Role of excitatory amino acid receptors in synaptic transmission in area CA1 of rat hippocampus. Proc R Soc Lond B Biol Sci. 1989 May 22;236(1285):373–384. doi: 10.1098/rspb.1989.0028. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Tonge D. A. The effects of tetanus toxin on neuromuscular transmission and on the morphology of motor end-plates in slow and fast skeletal muscle of the mouse. J Physiol. 1973 Jan;228(1):157–172. doi: 10.1113/jphysiol.1973.sp010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empson R. M., Amitai Y., Jefferys J. G., Gutnick M. J. Injection of tetanus toxin into the neocortex elicits persistent epileptiform activity but only transient impairment of GABA release. Neuroscience. 1993 Nov;57(2):235–239. doi: 10.1016/0306-4522(93)90058-n. [DOI] [PubMed] [Google Scholar]
- Empson R. M., Jefferys J. G. Synaptic inhibition in primary and secondary chronic epileptic foci induced by intrahippocampal tetanus toxin in the rat. J Physiol. 1993 Jun;465:595–614. doi: 10.1113/jphysiol.1993.sp019695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George G., Mellanby J. Memory deficits in an experimental hippocampal epileptiform syndrome in rats. Exp Neurol. 1982 Mar;75(3):678–689. doi: 10.1016/0014-4886(82)90034-6. [DOI] [PubMed] [Google Scholar]
- Goddard G. V., McIntyre D. C., Leech C. K. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969 Nov;25(3):295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Bigalke H., Bergey G. K., Neale E. A., Hardegree M. C., Nelson P. G. Tetanus toxin in dissociated spinal cord cultures: long-term characterization of form and action. J Neurochem. 1986 Sep;47(3):930–937. doi: 10.1111/j.1471-4159.1986.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G., Empson R. M. Development of chronic secondary epileptic foci following intrahippocampal injection of tetanus toxin in the rat. Exp Physiol. 1990 Sep;75(5):733–736. doi: 10.1113/expphysiol.1990.sp003455. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G., Evans B. J., Hughes S. A., Williams S. F. Neuropathology of the chronic epileptic syndrome induced by intrahippocampal tetanus toxin in rat: preservation of pyramidal cells and incidence of dark cells. Neuropathol Appl Neurobiol. 1992 Feb;18(1):53–70. doi: 10.1111/j.1365-2990.1992.tb00764.x. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G., Williams S. F. Physiological and behavioural consequences of seizures induced in the rat by intrahippocampal tetanus toxin. Brain. 1987 Apr;110(Pt 2):517–532. doi: 10.1093/brain/110.2.517. [DOI] [PubMed] [Google Scholar]
- Jordan S. J., Jefferys J. G. Sustained and selective block of IPSPs in brain slices from rats made epileptic by intrahippocampal tetanus toxin. Epilepsy Res. 1992 Apr;11(2):119–129. doi: 10.1016/0920-1211(92)90046-v. [DOI] [PubMed] [Google Scholar]
- Kamphuis W., Gorter J. A., da Silva F. L. A long-lasting decrease in the inhibitory effect of GABA on glutamate responses of hippocampal pyramidal neurons induced by kindling epileptogenesis. Neuroscience. 1991;41(2-3):425–431. doi: 10.1016/0306-4522(91)90338-o. [DOI] [PubMed] [Google Scholar]
- Manning K. A., Erichsen J. T., Evinger C. Retrograde transneuronal transport properties of fragment C of tetanus toxin. Neuroscience. 1990;34(1):251–263. doi: 10.1016/0306-4522(90)90319-y. [DOI] [PubMed] [Google Scholar]
- Mellanby J., George G., Robinson A., Thompson P. Epileptiform syndrome in rats produced by injecting tetanus toxin into the hippocampus. J Neurol Neurosurg Psychiatry. 1977 Apr;40(4):404–414. doi: 10.1136/jnnp.40.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby J., Green J. How does tetanus toxin act? Neuroscience. 1981;6(3):281–300. doi: 10.1016/0306-4522(81)90123-8. [DOI] [PubMed] [Google Scholar]
- Mellanby J., Renshaw M., Cracknell H., Rands G., Thompson P. Long-term impairment of learning ability in rats after an experimental hippocampal epileptiform syndrome. Exp Neurol. 1982 Mar;75(3):690–699. doi: 10.1016/0014-4886(82)90035-8. [DOI] [PubMed] [Google Scholar]
- Mody I. The molecular basis of kindling. Brain Pathol. 1993 Oct;3(4):395–403. doi: 10.1111/j.1750-3639.1993.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Najlerahim A., Williams S. F., Pearson R. C., Jefferys J. G. Increased expression of GAD mRNA during the chronic epileptic syndrome due to intrahippocampal tetanus toxin. Exp Brain Res. 1992;90(2):332–342. doi: 10.1007/BF00227246. [DOI] [PubMed] [Google Scholar]
- Penner R., Neher E., Dreyer F. Intracellularly injected tetanus toxin inhibits exocytosis in bovine adrenal chromaffin cells. Nature. 1986 Nov 6;324(6092):76–78. doi: 10.1038/324076a0. [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Joubran C., Kesslak J. P., Bakay R. A. A selective decrease in the number of GABAergic somata occurs in pre-seizing monkeys with alumina gel granuloma. Epilepsy Res. 1989 Sep-Oct;4(2):126–138. doi: 10.1016/0920-1211(89)90017-x. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992 Oct 29;359(6398):832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Suda K., Thoenen H. Selective retrograde transsynaptic transfer of a protein, tetanus toxin, subsequent to its retrograde axonal transport. J Cell Biol. 1979 Sep;82(3):798–810. doi: 10.1083/jcb.82.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter R. S. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987 Jan 2;235(4784):73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the "dormant basket cell" hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991 Jan;1(1):41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]