Abstract
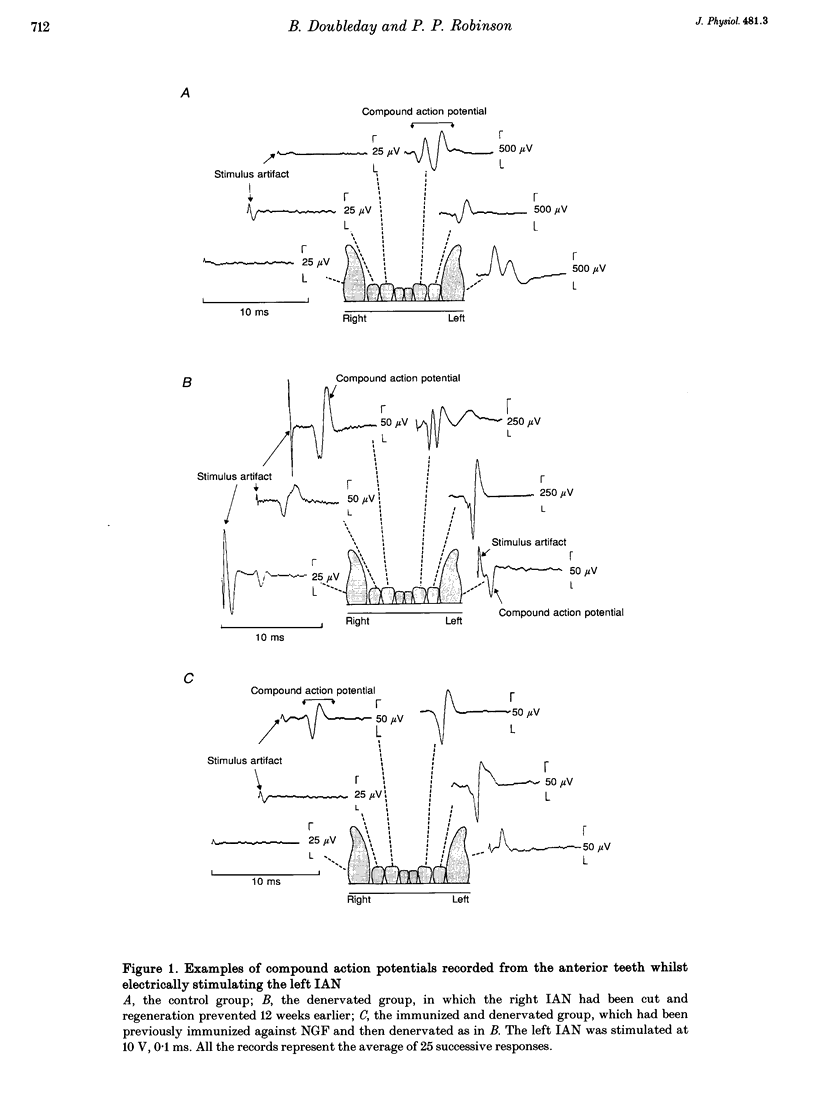
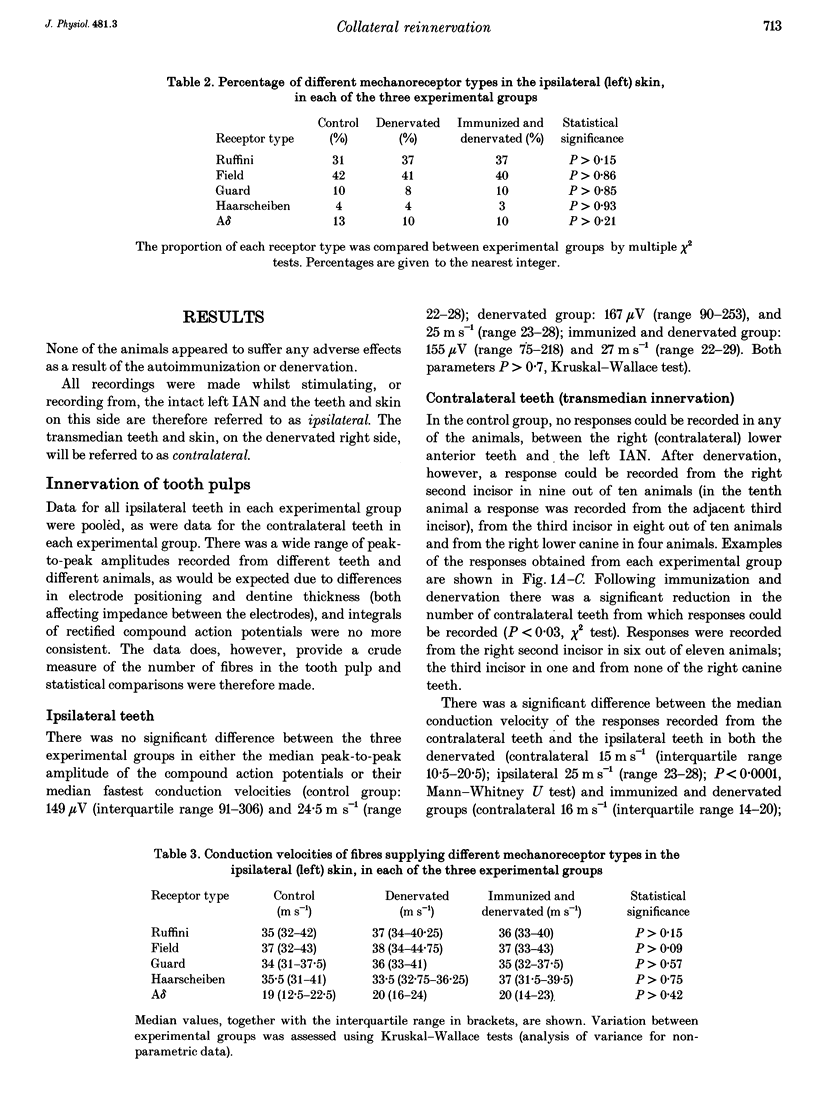
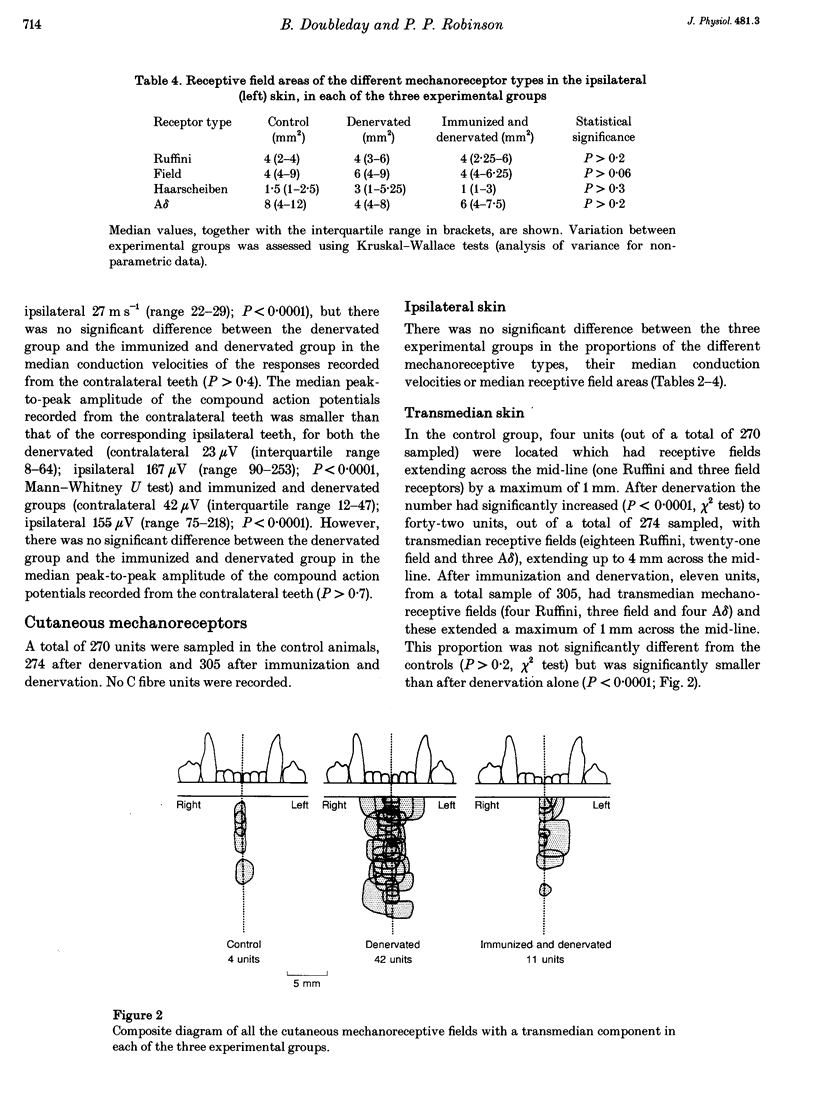
1. Electrophysiological experiments were carried out to determine whether or not collateral sprouting of cutaneous low-threshold mechanoreceptive fibres could be detected and to investigate the effect of nerve growth factor (NGF) deprivation on the sprouting of these fibres and the fibres innervating tooth pulps. 2. In twenty-one ferrets (eleven of which had been autoimmunized against NGF) the right inferior alveolar nerve (IAN) was sectioned and prevented from regenerating. After 12 weeks, transmedian innervation from the left IAN was determined by stimulating the nerve whilst recording from electrodes implanted in the contralateral anterior teeth and also by single unit recordings from the nerve whilst mechanically and electrically stimulating the skin. The results were compared with those from ten control animals. 3. Transmedian innervation of contralateral teeth was found in none of the control animals; in all ten of the animals which had undergone denervation without immunization (4/10 canines, 17/20 incisors); but in only six of the eleven immunized and denervated animals (0/11 canines, 7/22 incisors). 4. Of 270 cutaneous mechanoreceptive units sampled in the controls, only four units had transmedian receptive fields, extending a maximum of 1 mm across the mid-line. After denervation, significantly more units (42 of 274) crossed the mid-line and extended up to 4 mm. After immunization and denervation only eleven of 305 units crossed the midline by a maximum of 1 mm. 5. These data show that cutaneous low-threshold mechanoreceptive A beta and A delta fibres, as well as A delta tooth pulp fibres, are able to undergo collateral sprouting. This sprouting is partially blocked by NGF depletion, suggesting that NGF plays an essential role in the process.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson A., Barker P. A., Alderson R. F., Miller F. D., Murphy R. A. Detection of brain-derived neurotrophic factor-like activity in fibroblasts and Schwann cells: inhibition by antibodies to NGF. Neuron. 1991 Aug;7(2):265–275. doi: 10.1016/0896-6273(91)90265-2. [DOI] [PubMed] [Google Scholar]
- Anderson D. J., Hannam A. G., Mathews B. Sensory mechanisms in mammalian teeth and their supporting structures. Physiol Rev. 1970 Apr;50(2):171–195. doi: 10.1152/physrev.1970.50.2.171. [DOI] [PubMed] [Google Scholar]
- Bandtlow C. E., Heumann R., Schwab M. E., Thoenen H. Cellular localization of nerve growth factor synthesis by in situ hybridization. EMBO J. 1987 Apr;6(4):891–899. doi: 10.1002/j.1460-2075.1987.tb04835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers M. R., Matthews B. Autoradiographic demonstration of ipsilateral and contralateral sensory nerve endings in cat dentin, pulp, and periodontium. Anat Rec. 1981 Oct;201(2):249–260. doi: 10.1002/ar.1092010205. [DOI] [PubMed] [Google Scholar]
- Byers M. R. Segregation of NGF receptor in sensory receptors, nerves and local cells of teeth and periodontium demonstrated by EM immunocytochemistry. J Neurocytol. 1990 Oct;19(5):765–775. doi: 10.1007/BF01188044. [DOI] [PubMed] [Google Scholar]
- Byers M. R., Wheeler E. F., Bothwell M. Altered expression of NGF and P75 NGF-receptor by fibroblasts of injured teeth precedes sensory nerve sprouting. Growth Factors. 1992;6(1):41–52. doi: 10.3109/08977199209008870. [DOI] [PubMed] [Google Scholar]
- Cadden S. W., Lisney S. J., Matthews B. Thresholds to electrical stimulation of nerves in cat canine tooth-pulp with A beta-, A delta- and C-fibre conduction velocities. Brain Res. 1983 Feb 14;261(1):31–41. doi: 10.1016/0006-8993(83)91280-5. [DOI] [PubMed] [Google Scholar]
- Devor M., Schonfeld D., Seltzer Z., Wall P. D. Two modes of cutaneous reinnervation following peripheral nerve injury. J Comp Neurol. 1979 May 1;185(1):211–220. doi: 10.1002/cne.901850113. [DOI] [PubMed] [Google Scholar]
- Diamond J., Holmes M., Coughlin M. Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J Neurosci. 1992 Apr;12(4):1454–1466. doi: 10.1523/JNEUROSCI.12-04-01454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubleday B., Robinson P. P. The role of nerve growth factor in collateral reinnervation by cutaneous C-fibres in the rat. Brain Res. 1992 Oct 16;593(2):179–184. doi: 10.1016/0006-8993(92)91306-y. [DOI] [PubMed] [Google Scholar]
- Ebendal T., Olson L., Seiger A., Hedlund K. O. Nerve growth factors in the rat iris. Nature. 1980 Jul 3;286(5768):25–28. doi: 10.1038/286025a0. [DOI] [PubMed] [Google Scholar]
- Edin B. B., Abbs J. H. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol. 1991 Mar;65(3):657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Wall P. D., Goedert M., Emson P. C. Nerve growth factor counteracts the neurophysiological and neurochemical effects of chronic sciatic nerve section. Brain Res. 1985 Apr 15;332(1):131–141. doi: 10.1016/0006-8993(85)90396-8. [DOI] [PubMed] [Google Scholar]
- Fried K., Risling M. Nerve growth factor receptor-like immunoreactivity in primary and permanent canine tooth pulps of the cat. Cell Tissue Res. 1991 May;264(2):321–328. doi: 10.1007/BF00313969. [DOI] [PubMed] [Google Scholar]
- Gorin P. D., Johnson E. M., Jr Effects of long-term nerve growth factor deprivation on the nervous system of the adult rat: an experimental autoimmune approach. Brain Res. 1980 Sep 29;198(1):27–42. doi: 10.1016/0006-8993(80)90341-8. [DOI] [PubMed] [Google Scholar]
- Holland G. R., Robinson P. P. Reinnervation of the canine tooth pulp after section of the inferior alveolar nerve in the cat. Brain Res. 1985 Mar 11;329(1-2):300–303. doi: 10.1016/0006-8993(85)90538-4. [DOI] [PubMed] [Google Scholar]
- Holland G. R., Robinson P. P. The number and size of axons central and peripheral to inferior alveolar nerve injuries in the cat. J Anat. 1990 Dec;173:129–137. [PMC free article] [PubMed] [Google Scholar]
- Horch K. W., Tuckett R. P., Burgess P. R. A key to the classification of cutaneous mechanoreceptors. J Invest Dermatol. 1977 Jul;69(1):75–82. doi: 10.1111/1523-1747.ep12497887. [DOI] [PubMed] [Google Scholar]
- Horch K. Absence of functional collateral sprouting of mechanoreceptor axons into denervated areas of mammalian skin. Exp Neurol. 1981 Oct;74(1):313–317. doi: 10.1016/0014-4886(81)90170-9. [DOI] [PubMed] [Google Scholar]
- Inbal R., Rousso M., Ashur H., Wall P. D., Devor M. Collateral sprouting in skin and sensory recovery after nerve injury in man. Pain. 1987 Feb;28(2):141–154. doi: 10.1016/0304-3959(87)90112-6. [DOI] [PubMed] [Google Scholar]
- Jackson P. C., Diamond J. Temporal and spatial constraints on the collateral sprouting of low-threshold mechanosensory nerves in the skin of rats. J Comp Neurol. 1984 Jul 1;226(3):336–345. doi: 10.1002/cne.902260304. [DOI] [PubMed] [Google Scholar]
- Kinnman E. Collateral sprouting of sensory axons in the hairy skin of the trunk: a morphological study in adult rats. Brain Res. 1987 Jun 30;414(2):385–389. doi: 10.1016/0006-8993(87)90021-7. [DOI] [PubMed] [Google Scholar]
- Lewin G. R., McMahon S. B. Physiological properties of primary sensory neurons appropriately and inappropriately innervating skin in the adult rat. J Neurophysiol. 1991 Oct;66(4):1205–1217. doi: 10.1152/jn.1991.66.4.1205. [DOI] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Mitsiadis T. A., Dicou E., Joffre A., Magloire H. Immunohistochemical localization of nerve growth factor (NGF) and NGF receptor (NGF-R) in the developing first molar tooth of the rat. Differentiation. 1992 Jan;49(1):47–61. doi: 10.1111/j.1432-0436.1992.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Negro A., Corsa V., Skaper S. D., Callegaro L. Nerve growth factor antibodies recognize neurotrophin-3. Neurochem Res. 1993 Jun;18(6):705–709. doi: 10.1007/BF00966785. [DOI] [PubMed] [Google Scholar]
- Owen D. J., Logan A., Robinson P. P. A role for nerve growth factor in collateral reinnervation from sensory nerves in the guinea pig. Brain Res. 1989 Jan 9;476(2):248–255. doi: 10.1016/0006-8993(89)91245-6. [DOI] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A., Kenigsberg R. L., Cuello A. C. Light and electron microscopic distribution of nerve growth factor receptor-like immunoreactivity in the skin of the rat lower lip. Neuroscience. 1991;43(2-3):631–646. doi: 10.1016/0306-4522(91)90322-f. [DOI] [PubMed] [Google Scholar]
- Rich K. M., Alexander T. D., Pryor J. C., Hollowell J. P. Nerve growth factor enhances regeneration through silicone chambers. Exp Neurol. 1989 Aug;105(2):162–170. doi: 10.1016/0014-4886(89)90115-5. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Ebendal T. Nerve growth activities in rat peripheral nerve. Brain Res. 1982 Aug 19;246(1):57–64. doi: 10.1016/0006-8993(82)90141-x. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Riopelle R. J. Uptake of nerve growth factor along peripheral and spinal axons of primary sensory neurons. J Neurosci. 1984 Jul;4(7):1683–1689. doi: 10.1523/JNEUROSCI.04-07-01683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. P. A peripheral stimulus initiates the collateral reinnervation of cat teeth. Brain Res. 1986 Feb 26;366(1-2):397–400. doi: 10.1016/0006-8993(86)91327-2. [DOI] [PubMed] [Google Scholar]
- Robinson P. P. An electrophysiological study of the reinnervation of reimplanted and autotransplanted teeth in the cat. Arch Oral Biol. 1983;28(12):1139–1147. doi: 10.1016/0003-9969(83)90172-3. [DOI] [PubMed] [Google Scholar]
- Robinson P. P. Observations on the recovery of sensation following inferior alveolar nerve injuries. Br J Oral Maxillofac Surg. 1988 Jun;26(3):177–189. doi: 10.1016/0266-4356(88)90161-1. [DOI] [PubMed] [Google Scholar]
- Robinson P. P. Reinnervation of teeth, mucous membrane and skin following section of the inferior alveolar nerve in the cat. Brain Res. 1981 Sep 14;220(2):241–253. doi: 10.1016/0006-8993(81)91215-4. [DOI] [PubMed] [Google Scholar]
- Seilheimer B., Schachner M. Regulation of neural cell adhesion molecule expression on cultured mouse Schwann cells by nerve growth factor. EMBO J. 1987 Jun;6(6):1611–1616. doi: 10.1002/j.1460-2075.1987.tb02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfath M., Chen Y. Y., Lavický J., Magnussen O., Nose M., Rosswag S., Schmitz W., Scholz H. Cardiac alpha 1-adrenoceptor densities in different mammalian species. Br J Pharmacol. 1992 Sep;107(1):185–188. doi: 10.1111/j.1476-5381.1992.tb14484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M., Clark H. B., Johnson E. M., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantini G. The pharmacological potential of neurotrophins: a perspective. Psychoneuroendocrinology. 1992 Aug;17(4):401–410. doi: 10.1016/0306-4530(92)90045-9. [DOI] [PubMed] [Google Scholar]
- Vos P., Stark F., Pittman R. N. Merkel cells in vitro: production of nerve growth factor and selective interactions with sensory neurons. Dev Biol. 1991 Apr;144(2):281–300. doi: 10.1016/0012-1606(91)90422-y. [DOI] [PubMed] [Google Scholar]




