Abstract
Background
Fever is a common condition in intensive care unit (ICU) patients, with an incidence between 30 and 50% in non-neurological ICU patients and up to 70–90% in neurological ICU patients. We aim to perform systematic review and meta-analysis of current literature to assess impact of fever on neurological outcomes and mortality of acute brain injury patients.
Methods
We searched PubMed/Medline, Scopus and Embase databases following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, and we included both retrospective and prospective observational studies, interventional studies, and randomized clinical trials that had data on body temperature and fever during ICU admission. The primary endpoints were neurological outcome and mortality at any time. Secondary outcomes included: early neurological deterioration, delayed cerebral ischemia (DCI, only for patients with subarachnoid hemorrhage), large infarct or hemorrhage size, hemorrhagic transformation (only for patients with ischemic stroke). This study was registered in PROSPERO (CRD42020155903).
Results
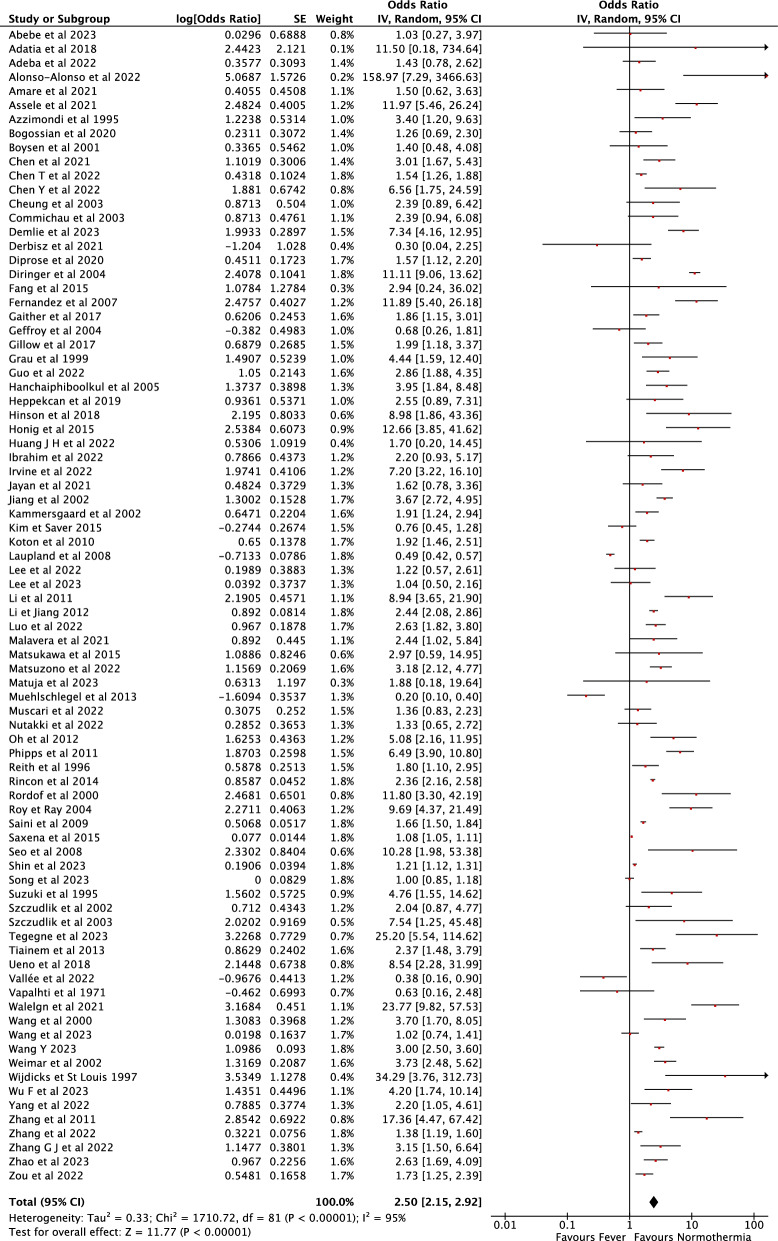
180 studies from 14692 records identified after the initial search were included in the final analysis, for a total of 460,825 patients. Fever was associated with an increased probability of unfavorable neurological outcome (pooled OR 2.37 [95% CI 2.08–2.71], I2:92%), death (pooled OR 1.31 [95% CI 1.28–1.34], I2:93%), neurological deterioration (pooled OR 1.10 [95% CI 1.05–1.15]), risk of DCI (pooled OR 1.96 [95% CI 1.73–2.22]), large infarct size (pooled OR 2.94 [95% CI 2.90–2.98]) and hemorrhagic transformation (pooled OR 1.63 [95% CI 1.34–1.97]) and large hemorrhagic volume (pooled OR 2.38 [95% CI 1.94–2.93]).
Conclusion
Fever was associated with poor neurological outcomes and mortality in patients with acute brain injury. Whether normothermia should be targeted in the management of all neuro critically ill patients warrants specific research.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05132-6.
Keywords: Stroke, Traumatic brain injury, Pyrexia, Outcome
Introduction
Fever is an innate response of the immune system, led by pyrogenic cytokines, that causes an increment in the body temperature and can have infective and non-infective causes [1]. It is a common condition in intensive care unit (ICU) patients, with an incidence between 30 and 50% in non-neurological ICU patients, and up to 70–90% in neurological-ICU patients [2–4].
It is known that fever has some potential protective functions [5]. Despite this, fever shows detrimental effects, especially in patients with non-infectious neurologic injuries [6–8]. There are several mechanisms proposed to explain this detrimental effect including endothelial damage and consequent blood brain barrier breakdown, which can cause cerebral edema and increase in intracranial pressure; increase in metabolic demand, potentially augmenting cerebral blood flow and blood volume promoting cerebral edema; ischemic reperfusion injury; release of excitotoxic neurotransmitters, such as glutamate; neuro-inflammation and apoptosis barrier [9–12].
Indeed, a comprehensive meta-analysis conducted by Greer et al. [13] in 2008 showed that in acute brain injury patients, fever was associated with poor outcomes, such as increased ICU mortality, longer ICU stay, and worse functional outcomes. Since then, there has been an increased interest and published studies on the role of temperature targeted management (TTM), especially active normothermia, in the management of acute non-anoxic brain injury patients [14–16]. A recent systematic review without quantitative analysis, which assessed the impact of fever and TTM in acute brain injury patients excluding patients suffering from post-anoxic encephalopathy, suggested that fever control may be beneficial in traumatic brain injury (TBI) patients, but there is still a considerable lack of evidence [17]. In the last 15 years, management of acute brain injury patients has evolved, the prognosis of these patients has somewhat improved [18–20]. Therefore, an updated systematic review and meta-analysis is of interest to summarize the current evidence regarding the impact of fever on the neurological outcome and mortality of patients with acute brain injury due to stroke (all types) and TBI.
Methods
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [21]. The protocol of this study was registered with the International Prospective Register of Systematic Reviews (PROSPERO) and last edited on April 28th, 2020 (CRD42020155903).
Data sources and study selection
We conducted a systematic review and meta-analyses of both retrospective and prospective observational studies, interventional studies and randomized clinical trials. The PubMed/Medline, Scopus and Embase databases were last searched on January 31st, 2024, including publications of adult human studies without date restriction. We used the PICO strategy to formulate our search as follows: Population adult patients (i.e., age > 18 years), admitted to the hospital due to non-cardiac arrest acute brain injury including stroke (ischemic, hemorrhagic), subarachnoid hemorrhage and traumatic brain injury; Intervention: fever, as defined by the authors of each study; Control: normothermia; Outcome: primary outcomes were unfavorable neurological outcome and mortality at any time point as defined by the authors. The research strategy with the string for each database is shown in the Supplemental Electronic Material S1.
We considered the following criteria for study inclusion: (1) full-length reports published in peer-reviewed journals in English; (2) randomized clinical trial, interventional studies, observational cohorts, case control studies of adult human patients; (3) studies that assessed body temperature and reported the occurrence and definition of fever (e.g. when “hyperthermia” was used, this was considered as “fever”); (4) studies that included outcomes measures (i.e., mortality at any time point, unfavorable neurological outcome at any time point; neurological deterioration during hospitalization and stroke progression) in acute brain injury patients. Studies conducted in hypoxic ischemic encephalopathy post cardiac arrest patients, children, healthy volunteers, or in animal models were excluded. We also excluded studies that compared hypothermia to normothermia without reporting the occurrence of fever. Editorials, commentaries, letters to editor, opinion articles, reviews, meeting abstracts and case reports were also excluded. When multiple publications of the same research group/center described case series with potential overlap, the more recent or larger publication, if eligible, was considered.
Four investigators (MS, EGB, MF, MT) performed the study selection process, including the initial search for the identification of references, the selection of potentially relevant titles for review of abstracts and, among them, of those chosen for review of the full-length reports. All selections were decided by consensus.
Data extraction, synthesis and outcomes
Three investigators (MS, EGB, SF) independently extracted information from the selected articles using a standardized data collection system. The following data fields were collected (whenever available): study location, period of enrollment, patient enrollment criteria, number of patients enrolled, definition of fever/hyperthermia and time of assessment, rates of mortality, unfavorable neurological outcome, neurological deterioration, delayed cerebral ischemia (DCI) and stroke progression (hemorrhagic and ischemic). All selected studies were included in the qualitative synthesis and their characteristics and results summarized in a table. We also performed a quantitative synthesis through a meta-analysis. The primary outcome of the meta-analysis was the occurrence of unfavorable neurological outcome and mortality at any time point. If more than one time point for each study was available, we used the longest follow-up time point.
Unfavorable neurological outcome could be defined by Glasgow Outcome Scale [22], extended Glasgow outcome scale (GOSE) [23], modified Rankin scale [24], Barthel Index [25] or any functional scale chosen by the authors of the original articles that could be dichotomized into favorable and unfavorable neurological outcome. Secondary outcomes were early neurological deterioration defined as a drop of 2 or more points in Glasgow coma scale (GCS) [26] in patients with TBI and subarachnoid hemorrhage (SAH) or more than 2–4 points increase in the National Institute of Health stroke scale (NIHSS) [27] in patients with acute ischemic stroke (AIS) or intracerebral hemorrhage (ICH) in the first 24-72h; symptomatic vasospasm/ DCI as defined by the original studies in SAH patients; infarct size/progression and symptomatic hemorrhagic transformation as defined by the authors of the original studies in AIS patients; hematoma volume/expansion in intracranial in ICH and whenever this was collected. Pre-defined analyses were performed in subgroups of studies: (a) studies that included only TBI; (b) studies that included only AIS; (c) studies that included only ICH; (d) studies that included only SAH.
Risk of bias assessment and quality of evidence
To assess the methodological quality of the studies, we used the Cochrane risk of bias tool (Risk of bias 2—RoB 2) [28] for studies designed as randomized clinical trials. We considered RCT as having a low risk of bias if all 5 domains of the tool was classified as low risk; the RCT was judged to have “some concerns” (moderate risk of bias) if at least one domain was classified as some concerns and no domains were classified as high risk of bias; the study was considered as having high risk of bias if it was classified as such in at least one domain or if it was judged to have some concerns in multiple (> 2) domains. The Newcastle–Ottawa Quality Assessment Scale (NOS) [29] was used to assess the quality of cohort and case control studies and secondary or post hoc analyses of randomized clinical trials. Observational studies were considered to have poor quality if 0 or 1 star in selection domain or, 0 stars in comparability domain or, 0 or 1 stars in outcome/exposure domain; fair quality if 2 stars in selection domain and 1 or 2 stars in comparability domain and 2 or 3 stars in outcome/exposure domain and, good quality if 3 or 4 stars in selection domain and 1 or 2 stars in comparability domain and 2 or 3 stars in outcome/exposure domain.
This assessment was performed by two independent reviewers (EGB and MS) and in case of discordant analysis a third investigator (FST) made the final decision. We only included articles that had a low or moderate risk of bias. Studies with poor quality and high risk of bias were not included in the quantitative synthesis. We determined the level of evidence using the GRADE classification system [30].
Statistical analysis
We performed the meta-analysis using the random effect inverse variance method. The results were pooled together in a Forest Plot. We computed pooled odds ratio (OR) with 95% confidence intervals (CI) for dichotomic outcomes. We extracted the respective covariate adjusted OR and 95% CI from each study. We also calculated unadjusted OR 95% CI in studies that did not report multivariable analysis. Beta coefficients were exponentiated to obtain the OR. If the study presented the results as risk ratio (RR), we estimated the equivalent OR following the recommendations and formulas available in the Cochrane Handbook [31]. If the study presented hazard ratios (HR), we first estimated the RR and then the OR [32]. Standard mean differences and correlation coefficient r were also converted to logOR and then to OR using previously described formulas [31, 33]. Standard errors of logOR, coefficient r and SMD were converted into confidence intervals [31]. If data necessary to obtain OR was unavailable in the published manuscript and electronic supplementary material, we contacted the authors and requested said data. Heterogeneity was assessed by means of the I2 statistic, which reflects the amount of between-study heterogeneity over and above the sampling variation and is robust to the number of studies and choice of effect measure. We assessed the potential of publication bias through funnel plot generation. We performed a meta-regression moderated for BT used to define fever and time of endpoint in days for both neurological outcome and mortality. We performed all analyses using Review Manager version 5.4 and STATA 17.0.
Results
Study selection
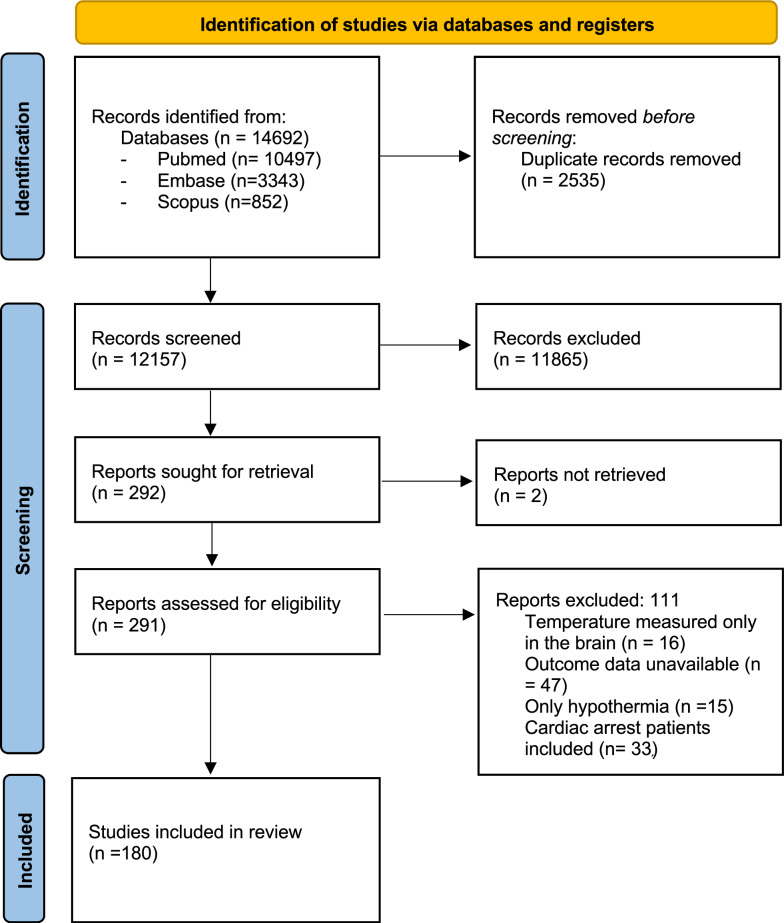
A total of 180 studies from 14,692 records identified after the initial search were included in the final analysis (Fig. 1), resulting in 460,846 studied patients. Nine studies did not report or provide data for the calculation of the prevalence of fever; the remaining 171 included 259,231 patients, of which 80,984 experienced fever (e.g. prevalence of 18%).
Fig. 1.
Flow diagram of the systematic review and meta-analysis
Study characteristics
The characteristics of the selected studies are summarized in Table 1. We identified 1 RCTs, 22 observational prospective studies, and 157 retrospective studies. The risk of bias for the RCT was some concern, as shown in Supplementary Table S1. For cohort, case–control studies and post hoc or secondary analysis of RCT, the risk of bias was moderate in 142 studies and low in 15 studies (Supplementary Table S2). The level of evidence assessed by the GRADE scale was moderate in the RCT (Supplemental Table S1). The observational studies were graded in their majority as low quality of evidence (155/179, 87%), 2 (1%) were very low quality of evidence, and 22 (12%) had moderate quality of evidence (Supplemental Table S2).
Table 1.
Characteristics and results of the included studies in the systematic review
| Author year | Study characteristics | Population |
|---|---|---|
| Abebe et al. [90] |
Retrospective single center cohort study Ethiopia 2018–2021 |
N = 912 Adult stroke patients admitted to the hospital |
| Adatia et al. [38] |
Retrospective single center study of prospectively collected data USA 2013–2017 |
N = 85 Comatose patients (GCS < 8) due to primary brain injury monitored with NIRS and central temperature probe |
| Addis et al. [91] |
Retrospective single center study of prospectively collected data Austria 2010–2016 |
N = 46 Adult poor grade SAH patients who underwent invasive multimodal monitoring |
| Adeba et al. [92] |
Retrospective single center study Ethiopia 2016–2018 |
N = 251 Adult (> 18 yo) with diagnosis of stroke (AIS or ICH) confirmed by imaging |
| Alexandrov et al. [93] |
Observational prospective multicentric study USA Study period not reported |
N = 235 Adult Acute stroke (AIS or ICH) patients |
| Alonso-Alonso et al. [94] |
Retrospective single center study of prospectively collected data Spain 2008–2017 |
N = 119 AIS with total anterior circulation or MCA infarct |
| Alonso- Alonso et al. [95] |
Retrospective single center study of prospectively collected data Spain 2008–2017 |
N = 4989 Adult stroke patients (ICH and AIS) except wake up stroke who underwent temperature control |
| Amare et al. [96] |
Retrospective single center cohort study Ethiopia 2015–2019 |
N = 372 TBI patients, aged > 15 yo |
| Andrews et al. [39] |
Prospective single center cohort study 1989–1991 Scotland |
N = 124 (69 included in the outcome analysis) TBI patients > 16 years old admitted to the ICU with GCS < 13 or GCS > 12 with ISS > 15 |
| Assele et al. [97] |
Retrospective cohort single center study Ethiopia 2017–2019 |
N = 1159 All patients admitted to the hospital due to TBI with complete medical history |
| Azzimondi et al. [98] |
Prospective single center cohort study Italy 1993 a |
N = 183 Adult stroke patients (AIS and ICH) |
| Bao et al. [99] |
Retrospective single center cohort study China 2010–2012 |
N = 355 TBI patients > 18 yo admitted to hospital within 24 h of injury with GCS 3–14 |
| Barber et al. [100] |
Case–control single center study UK 2000–2001 |
N = 392 AIS admitted within 24 h of ictus |
| Barrow et al. [101] |
Post hoc analysis of prospectively collected data of patients included in a RCT 70 centers in 8 European countries |
N = 437 AIS with unknown time of symptoms with acute ischemic lesions on diffusion-weighted imaging with no marked parenchymal hyperintensity on fluid-attenuated inversion recovery, suggesting time of onset < 4.5 h |
| Bill et al. [102] |
Single center retrospective analysis of prospectively collected data Switzerland 2004–2010 |
N = 243 Severe AIS patients (NIHSS > 20) admitted to hospital within 24 h since last seen normal |
| Blanco et al. [103] |
Retrospective single center cohort study of prospectively collected data Spain 1997–1999 |
N = 113 Lacunar AIS admitted to hospital within 24 h from symptoms |
| Blanco et al. [104] |
Prospective single center cohort study Spain 2004–2010 |
N = 2931 Consecutive adult stroke (AIS and ICH) patients |
| Bonds et al. [105] |
Retrospective single center cohort study USA 2008–2010 |
N = 50 Adult (> 17 yo) Severe TBI (patients (GCS < 9) confirmed by neuroimaging |
| Boysen et al. [106] |
Prospective single center cohort study Denmark 1998–2000 |
N = 725 Adult patients with acute stroke (AIS or ICH) admitted within 6 h form ictus |
| Burkot et al. [107] |
Prospective single center cohort study Poland 2011–2014 |
N = 566 Adult patients admitted to the stroke unit within 24 h of ictus due to AIS |
| Bush et al. [108] |
Prospective single center cohort study USA 2011–2015 |
N = 106 Adult patients with spontaneous ICH |
| Campos et al. [109] |
Case – control single center study Spain 2009–2012 |
N = 200 Adult Acute stroke patients (AIS or ICH) |
| Carlson et al. [110] |
Retrospective single center study USA 2002–2003 |
N = 169 Patients aged ≥ 13 yo with severe TBI who stayed at least 24 h in the ICU |
| Castellanos et al. [111] |
Multicentric study of retrospective study of prospectively collected data Spain 1999 to 2001 |
N = 138 Patients with spontaneous hemispheric ICH > 20 ml. They were non- surgically treated and were admitted consecutively to 15 hospitals within the first 12 h of symptom onset |
| Castillo et al. [112] |
Prospective single center cohort study Spain 1992–1994 |
N = 128 First-ever hemispheric ischemic stroke; admission within 24 h after the onset of symptoms |
| Castillo et al. [113] |
Prospective single center cohort study Spain Study period not reported |
N = 260 Acute hemispheric ischemic stroke admitted to the hospital within 24 h form ictus |
| Castillo et al. [50] |
Prospective single center cohort study Spain 1992–1994 |
N = 128 First-ever hemispheric ischemic stroke and admission within 24 h after the onset of symptoms |
| Chen et al. [114] |
Retrospective analysis of prospective collected data, single center China 2015–2019 |
N = 258 Adult (> 18 yo) acute ischemic stroke patients with large vessel occlusion that underwent mechanical thrombectomy |
| Chen et al. [115] |
Retrospective single center cohort study China 2018–2020 |
N = 244 Adults (> = 18 yo) TBI patients admitted within 72 h from injury and required surgical intervention |
| Chen et al. [116] |
Retrospective single center study using the MIMIC database USA 2001–2019 |
N = 2085 Adult patients admitted to the ED or ICU of Beth Israel Deaconess Medical Center due to ischemic stroke who had admission creatine and BUN levels |
| Chen et al. [117] |
Retrospective single center study China 2018–2021 |
N = 89 Large vessel occlusion (ICA, MCA M1 and m2, basilar) stroke in adult (> 18 yo) patients who underwent EVT within 24 h of symptoms |
| Cheung et al. [118] |
Single center retrospective cohort study Hong Kong (China) 1999 |
N = 141 Spontaneous ICH admitted to the emergency department |
| Christensen et al. [119] |
Prospective single center cohort study Denmark 1999–2001 |
N = 896 Consecutive acute ischemic stroke admitted to stroke unit within 24 h from ictus |
| Cisse et al. [120] |
Retrospective cohort study single center Guinea 2015–2021 |
N = 1018 Ischemic and hemorrhagic stroke (SAH not included) stroke patients admitted within 24 h |
| Commichau et al. [61] |
Prospective single center cohort study USA 1999 |
N = 387 Patients admitted to neuro-intensive care unit |
| Dávalos et al. [121] |
Prospective single center prospective cohort study Spain 1992–1994 |
N = 128 First-ever hemispheric ischemic stroke and admission within 24 h after the onset of symptoms |
| Dehkharghani et al. [122] |
Retrospective single center study of prospectively collected data USA 2010–2014 |
N = 129 Acute ischemic stroke patients due to large vessel occlusion who underwent successful endovascular reperfusion therapy |
| Demlie et al. [123] |
Retrospective multicentric follow up study Ethiopia 2021 |
N = 544 All adult TBI patients admitted to the comprehensive specialized hospitals of the Amhara region during the study period that had complete medical records |
| Den Hertog et al. [124] |
Multicentric phase 3 RCT placebo controlled Netherlands 2003–2008 |
N = 1400 Stroke (AIS or ICH) patients admitted with BT between 36 °C and 39 °C |
| Derbisz et al. [125] |
Retrospective single center study of prospectively collected data Poland 2014–2018 |
N = 362 Acute ischemic stroke patients who underwent intravenous thrombolysis with or without EVT |
| Dicpinigaitis et al. [126] |
Retrospective cohort study, multicenter USA 2015–2018 |
N:5580 Adult patients admitted with traumatic intracerebral hemorrhage, who underwent DSA |
| Diprose et al. [127] |
Retrospective analysis of prospectively collected data New Zealand 2011–2019 |
N = 432 AIS patients that underwent EVT for large vessel occlusion |
| Diringer et al. [2] |
Retrospective single center study of prospectively collected data USA 1996–2001 |
N = 4295 All adult (> 18 yo) neuro critically ill patients who stayed at least 24 h in the ICU |
| Dowlati et al. [128] |
Retrospective bicentric cohort study 2017–2020 USA |
N = 151 aSAH who underwent endovascular treatment (angioplasty or intraarterial infusion of vasodilatory agents) for radiographic vasospasm |
| Dzierzęcki et al. [129] |
Prospective observational cohort study single center Poland (Study period not described) |
N = 60 Severe TBI patients (GCS < 9) |
| Eagles et al. [130] |
Post hoc analysis of prospectively collected data of patients included in the CONSCIOUS I RCT 2005–2006 Europe, UK, Canada, USA |
N = 301 Patients aged between 18 to 70 years old with aneurysmal SAH (saccular aneurysm) confirmed by DSA with good grade at admission (WFNS 1–2) |
| Elf et al. [131] |
Retrospective single center study Sweden 1998–2002 |
N = 53 TBI patients with 16 yo to 79 yo admitted to neuro ICU monitored with at least 54 h of monitored physiological data in the first 5 days of admission |
| Fan et al. [132] |
Retrospective single center cohort study Taiwan 2002–2009 |
N = 619 Consecutive spontaneous ICH patients admitted to the ED within 12 h from ictus and an initial GCS > 12 |
| Fang et al. [133] |
Prospective multicentric cohort study US millitary hospitals in Afeganistan and Iraq 2009–2010 |
N = 99 (65 had temperature data) Combat related TBI with GCS < 13 including penetrating TBI |
| Ferguson et al. [134] |
Post hoc analysis of prospectively collected data of patients included in 4 multicentric RCTs Europe, Australia, New Zealand, the United States, Canada, Mexico, and South Africa 1991–1997 |
N = 2741 Adult (> 18 yo) SAH patients admitted to the hospital within 48 h of ictus with confirmed aneurysm on angiogram |
| Fernandez et al. [135] |
Prospective observational single center cohort study USA 1996–2002 |
N = 353 Adult (> 17 yo) aneurysmal SAH patients |
| Fu et al. [136] |
Retrospective single center cohort study USA 2014–2017 |
N = 276 Adult (> 18 yo) spontaneous ICH patients with admission data on liver function |
| Fukuda et al. [137] |
Retrospective single center cohort study Japan 1993–1998 |
N = 183 Consecutive Acute ischemic stroke admitted to the hospital within 34 h of ictus |
| Gaither et al. [138] |
Retrospective multicentric cohort study USA 2007–2012 |
N = 11,877 Moderate to Severe TBI patients |
| Geffroy et al. [139] |
Retrospective single center study France 1999–2001 |
N = 101 Severe TBI or moderate TBI with deterioration |
| Georgilis et al. [140] |
Retrospective single center study Greece 1992–1994 |
N = 330 Acute (< 48 h) stroke (AIS and ICH) patients |
| Geurts et al. [141] |
Retrospective multicentric study of prospectively collected data Netherlands 2009–2013 |
N = 419 Adult patients with acute ischemic stroke with symptom duration < 9 h, and NIHSS ≥ 2, or ≥ 1 if (IV-rtPA) was indicated |
| Gillow et al. [142] |
Retrospective single center cohort study USA 2009–2010 |
N = 351 Consecutive adult (> = 18yo) patients with Spontaneous ICH confirmed by CT |
| Gouvea Bogossian et al. [143] |
Retrospective single center study Belgium 2011–2016 |
N = 248 Adult patients (> 17 yo) non traumatic SAH Who stayed in ICU for at least 24 h |
| Grau et al. [144] |
Single center observational cohort study Germany Study period not reported |
N = 119 Acute ischemic stroke admitted within 24 h |
| Guo et al. [145] |
Retrospective Single center study China 2018–2019 |
N = 751 Consecutive adult patients with spontaneous intracerebral hemorrhage confirmed by neuroimaging |
| Guth et al. [146] |
Retrospective single center study of prospectively collected data study USA 2006–2012 |
N = 235 Spontaneous ICH admitted to neuro ICU |
| Hanchaiphiboolkul [147] |
Retrospective single center cohort study Thailand 2002–2003 |
N = 332 Acute ischemic stroke confirmed by neuroimaging patients admitted within 48 h of symptoms |
| He Lee et al. [148] |
Post hoc analysis of prospectively collected data of patients included in a RCT Canada and USA 2014–2017 |
N = 248 Adult patients with hypertensive supratentorial ICH without EVD |
| Heppekcan et al. [149] |
Single center retrospective study Turkey 2015–2018 |
N = 100 Patients older than 15 with severe TBI that stayed at least 48 h in the ICU Patients who died “cardiac death” were excluded |
| Hifumi et al. [150] |
Post-hoc analysis of prospectively collected data of patients included in the B-HYPO RCT Japan 2002–2008 |
N = 130 Severe TBI (GCS 4–8 on admission) patient aged between 15 and 69 years |
| Hindfelt [151] |
Retrospective single center cohort study Sweden |
N = 110 Acute ischemic stroke admitted within 24 h of symptoms |
| Hinson et al. [152] |
Prospective single center cohort study USA 2013–2015 |
N = 158 Acute isolated TBI (n = 97) and Polytrauma with TBI (n = 59) |
| Honig et al. [62] |
Retrospective single center study of prospectively collected data Israel 2009–2010 |
N = 95 Spontaneous ICH confirmed by neuroimaging admitted for > 24 h in the ICU with temperature data for 1 week |
| Hu et al. [153] |
Retrospective cohort study single center China 2018–2020 |
N = 120 Severe TBI (GCS < 9) adult (20–70 yo) patients with brain herniation admitted to the hospital within 6 h of injury with CT scan showing midline shift and compression of ventricles on admission |
| Huang et al. [154] |
Retrospective single center study Taiwan 2008–2014 |
N = 93 Adult > 20 yo patients s/p post craniotomy due to acute TBI |
| Huang et al. [155] |
Retrospective multicentric cohort study China |
N = 835 Adult (> 18 yo) spontaneous ICH patients with confirmatory CT scan within 6 h from ictus |
| Hulscher et al. [156] |
Retrospective single center cohort study Belgium 2018–2021 |
N = 61 Acute ischemic stroke patients who underwent mechanical thrombectomy for distal medium vessel occlusion |
| Ibrahim et al. [157] |
Retrospective single center study Nigeria 2015–2019 |
N = 276 Adult stroke patients admitted to the emergency department with confirmatory CT that had complete medical records |
| Iglesias- Rey et al. [158] |
Retrospective analysis of prospectively collected data single center study Spain 2015–2018 |
N = 663 Adult patients with ischemic stroke admitted to the stroke unit < 12 h confirmed by neuroimaging with baseline mRankin < 3 and no comorbidities associated with life expectancy less than 3 months |
| Iglesias-Rey et al. [159] |
Retrospective single center analysis of prospectively collected data Spain 2008–2017 |
N = 887 Spontaneous ICH patients confirmed by neuroimaging who were previously independent |
| Irvine et al. [160] |
Retrospective bicentric study of prospectively collected USA 2018–2020 |
N = 234 (non-covid patients) Acute ischemic stroke patients who underwent thrombectomy |
| Jacome et Tatum [161] |
Retrospective single center cohort study USA 2009–2014 |
N = 330 Adult patients (> 18 yo) with isolated non penetrating TBI |
| Jayan et al. [162] |
Retrospective single center cohort study India 2012 |
N = 243 Adult moderate to severe TBI patients admitted to the ICU |
| Jeong et al. [163] |
Retrospective single center study of a prospectively collected data Korea 2013–2014 |
N = 246 Adult patients with acute ischemic stroke admitted to stroke unit within 7 days of ictus stayed for at least 12 h in the unit and had 3 months follow up |
| Jiang et al. [164] |
Retrospective single center study China 1991–1998 |
N = 846 Severe TBI patients pediatric and adult (GCS < 8) |
| Jorgensen et al. [35] |
Single center retrospective analysis of prospectively collected data (the Copenhagen Stroke study) Denmark 1991–1993 |
N = 84 Acute stroke patients with SSS < 15 on admission who survived |
| Jorgensen et al. [165] |
Retrospective single center study of prospectively collected data (the Copenhagen Stroke study) Denmark 1991–1993 |
N = 396 Consecutive acute stroke patients admitted within 6 h of onset |
| Kammersgaard et al. [166] |
Single center prospective study (the Copenhagen Stroke study) Denmark 1991–1993 |
N = 390 Consecutive acute stroke patients (AIS or ICH) admitted within 6 h of onset |
| Karaszewski et al. [167] |
Prospective single center cohort study Scotland 2007–2009 |
N = 48 Adult (> = 18 yo) patients with potentially disabling acute ischemic stroke patients who underwent MRI |
| Karaszewski et al. [36] |
Prospective single center cohort study Scotland Study period not reported |
N = 40 Acute ischemic stroke patients who did not receive thrombolytic treatment and could undergo MRI |
| Kim et Saver 168] |
Retrospective analysis of patients included in a RCT (The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke.) USA Study period not reported |
N = 595 Acute ischemic stroke patients that were randomized to undergo thrombolysis with rt-PA or placebo and had admission temperature recordings |
| Koton et al. [169] |
Retrospective multicentric study of prospectively cross sectional collected data Israel 2004 |
N = 1079 First ever acute ischemic stroke in adult patients (> 18 yo) confirmed by neuroimaging |
| Kramer et al. [170] |
Retrospective single center study USA 2001–2013 |
N = 584 Consecutive adult aSAH patients |
| Kvistad et al. [171] |
Single center retrospective analysis of prospectively collected data study Norway 2006–2013 |
N = 172 Acute ischemic stroke treated with tPA with normal CTA of the cerebral arteries |
| Kvistad et al. [172] |
Retrospective single center study of prospectively collected data study Norway 2006–2012 |
N = 88 Ischemic stroke or transient ischemic attack (TIA) treated with tPA |
| Lai et al. [173] |
Retrospective single and prospective cohort study USA 2015–2017 |
N = 44 aSAH confirmed by DSA or CTA |
| Laupland et al. [174] |
Retrospective single center study Canada 2000–2006 |
N = 24,204 (3748 trauma/ neuro) All adult (≥ 18yo) patients admitted to ICU (including Trauma/ Neuro) |
| Lee et al. [175] |
Retrospective single center study Korea 2015–2020 |
N = 690 Adult patients presenting to ED within 24 h of TBI |
| Lee et al. [176] |
Retrospective cohort single center study Korea 2019–2020 |
N = 248 TBI (age > 18 yo) patients admitted to the pre-hospital emergency medical system with AIS > 2 |
| Leira et al. [177] |
Prospective multicentric cohort study Spain 1999–2001 |
N = 266 Consecutive spontaneous supratentorial ICH admitted within 12 h of ictus and absence of stupor or coma |
| Leira et al. [178] |
Retrospective multicentric study of prospectively collected data Spain 1999–2001 |
N = 229 First ever hemispheric acute ischemic stroke admitted within 24 h of symptoms who survived the first 48 h after stroke |
| Leira et al. [179] |
Secondary analysis of an observational multicentric study Spain 1999–2001 |
N = 161 Acute ischemic stroke of less than 12 h from stroke onset without previous disability untreated with r-tpa |
| Li et al. [180] |
Single center prospective observational cohort study China 2004–2008 |
N = 662 First ever acute ischemic stroke patients confirmed in neuroimaging admitted to the hospital within 24 h of symptoms |
| Li et Jiang 181] |
Multicentric prospective cohort study China 2008–2009 |
N = 7145 Acute traumatic brain injury |
| Lin et al. [182] |
Retrospective single center cohort study China 2012–2020 |
N = 426 aSAH in adult patients over 60 yo |
| Liu et al. [183] |
Retrospective single center study China 2008–2013 |
N = 339 Patients with severe (GCS < 9) isolated TBI aged from 16 to 65 yo without risk factors for cerebrovascular disease who stayed at least 96 h in the ICU |
| Liu et al. [184] |
Retrospective multicentric cohort study China 2019–2020 |
N = 789 Acute ischemic stroke with dysphagia |
| Lord et al. [185] |
Retrospective cohort study of placebo patients in intracerebral hemorrhage RCT (VISTA database) International Study period not reported |
N = 376 Spontaneous ICH patients who had CT scan performed within 3 h of symptom onset, follow-up CT scan at 24 and 72 h, and GCS and NIHSS performed at baseline, 1 h, 1 day, 2 days, 3 days, and 15 days, and available 3-month modified Rankin Scale score |
| Luo et al. [186] |
Retrospective single center study of prospectively collected data China 2016–2020 |
N:406 Acute ischemic stroke patients who underwent thrombectomy for large vessel occlusion (internal carotid artery, the anterior cerebral artery, and/or the middle cerebral artery (M1 and/or M2 segments) or vertebrobasilar circulation) |
| Maas et al. [34] |
Retrospective single center study of prospectively collected data study USA 2006–2012 |
N = 234 Spontaneous ICH admitted to neuro ICU |
| Malavera et al. [187] |
Post hoc analysis of prospectively collected data of patients included in a RCT 2008–2012 21 countries in North and south America, Europe, Asia and Oceania |
N = 2792 Adult patients with ICH admitted within 6 h of symptoms onset who had baseline temperature recorded |
| Matsukawa et al. [188] |
Retrospective single center retrospective study Japan 2003–2013 |
N = 118 Consecutive isolated non traumatic pontine hemorrhage patients |
| Matsuzono et al. [189] |
Prospective cohort bicentric center study Japan 2016–2020 |
N = 1116 Consecutive Acute ischemic stroke admitted within one week of ictus |
| Matuja et al. [190] |
Prospective single center cohort study Tanzania 2022 |
N = 135 Consecutive stroke (ischemic and hemorrhagic) Admitted to the hospital in a 4-month period |
| Melmed et al. [191] |
Retrospective single center cohort study of prospective and retrospective collected data USA 2013–2020 |
N = 83 Adult patients admitted due to spontaneous Intracerebral hemorrhage with at least 2 CT with 24 h of admission |
| Middleton et al. [192] |
Secondary data analysis from a single-blind cluster multicentric randomized trial (Quality in Acute stroke care trial) Australia 2005–2010 |
N = 970 Patients > 18 years with stroke (AIS or ICH) participating stroke units < 48 h of stroke onset |
| Millán et al. [193] |
Retrospective multicentric center study Spain Study period not reported |
N = 254 Consecutive patients with acute ischemic stroke treated with tPA within 3 h from stroke onset |
| Mohamed et al. [194] |
Prospective single center cohort study Egypt Study period not reported |
N = 80 Adult (> 18 yo) patients with spontaneous ICH admitted to the stroke unit within 24 h of ictus |
| Muehlschlegel et al. [195] |
Prospective single center cohort study USA 2009–2012 |
N = 213 Consecutive patients with moderate to severe TBI |
| Muscari et al. [196] |
Retrospective cross sectional single center study Germany 2011–2015 |
N = 1209 Adult stroke (AIS or ICH) patients with fasting blood glucose measured the morning after admission to stroke unit |
| Naess et al. [197] |
Prospective single center cohort study Norway 2006–2009 |
N = 250 First ever acute ischemic stroke admitted within 6 h of symptoms onset |
| Naidech et al. [198] |
Prospective single center prospective cohort study USA 2006–2007 |
N = 94 Non traumatic subarachnoid hemorrhage |
| Nutakki et al. [199] |
Retrospective single center cohort study Zambia 2018–2019 |
N = 324 Adult patients with stroke (AIS and ICH) |
| Oh et al. [200] |
Retrospective single center cohort study South Korea 20,004–2008 |
N = 126 Acute brain injury patients > 18 yo admitted to neuroICU with available temperature, BP, ICP and GCS score data for the first 72 h after ICU admission and no ongoing infection |
| Oliveira-Filho et al. [8] |
Prospective single center study USA 1998–199 |
N = 92 Non traumatic SAH |
| Ostini et al. [201] |
Retrospective single center study of prospectively collected data Switzerland 2014–2018 |
N = 97 aSAH adult (> 18 yo) patients who underwent aneurysm occlusion with endovascular or surgical approach |
| Park et al. [202] |
Retrospective single center cohort study South Korea 2007–2016 |
N = 412 aneurysmal SAH who stayed at least 14 days in hospital and has complete follow-up |
| Pegoli et al. [203] |
Retrospective single center retrospective study USA 2001–2013 |
N = 373 Consecutive aSAH patients with follow up within 1 year |
| Phipps et al. [204] |
Retrospective multicentric cohort study USA 1998–2003 |
N = 1361 Adult (> = 18 yo) with acute ischemic stroke admitted within 2 days of symptoms onset and NIHSS ≥ 2 |
| Reith et al. [205] |
Retrospective single center study of prospectively collected data (the Copenhagen Stroke study) Denmark 1991–1993 |
N = 390 Consecutive acute stroke patients admitted within 6 h of onset |
| Rincon et al. [206] |
Retrospective multicentric cohort study of patients in the control arm in the intracerebral hemorrhage trials (VISTA prospective database) International Study period not reported |
N = 330 Spontaneous ICH patients (controls only) confirmed with neuroimaging within 6 h of symptoms onset |
| Rincon et al. [207] |
Retrospective multicentric cohort study USA 2003–2008 |
N = 13,587 Consecutive adult patients (> = 18 yo) admitted to ICU with acute ischemic stroke, hemorrhagic stroke, subarachnoid hemorrhage or TBI |
| Rordof et al. [208] |
Retrospective single center study USA 1992–1995 |
N = 63 Acute ischemic stroke |
| Rosengart et al. [209] |
Post hoc analysis of prospectively collected data of patients included in 4 RCT of tirilazad Europe, Australia, North America and Africa 1991–1997 |
N = 2695 Adult patients ≥ 18 yo with SAH confirmed by neuroimaging or lumbar puncture due to saccular aneurysm confirmed by DSA with complete follow up data |
| Roy et Ray 210] |
Single center observational study India Study period not reported |
N = 200 Ischemic and hemorrhagic stroke confirmed by neuroimaging |
| Ryttlefors et al. [211] |
Retrospective single center cohort study 1999–2002 Sweden |
N = 99 aSAH with a minimum of 120 H of valid multimodality monitoring in the first 10 days of hospitalization |
| Saini et al. [212] |
Retrospective multicentric cohort study of patients in the control arm in the Acute ischemic stroke trials (VISTA prospective database) International Study period not reported |
N = 5305 Acute ischemic stroke (first ever) in adult patients confirmed by neuroimaging, |
| Saripalli et al. [213] |
Retrospective single center cohort study of prospectively collected data Australia 2015–2020 |
N = 175 Consecutive Adult (> 18 yo) patients admitted due to aneurysmal SAH who survived at least until day 4 |
| Saxena et al. [6] |
Retrospective multicentric cohort study UK, New Zealand, and Australia 2005–2013 |
N = 110 638 TBI, acute ischemic stroke, ICH, SAH and CNS infection admitted to the ICU without cardiac arrest in the previous 24 h |
| Schirmer-Mikalsen et al. [214] |
Prospective single center cohort stud Norway 2004–2009 |
N = 133 Severe (GCS < 9)TBI patients excluding patients who underwent limitation / withdrawal of care |
| Schwarz et al. [215] |
Single center retrospective study Germany 1992–1996 |
N = 251 Consecutive supra-tentorial spontaneous ICH patients admitted within 24 h of symptoms |
| Seo and Oh 41] |
Prospective single center cohort study South Korea 2002–2004 |
N = 190 Adult patients with Hemorrhagic stroke or TBI admitted within 24 h of ictus/injury |
| Seo et al. [216] |
Retrospective single center study South Korea 2004–2006 |
N = 150 Acute ischemic stroke within 24 h of symptoms onset |
| Shin et al. [217] |
Retrospective multicentric cohort study Korea 2014–2016 |
N = 207,371 Adult (> 18 yo) patients with cerebral vascular accident (N = 125,556) or TBI (N = 81,815) presenting directly to the ED |
| Song et al. [218] |
Retrospective analysis of a multicentric prospective observational study Asia 2015–2020 |
N = 6540 TBI (> 15yo) patients transported to ED department by EMS |
| Springer et al. [219] |
Prospective single center cohort study USA 1996–2002 |
N = 232 Adult (> 18 yo) aSAH patients |
| Stochetti et al. [4] |
Retrospective single center study Italy 1996–1997 |
N = 110 TBI patients older than 13 yo |
| Stosser et al. [220] |
Retrospective single center study Germany 2016 |
N = 923 Adult patients admitted with ischemic or hemorrhagic stroke within 7 days of the ictus |
| Suehiro et al. [221] |
Prospective single center cohort study Japan 2012–2013 |
N = 62 aSAH who underwent coiling or clipping within 72 h from ictus |
| Suzuki et al. [222] |
Retrospective single center cohort study USA 1989–1993 |
N = 82 Consecutive spontaneous non lobar ICH admitted within 72 h of bleed |
| Swor et al. [223] |
Prospective single center cohort study USA 2006–2016 |
N = 248 Consecutive spontaneous ICH who presented directly to the emergency department and were in sinus rhythm |
| Szczudlik et al. [224] |
Prospective single center study Poland 4 years |
N = 152 Spontaneous non-surgical supratentorial ICH confirmed by neuroimaging and admitted within 24 h of symptoms |
| Szczudlik et al. [225] |
Prospective single center study Poland 1 year |
N = 60 Consecutive first ever AIS admitted to hospital within 24 h of symptoms |
| Tanaka et al. [226] |
Retrospective multicentric analysis of prospective collected data Japan 2009–2011 |
N = 195 Mild TBI at arrival (GCS > = 13) that required neurosurgical intervention |
| Tegegne et al. [227] |
Retrospective single center cohort study Ethiopia 2017–2022 |
N = 382 Adult TBI patients who either underwent surgery or were admitted to the ICU during the study period |
| Tiainen et al. [228] |
Retrospective single center study of prospectively collected data Finland 1995–2008 |
N = 985 Acute ischemic stroke patients who underwent IV thrombolysis |
| Todd et al. [229] |
Post hoc analysis of prospectively collected data of patients included in the IHAST RCT 2000–2003 USA, Canada, UK, Germany, Austria, Australia and New Zealand |
N = 1000 aSAH WFNS 1–3 undergoing clipping |
| Tseng et al. [230] |
Prospective single center cohort study Taiwan 2019–2021 |
N = 100 Consecutive adults (> 20 yo) primary ICH patients undergoing rehabilitation post stroke who completed 1 year follow up |
| Ueno et al. [231] |
Retrospective single center cohort study with nested case control propensity matched study Japan 2009–2017 |
N = 120 (cohort) Consecutive acute ischemic stroke treated with rtpa |
| Vallée et al. [232] |
Retrospective multicentric study France 2005–2007 |
N = 207 Severe TBI (GCS 8 after correction of hypotension) patients older than 15 years who were admitted alive, stabilized over the first 24 h and monitored (within 12 h) with ICP in ICU |
| Vapalhti et al. [233] |
Prospective single center observational study Finland 1967–1969 |
N = 50 Severe Traumatic brain injury (patients monitored with ICP) |
| Volbers et al. [37] |
Retrospective single center cohort study from prospectively collected data Germany 2006–2010 |
N = 220 Spontaneous supratentorial ICH |
| Volbers et al. [234] |
Retrospective single center cohort study from prospectively collected data Germany 2006–2014 |
N = 292 Spontaneous supratentorial ICH |
| Walelgn et al. [235] |
Retrospective single center cohort study Ethiopia 2014–2019 |
N = 368 Patients aged > 15 yo admitted with stroke (AIS or ICH) confirmed by neuroimaging |
| Wang et al. [236] |
Retrospective single center study Australia 1995–1997 |
N = 509 Consecutive acute stroke (AIS or ICH) patients |
| Wang et al. [40] |
Retrospective single center study Australia 1995–1997 |
N = 223 Consecutive acute ischemic stroke |
| Wang et al. [237] |
Retrospective single center study USA 2001–2012 |
N = 1123 TBI patients with age ≥ 65 yo with Abbreviated Injury Score-head ≥ 3 |
| Wang et al. [238] |
Retrospective single center study USA 2008–2019 |
N = 2990 Adult hemorrhagic stroke patients who stayed at least 24 h in the ICU |
| Wartenberg et al. [239] |
Prospective single center cohort study USA 1996–2002 |
N = 576 Adult patients (> 17 yo) non traumatic SAH |
| Weimar et al. [37] |
Multicentric retrospective study of prospectively collected data Germany 1998–1999 |
N = 1754 Acute ischemic stroke |
| Weimer et al. [240] |
Single center prospective study USA 2008–2011 |
N = 116 Patients aged > 17 years, diagnosis of SDH based on admission CT scan data |
| Wettervik et al. [52] |
Retrospective single center study Sweden 2008–2018 |
N = 115 Severe TBI (motor score < 6) who underwent invasive neuromonitoring |
| Wettervik et al. [241] |
Retrospective single center cohort study 2008–2018 Sweden |
N = 166 aSAH patients who underwent invasive neuromonitoring |
| Wijdicks et St Louis 242] |
Retrospective single center cohort study USA 1976–1996 |
N = 38 Primary pontine hemorrhage |
| Wu et al. [243] |
Retrospective single center study China 2017–2022 |
N = 308 Adult (> 18 yo) spontaneous ICH patients who underwent hematoma evacuation |
| Wu F et al. [244] |
Retrospective single center cohort study China 2020–2021 |
N = 195 Consecutive adult ICH patients who underwent minimally invasive surgery |
| Yamamoto et al. [245] |
Retrospective single center study Japan 1993–2000 |
N = 22 TBI patients who underwent mild hypothermia and had invasive neuro-monitoring |
| Yang et al. [246] |
Retrospective analysis of multicentric cohort database USA 2014–2015 |
N = 6201 Stroke (ischemic and hemorrhagic) patients admitted to the ICU with LOS > 24 h |
| Yokobori et al. [247] |
Retrospective multicentric cohort study of prospectively collected data Japan 1998–2018 |
N = 1458 Elderly patients (≥ 65 yo) with severe TBI (initial GCS 8) or TBI with a decrease in GCS score within 48 h of injury onset and the start of treatment |
| Zhang et al. [248] |
Retrospective single center study China 2008–2009 |
N = 155 Adult Patient (≥ 18 yo) non-traumatic SAH, admitted to hospital within 7 days after onset |
| Zhang et al. [249] |
Retrospective analysis of prospectively collected data. Multicentric China 2014–2019 |
N = 664 Adult patients with AIS due to basilar artery occlusion |
| Zhang et al. [250] |
Retrospective single center cohort study China 2011–2018 |
N = 1036 Patients admitted to hospital due to spontaneous ICH within 6 h from onset |
| Zhao et al. [251] |
Retrospective single center cohort study China 2016–2020 |
N = 515 aSAH who underwent aneurysm treatment |
| Zhou et al. [252] |
Retrospective single center cohort study China 2015–2021 |
N = 150 Adult (> 18yo) Poor grade SAH patients treated with clipping |
| Zou et al. [253] |
Retrospective analysis of a single center database USA 2001–2012 |
N = 623 Adult ICH patients with at least 24 h of ICU LOS |
| Fever/hyperthermia definition | Outcome measures | Main results |
|---|---|---|
|
BT > 37.5 °C Number of patients with fever: 231 Prevalence of fever: 25% |
In hospital mortality | The risk score for in hospital mortality of stroke patients which included BT > 37.5 °C had good discrimination ability |
|
BT increase > 1 °C Number with increase in BT: 9 Prevalence of fever: 11% |
mRankin and mortality at discharge, 3 and 6 months UO defined as mRankin 4–6 |
There was no association between BT increase and outcome |
|
Core BT > 38.3 °C Number of patients with fever: 28 Prevalence of fever: 61% |
GOS at 3 months UO was defined as GOS 1–3 |
A higher difference between core BT and brain temperature was associated with improved outcome at 3 months BT was not associated with outcome |
|
BT ≥ 37.5 °C Number of patients with fever: 60 Prevalence of fever: 24% |
Hospital mortality | BT ≥ 37.5 was not associated with mortality in a multivariable model |
|
Poor temperature control defined as BT > 38 °C during the first 5 days of admission Number of patients with poor temperature control (fever): 24 Prevalence of poor fever control: 10% |
mRankin at discharge. UO was defined as mRankin 3–6 | AIS and ICH patient with poor fever control had worse neurological outcome at discharge |
|
Increase in BT in the first 24 h after admission Number of patients with BT > 38.0 °C at 24 h of admission:73 Prevalence of fever:61% |
Mortality at 3 months | BT increase in the first 24 h was associated with a higher mortality in a multivariable model |
|
BT on admission Number of patients with fever calculated as BT > 37 °C: 1248 Prevalence of fever:25% |
mRankin at discharge and at 3 months UO was defined as mRankin 2–6 |
Higher admission BT was significantly associated with poor outcomes at 3 months |
|
Admission BT > 37.5 °C Number of patients with fever: 67 Prevalence of fever: 18% |
Hospital mortality | BT < 36.5 °C below but not fever was associated with hospital mortality |
|
BT ≥ 38 °C during ICU stay Number of patients with fever not reported Median time with BT ≥ 38 °C = 254 min, median time with BT ≥ 39 °C =195 min Median time with BT ≥ 40 °C =104 min |
GOS and mortality at 12 months UO was defined as GOS 1–3 |
Duration of pyrexia was associated mortality at 12 months |
|
BT > 38 °C during hospital stay Number of patients with hyperthermia: 161 Prevalence of fever: 14% |
Hospital Mortality | Hyperthermia during hospitalization was associated with mortality |
|
BT ≥ 37.9 °C during the first 7 days of admission Number of patients with fever = 132 Prevalence of fever: 73% |
30-day Mortality | The maximum BT measured during the first 7 days of admission was independently associated with mortality |
|
Fever burden was defined as a BT > 37 °C, and was quantified as the highest axillary temperature reached during the day minus 37 C. The total fever burden was defined as the arithmetic sum of the fever burdens during the 14 days, expressed as °C-days Number of patients with fever = 274 Prevalence of fever: 77% |
GOS at 6 months UO was defined as GOS 1–3 |
Fever burden might be an independent predictor of TBI prognosis, especially in the early stages of the disease course |
|
BT > 37 °C during the first 72 h of hospitalization Number of patients with hyperthermia: 161 Prevalence of fever: 33% |
Stroke progression in the first 72 h of ictus | Elevated BT was not associated with stroke progression |
|
Admission BT Number of patients with elevated BT calculated as BT > 37 °C = 294 Prevalence of fever: 67% |
mRankin at 90 days UO was defined as mRankin 3–6 |
Low BT on admission was associated with unfavorable outcome. Elevated BT was not associated with outcome |
|
Acute BT Number of patients with Elevated BT calculated as acute BT > 37 °C =39 Prevalence of fever: 16% |
mRankin at 3 months. UO was defined as mRankin 4–6 | Elevated BT was associated with poor outcome at 3 months |
|
BT at admission and in the first 72 h Number of patients with elevated BT on admission calculated as BT > 37 °C = 30 Number of patients with elevated BT in the first 72 h calculated as BT > 37 °C = 37 Prevalence of fever: 33% |
BI at 3 months UO was defined as BI < 85 |
Patients with unfavorable had higher incidence of elevated BT at baseline but not at 72 h compared to those with with favorable outcome |
|
BT in the first 72 h (high BT: BT > 37 °C) Number of patients with high temperature = 890 Prevalence of fever: 30% |
mRankin at 3 months. UO was defined as mRankin 3–6 | BT increases in patients with stroke in the first 72 h, with the harmful effect of high BT on outcome occurring in the first 48 h |
|
Hyperthermia was defined as BT < 38.5 °C assessed during the first 5 days of admission Number of patients with hyperthermia = 31 Prevalence of fever: 62% |
GOSE at 6 months UO was defined as GOSE 1–4 |
Hyperthermia combined with traumatic intracerebral hemorrhage were shown to be significant prognostic indicators of future poor neurologic outcomes |
|
Admission BT > 37.5 °C Number of patients with elevated BT: N = 35 Prevalence of fever: 5% |
mRankin at 3 months and mortality at 3 months UO was defined as mRankin 4–6 |
Elevated BT on admission within 6 h of stroke onset had no prognostic influence on stroke outcome at 3 months |
|
BT > 38 °C on the first day of hospitalization Number of patients with fever: 69 Prevalence of fever: 12% |
mRankin at 1 month UO was defined mRankin 3–6 |
Patients with fever on the first day of admission had worse functional outcome at 30 days |
|
Fever burden was defined as number of days with BT > 38 °C Number of patients with fever = 42 Median fever burden = 3 (1–6) days Prevalence of fever: 37% |
Neuro-QoL domains of Cognitive Function and Mobility at 28 days, 3 months, and 1 year | Each additional day with a fever was predictive of worse HRQoL domains of Cognitive Function and Mobility after ICH up to 1 year |
|
Hyperthermia was defined as BT ≥ 37.5 °C on day 1 Number of patients with hyperthermia = 100 Prevalence of fever: 50% |
mRankin at 3 months UO was defined as mRankin 3–6 | Hyperthermia is associated with poor outcomes in stroke |
|
Number of days with BT > 38.5 °C in the first 19 days of hospitalization Number of patients with fever not reported Mean number of days with fever = 4.7 |
GCS, GOS, FIM and RLA at hospital discharge; GOS and FIM long-term follow-up | Days with fever was correlated with short term outcome measures and with long term GOS |
|
Admission BT Number of patients with elevated BT calculated as BT > 37.5 °C =16 Prevalence of fever: 12% |
mRankin at 3 months UO was defined as mRankin 3–6 |
The incidence of elevated admission BT was higher in patients with poor outcome compared to those with good outcome |
|
Admission BT Number of patients with elevated admission BT calculated as BT > 37 °C = 43 Prevalence of fever: 34% |
Stroke progression | Elevated BT was associated with ischemic stroke progression |
|
Hyperthermia was defined as BT > 37.5 °C on two measurements in the first 3 days from ictus Number of patients with hyperthermia = 158. Prevalence of fever: 61% |
Neurological Outcome at 3 months defined as Canadian Stroke Scale (poor outcome: < 7 points), Barthel Index (poor outcome: < 60 points) and infarct volume (large: > 30 cm [3]) | The relationship between brain damage and high BT is greater the earlier the increase in temperature occurs. However, only BT within the first 24 h from stroke onset was associated with poor outcome and large cerebral infarcts |
|
Elevated BT was defined as BT on admission > 37.5 °C Number of patients with elevated BT = 33 Prevalence of fever: 26% |
Stroke progression and infarct volume | Elevated BT was not significantly related to infarct volume |
|
Pyrexia was defined as BT ≥ 37.5 °C within 24 h of admission Number of patients with pyrexia = 152 Prevalence of fever: 59% |
mRankin at 3 months. UO was defined as mRankin 3–6 Mortality in the hospital and at 3 months, intracranial hemorrhage transformation (HT), early neurological deterioration represented the secondary outcomes |
Pyrexia is associated with poor outcome at 3 months |
|
BT on admission Number of patients with elevated BT not reported |
Post operative progressive hemorrhagic injury defined as new intracranial hemorrhage or 25% increase in the original hemorrhage | Elevated BT was associated with postoperative hemorrhagic progression in TBI |
|
BT on admission Number of patients with fever calculated as BT > 38 °C= 584 Prevalence of fever: 28% |
In hospital death | Both Lower admission body temperature and fever on admission (BT > 38 °C were also associated with increased risk of death |
|
Elevated peak BT ≥ 37.3 °C. within 24 h of EVT Fever was defined as peak BT ≥ 38 °C) within 24 h of EVT Number of patients with elevated BT = 55 Number of patients with fever = not reported Prevalence of fever: 62% |
mRankin at 3 months mortality at 3 months Hospital mortality and mRankin at discharge UO was defined as mRankin 3–6 |
Fever within 24 h of EVT was significantly associated with an increased incidence of symptomatic intracranial hemorrhage, discharge to hospice or inpatient death, poorer clinical outcome and 3-month mortality, and with less functional independence |
|
BT on admission Fever was calculated as BT ≥ 37.5 °C Number of patients with fever = 22 Prevalence of fever: 16% |
mRankin at 30 days UO was defined as mRankin 3–6 |
High body temperature was independently associated with poor outcome at 30 days but not with mortality |
|
BT on admission Number of patients with elevated BT calculated as BT > 37 °C= 18 Prevalence of fever: 2% |
Neurological deterioration in the first 72 h after ictus | Elevated BT on admission was not associated with neurological deterioration |
|
Fever was defined as BT ≥ 38 °C Number of patients with fever = 170 Prevalence of fever: 17% |
mRankin at discharge UO was defined as mRankin 3–6 |
Fever was associated with unfavorable outcome at hospital discharge |
|
Fever was defined as BT ≥ 38.3 °C Number of patients with fever = 87 Prevalence of fever: 22% |
Hospital mortality | Fever was not associated with hospital mortality in a mixed population of neuro ICU patients |
|
Elevated BT was defined as BT on admission > 37 °C Number of patients with elevated BT = 43 Prevalence of fever: 34% |
Stroke progression | Elevated BT was associated with ischemic stroke progression |
|
Fever was defined as BT > 37.5 °C Number of patients with fever not reported |
mRankin at 90 days. UO was defined as mRankin 3–6 | BT > 37.5 °C correlated with relative infarction growth and was associated with poor outcome at 90 days |
|
No definition of fever Number of patients with fever = 221 Prevalence of fever: 41% |
Mortality at 3 months | The overall incidence of mortality was found to be high. Fever was associated with death |
|
BT measured on admission and at 24 h Fever defined as BT > 37 °C Number of patients with fever:661 Prevalence of fever: 47% |
Improvement in mRankin at 3 months (was defined as a score on the mRS lower than the median grade of patients with a similar prognostic index.) | Increase in BT measured 24 h after randomization was associated with a lack of improvement at 3 months |
|
BT > 37 °C on the first 24 h of admission Number of patients with fever = 31 Prevalence of fever: 9% |
Hemorrhagic transformation, unfavorable outcome at discharge defined as mRankin 3–6, hospital mortality |
LVO in patients treated by IVT or IVT and MT increases the risk of hemorrhagic and unfavorable short-term outcome but not in-hospital mortality Body temperature > 37 in the first 24 h of admission was not associated with outcome |
|
Fever not defined Number of patients with fever = 215 Prevalence of fever: 4% |
Traumatic Vasospasm |
Fever was associated with the development of traumatic vasospasm Vasospasm was associated with lower likelihood of routine discharge and an extended LOS |
|
BT before and after EVT Number of patients with fever calculated as BT > 37.5 °C =108 Prevalence of fever: 25% |
Functional independence at 3 months defined as mRankin 0 -2. Hemorrhagic transformation and mortality at 3 months |
Higher BT during both the intra-ischemic and post-ischemic phases were associated with poorer clinical outcome |
|
Low fever was defined a BT between 37.5 and 38.4 moderate fever as BT between 38.5 and 39.0 °C; high fever as BT > 39.0 °C Number of patients with fever = 3027 (low:1591; moderate:719, high:717) Prevalence of fever: 70% |
Hospital Mortality; ICU and hospital LOS Prevalence of fever: 47% |
Elevated BT was associated with a longer ICU and hospital LOS, higher mortality rate, and worse outcome |
|
Fever was defined as BT > 38.2 °C in the first 5 days of hospital stay Number of patients with fever: 79 Prevalence of fever: 52% |
Refractory vasospasm, DCI | Early fevers may be predictive of need for multiple endovascular interventions in refractory cerebral vasospasm after aSAH |
|
BT measured by temporal artery temperature at day 30, day 31, day 32 and day 33 post hospital admission Number of patients with fever calculated as BT > 37.5 °C: 29 Prevalence of fever: 48% |
GOS at hospital discharge. UO was defined as GOS 1–3 | BT was not associated with outcome |
|
Maximum BT Number of patients with fever calculated as BT ≥ 38 °C:118 Prevalence of fever: 39% |
mRankin at 3 months. UO defined as 3–6 | Maximum BT was an independent factor associated with poor outcome |
|
Hyperthermia was defined as BT > 38 °C Number of patients with hyperthermia: 44 Prevalence of fever: 83% |
GOS 6 months. UO was defined as GOS 1–3 | Hyperthermia was associated with poor outcome at 6 months |
|
Fever was defined as BT > 37.0 °C Number of patients with fever:193 Prevalence of fever: 31% |
Early neurological deterioration | Fever on admission was associated with early neurological deterioration in stroke |
|
Hyperthermia was defined as BT > 38.6 °C Number of patients with hyperthermia: 10 On admission:3 Prevalence of fever: 10% |
Early mortality (first 72 h) | Hyperthermia was not associated with early mortality |
|
Fever was defined as BT > 38 °C on admission and on day 8 Number of patients with fever on admission = 208 Prevalence of fever on admission: 8% Number of patients with fever on day 8 = 1215 Prevalence of fever: 44% |
Cerebral infarction and GOS at 3 months. UO was defined as 1–3 | Fever at day 8 was associated with UO at 3 months. Fever at day 8 was also associated with cerebral infarction |
|
Fever was defined as maximum BT in the first 10 days of hospitalization > 38.3 °C Number of patients with fever: 254 Prevalence of fever: 72% |
Mortality at 90 days Unfavorable neuro outcome at 90 days (mRankin 4–6) Lawton instrumental activities of daily living scale at 90 days (poor outcome > 8) Telephone Interview of Cognitive Status (impaired if TICS 30) Sickness Impact Profile Quality of life (QoL < the median) |
Refractory fever during the first 10 days after subarachnoid hemorrhage is associated with increased mortality and more functional disability and cognitive impairment among survivors |
|
Admission BT Number of patients with fever calculated as BT > 37.5 °C =138 Prevalence of fever: 50% |
mRankin scale at discharge | Admission BT had an indirect significant correlation with mRankin scale at discharge |
|
Maximum BT recorded in the first 7 days of hospitalization Number of patients with fever calculated as BT > 37.5 °C =91 Prevalence of fever: 50% |
mRankin scale at 2 months. UO was define as mRankin 5–6 | BT correlated well with both functional outcome and infarct size in patients with an acute cerebral infarction |
|
Initial trauma center BT (ICTC) elevated if ≥ 38 °C Number of patients with fever = 177 Prevalence of fever: 1.5% |
In Hospital mortality | Elevated BT immediately following pre-hospital transport was associated with higher mortality |
|
Early hyperthermia (EH) if BT > 38.5 °C at least 1 time within the first 2 days Number of patients with hyperthermia = 44 Prevalence of fever: 44% |
ICU mortality GOS at 6 months. UO was defined as GOS 1–3 |
Patients who experienced early hyperthermia had worse neurological outcome ta 6 months |
|
Fever was defined as BT > 37.5 °C in > 2 measurements in 48 h Number of patients with fever = 124 Prevalence of fever: 38% |
mRankin at discharge. UO was defined as mRankin 4–6 Barthel Index at discharge Severe disability was defined as BI < 40 |
Stroke patients that develop fever have worse outcomes than those with normothermia. They also have higher rate of hemorrhagic transformation and bigger infarct size and hematoma volume |
|
Peak BT defined as the highest BT on days one to three after admission Number of patients with fever calculated as peak BT > 37.5 °C = 142 Prevalence of fever: 34% |
Infarct volume at day 3 mRankin at 90 days. UO was defined as mRankin 3–6 |
Higher peak BT during the first days after ischemic stroke, rather than on admission, are associated with larger infarct size and poor functional outcome |
|
Fever was defined as BT > 38.3 °C during hospitalization Number of patients with fever = 136 Prevalence of fever: 39% |
In hospital mortality | Patients with fever have higher in hospital mortality |
|
Fever was defined as any episode of BT > 38.0 °C during ICU stay Number of patients with fever = 64 Prevalence of fever: 26% |
GOS at 3 months, DCI and in hospital mortality UO was defined as GOS 1–3 |
Fever was associated with the development of DCI and poor neurological outcome but not mortality |
|
Fever was defined as BT ≥ 38 °C within 48 h of stroke Number of patients with fever = 30 Prevalence of fever: 25% |
Barthel index after 3 months. UO was defined as BI < 70 Mortality at 3 months |
Patients who experienced fever in the first 48 h after stroke had higher mortality rates at 3 months and worse functional outcome at 3 months |
|
BT on admission Number of patients with fever was calculated as BT > 37 °C:138 Prevalence of fever: 18% |
90 days mortality and neurological outcome (mRankin). UO was defined as mRankin 3–6 | BT was associated with 90-day mortality and neurological outcome |
|
Fever was defined a BT > 38 °C during the first 14 days of admission Number of patients with fever = 137 Prevalence of fever: 58% |
mRankin at 3 months UO was defined as mRankin 4–6 |
Fever has deleterious effects on outcome of ICH patients with and without subarachnoid extension of blood |
|
Fever was defined as BT > 37.5 °C in the first 72 h Number of patients with fever = 88 Prevalence of fever: 27% |
In hospital mortality | Fever was associated with in hospital mortality |
|
Baseline BT Number of patients with fever calculate as BT on admission ≥ 37.5 °C = 40 Prevalence of fever: 16% |
mRankin at 30 and 90 days UO was defined as mRankin 4–6 |
No association between fever ad outcome at 30 and 90 days |
|
Hyperthermia in the first 24 h was defined as BT > 38 °C Number of patients with fever = 11 Prevalence of fever: 11% |
Brain death | Hyperthermia in the first 24 h was not associated with brain death |
|
Core Temperature on admission Number of patients with elevated core Temperature calculated as ≥ 37 °C = 24 Prevalence of fever: 18% |
GOS at 6 months UO was defined as GOS 1–3 |
Elevated BT on admission was associated with poor outcome |
|
BT measured twice daily in the first week subfebrile if BT > 37.5 (n = 31) and fever if BT > 38 °C (n = 17) Prevalence of fever: 15% |
Recovery ratio assessed at 2, 5, 7 and 60 days | An elevation of BT during the first week of an ischemic stroke is an unfavorable prognostic sign |
|
Early fever defined as BT > 38.3 °C in the first 48 h Number of patients with fever = 42 Prevalence of fever: 27% |
GOSE at discharge and Hospital mortality UO was defined as GOSE 1–4 |
Early onset fever is associated with death and worse neurological outcome at discharge |
|
Fever was defined as BT ≥ 38.3 °C (central and infectious) Number of patients with fever = 39 Prevalence of fever: 41% |
mRankin at 90 days UO was defined 3–6 |
sICH patients with central fever compared to patients without fever had higher mortality rates and worse neurological outcome at 3 months |
|
BT at admission Number of patients with fever calculated as BT > 37.5 = 60 Prevalence of fever: 50% |
28-day survival cerebral infarction during hospital stay |
BT was not associated with cerebral infarction |
|
Fever was defined as BT > 38.0 °C at least two measurements for seven consecutive days after admitted to the ICU Number of patients with fever = 76 Prevalence of fever: 82% |
GOS at hospital discharge. UO was defined as GOS1-3 Hospital Mortality |
A significant portion of patients developed a fever during the first post-craniotomy week. Fever was associated with poor outcome at hospital discharge but not mortality |
|
Admission BT Number of patients with fever calculated as BT > 37 °C = 209 Prevalence of fever: 25% |
mRankin at 90 days UO was defined mRankin 4–6 |
BT on admission was not associated with outcome |
|
BT on admission Number of patients with fever calculated as BT > 37 °C = 15 Prevalence of fever: 25% |
Neurological outcome at 3 months UO was defined mRankin 3–6 |
Admission BT and fever were not associated with outcome |
|
Hyperthermia was defined as BT > 37.2 °C on admission Number of patients with hyperthermia = 53 Prevalence of fever: 19% |
Emergency department mortality in the first 7 days | The 7-day fatality was 10.1%. Hyperthermia was not associated with 7-day fatality |
|
BT on admission Number of patients with fever calculated as BT > 37.5 °C = 133 Prevalence of fever: 20% |
Neurological outcome at 3 months. UO was defined as mRankin > 2 | Patients who had poor outcome had higher admission BT than those with favorable outcome |
|
Hyperthermia was defined as BT ≥ 37.5 °C during the first 24 h Number of patients with fever = 165 Prevalence of fever: 19% |
mRankin scale at 3 months. UO was defined as mRankin 3–6 Early neuro deterioration |
Hyperthermia is associated with poor outcome, especially in ICH of hypertensive origin. There is a probable relationship between edema volume and elevated body temperature in the first 24 h in hypertensive patients with ICH |
|
Fever on admission was defined as BT > 38 °C Number of patients with fever = 74 Prevalence of fever: 32% |
In hospital mortality | Markers of infection and inflammation, including fever, are associated with in hospital mortality after thrombectomy |
|
Fever was defined as BT < 38 °C (SIRS definition) Number of patients with fever = 7 Prevalence of fever: 2% |
GOS at discharge. UO was defined as GOS 1–3 | Hyperthermia compared to normothermia was not associated with unfavorable outcome in a multivariate model |
|
Hyperthermia was defined as BT > 37.5 °C Number of patients with fever = 64 Prevalence of fever: 26% |
In hospital mortality | Hyperthermia was not associated with mortality in a multivariate model |
|
Fever was defined as T > 37.5 °C Number of patients with fever = 67 Prevalence of fever: 27% |
mRankin scale at 3 months. UO was defined as mRankin 3–6 | Patients with poor outcome at 3 months had higher frequency of fever episodes |
|
Hyperthermia was defined as BT ≥ 37 °C. in the first 72 h of admission Number of patients with fever = 567 Prevalence of fever: 67% |
GOS at 1 year. UO was defined as GOS 1–3 | Hyperthermia was associated with unfavorable outcome |
|
Hyperthermia was defined as BT > 37.5 °C Number of patients with fever calculated as BT > 37.5 °C = 46 Prevalence of fever: 55% |
Neurological outcome assessed by Barthel index (UO < 50) after rehabilitation | Increase in admission BT was associated with poor outcome |
|
Elevated BT defined as BT on admission > 37 °C Number of patients with fever = 49 Prevalence of fever: 12% |
Scandinavian stroke scale at discharge. (UO was defined as SSS > 30, death or severe disability) | Elevated BT was associated with poor neurological outcome at discharge |
|
Fever was defined as BT on admission > 37 °C Number of patients with fever = 211 Prevalence of fever: 54% |
Mortality at 3 months and 5 years | Patients with fever had higher mortality rates at 5 years |
|
Pyrexia was defined as BT ≥ 37.5 °C from hospital arrival to 120 h Number of patients with pyrexia = 16 Prevalence of fever: 33% |
mRankin at 3 months. UO was defined as mRankin 3–6 | Pyrexia was not associated with poor neurological outcome at 3 months |
|
Pyrexia was defined as BT ≥ 37.5 °C from hospital arrival to 120 h Number of patients with pyrexia = 12 Prevalence of fever: 30% |
mRankin at 3 months. UO was defined as mRankin 3–6 | Pyrexia in the first 120 h of hospitalization was associated with 3 months poor outcome |
|
High BT was defined as BT > 37 °C Number of patients with high BT = 140 Prevalence of fever: 24% |
Early neuro deterioration (24 h); Symptomatic hemorrhagic transformation within 36 h; 3-month CT infarct volume; global functional outcome assessed by the mRankin Scale at 3 months (UO = 2–6); and mortality at 3 months |
In patients with hyperacute stroke, higher presenting BT are associated with less severe neurological deficits and reduced final infarct volumes |
|
BT on admission Number of patients with fever calculated as BT > 37.5 °C = 34 Prevalence of fever: 3% |
Mortality at 1 month and 3 years | Higher BT on admission was associated with 30-day mortality but not with 3-year mortality |
|
Fever was defined as core temperature ≥ 38.3 °C on 2 consecutive days Number of patients with fever = 281 Prevalence of fever: 48% |
Neurological outcome at 6 and 12 months. UO was defined as mRankin 3–6 | The number of days spent with fever was associated with poor neurological outcome at 6–12 months |
|
High admission BT was defined as BT ≥ 37 °C Number of patients with high BT = 48 Prevalence of fever: 28% |
mRankin on day 7 of hospitalization or discharge. FO was defined as mRankin 0–1 | High admission BT was associated with favorable outcome |
|
High admission BT was defined as BT ≥ 37.5 °C Number of patients with high BT = 5 |
Early neurological improvement at 24 h (decrease in 8 points in the NIHSS) | Higher body temperature was associated with early neurological improvement |
|
Noninfectious fever was defined as BT > 38.6 °C assessed daily Number of patients with fever = 22 |
Vasospasm, DIND, DCI and mRankin at 6 months to 2 years. UO was defined as 3–6 |
Non-infectious fever was associated with angiographic and TCD- assessed vasospasm |
|
Fever was defined by temperature ≥ 38.3 °C and high fever by ≥ 39.5 °C during ICU stay Number of patients with fever:10,730 (2443 in trauma/neuro) |
ICU mortality | Fever was associated with ICU survival in trauma/neuro patients |
|
Fever was defined as BT > 38 °C in the first 5 min of admission to ED Number of patients with fever = 129 Prevalence of fever: 19% |
Hospital mortality and mRakin at discharge. UO was defined as mRankin 4–6 |
TBI fever was not associated with mortality or poor neuro outcome; however, in elderly patients (> 65 yo) with TBI, fever was associated with increased in- hospital mortality |
|
BT on pre-hospital setting Fever calculated as BT > 37 °C = 62 Prevalence of fever: 25% |
In-hospital mortality | Pre-hospital fever was not associated with in hospital mortality |
|
Fever was defined as BT on admission ≥ 37.5 °C Number of patients with fever = 16 Prevalence of fever: 6% |
Early neurological deterioration | The presence of fever on admission was associated with early neurological deterioration |
|
Hyperthermia was defined as BT ≥ 37.5 °C on admission Number of patients with hyperthermia = 81 Prevalence of fever: 35% |
Infarct volume; Canadian Stroke Scale (CSS) at 3 month. CSS < 7 was considered poor outcome | Hyperthermia in the first 24 h was associated with poor outcome at 3 months |
|
Hyperthermia was defined as mean BT ≥ 37.5 °C in the first 72 h Number of patients with hyperthermia = 61 Prevalence of fever: 38% |
Hemorrhagic transformation | Mean BT in the first 24 h was associated with hemorrhagic transformation |
|
Pyrexia was defined as BT ≥ 37.5 °C Number of patients with pyrexia = 235 Prevalence of fever: 36% |
Barthel index at 3 months Mortality at 3 months UO was defined as BI < 60 |
Patients with mild to moderate neurogenic fever and those with infectious fever had worst functional outcome at 3 months compared to those without fever Fever regardless of etiology was associated with 3 months mortality |
|
Fever in the first 72 h was defined as BT > 38 °C Number of patients with fever = 916 Prevalence of fever: 13% |
GOS at hospital discharge. UO was defined as GOS 1–3 |
The mortality rate in patients with fever for 3 days was higher than in patients with 1 or 2 days of fever Patients with fever had higher mortality rates and higher rates of UO than those with normothermia |
|
BT on admission Fever was calculated as BT > 37.5 °C = 35 Prevalence of fever: 8% |
DCI with cerebral infarction | Fever was not associated with DCI with cerebral infarction in elderly patients |
|
Hyperthermia was defined as BT ≥ 38.5 °C Number of patients with hyperthermia = 146Prevalence of fever: 43% |
Post-traumatic cerebral infarction | Hyperthermia was a risk factor for cerebral infarction after severe head trauma |
|
Fever was defined as BT ≥ 37.5 °C Number of patients with fever = 19 Prevalence of fever: 2% |
mRankin at 3 months UO was defined as mRankin 3–6 |
Patients with good nutritional status had better prognosis than mal nourished patients. Low BT was associated with unfavorable outcome |
|
Fever was not defined Number of patients with fever = 63 Prevalence of fever: 17% |
Neurological deterioration (≥ 2 points decrease in GCS or a ≥ 4 points increase in the NIHSS score.) | Fever was associated with subacute neurological deterioration (day 1 to day 3) |
|
Fever: was defined as BT > 37.5 °C Number of patients with fever = 158 Prevalence of fever: 39% |
Early neurological deterioration which was defined as an increase of four or more points in the total NIHSS score within 24 h after EVT compared with the NIHSS score at admission mRankin at 3 months. UO was defined as mRankin 3–6 In hospital death |
Patients with fever had higher rates of early neurological deterioration, worse neurological outcome at 3 months and higher in hospital and 3 months mortality rate |
|
Fever was defined a BT > 38 °C during the first 14 days of admission Number of patients with fever = 137 Prevalence of fever: 56% |
mRankin at 3 months Subarachnoid extension of ICH |
Fever was more frequent in patients with subarachnoid extension of ICH |
|
Early pyrexia was defined as BT ≥ 37.5 °C at baseline Number of patients with early pyrexia = 39 Prevalence of fever: 1.4% |
Death at 90 days mRankin scale (UO = 3–6) at 90 days and mRankin (UO = 3–5) at 90 days |
Early pyrexia in mild to moderate ICH is associated with greater mortality at 90 days and larger PHE volume but not neurological outcome at 90 days |
|
Hyperthermia was defined as a core temperature of ≥ 39 °C Number of patients with hyperthermia = 9 Prevalence of fever: 8% |
6 months mortality rate | Hyperthermia was independently associated with death at 6 months after non-traumatic pontine hemorrhage |
|
BT on admission Number of patients with fever calculated as BT > 37 °C = 293 Prevalence of fever: 26% |
1 year mortality | Patients who were deceased at 1 year mark after stroke had higher admission temperature than survivors |
|
Fever was defined as BT > 38 °C lasting > 24 h Number of patients with fever = 63 Prevalence of fever: 47% |
30 days mortality | Overall mortality rate was 37%. Fever was not independently associated with 30 days mortality |
|
BT on admission Number of patients with fever was calculated as BT > 37.5 °C = 13 Prevalence of fever: 17% |
Hematoma expansion between the first and second CT scan within 24 h, which was defined as relative enlargement 33% or absolute growth 6 mL mRakin at discharge and 90 days. UO was calculated as mRankin of 3–6 |
SIRS is associated with hematoma expansion of ICH within the first 24 h, and hematoma expansion mediates the effect of SIRS on poor outcome |
|
Fever was defined an BT > 37.5 °C in the first 72 h of hospitalization Number of patients with fever = 219 Prevalence of fever: 23% |
90 days mRankin scale. UO was defined as mRankin 2–6 | Fever was associated with functional dependency at 90 days |
|
Elevated BT defined as BT > 37.0 °C in the first 24 h of hospitalization Number of patients with elevated BT = 114 Prevalence of fever: 45% |
mRankin at 3 months. UO was defined as mRankin 3–6 | Elevated BT in the first 24 h of admission was independently associated with poor outcome at 3 months |
|
Elevated admission BT was defined as BT > 37.0 °C Number of patients with elevated BT = 40 Prevalence of fever: 50% |
Early neurological deterioration at (four points or a greater increase in the NIHSS score and/or two points or a greater decrease in GCS or death at day 7) | Elevated BT was not associated with END |
|
Fever was defined as BT ≥ 38.3 °C Number of patients with fever = 132 Prevalence of fever: 62% |
In hospital mortality GOS at discharge. UO was defined as GOS 1–3 |
Fever was associated with hospital survival but not with neurological outcome |
|
Fever was defined as BT ≥ 37.5 °C Number of patients with fever = 375 Prevalence of fever: 31% |
mRankin scale on discharge. UO was defined as mRankin 3–6 Stroke unit mortality at discharge |
Fever was not independently associated with outcome |
|
BT on admission Number of patients with fever not reported |
1-week neurological outcome mRankin. UO was defined as mRankin 3–6 | In patients who underwent thrombolysis higher BT on admission was independently associated with favorable outcome while in patients who did not receive thrombolysis, higher body temperature was independently associated with unfavorable outcome at 7 days from ictus |
|
Daily higher core temperature from day 0 to day 13 Fever burden was defined as the daily highest core temperature minus 38 °C summed from admission through day 13 Number of patients with fever not reported |
14 days, 28 days and 3 months mRankin | Cumulative fever burden was associated with worse outcomes in good-grade patients and potential late recovery in poor-grade patients |
|
Fever: no definition provided Number of patients with fever = 50 Prevalence of fever: 15% |
In hospital and 90 days mortality | Patients with fever had higher in hospital and 90 days mortality rates compared to those without fever; however, fever was not independently associated with outcome in a multivariable analysis |
|
Hyperthermia was defined as BT > 38 °C in the first 72 h Number of patients with hyperthermia = 32 Prevalence of fever: 25% |
In hospital mortality | Hyperthermic patients had higher in hospital mortality rate than non-hyperthermic patients |
|
Fever was defined as BT > 38.3 °C for 2 or more consecutive days Number of patients with fever = 38 Prevalence of fever: 41% |
mRankin on discharge. UO was defined as mRankin 3–6 | The higher the number of days febrile the higher chance of poor outcome at discharge |
|
Fever was defined as core temperature > 38.2 °C Calculated number of patients with fever: 81 Prevalence of fever: 84% Mean number of days with fever: 2 (± 3) |
mRankin at discharge. UO was defined as mRankin 4–6 DCI |
Days of fever was not independently associated with outcome |
|
Fever was defined as BT > = 38 °C for 2 consecutive days or for more than 3 days within 2 weeks from the bleed Number of patients with fever = 203 Prevalence of fever: 49% |
DCI mRankin at 3 months. UO was defined as GOS 3–6 |
Fever was an independent risk factor for DCI and unfavorable outcomes after aneurysmal SAH |
|
Fever load was defined as the number of hours with temperature greater than 38 °C while in the intensive care unit Calculated number of patients with fever:112 Prevalence of fever: 30% Overall fever burden = 32 (± 56) hours |
mRankin at 1 year. Excellent outcome was defined as mRankin 0–1 | Patients with unfavorable outcome (mRankin 2–6) had higher fever burden than those with excellent outcome (mRankin 0–1) |
|
Fever was defined as BT ≥ 37.8 °C. A fever event was defined as a period with BT ≥ 37.8 °C, where the event ended when the temperature fell below 37.8 °C for ≥ 24 h. Fever burden was defined as the maximum temperature measured during hospitalization (Tmax) minus 37.8 °C, multiplied by the number of days with a temperature ≥ 37.8 °C Number of patients with fever = 483 Prevalence of fever: 35% |
Combined endpoint of in hospital mortality or discharge to hospice | Any episode of fever and medium or high burden of fever were independently associated with outcome |
|
Hyperthermia was defined as BT > 37.5 °C Number of patients with fever = 97 Prevalence of fever: 25% |
In hospital mortality and neurological outcome | Hyperthermia was associated with stroke severity, stroke size, in hospital mortality and poor neuro outcome in survivors |
|
Fever was defined as any daily recorded maximal temperature BT ≥ 37.5 °C at baseline, 24, 48, 72, or 168 h after onset of ICH symptoms Delta temperature: linear variation of each subject’s temperature by subtracting 37 °C from the maximal daily recorded temperature Number of patients with fever = 288 Prevalence of fever: 87% |
Hematoma growth Functional outcome in 90 days |
Cumulative delta temperature variation was independently associated with hematoma growth at 72 h, moderately severe disability and severe disability at 90 days |
|
Fever was defined as any BT ≥ 37.5 °C within the first 24 h of admission to the ICU Sensitivity analysis with fever define as BT ≥ 38.3 °C Number of patients with fever = 6965 Prevalence of fever: 51% |
In hospital mortality | Both early spontaneous fever and hypothermia conferred a higher risk of in-hospital death after brain injury |
|
BT on admission Number of patients with fever calculated as BT > 37.5 °C = 17 Prevalence of fever: 27% |
In hospital mortality | Elevated BT on admission was independently associated with ICU death in acute ischemic stroke patients |
|
Fever was defined as admission and day 8 BT > 38 °C Number of patients with fever on admission = 207 Number of patients with fever on day 8 = 1200 Prevalence of fever: 45% |
GOS at 3 months. UO was defined as GOS 1–3 | Fever on day 8 was independently associated with poor neurological outcome at 3 months |
|
Fever was defined as BT ≥ 37.5 °C measured in the first 4-12 h of hospitalization Number of patients with fever = 41 Prevalence of fever: 21% |
Mortality at 30 days | Hyperthermic patients had higher 30 days mortality rate than normothermic patients |
|
BT in the firs 10 days of the initial hemorrhage. Fever was defined as BT ≥ 38 °C and severe fever as BT ≥ 39 °C Number of patients with severe fever = 11 Prevalence of fever: 11% All patients spent at least 5% of GMT with BT ≥ 38 °C |
GOS (UO = 1–3) and GOSE (UO = 1–4) at 6 months Neurological deterioration (death or worse GCS at discharge) |
Fever had no impact on outcome nor on neurological deterioration |
|
Hyperthermia was defined as BT > 37.2 °C Number of patients with fever measured at baseline = 817, at 8 h = 188, at 24 h = 521, at 48 h = 323, at 72 h = 328 and 7 days = 152 Prevalence of fever: 15% |
mRankin at 3 months. UO was defined as mRankin 3–6 Mortality at 3 months |
Hyperthermia, in acute ischemic stroke, is associated with a poor clinical outcome. The later the hyperthermia occurs within the first week, the worse the prognosis |
|
Fever was defined as BT > 38 °C measured every 2 h from ictus to 14 days Number of patients with fever = 116 Prevalence of fever: 66% |
DCI | Temperature elevation ≥ 2.5 °C on day 4 or 5 compared with baseline is independently associated with DCI |
|
Fever in the first 24 h was defined as BT ≥ 37.5 °C Number of patients with fever = 18,305 Prevalence of fever: 17% |
Hospital mortality | In stroke and TBI patients but not CNS infection patients, BT below 37 °C and above 39 °C was associated with an increased risk of death |
|
Hyperthermia was defined as BT ≥ 38 °C Number of patients with fever = 93 Prevalence of fever: 70% |
GOSE (UO = 1–4) at 1 year | Hyperthermia was not independently associated with 1-year unfavorable outcome in severe TBI patients |
|
Subfebrile patients were defined by 37.5 °C < BT < 38.5 °C in the first 72 h of hospitalization Fever was defined as BT ≥ 38.5 °C in the first 72 h of hospitalization Number of subfebrile patients = 45 Number of febrile patients = 2 Prevalence of fever (subfebrile + febrile): 19% |
GOS at hospital discharge. UO was defined as 1–2 | In patients surviving the first 72 h after hospital admission, the duration of fever is associated with poor outcome and seems to be an independent prognostic factor in these patients |
|
BT on admission Number of patients with fever not reported |
Mortality and functional outcome (Rappaport Disability Rating) at 6 months | Elevated BT was independently associated with functional disability in TBI patients |
|
Hyperthermia was defined a BT ≥ 37.6 °C in two consecutive measures and severe hyperthermia as BT ≥ 38.0 °C Number of patients with hyperthermia = 56 (40 had severe hyperthermia) Prevalence of fever: 37% |
In Hospital mortality | Severe hyperthermia was independently associated with hospital mortality |
|
Elevated BT was defined as BT > 37.0 °C on admission Number of patients with elevated BT = 23,444 Prevalence of fever: 11% |
In hospital mortality | In CVA patients but not in TBI patients elevated BT was associated with all cause in hospital mortality |
|
BT on admission to ED Number of patients with elevated BT calculated as BT > 37 °C = 1046 Prevalence of fever: 16% |
In hospital mortality | Low BT (BT 36.5) but not elevated BT was associated with hospital mortality |
|
Fever was defined as any episode of BT ≥ 38.6 °C Number of patients with fever = 104 Prevalence of fever: 45% |
Telephone Interview for Cognitive Status at 3 and 12 months (Cognitive impairment 30) | Fever was independently associated with global cognitive impairment in survivors at 1 year after aSAH |
|
Pyrexia was defined as an BT higher than 38 °C or core temperature higher than 38.4 °C Number of patients with pyrexia = 80 Prevalence of fever: 73% |
GOS at 6 months UO was calculated as GOS 1–3 |
The study did not find any relationship between outcome at 6 months and presence or duration of pyrexia |
|
Fever was defined as at least one recorded BT > 38 °C within 5 days of admission Number of patients with fever = 127 Prevalence of fever: 14% |
NIHSS at discharge | Post stroke fever was associated with higher NIHSS at discharge |
|
Mean BT from day 4 to day 14 Fever was calculated as mean BT ≥ 38 °C from day 4 to day 14 Number of patients with fever = 10 Prevalence of fever: 16% |
DCI; GOS at discharge. UO was defined as GOS 1–3 |
Patients with DCI had higher incidence of fever. Fever was independently associated with outcome at discharge |
|
Admission BT Fever was calculated as BT > 37 °C = 17 Prevalence of fever: 21% |
Functional outcome at 30 days divided as death, severe disability moderate disability and minor disability measured by GOS. UO was defined as death, severe and moderate disability 30-day Mortality Hematoma volume |
Admission BT was not associated with death. However, fever on admission was associated with 30-day mortality. Fever on admission was not associated with UO |
|
Fever was defined as BT ≥ 38 °C in the first 14 h of hospital stay. Fever burden was defined as number of days with BT ≥ 38 °C Number of patients with fever = 132 Prevalence of fever: 53% |
mRankin at 3 months. UO was defined as 4–6 | Fever in the first 14 days of hospital stay and fever burden were independently associated with poor outcome at 3 months |
|
Hyperthermia was defined as BT > 37.5 °C on day 1 Number of patients with hyperthermia = 49 Prevalence of fever: 32% |
30 days mortality and Barthel Index (UO calculated as BI < 80) |
Hyperthermia on day 1 was not independently associated with 30 days mortality Hyperthermic patients on day 1 had worse functional outcome at 30 days than those who were normothermic |
|
Hyperthermia was defined as BT > 37.5 °C in the first 48 h Number of patients with hyperthermia = 15 Prevalence of fever: 25% |
90 days and 12 months Barthel Index (UO calculated as BI < 80) 90 days and 12 months mortality |
Barthel Index at 90 days and one year was similar between patients with hyperthermia in the first 48 h of hospitalization and those who were normothermic. Hyperthermic patients had higher 90 days and 1 year mortality rates than normothermic patients |
|
Admission BT Elevated BT calculated as BT > 37 °C Number of patients with elevated BT = 45 Prevalence of fever: 23% |
GOS at discharge. UO was defined as GOS 1–3 | Elevated BT was not associated with neurological outcome in mild TBI patients |
|
Hyperthermia was defined as BT ≥ 38 °C during hospitalization Number of patients with hyperthermia = 107 Prevalence of fever: 28% |
In hospital mortality | Hyperthermia was associated with in hospital mortality |
|
Fever was defined as BT > 37.5 °C on day 1 Number of patients with fever = 164 Prevalence of fever: 17% |
mRankin at 3 months. UO was defined as mRankin 3–6 Mortality at 3 months Symptomatic Hemorrhagic transformation Neurological deterioration |
Fever on day 1 was independently associated with poor outcome at 3 months and 3 months mortality rate Fever on day 1 was also associated with symptomatic hemorrhagic transformation Fever on day 1 was associated with neuro deterioration |
|
Fever was defined as BT ≥ 38.5 °C Number of patients with fever = 410 Prevalence of fever: 44% Prevalence of fever: 41% |
GOS (UO defined as 1–4) at 3 months; Rankin Disability Score (UO defined as 2–6) at 3 months; Barthel Index (UO defined as < 95) at 3 months; NIHSS at 3 months and DIND |
Fever is associated with DIND and worsened outcome in good grade surgical subarachnoid hemorrhage patients |
|
Early fever defined as BT ≥ 38 °C on admission Number of patients with fever = 16 Prevalence of fever: 16% |
mRankin and Barthel Index at 3, 6 and 12 months. UO was defined as mRankin 3–6 or BI 90 | Early fever was independently associated with unfavorable outcome at 1 year assessed by mRankin |
|
Hyperthermia was defined as BT ≥ 38 °C within 72 h from thrombolysis Number of patients with hyperthermia = 48 Prevalence of fever: 40% |
mRankin scale at end of rehabilitation or home discharge 90 days mortality |
Hyperthermia was associated with 90 days mortality and worse neurological outcome |
|
BT in the first 72 h of hospitalization Number of patients with elevated BT calculated as BT > 37.5 °C = 60 Prevalence of fever: 29% |
Mortality at 28 days GOSE at 3 years UO was defined as GOSE 1- 4 |
Elevated BT was associated with 28 days survival but not with UO at 3 years |
|
Fever was defined as BT > 39 °C Number of patients with fever = 14 Prevalence of fever: 28% |
Outcome defined as recovered, vegetative state and death at discharge | Fever was associated with poor outcome |
|
Fever burden was defined as number of days with peak BT > 37.5 °C until day12 Mean fever burden (considering BT > 38 °C) = 4.1(± 3.9) days |
mRankin at discharge. UO was defined mRankin 4–6 | Fever burden was independently associated with poor outcome at discharge |
|
Fever burden was defined as number of days with peak temperature ≥ 38 °C Number of patients with fever calculated as number of patients with fever burden > 0 = 193 Prevalence of fever: 66% |
mRankin at 90 days. UO was defined as mRankin 4–6 |
Patients with unfavorable outcome at 90 days had higher fever burden than those with good outcome Any episode of fever was associated with poor outcome at 90 days |
|
Elevated BT was defined as BT > 37.1 °C in the first 24 h of admission Number of patients with elevated BT = 131 Prevalence of fever: 36% |
In hospital mortality | Elevated BT in the first 24 h was independently associated with in-mortality |
|
Hyperthermia was defined as admission BT > 37.5 °C Number of patients with hyperthermia = 74 Prevalence of fever: 15% |
In hospital and 1 year mortality |
Hyperthermia was associated with 1 year mortality but not with in hospital mortality in acute ischemic stroke patients In hemorrhagic stroke patients, hyperthermia was not associated with mortality |
|
Hyperthermia was defined as admission BT > 37.5 °C Number of patients with hyperthermia = 36 Prevalence of fever: 16% |
1 year mortality | Hyperthermia was independently associate with 1 year mortality in acute ischemic stroke patients |
|
BT on admission Number of patients with elevated BT calculated as BT > 37.2 °C on admission = 281 Prevalence of fever: 25% |
30 days mortality | Low BT was independently associated with 30 mortality, whereas elevated BT was not associated with mortality |
|
BT on admission Number of patients with elevated BT calculated as BT > 37 °C on admission = 898 Prevalence of fever: 30% |
7 day and 28-day Mortality | Elevated BT was associated with 28 days mortality |
|
Fever was defined as BT > 38.3 °C Number of patients with fever = 309 Prevalence of fever: 54% |
mRankin at 3 months. UO was defined as 4–6 | Fever was significantly associated poor neurological outcome |
|
Fever was defined as BT > 38 °C in the first 72 h of hospitalization Number of patients with fever: 219 Prevalence of fever: 13% |
Functional independence was defined as a BI ≥ 95 after 100 days | Fever was independently associated with the combined outcome of poor functional outcome assessed by the Barthel index and mortality |
|
Fever was defined as BT > 38.3 °C during hospitalization Number of patients with fever = 11 Prevalence of fever: 10% |
mRankin at 3 months. UO was defined as 4–6 | Fever was independently associated with poor outcome at 3 months |
|
Hyperthermia was defined as BT > 38 °C during the first 10 days of admission Burden of hyperthermia was defined as the % of good monitoring time spent with BT > 38 °C Number of patients with hyperthermia = 108 Prevalence of fever: 94% |
GOSE 6 months UO was defined as GOSE 1–4 |
No association between hyperthermia or burden of hyperthermia and worse clinical outcome |
|
Hyperthermia was defined as BT > 38 °C Number of patients with hyperthermia in the early phase = 23, and in the vasospasm phase = 87 Prevalence of fever: 66% |
GOSE at 12 months. UO was defined as1-4 |
Hyperthermia was not independently associated with poor neurological recovery at 1 year |
|
Hyperthermia was defined as BT > 39 °C within 12 h from admission Number of patients with hyperthermia = 14 Prevalence of fever: 37% |
Mortality 3- 12 months Functional outcome described as death, moderate or severe disability and good recovery UO was defined death and moderate to severe disability |
Hyperthermia was independently associated with death at 3–12 months, but not with unfavorable outcome |
|
Fever was defined as BT > 37.5 °C at any time within 24 h after surgery Number of patients with fever = 219 Prevalence of fever: 71% |
Early neurological deterioration (defined as a decrease in the GCS score by ≥ 2 points within 24 h after surgery compared to that at admission); mRankin at 3 months. UO was defined as mRankin 3–6 |
The presence of fever was associated with unfavorable outcome but not with early neurological deterioration |
|
BT on admission, before and after minimally invasive surgery, day 1, day 2, day e and at discharge Number of patients with elevated BT defined as BT ≥ 36.95 not reported |
In hospital mortality, 3 months and 1 year mortality | Elevated BT at discharge was associated with 3 months and 1 year mortality |
|
Fever was defined as admission BT > 38 °C Number of patients with fever = 2 Prevalence of fever: 9% |
GOS at 3 months. UO was defined as 1–3) | Fever on admission was not associated with poor outcome |
|
Fever: no definition provided Number of patients with fever = 245 Prevalence of fever: 4% |
Hospital mortality | Fever was associated with in hospital mortality in a univariate analysis but not in multivariate analysis |
|
BT on admission Number of patients with elevated BT calculated as admission BT ≥ 37.5 °C = 233 Prevalence of fever: 16% |
GOS at 6 months. UO was defined as GOS 1–3 | Elevated admission BT was not independently associated with outcome |
|
Fever was defined as BT exceeding 38.3 °C at least in two different days Number of patients with fever = 63 Prevalence of fever: 41% |
In hospital mortality | Fever is independently associated with in-hospital mortality after SAH |
|
Peak BT Elevated BT was calculated as peak BT > 38.3 °C = 166 Prevalence of fever: 25% |
mRankin at 90 days; mortality at discharge and 90 days; hemorrhagic transformation UO was defined as mRankin 3–6 |
Elevated BT was an independent predictor of mortality, unfavorable outcome and hemorrhagic transformation in patients with acute basilar artery occlusion |
|
Fever was defined as admission BT ≥ 37.5 °C Number of patients with fever = 72 Prevalence of fever: 7% |
90 days mortality | Fever was independently associated with 90 days mortality |
|
Admission BT Elevated BT calculated as admission BT ≥ 37.5 °C = 100 Prevalence of fever: 19% |
Hospital mortality | Elevated admission BT was associated with in hospital mortality |
|
Refractory hyperpyrexia defined as BT > 38.3 °C despite pharmacological and physical cooling Number of patients with refractory fever = 75 Prevalence of fever: 50% |
GOS at 6 months UO was defined as GOS 1–3 |
Refractory fever was independently associated with poor outcome at 6 months |
|
BT on admission Elevated BT calculate as BT on admission ≥ 37.5 °C = 156 Prevalence of fever:25% |
30-day mortality | Elevated admission BT was independently associated with 30-day mortality |
BT Body temperature, aSAH (aneurysmal) subarachnoid hemorrhage, AIS Acute ischemic stroke, ICH Intracerebral hemorrhage, TBI Traumatic brain injury, GOS Glasgow outcome scale, GOSE Extended Glasgow outcome scale, mRankin modified Rankin scale, UO unfavorable neurological outcome, BI Barthel Index, GCS Glasgow coma scale, FIM Functional Independence Measures, RLA Ranchos Los Amigos Score, NIRS Near infrared spectroscopy, yo years old, MCA Middle cerebral artery, ISS:NIHSS National Institute of Health Stroke Scale, ISS Injury severity score, QoL Quality of life, END Early neurological deterioration, DIND Delayed ischemic neurological deficit, LOS Length of stay, ICU Intensive care unit, SDH Subdural hematoma
Neurological outcome at any time point
We identified 109 studies that reported neurological outcome according to the presence of fever, of which 104 were included in the meta-analysis with fever as a dichotomous variable. Four studies [34–37] were not included because they were from the same group with overlap of patients, while one study reported no association between admission body temperature and outcome but did not provide with sufficient data to be included in the meta-analysis [38]. Fever was independently associated with poor neurological outcome with a pooled OR of 2.37 (95% CI 2.08–2.71), as shown in Fig. 2A. The funnel plots to assess the risk of publication bias are presented in Fig. 2B, which shows an asymmetry toward the publication of positive studies. Heterogeneity was high among the included studies.
Fig. 2.
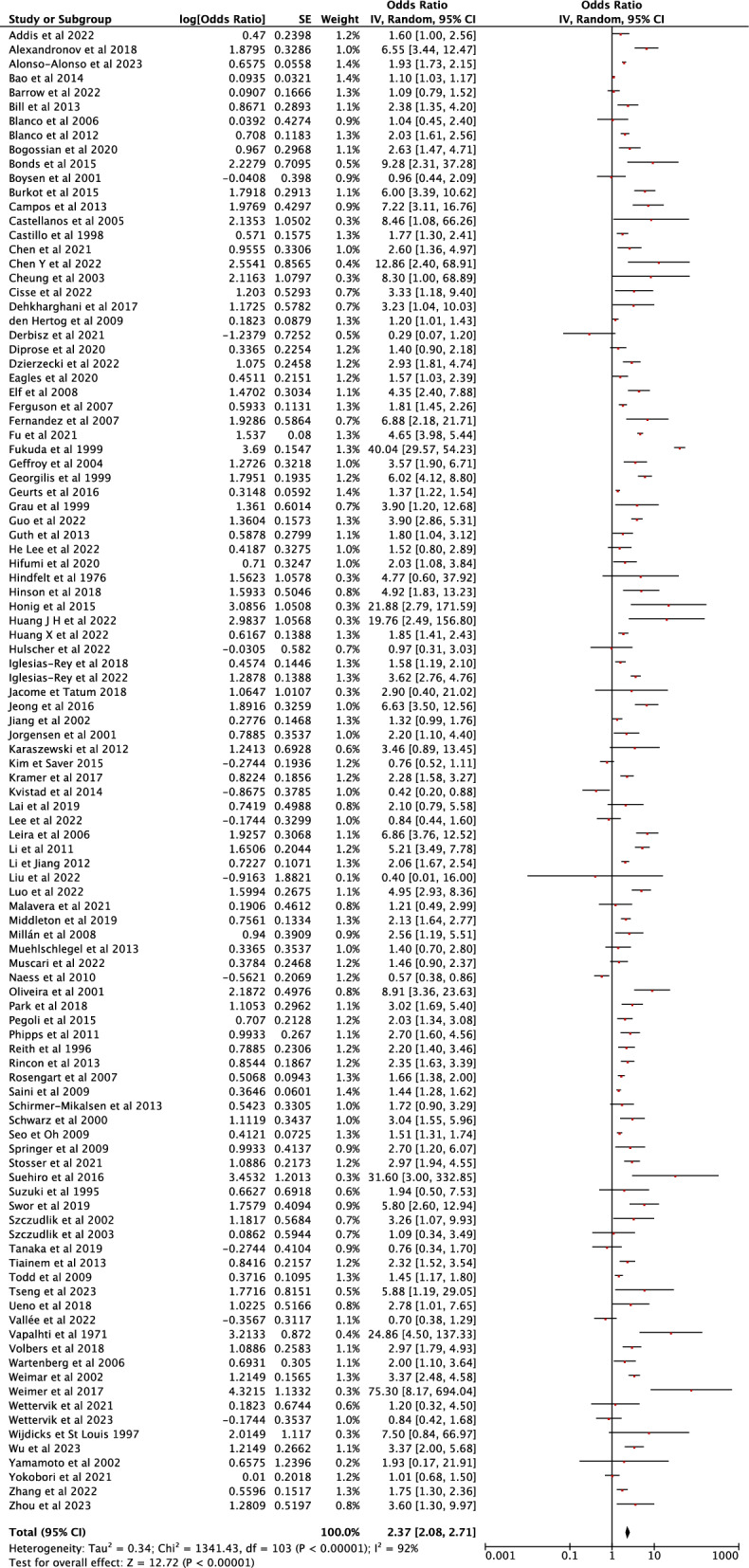
Fixed effects meta-analysis assessing the impact of fever/hyperthermia on neurological outcome at any time point compared to normothermia. Fever was associated with increased chance of unfavorable neurological outcome (pooled OR 1.72 (95% CI 1.67–1.78). Panel A Forest plot. Panel B Funnel plot
The burden of fever was reported in 15 studies. However, one study that reported an association between longer duration of fever and poor outcome did not provide enough data to allow meta-analysis calculations [39]. The pooled OR of the remaining 14 studies was 1.44 (95% CI 1.26–1.65), indicating that for each increase in 1 point in the fever burden there was an expected increase of 44% in the probability of poor outcome, as shown in the Supplemental Fig. S1.
In TBI patients, the presence of fever was independently associated with unfavorable neurological outcome (pooled OR 2.03; 95% CI 1.57–2.63), as it was in SAH patients (pooled OR 2.3; 95% CI 1.85–2.99), in AIS patients (pooled OR 2.75, 95% CI 2.08–3.64), in ICH patients (pooled OR 2.81; 95% CI 2.01–3.94) and in a mixed population of stroke patients (pooled OR 2.45; 95% CI 1.80–3.30), as shown in the Supplementary Figure S2.
Mortality at any time point
Specific data on mortality was reported in 87 studies, of which 84 were included in the meta-analysis. One study [40] was not included because it was from the same group with overlap of patients. One study reported an association between a longer duration of fever and mortality but did not provide enough data to allow meta-analysis calculations [39]. One study reported no association between elevated body temperature and mortality, but did not provide numeric data to be meta-analyzed [41]. Fever was independently associated with an increased risk of mortality with a pooled OR of 2.50 (95% CI 2.15–2.92), as shown in Fig. 3A. The funnel plot (Fig. 3B) analysis showed an asymmetry towards the publication of positive studies. Heterogeneity was high among the included studies.
Fig. 3.
Fixed effects meta-analysis assessing the impact of fever/hyperthermia on mortality at any time point compared to normothermia in acute brain injury patients. Fever was associated with increased chance of death (pooled OR 1.72 (95% CI 1.67–1.78). Panel A Forest plot. Panel B Funnel plot
The presence of fever was independently associated with mortality in TBI patients (pooled OR 1.88; 95% CI 1.23–2.89), in SAH patients (pooled OR 4.58; 95% CI 1.61–13.01), in AIS patients (pooled OR 2.71; 95% CI 2.20–3.35), in ICH patients (pooled OR 2.81; 95% CI 2.01–3.94), in a mixed population of stroke patients (pooled OR 2.20; 95% CI 1.52–3.75) and in a mixed population of neurocritical care patients (pooled OR 2.35; 95% CI 1.21–4.57), as shown in the Supplementary Figure S3.
Secondary outcomes
Fever was associated with an increased probability of early neurological deterioration (pooled OR 2.96; 95% CI 1.59–2.94) in acute brain injury patients, as shown in the Supplemental Figure S4. In SAH patients, fever was also associated with an increased risk of symptomatic vasospasm/DCI (pooled OR 2.57; 95% CI 1.83–3.61); in AIS patients, fever was associated with large infarct size (pooled OR 2.50; 95% CI 1.75–3.57) and with hemorrhagic transformation (pooled OR 1.75; 95% CI 1.21–2.54); in ICH patients, fever was associated with hematoma expansion (pooled OR 2.45; 95% CI 1.83–3.29), as presented in Supplemental Figure S5.
Meta regression
The shorter the follow-up time the more significant was the association between fever and neurological outcome (log OR − 0.0011175, 95% CI − 0.0021813 to − 0.0000537—Supplemental Figure S6A) was observed. The higher the BT threshold used to define fever the more significant association between fever and neurological outcome (log OR 0.565981, 95% CI 0.2562389 to 0.8757231—Supplemental Figure S6B) was observed. The moderator effect remained when both variables were included in the model (follow-up time: log OR − 0.0011487, 95% CI − 0.0021817 to − 0.0001156; BT threshold: log OR 0.5674159, 95% CI 0.2625423–0.8722895). However, heterogeneity remained high (I2 = 93%).
Neither follow-up time (log OR − 0.000279, 95% CI − 0.0010043 to 0.0004463—Supplemental Figure S7A) nor the BT threshold (log OR 0.2400004, 95% CI − 0.197733 to 0.6777338—Supplemental Figure S7B) moderated the effect between fever and mortality. The inclusion of both moderators in the model did not impact the association between fever and mortality (follow-up time: log OR − 0.0002072, 95% CI − 0.0009532 to 0.0005387; BT threshold: log OR 0.215909, 95% CI − 0.2333637 to 0.6651816).
Discussion
This systematic review and meta-analysis investigated the impact of fever on the neurological outcome and mortality in acute brain injury patients. We found that fever was independently associated with unfavorable neurological outcome and mortality in neurocritical care patients, including those with TBI, SAH and stroke. Moreover, fever was also associated with an increased risk of neurological deterioration, stroke progression, hemorrhagic transformation and occurrence of DCI.
In 2008, Greer et al. [13] published an extensive meta-analysis of 44 studies including 14,431 patients, showing a significant association between fever and poor outcomes (e.g. unfavorable neurological outcome, mortality, ICU and hospital length of stay) in acute brain injury patients. Sixteen years later, we performed this systematic review and meta-analysis of 180 studies including 460,846 patients, a considerably larger sample size which further reinforced the importance of fever as a potential contributor to secondary brain injury. Importantly, there were only few patients suffering from subarachnoid hemorrhage in the original meta-analysis; in the present study, we provided a significant amount of data also on the association of fever and outcome in this subgroup of patients. We also explored the impact of fever on the occurrence of complications that occurred during the hospital course, such as delayed cerebral ischemia in subarachnoid hemorrhage, neurological deterioration in acute brain injury patients, stroke progression in ischemic and hemorrhagic stroke, including increase in infarct size and hemorrhage volume. All these events could contribute to further worsen the outcome of brain injured patients and had not been previously explored by Greer et al. [13].
In our study, the pooled prevalence of fever was 18% which is lower than reported in some studies [2–4]. Older studies tended to have higher prevalence of fever compared to more recent ones. This may be explained due to the advances in temperature control methods, including physical methods with controlled temperature-feedback [42] and improvements in hospital infection control [43]. Additionally, studies that measured BT on admission presented with lower prevalences of fever compared to those who measured on day 8 or during ICU stay. In fact, the length of stay influences the prevalence of fever, as shown in a study conducted in a neuro-ICU that reported a prevalence of fever of 15% in patients who stayed in the ICU less than 24 h, but in 93% of those who remained longer than 14 days [44].
Fever is an important cause of secondary brain injury, causing direct cytotoxic damage and indirectly promoting neuronal dysfunction [45] by increasing systemic and neuroinflammation mechanisms of secondary injury, which leads to leucocyte recruitment and activation of the coagulation cascade, excitotoxicity, free radical production, and blood–brain barrier dysfunction with increasing permeability and cerebral edema [46–50]. Fever also increases cerebral blood flow [51] and metabolic demand which may lead to an imbalance between oxygen delivery and consumption and ICP elevation in an already injured brain [9, 52, 53]. Interestingly, this negative impact of fever seems to be less relevant in patients with primary CNS infection [6], where it may provide some neuroprotective actions [54] and temperature control may be less restrictive. Moreover, body temperature is a commonly used clinical marker to assess response to antimicrobial treatment in CNS infection [55].
Fever may have different etiologies in ABI patients including infection, drug related fever, thromboembolism and neurogenic fever. Acute brain injury can trigger systemic immune-suppression, leaving patients vulnerable to hospital acquired infections [56]. These patients are often comatose or sedated under mechanical ventilation, requiring central venous catheters for hemodynamic monitoring and administration of pharmacological therapies, commonly develop dysphagia requiring nasogastric tubes for feeding, and external ventricular drains for cerebral spinal fluid diversion and ICP monitoring, all of which considerably increases the risk of infection [57], in particular following stroke and TBI [58–60]. Moreover, neurogenic fever is common in neuro-critically ill patients accounting for 28–50% of fever etiology [44, 61–63]. Importantly neurogenic fever is usually resistent to pharmacological therapy and require more advanced methods ot temperature management [64]. Identifying and treating the cause of fever is imperative because of the consequences of failing to identify a treatable condition such as infection and sepsis; conversely, failing to identify a non infectious etiology of fever can least to antibiotic overuse, adverse events related to antibiotic use and selection of multi drug resistant organisms [65].
In this study we also performed meta-regression analysis, which has yielded interesting results. We found that the higher the threshold used to define fever, the greater the probability of a poor outcome. This finding may guide the design of future interventional studies and assist clinicians in making decisions about when and how to intervene. Unfortunately, there is no commonly agreed threshold to start fever control measures and, practices vary greatly among specialized centers [66]. Based on the results of the meta-regression for neurological outcome, a general target of body temperature above 38 °C seems reasonable to initiate therapies. However, ideal body temperature targets may differ depending on the clinical severity, etiology of brain injury and the brain physiology [10] and should be individualized. Patients with severe brain injury may benefit from maintaining strict normothermia (e.g. core BT of 37 °C) [67] through the use of advanced temperature management devices with closed loop feedback, while patients with mild to moderate brain injury may tolerate higher BT without significant detrimental to brain function [10]. In this setting multimodal neuromonitoring may help titrate body temperature according to brain physiology by assessing the variation of brain hemodynamics, ICP, brain tissue oxygenation, electroencephalogram in response to different BT thresholds, thus tailoring temperature management to patient’s specific clinical status. Additionally, low body temperature and spontaneous hypothermia is also associated with poor outcome in acute brain injury patients [68–71]. This apparent U-shaped effect of spontaneous BT on outcome may suggest TTM as a potential strategy for prevention of secondary brain injury; however, whether normothermia should be actively targeted in the management of all brain-injured critically ill patients remains unknown.
When considering TTM, brain temperature is the ideal target for neuroprotection; indeed, brain temperature is usually higher than BT and is influenced by brain metabolic activity [72]. However, brain temperature is not easily available in all centers, while body temperature is routinely measured and shows an important association with poor outcomes, as suggested in this study. In this meta-analysis, the definition of fever included both peripheral and core BT. Of note, core BT is preferred over peripheral BT since it is closer to the actual brain temperature [73–75]. Additionally, BT should be measured continuously to allow adequate management and prevention of secondary brain injury [76], which was not the case in most of the studies included in this review.
Another important aspect when performing temperature control is choosing the adequate method to rapidly decrease body temperature within targets. Non-pharmacological interventions to control fever (e.g. surface cooling using of cold water or air and/or intravascular devices, both with automated temperature feedback control) [77, 78] are more effective than basic passive strategies (e.g. cold packs, cold air). This has been shown in a recently published randomized clinical trial [67[ including stroke patients, reporting that automated surface cooling devices with closed loop feedback targeting strict normothermia successfully reduced the burden of fever compared to standard of care, which consisted of the a tiered approach initiated by the detection of fever (BT > 38 °C) and including the use of use of antipyretic agents, basic external cooling strategies (ice packs, tepid baths, fans), cooling blankets or advanced cooling strategies. Of note, the reduction in fever burden did not improve outcomes at 6 months; however, the study was underpowered to detect such changes. Regarding TBI patients, the European Society of Intensive Care Medicine [15] recommends to target an initial core BT of below 38 °C; however, in patients with intracranial hypertension, this target could be lower (e.g. 36.0–37.5 °C) and, in the event of refractory intracranial hypertension, physicians could consider mild hypothermia (e.g. 35.0–36.0 °C). Despite lack of high grade level evidence temperature management is an available and effective strategy in our toolbox to control intracranial hypertension crisis and reverse brain tissue hypoxia [79, 80] although the impact on outcome remains to be elucidated.
Another important finding of the meta-regression analysis is that the association between fever and outcome was more consistent in short to middle term follow up time, specially between 90 and 180 days. In this meta-analysis fever was often assessed at admission or in the first week of ICU stay in the acute and subacute phase of brain injury, when the brain is especially vulnerable to secondary injury and the more significant is the impact on outcome. Importantly, neurological recovery is often a long-term process with considerable improvement in the setting of adequate rehabilitation and post ICU care [15, 81–83] which may explain our results.
Finally, we did not include patients with hypoxic ischemic injury due to cardiac arrest. The physiopathology of brain injury in these patients involves global ischemia (primary) and reperfusion injuries (secondary) which trigger neuroinflammation with possible advent of fever [84]. In fact, in this group of patients, fever is a marker of severity of ischemic—reperfusion injury and an important factor associated with poor outcome [85]. TTM targeting both hypothermia and strict normothermia have been extensively studied [86] and, currently, the International Liaison Committee on Resuscitation (ILCOR) recommends active prevention of fever for ≥ 72 h by targeting a temperature ≤ 37.5 °C [87] in survivors of cardiac arrest. Interestingly, with the advent of reperfusion techniques including thrombolysis and endovascular thrombectomy, acute ischemic stroke patients often experience reperfusion injury which causes oxidative stress, leukocyte infiltration, mitochondrial dysfunction, platelet activation and aggregation, complement activation, and blood–brain-barrier (BBB) disruption leading to brain edema or hemorrhagic transformation potentially causing significant neuron death and poor neurological outcome [88]. Moreover, SAH patients may also experience transient brain circulatory arrest and hypoperfusion due to the rapid increase in ICP following extravasation of blood into the subarachnoid space, followed by reperfusion and becoming susceptible to ischemic reperfusion injury which is one of the mechanisms of early brain injury [89]. Ischemic reperfusion injury causes neuroinflammation which contributes to the high prevalence of fever in stroke patients.
This study has some limitations. To include a maximum number of studies possible we chose as primary endpoints neurological outcome and mortality at any time point which led to high heterogeneity of the time of assessment varying from 7 days up to 5 years follow up. Similarly, we included studies with any definition of fever, that varied from > 36.95 °C to > 40 °C. The meta-regression demonstrated that both follow-up time and BT threshold used to define fever were moderators of the effect of fever on neurological outcome, but heterogeneity remained high. In fact, other factors regarding temperature assessment and management differed considerably among studies and were not included in the meta-regression explaining the persistently high heterogeneity in this meta-analysis. For example, studies had highly variable frequency of temperature measurements (intermittent, continuous) which could have influenced the recording of the duration and the number of episodes of fever. Studies used different sites of BT and core BT measurements (e.g. tympanic, axillary, bladder, esophageal, rectal, forehead), which could lead to a difference in measurement of at least 1 °C. The assessment and definition of fever burden also diverged among studies and included number of days with fever, time in hours spent above a certain threshold, fever intensity and fever duration. Moreover, fever management strategies were frequently not reported and those that were described varied greatly. Additionally, several studies included a mixed population of acute brain injury with different pathophysiology. However, to minimize this bias we performed subgroup analysis for each pathology. Also, despite only including studies with low or moderate risk of bias, the quality of evidence of the studies assessed by GRADE was usually low to moderate, which can limit the clinical impact of this meta-analysis. There was overall bias of publication towards articles that showed the positive association between fever and outcomes. We also did not compare different causes of fever such as infectious and non-infectious fever.
Conclusions
Fever was associated with an increased risk of unfavorable neurological outcome and reduced survival in acute brain injured patients. Fever management should be regarded as an important aspect of high-quality care for these patients; further research is essential to determine the most appropriate temperature target management strategies in the different populations of acute brain injury.
Supplementary Information
Acknowledgements
None
Author contributions
EGB, MS and MF conceived the study; MS, EGB, MT,CP, MF performed the search and screening process; EGB, MS, SF performed data extraction and curation; EGB and FST conducted the statistical analysis; EGB and MS wrote the first draft of the paper; FST, SS, MF revised the text for intellectual content. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data is provided within the manuscript or supplementary information files.
Declarations
Ethical approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elisa Gouvêa Bogossian and Michele Salvagno equally contributed as first author.
References
- 1.O’Grady NP, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36:1330–49. 10.1097/CCM.0b013e318169eda9. [DOI] [PubMed] [Google Scholar]
- 2.Diringer MN, Reaven NL, Funk SE, Uman GC. Elevated body temperature independently contributes to increased length of stay in neurologic intensive care unit patients. Crit Care Med. 2004;32:1489–95. 10.1097/01.ccm.0000129484.61912.84. [DOI] [PubMed] [Google Scholar]
- 3.Niven DJ, Laupland KB. Pyrexia: aetiology in the ICU. Crit Care. 2016;20:247. 10.1186/s13054-016-1406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stocchetti N, et al. Pyrexia in head-injured patients admitted to intensive care. Intensive Care Med. 2002;28:1555–62. 10.1007/s00134-002-1513-1. [DOI] [PubMed] [Google Scholar]
- 5.Launey Y, Nesseler N, Mallédant Y, Seguin P. Clinical review: fever in septic ICU patients - friend or foe? Crit Care. 2011;15:222. 10.1186/cc10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saxena M, et al. Early temperature and mortality in critically ill patients with acute neurological diseases: trauma and stroke differ from infection. Intensive Care Med. 2015;41:823–32. 10.1007/s00134-015-3676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi S, Zanier ER, Mauri I, Columbo A, Stocchetti N. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J Neurol Neurosurg Psychiatry. 2001;71:448–54. 10.1136/jnnp.71.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira-Filho J, et al. Fever in subarachnoid hemorrhage: relationship to vasospasm and outcome. Neurology. 2001;56:1299–304. 10.1212/wnl.56.10.1299. [DOI] [PubMed] [Google Scholar]
- 9.Mrozek S, Vardon F, Geeraerts T. Brain temperature: physiology and pathophysiology after brain injury. Anesthesiol Res Pract. 2012;2012:989487. 10.1155/2012/989487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogossian EG, Taccone FS. Fever management in acute brain injury. Curr Opin Crit Care. 2022;28:130–7. 10.1097/MCC.0000000000000918. [DOI] [PubMed] [Google Scholar]
- 11.Thomas AJ, et al. Defining the mechanism of subarachnoid hemorrhage-induced pyrexia. Neurotherapeutics. 2020;17:1160–9. 10.1007/s13311-020-00866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stocchetti N, et al. Impact of pyrexia on neurochemistry and cerebral oxygenation after acute brain injury. J Neurol Neurosurg Psychiatry. 2005;76:1135–9. 10.1136/jnnp.2004.041269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke. 2008;39:3029–35. 10.1161/STROKEAHA.108.521583. [DOI] [PubMed] [Google Scholar]
- 14.Madden LK, et al. The implementation of targeted temperature management: an evidence-based guideline from the neurocritical care society. Neurocrit Care. 2017;27:468–87. 10.1007/s12028-017-0469-5. [DOI] [PubMed] [Google Scholar]
- 15.Lavinio A, et al. Targeted temperature control following traumatic brain injury: ESICM/NACCS best practice consensus recommendations. Crit Care. 2024;28:170. 10.1186/s13054-024-04951-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cariou A, et al. Targeted temperature management in the ICU: guidelines from a French expert panel. Anaesth Crit Care Pain Med. 2018;37:481–91. 10.1016/j.accpm.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Pegoli M, Zurlo Z, Bilotta F. Temperature management in acute brain injury: a systematic review of clinical evidence. Clin Neurol Neurosurg. 2020;197:106165. 10.1016/j.clineuro.2020.106165. [DOI] [PubMed] [Google Scholar]
- 18.Mahlamaki K, Rautalin I, Korja M. Case fatality rates of subarachnoid hemorrhage are decreasing with substantial between-country variation: a systematic review of population-based studies between 1980 and 2020. Neuroepidemiology. 2022;56:402–12. 10.1159/000526983. [DOI] [PubMed] [Google Scholar]
- 19.Luostarinen T, et al. Trends in mortality after intensive care of patients with traumatic brain injury in Finland from 2003 to 2019: a Finnish Intensive Care Consortium study. Acta Neurochir (Wien). 2022;164:87–96. 10.1007/s00701-021-05034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lackland DT, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–53. 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–4. 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 23.Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the glasgow outcome scale. J Neurol Neurosurg Psychiatry. 1981;44:285–93. 10.1136/jnnp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uk-Tia Study G. United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: interim results. UK-TIA Study Group Br Med J (Clin Res Ed). 1988;296:316–20. [PMC free article] [PubMed] [Google Scholar]
- 25.Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- 26.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4. [DOI] [PubMed] [Google Scholar]
- 27.National Institute of Neurological, D. & Stroke. NIH stroke scale. ([Bethesda, Md.?] : National Institute of Neurological Disorders and Stroke, Dept. of Health and Human Services, USA, [2011?], 2011).
- 28.Higgins JP, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, T. J., Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). (Cochrane, 2023).
- 32.VanderWeele TJ. Optimal approximate conversions of odds ratios and hazard ratios to risk ratios. Biometrics. 2020;76:746–52. 10.1111/biom.13197. [DOI] [PubMed] [Google Scholar]
- 33.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Hoboken: John Wiley & Sons; 2009. [Google Scholar]
- 34.Maas MB, et al. Subarachnoid extension of primary intracerebral hemorrhage is associated with poor outcomes. Stroke. 2013;44:653–7. 10.1161/STROKEAHA.112.674341. [DOI] [PubMed] [Google Scholar]
- 35.Jorgensen HS, et al. What determines good recovery in patients with the most severe strokes? The Copenhagen Stroke Study. Stroke. 1999;30:2008–12. 10.1161/01.str.30.10.2008. [DOI] [PubMed] [Google Scholar]
- 36.Karaszewski B, et al. Relationships between brain and body temperature, clinical and imaging outcomes after ischemic stroke. J Cereb Blood Flow Metab. 2013;33:1083–9. 10.1038/jcbfm.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volbers B, et al. Impact of perihemorrhagic edema on short-term outcome after intracerebral hemorrhage. Neurocrit Care. 2016;24:404–12. 10.1007/s12028-015-0185-y. [DOI] [PubMed] [Google Scholar]
- 38.Adatia K, et al. Effect of body temperature on cerebral autoregulation in acutely comatose neurocritically Ill patients. Crit Care Med. 2018;46:e733–41. 10.1097/CCM.0000000000003181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrews PJ, et al. Predicting recovery in patients suffering from traumatic brain injury by using admission variables and physiological data: a comparison between decision tree analysis and logistic regression. J Neurosurg. 2002;97:326–36. 10.3171/jns.2002.97.2.0326. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Lim LL, Heller RF, Fisher J, Levi CR. A prediction model of 1-year mortality for acute ischemic stroke patients. Arch Phys Med Rehabil. 2003;84:1006–11. 10.1016/s0003-9993(03)00032-7. [DOI] [PubMed] [Google Scholar]
- 41.Seo W, Oh H. Comparisons of acute physiological parameters influencing outcome in patients with traumatic brain injury and hemorrhagic stroke. Worldviews Evid Based Nurs. 2009;6:36–43. 10.1111/j.1741-6787.2008.00139.x. [DOI] [PubMed] [Google Scholar]
- 42.Calabró L, et al. Effect of different methods of cooling for targeted temperature management on outcome after cardiac arrest: a systematic review and meta-analysis. Crit Care. 2019;23:285. 10.1186/s13054-019-2567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gandra S, Ellison RT 3rd. Modern trends in infection control practices in intensive care units. J Intensive Care Med. 2014;29:311–26. 10.1177/0885066613485215. [DOI] [PubMed] [Google Scholar]
- 44.Kilpatrick MM, Lowry DW, Firlik AD, Yonas H, Marion DW. Hyperthermia in the neurosurgical intensive care unit. Neurosurgery. 2000;47:850–5. 10.1097/00006123-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Kim T, et al. Thermal effects on neurons during stimulation of the brain. J Neural Eng. 2022. 10.1088/1741-2552/ac9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badjatia N. Hyperthermia and fever control in brain injury. Crit Care Med. 2009;37:S250–7. [DOI] [PubMed] [Google Scholar]
- 47.Badjatia N. Fever control in the neuro-ICU: why, who, and when? Curr Opin Crit Care. 2009;15:79–82. 10.1097/MCC.0b013e32832922e9. [DOI] [PubMed] [Google Scholar]
- 48.Walter EJ, Carraretto M. The neurological and cognitive consequences of hyperthermia. Crit Care. 2016;20:199. 10.1186/s13054-016-1376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walter EJ, Hanna-Jumma S, Carraretto M, Forni L. The pathophysiological basis and consequences of fever. Crit Care. 2016;20:200. 10.1186/s13054-016-1375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castillo J, Davalos A, Noya M. Aggravation of acute ischemic stroke by hyperthermia is related to an excitotoxic mechanism. Cerebrovasc Dis. 1999;9:22–7. 10.1159/000015891. [DOI] [PubMed] [Google Scholar]
- 51.Svedung Wettervik T, et al. Cerebral blood flow and oxygen delivery in aneurysmal subarachnoid hemorrhage: relation to neurointensive care targets. Neurocrit Care. 2022;37:281–92. 10.1007/s12028-022-01496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Svedung Wettervik TM, et al. Systemic hyperthermia in traumatic brain injury-relation to intracranial pressure dynamics, cerebral energy metabolism, and clinical outcome. J Neurosurg Anesthesiol. 2021;33:329–36. 10.1097/ANA.0000000000000695. [DOI] [PubMed] [Google Scholar]
- 53.Wettervik TS, et al. Arterial oxygenation in traumatic brain injury-relation to cerebral energy metabolism, autoregulation, and clinical outcome. J Intensive Care Med. 2021;36:1075–83. 10.1177/0885066620944097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young PJ, et al. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med. 2012. 10.1007/s00134-012-2478-3. [DOI] [PubMed] [Google Scholar]
- 55.Hasbun R. Progress and challenges in bacterial meningitis: a review. JAMA. 2022;328:2147–54. 10.1001/jama.2022.20521. [DOI] [PubMed] [Google Scholar]
- 56.Santos Samary C, Pelosi P, Leme Silva P, Rieken Macedo Rocco P. Immunomodulation after ischemic stroke: potential mechanisms and implications for therapy. Crit Care. 2016;20:391. 10.1186/s13054-016-1573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma Z, et al. Prevalence, early predictors, and outcomes of sepsis in neurocritical illnesses: a prospective cohort study. Am J Infect Control. 2024;52:827–33. 10.1016/j.ajic.2024.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Sharma R, et al. Infections after a traumatic brain injury: the complex interplay between the immune and neurological systems. Brain Behav Immun. 2019;79:63–74. 10.1016/j.bbi.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 59.Badve MS, Zhou Z, van de Beek D, Anderson CS, Hackett ML. Frequency of post-stroke pneumonia: systematic review and meta-analysis of observational studies. Int J Stroke. 2019;14:125–36. 10.1177/1747493018806196. [DOI] [PubMed] [Google Scholar]
- 60.Bogossian EG, et al. The impact of extracerebral infection after subarachnoid hemorrhage: a single-center cohort study. World Neurosurg. 2020;144:e883–97. 10.1016/j.wneu.2020.09.102. [DOI] [PubMed] [Google Scholar]
- 61.Commichau C, Scarmeas N, Mayer SA. Risk factors for fever in the neurologic intensive care unit. Neurology. 2003;60:837–41. 10.1212/01.wnl.0000047344.28843.eb. [DOI] [PubMed] [Google Scholar]
- 62.Honig A, Michael S, Eliahou R, Leker RR. Central fever in patients with spontaneous intracerebral hemorrhage: predicting factors and impact on outcome. BMC Neurol. 2015;15:6. 10.1186/s12883-015-0258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rabinstein AA, Sandhu K. Non-infectious fever in the neurological intensive care unit: incidence, causes and predictors. J Neurol Neurosurg Psychiatry. 2007;78:1278–80. 10.1136/jnnp.2006.112730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meier K, Lee K. Neurogenic fever: review of pathophysiology, evaluation, and management. J Intensive Care Med. 2017;32:124–9. 10.1177/0885066615625194. [DOI] [PubMed] [Google Scholar]
- 65.Hocker SE, et al. Indicators of central fever in the neurologic intensive care unit. JAMA Neurol. 2013;70:1499–504. 10.1001/jamaneurol.2013.4354. [DOI] [PubMed] [Google Scholar]
- 66.Picetti E, Oddo M, Prisco L, Helbok R, Taccone FS. A survey on fever monitoring and management in patients with acute brain injury: the SUMMA study. J Neurosurg Anesthesiol. 2019;31:399–405. 10.1097/ANA.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 67.Greer DM, et al. Impact of fever prevention in brain-injured patients (INTREPID): study protocol for a randomized controlled trial. Neurocrit Care. 2021;35:577–89. 10.1007/s12028-021-01208-1. [DOI] [PubMed] [Google Scholar]
- 68.Rubiano AM, et al. The effect of admission spontaneous hypothermia on patients with severe traumatic brain injury. Injury. 2013;44:1219–25. 10.1016/j.injury.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeremitsky E, Omert L, Dunham CM, Protetch J, Rodriguez A. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J Trauma. 2003;54:312–9. 10.1097/01.TA.0000037876.37236.D6. [DOI] [PubMed] [Google Scholar]
- 70.Bukur M, et al. Pre-hospital hypothermia is not associated with increased survival after traumatic brain injury. J Surg Res. 2012;175:24–9. 10.1016/j.jss.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Thompson HJ, Kirkness CJ, Mitchell PH. Hypothermia and rapid rewarming is associated with worse outcome following traumatic brain injury. J Trauma Nurs. 2010;17:173–7. 10.1097/JTN.0b013e3181ff272e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang H, et al. Brain temperature and its fundamental properties: a review for clinical neuroscientists. Front Neurosci. 2014;8:307. 10.3389/fnins.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moran JL, et al. Tympanic temperature measurements: are they reliable in the critically ill? A clinical study of measures of agreement. Crit Care Med. 2007;35:155–64. 10.1097/01.CCM.0000250318.31453.CB. [DOI] [PubMed] [Google Scholar]
- 74.Lefrant JY, et al. Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med. 2003;29:414–8. 10.1007/s00134-002-1619-5. [DOI] [PubMed] [Google Scholar]
- 75.Shin J, Kim J, Song K, Kwak Y. Core temperature measurement in therapeutic hypothermia according to different phases: comparison of bladder, rectal, and tympanic versus pulmonary artery methods. Resuscitation. 2013;84:810–7. 10.1016/j.resuscitation.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 76.Andrews PJD, et al. Targeted temperature management in patients with intracerebral haemorrhage, subarachnoid haemorrhage, or acute ischaemic stroke: consensus recommendations. Br J Anaesth. 2018;121:768–75. 10.1016/j.bja.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 77.Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363:1256–64. 10.1056/NEJMct1002402. [DOI] [PubMed] [Google Scholar]
- 78.Calabro L, et al. Effect of different methods of cooling for targeted temperature management on outcome after cardiac arrest: a systematic review and meta-analysis. Crit Care. 2019;23:285. 10.1186/s13054-019-2567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hawryluk GWJ, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019;45:1783–94. 10.1007/s00134-019-05805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chesnut R, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020;46:919–29. 10.1007/s00134-019-05900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schäbitz M, et al. Long-term functional outcome and quality of life 2.5 years after thrombolysis in acute ischemic stroke. Neurol Res Pract. 2023;5:62. 10.1186/s42466-023-00291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kainz A, et al. Changes of health-related quality of life within the 1st year after stroke-results from a prospective stroke cohort study. Front Neurol. 2021;12:715313. 10.3389/fneur.2021.715313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roquer J, et al. Short- and long-term outcome of patients with aneurysmal subarachnoid hemorrhage. Neurology. 2020;95:e1819–29. 10.1212/WNL.0000000000010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sekhon MS, Ainslie PN, Griesdale DE. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care. 2017;21:90. 10.1186/s13054-017-1670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nolan JP, et al. European resuscitation council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021;47:369–421. 10.1007/s00134-021-06368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Granfeldt A, Holmberg MJ, Nolan JP, Soar J, Andersen LW. Targeted temperature management in adult cardiac arrest: systematic review and meta-analysis. Resuscitation. 2021;167:160–72. 10.1016/j.resuscitation.2021.08.040. [DOI] [PubMed] [Google Scholar]
- 87.Soar J, N. J. A. L., Böttiger BW, Couper K, Deakin CD, Drennan I, Hirsch KG, Hsu CH, Nicholson TC, O’Neil BJ, Paiva EF, Parr MJ, Reynolds JC, Sandroni C, Wang TL, Callaway CW, Donnino MW, Granfeldt A, Holmberg MJ, Lavonas EJ, Morrison LJ, Nation K, Neumar RW, Nikolaou N, Skrifvars MB, Welsford M, Morley PT, Berg KM. Temperature management in adult cardiac arrest consensus on science with treatment recommendations: international liaison committee on resuscitation (ILCOR) advanced life support task force (Brussels, Belgium, 2021).
- 88.Soldozy S, et al. Reperfusion injury in acute ischemic stroke: tackling the irony of revascularization. Clin Neurol Neurosurg. 2023;225:107574. 10.1016/j.clineuro.2022.107574. [DOI] [PubMed] [Google Scholar]
- 89.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–53. 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 90.Abebe TG, Feleke SF, Dessie AM, Anteneh RM, Anteneh ZA. Development and internal validation of a clinical risk score for in-hospital mortality after stroke: a single-centre retrospective cohort study in Northwest Ethiopia. BMJ Open. 2023;13:e063170. 10.1136/bmjopen-2022-063170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Addis A, et al. Brain temperature regulation in poor-grade subarachnoid hemorrhage patients - a multimodal neuromonitoring study. J Cereb Blood Flow Metab. 2021;41:359–68. 10.1177/0271678X20910405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sahle Adeba T, Mekonen H, Alemu T, Alate T, Melis T. Survival status and predictor of mortality among adult stroke patients in Saint Paul’s hospital millennium medical college, Addis Ababa, Ethiopia. SAGE Open Med. 2022;10:20503121221112484. 10.1177/20503121221112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alexandrov AW, et al. Back to basics: adherence with guidelines for glucose and temperature control in an american comprehensive stroke center sample. J Neurosci Nurs. 2018;50:131–7. 10.1097/JNN.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 94.Alonso-Alonso ML, et al. Antihyperthermic treatment in the management of malignant infarction of the middle cerebral artery. J Clin Med. 2022. 10.3390/jcm11102874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alonso-Alonso ML, et al. Influence of temperature chronobiology on stroke outcome. Int J Mol Sci. 2023. 10.3390/ijms24043746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amare AT, et al. Survival status and predictors of mortality among traumatic brain injury patients in an Ethiopian hospital: a retrospective cohort study. Afr J Emerg Med. 2021;11:396–403. 10.1016/j.afjem.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Assele DD, Lendado TA, Awato MA, Workie SB, Faltamo WF. Incidence and predictors of mortality among patients with head injury admitted to Hawassa University Comprehensive Specialized Hospital, Southern Ethiopia: a retrospective follow-up study. PLoS One. 2021;16:e0254245. 10.1371/journal.pone.0254245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Azzimondi G, et al. Fever in acute stroke worsens prognosis. A prospective study. Stroke. 1995;26:2040–3. 10.1161/01.str.26.11.2040. [DOI] [PubMed] [Google Scholar]
- 99.Bao L, Chen D, Ding L, Ling W, Xu F. Fever burden is an independent predictor for prognosis of traumatic brain injury. PLoS One. 2014;9:e90956. 10.1371/journal.pone.0090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barber M, Wright F, Stott DJ, Langhorne P. Predictors of early neurological deterioration after ischaemic stroke: a case-control study. Gerontology. 2004;50:102–9. 10.1159/000075561. [DOI] [PubMed] [Google Scholar]
- 101.Barow E, et al. Association of white blood cell count with clinical outcome independent of treatment with alteplase in acute ischemic stroke. Front Neurol. 2022;13:877367. 10.3389/fneur.2022.877367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bill O, Zufferey P, Faouzi M, Michel P. Severe stroke: patient profile and predictors of favorable outcome. J Thromb Haemost. 2013;11:92–9. 10.1111/jth.12066. [DOI] [PubMed] [Google Scholar]
- 103.Blanco M, et al. High blood pressure and inflammation are associated with poor prognosis in lacunar infarctions. Cerebrovasc Dis. 2006;22:123–9. 10.1159/000093240. [DOI] [PubMed] [Google Scholar]
- 104.Blanco M, et al. Neuroprotection or increased brain damage mediated by temperature in stroke is time dependent. PLoS One. 2012;7:e30700. 10.1371/journal.pone.0030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bonds BW, et al. Predictive value of hyperthermia and intracranial hypertension on neurological outcomes in patients with severe traumatic brain injury. Brain Inj. 2015;29:1642–7. 10.3109/02699052.2015.1075157. [DOI] [PubMed] [Google Scholar]
- 106.Boysen G, Christensen H. Stroke severity determines body temperature in acute stroke. Stroke. 2001;32:413–7. 10.1161/01.str.32.2.413. [DOI] [PubMed] [Google Scholar]
- 107.Burkot J, Kopec G, Pera J, Slowik A, Dziedzic T. Decompensated heart failure is a strong independent predictor of functional outcome after ischemic stroke. J Card Fail. 2015;21:642–6. 10.1016/j.cardfail.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 108.Bush RA, Beaumont JL, Liotta EM, Maas MB, Naidech AM. Fever burden and health-related quality of life after intracerebral hemorrhage. Neurocrit Care. 2018;29:189–94. 10.1007/s12028-018-0523-y. [DOI] [PubMed] [Google Scholar]
- 109.Campos F, et al. Hyperthermia in human ischemic and hemorrhagic stroke: similar outcome, different mechanisms. PLoS One. 2013;8:e78429. 10.1371/journal.pone.0078429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carlson AP, Schermer CR, Lu SW. Retrospective evaluation of anemia and transfusion in traumatic brain injury. J Trauma. 2006;61:567–71. 10.1097/01.ta.0000231768.44727.a2. [DOI] [PubMed] [Google Scholar]
- 111.Castellanos M, et al. Predictors of good outcome in medium to large spontaneous supratentorial intracerebral haemorrhages. J Neurol Neurosurg Psychiatry. 2005;76:691–5. 10.1136/jnnp.2004.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Castillo J, Davalos A, Noya M. Progression of ischaemic stroke and excitotoxic aminoacids. Lancet. 1997;349:79–83. 10.1016/S0140-6736(96)04453-4. [DOI] [PubMed] [Google Scholar]
- 113.Castillo J, Davalos A, Marrugat J, Noya M. Timing for fever-related brain damage in acute ischemic stroke. Stroke. 1998;29:2455–60. 10.1161/01.str.29.12.2455. [DOI] [PubMed] [Google Scholar]
- 114.Chen M, et al. Association between hyperpyrexia and poststroke outcomes in patients with recanalization after mechanical thrombectomy: a retrospective cohort study. BMC Neurol. 2021;21:365. 10.1186/s12883-021-02400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen T, et al. A predictive model for postoperative progressive haemorrhagic injury in traumatic brain injuries. BMC Neurol. 2022;22:16. 10.1186/s12883-021-02541-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen T, et al. The association of blood urea nitrogen to creatinine ratio and the prognosis of critically Ill patients with cerebral infarction: a cohort study. Mediators Inflamm. 2022;2022:2151840. 10.1155/2022/2151840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen Y, et al. Association of early increase in body temperature with symptomatic intracranial hemorrhage and unfavorable outcome following endovascular therapy in patients with large vessel occlusion stroke. J Integr Neurosci. 2022;21:156. 10.31083/j.jin2106156. [DOI] [PubMed] [Google Scholar]
- 118.Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke. 2003;34:1717–22. 10.1161/01.STR.0000078657.22835.B9. [DOI] [PubMed] [Google Scholar]
- 119.Christensen H, Boysen G, Johannesen HH, Christensen E, Bendtzen K. Deteriorating ischaemic stroke. cytokines, soluble cytokine receptors, ferritin, systemic blood pressure, body temperature, blood glucose, diabetes, stroke severity, and CT infarction-volume as predictors of deteriorating ischaemic stroke. J Neurol Sci. 2002;201:1–7. 10.1016/s0022-510x(02)00160-0. [DOI] [PubMed] [Google Scholar]
- 120.Cisse FA, et al. Predictors of stroke favorable functional outcome in Guinea, results from the Conakry stroke registry. Sci Rep. 2022;12:1125. 10.1038/s41598-022-05057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dávalos A, Castillo J, Pumar JM, Noya M. Body temperature and fibrinogen are related to early neurological deterioration in acute ischemic stroke. Cerebrovasc Dis. 1997;7:64–9. 10.1159/000108169. [Google Scholar]
- 122.Dehkharghani S, et al. Body temperature modulates infarction growth following endovascular reperfusion. AJNR Am J Neuroradiol. 2017;38:46–51. 10.3174/ajnr.A4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Demlie TA, Alemu MT, Messelu MA, Wagnew F, Mekonen EG. Incidence and predictors of mortality among traumatic brain injury patients admitted to Amhara region Comprehensive Specialized Hospitals, northwest Ethiopia, 2022. BMC Emerg Med. 2023;23:55. 10.1186/s12873-023-00823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.den Hertog HM, et al. The Paracetamol (Acetaminophen) In Stroke (PAIS) trial: a multicentre, randomised, placebo-controlled, phase III trial. Lancet Neurol. 2009;8:434–40. 10.1016/S1474-4422(09)70051-1. [DOI] [PubMed] [Google Scholar]
- 125.Derbisz JM, et al. The prognostic significance of large vessel occlusion in stroke patients treated by intravenous thrombolysis. Pol J Radiol. 2021;86:e344–52. 10.5114/pjr.2021.107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dicpinigaitis AJ, et al. Development of cerebral vasospasm following traumatic intracranial hemorrhage: incidence, risk factors, and clinical outcomes. Neurosurg Focus. 2022;52:E14. 10.3171/2021.12.FOCUS21668. [DOI] [PubMed] [Google Scholar]
- 127.Diprose WK, et al. Impact of body temperature before and after endovascular thrombectomy for large vessel occlusion stroke. Stroke. 2020;51:1218–25. 10.1161/STROKEAHA.119.028160. [DOI] [PubMed] [Google Scholar]
- 128.Dowlati E, et al. Early fevers and elevated neutrophil-to-lymphocyte ratio are associated with repeat endovascular interventions for cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2022;36:916–26. 10.1007/s12028-021-01399-7. [DOI] [PubMed] [Google Scholar]
- 129.Dzierzecki S, Zabek M, Zapolska G, Tomasiuk R. The S-100B level, intracranial pressure, body temperature, and transcranial blood flow velocities predict the outcome of the treatment of severe brain injury. Medicine (Baltimore). 2022;101:e30348. 10.1097/MD.0000000000030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eagles ME, Tso MK, Ayling OGS, Wong JH, MacDonald RL. Unfavorable outcome after good grade aneurysmal subarachnoid hemorrhage: exploratory analysis. World Neurosurg. 2020;144:e842–8. 10.1016/j.wneu.2020.09.079. [DOI] [PubMed] [Google Scholar]
- 131.Elf K, Nilsson P, Ronne-Engstrom E, Howells T, Enblad P. Temperature disturbances in traumatic brain injury: relationship to secondary insults, barbiturate treatment and outcome. Neurol Res. 2008;30:1097–105. 10.1179/174313208X319125. [DOI] [PubMed] [Google Scholar]
- 132.Fan JS, et al. Emergency department neurologic deterioration in patients with spontaneous intracerebral hemorrhage: incidence, predictors, and prognostic significance. Acad Emerg Med. 2012;19:133–8. 10.1111/j.1553-2712.2011.01285.x. [DOI] [PubMed] [Google Scholar]
- 133.Fang R, et al. Early in-theater management of combat-related traumatic brain injury: a prospective, observational study to identify opportunities for performance improvement. J Trauma Acute Care Surg. 2015;79:S181-187. 10.1097/TA.0000000000000769. [DOI] [PubMed] [Google Scholar]
- 134.Ferguson S, Macdonald RL. Predictors of cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;60:658–67. 10.1227/01.NEU.0000255396.23280.31. [DOI] [PubMed] [Google Scholar]
- 135.Fernandez A, et al. Fever after subarachnoid hemorrhage: risk factors and impact on outcome. Neurology. 2007;68:1013–9. 10.1212/01.wnl.0000258543.45879.f5. [DOI] [PubMed] [Google Scholar]
- 136.Fu K, Garvan CS, Heaton SC, Nagaraja N, Dore S. Association of serum bilirubin with the severity and outcomes of intracerebral hemorrhages. Antioxidants (Basel). 2021. 10.3390/antiox10091346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fukuda H, Kitani M, Takahashi K. Body temperature correlates with functional outcome and the lesion size of cerebral infarction. Acta Neurol Scand. 1999;100:385–90. 10.1111/j.1600-0404.1999.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 138.Gaither JB, et al. Body temperature after EMS transport: association with traumatic brain injury outcomes. Prehosp Emerg Care. 2017;21:575–82. 10.1080/10903127.2017.1308609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Geffroy A, et al. Severe traumatic head injury in adults: which patients are at risk of early hyperthermia? Intensive Care Med. 2004;30:785–90. 10.1007/s00134-004-2280-y. [DOI] [PubMed] [Google Scholar]
- 140.Georgilis K, Plomaritoglou A, Dafni U, Bassiakos Y, Vemmos K. Aetiology of fever in patients with acute stroke. J Intern Med. 1999;246:203–9. 10.1046/j.1365-2796.1999.00539.x. [DOI] [PubMed] [Google Scholar]
- 141.Geurts M, et al. Temporal profile of body temperature in acute ischemic stroke: relation to infarct size and outcome. BMC Neurol. 2016;16:233. 10.1186/s12883-016-0760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gillow SJ, Ouyang B, Lee VH, John S. Factors associated with fever in intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2017;26:1204–8. 10.1016/j.jstrokecerebrovasdis.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 143.Bogossian EG, et al. The impact of extra-cerebral infection after subarachnoid hemorrhage: a single center cohort study. World Neurosurg. 2020. 10.1016/j.wneu.2020.09.102. [DOI] [PubMed] [Google Scholar]
- 144.Grau AJ, et al. Fever and infection early after ischemic stroke. J Neurol Sci. 1999;171:115–20. 10.1016/s0022-510x(99)00261-0. [DOI] [PubMed] [Google Scholar]
- 145.Guo R, et al. Machine learning-based approaches for prediction of patients’ functional outcome and mortality after spontaneous intracerebral hemorrhage. J Pers Med. 2022. 10.3390/jpm12010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guth JC, et al. Subarachnoid extension of primary intracerebral hemorrhage is associated with fevers. Neurocrit Care. 2014;20:187–92. 10.1007/s12028-013-9888-0. [DOI] [PubMed] [Google Scholar]
- 147.Hanchaiphiboolkul S. Body temperature and mortality in acute cerebral infarction. J Med Assoc Thai. 2005;88:26–31. [PubMed] [Google Scholar]
- 148.Lee KH, Lioutas VA, Marchina S, Selim M, iDEF Investigators. The prognostic roles of perihematomal edema and ventricular size in patients with intracerebral hemorrhage. Neurocrit Care. 2022;37:455–62. 10.1007/s12028-022-01532-0. [DOI] [PubMed] [Google Scholar]
- 149.Heppekcan D, Ekin S, Çivi M, Aydın Tok D. Impact of secondary insults in brain death after traumatic brain injury. Transpl Proceed. 2019;51:2186–8. 10.1016/j.transproceed.2019.01.176. [DOI] [PubMed] [Google Scholar]
- 150.Hifumi T, et al. High early phase hemoglobin level is associated with favorable neurological outcome in patients with severe traumatic brain injury. Am J Emerg Med. 2021;44:373–7. 10.1016/j.ajem.2020.04.065. [DOI] [PubMed] [Google Scholar]
- 151.Hindfelt B. The prognostic significance of subfebrility and fever in ischaemic cerebral infarction. Acta Neurol Scand. 1976;53:72–9. 10.1111/j.1600-0404.1976.tb04326.x. [DOI] [PubMed] [Google Scholar]
- 152.Hinson HE, Rowell S, Morris C, Lin AL, Schreiber MA. Early fever after trauma: does it matter? J Trauma Acute Care Surg. 2018;84:19–24. 10.1097/TA.0000000000001627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu X, et al. Predictive role of shock index in the early formation of cerebral infarction in patients with TBI and cerebral herniation. Front Neurol. 2022;13:956039. 10.3389/fneur.2022.956039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang JH, Wang TJ, Wu SF, Liu CY, Fan JY. Post-craniotomy fever and its associated factors in patients with traumatic brain injury. Nurs Crit Care. 2022;27:483–92. 10.1111/nicc.12640. [DOI] [PubMed] [Google Scholar]
- 155.Huang X, et al. Development and validation of a clinical-based signature to predict the 90-day functional outcome for spontaneous intracerebral hemorrhage. Front Aging Neurosci. 2022;14:904085. 10.3389/fnagi.2022.904085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hulscher F, et al. Predictors of good clinical outcome after thrombectomy for distal medium vessel occlusions. World Neurosurg. 2022;160:e566–72. 10.1016/j.wneu.2022.01.067. [DOI] [PubMed] [Google Scholar]
- 157.Ibrahim AO, Shabi OM, Agbesanwa TA, Olowoyo P. Five-year analysis of clinical presentations and predictors of stroke mortality in rural Southwestern Nigeria: a retrospective observational study. Afr J Emerg Med. 2022;12:12–8. 10.1016/j.afjem.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Iglesias-Rey R, et al. Neurological instability in ischemic stroke: relation with outcome, latency time, and molecular markers. Transl Stroke Res. 2022;13:228–37. 10.1007/s12975-021-00924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Iglesias-Rey R, et al. Inflammation, edema and poor outcome are associated with hyperthermia in hypertensive intracerebral hemorrhages. Eur J Neurol. 2018;25:1161–8. 10.1111/ene.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Irvine H, et al. Markers of infection and inflammation are associated with post-thrombectomy mortality in acute stroke. Clin Neurol Neurosurg. 2022;222:107467. 10.1016/j.clineuro.2022.107467. [DOI] [PubMed] [Google Scholar]
- 161.Jacome T, Tatum D. Systemic inflammatory response syndrome (SIRS) score independently predicts poor outcome in isolated traumatic brain injury. Neurocrit Care. 2018;28:110–6. 10.1007/s12028-017-0410-y. [DOI] [PubMed] [Google Scholar]
- 162.Jayan M, Shukla D, Devi BI, Bhat DI, Konar SK. Development of a prognostic model to predict mortality after traumatic brain injury in intensive care setting in a developing Country. J Neurosci Rural Pract. 2021;12:368–75. 10.1055/s-0041-1726623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jeong HG, et al. Tachycardia burden in stroke unit is associated with functional outcome after ischemic stroke. Int J Stroke. 2016;11:313–20. 10.1177/1747493016631357. [DOI] [PubMed] [Google Scholar]
- 164.Jiang JY, Gao GY, Li WP, Yu MK, Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma. 2002;19:869–74. 10.1089/08977150260190456. [DOI] [PubMed] [Google Scholar]
- 165.Jorgensen HS, et al. Potentially reversible factors during the very acute phase of stroke and their impact on the prognosis: is there a large therapeutic potential to be explored? Cerebrovasc Dis. 2001;11:207–11. 10.1159/000047640. [DOI] [PubMed] [Google Scholar]
- 166.Kammersgaard LP, et al. Admission body temperature predicts long-term mortality after acute stroke: the Copenhagen Stroke Study. Stroke. 2002;33:1759–62. 10.1161/01.str.0000019910.90280.f1. [DOI] [PubMed] [Google Scholar]
- 167.Karaszewski B, Thomas RG, Dennis MS, Wardlaw JM. Temporal profile of body temperature in acute ischemic stroke: relation to stroke severity and outcome. BMC Neurol. 2012;12:123. 10.1186/1471-2377-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim SH, Saver JL. Initial body temperature in ischemic stroke: nonpotentiation of tissue-type plasminogen activator benefit and inverse association with severity. Stroke. 2015;46:132–6. 10.1161/STROKEAHA.114.006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Koton S, Tanne D, Green MS, Bornstein NM. Mortality and predictors of death 1 month and 3 years after first-ever ischemic stroke: data from the first national acute stroke Israeli survey (NASIS 2004). Neuroepidemiology. 2010;34:90–6. 10.1159/000264826. [DOI] [PubMed] [Google Scholar]
- 170.Kramer CL, Pegoli M, Mandrekar J, Lanzino G, Rabinstein AA. Refining the association of fever with functional outcome in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2017;26:41–7. 10.1007/s12028-016-0281-7. [DOI] [PubMed] [Google Scholar]
- 171.Kvistad CE, et al. Is higher body temperature beneficial in ischemic stroke patients with normal admission CT angiography of the cerebral arteries? Vasc Health Risk Manag. 2014;10:49–54. 10.2147/VHRM.S55423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kvistad CE, Thomassen L, Waje-Andreassen U, Logallo N, Naess H. Body temperature and major neurological improvement in tPA-treated stroke patients. Acta Neurol Scand. 2014;129:325–9. 10.1111/ane.12184. [DOI] [PubMed] [Google Scholar]
- 173.Lai PMR, et al. Noninfectious fever in aneurysmal subarachnoid hemorrhage: association with cerebral vasospasm and clinical outcome. World Neurosurg. 2019;122:e1014–9. 10.1016/j.wneu.2018.10.203. [DOI] [PubMed] [Google Scholar]
- 174.Laupland KB, et al. Occurrence and outcome of fever in critically ill adults. Crit Care Med. 2008;36:1531–5. 10.1097/CCM.0b013e318170efd3. [DOI] [PubMed] [Google Scholar]
- 175.Lee D, Ryu H, Jung E. Effect of fever on the clinical outcomes of traumatic brain injury by age. Medicina (Kaunas). 2022. 10.3390/medicina58121860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee J, Lee D, Lee B, No E. Association between pre-hospital National early warning score and in-hospital mortality in patients with traumatic brain injury. Ulus Travma Acil Cerrahi Derg. 2023;29:292–6. 10.14744/tjtes.2022.96809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Leira R, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461–7. 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 178.Leira R, et al. Hyperthermia is a surrogate marker of inflammation-mediated cause of brain damage in acute ischaemic stroke. J Intern Med. 2006;260:343–9. 10.1111/j.1365-2796.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- 179.Leira R, et al. A higher body temperature is associated with haemorrhagic transformation in patients with acute stroke untreated with recombinant tissue-type plasminogen activator (rtPA). Clin Sci (Lond). 2012;122:113–9. 10.1042/CS20110143. [DOI] [PubMed] [Google Scholar]
- 180.Li G, et al. Mild-to-moderate neurogenic pyrexia in acute cerebral infarction. Eur Neurol. 2011;65:94–8. 10.1159/000322803. [DOI] [PubMed] [Google Scholar]
- 181.Li J, Jiang JY. Chinese head trauma data bank: effect of hyperthermia on the outcome of acute head trauma patients. J Neurotrauma. 2012;29:96–100. 10.1089/neu.2011.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lin H, et al. Lower body temperature independently predicts delayed cerebral infarction in the elderly with ruptured intracranial aneurysm. Front Neurol. 2021;12:763471. 10.3389/fneur.2021.763471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu S, et al. Posttraumatic cerebral infarction in severe traumatic brain injury: characteristics, risk factors and potential mechanisms. Acta Neurochir (Wien). 2015;157:1697–704. 10.1007/s00701-015-2559-5. [DOI] [PubMed] [Google Scholar]
- 184.Liu T, et al. Influence of nutritional status on prognosis of stroke patients with dysphagia. Altern Ther Health Med. 2022;28:26–33. [PubMed] [Google Scholar]
- 185.Lord AS, Gilmore E, Choi HA, Mayer SA, Collaboration V-I. Time course and predictors of neurological deterioration after intracerebral hemorrhage. Stroke. 2015;46:647–52. 10.1161/STROKEAHA.114.007704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Luo Y, et al. Relationship between body temperature and early neurological deterioration after endovascular thrombectomy for acute ischemic stroke with large vessel occlusion. Neurocrit Care. 2022;37:399–409. 10.1007/s12028-021-01416-9. [DOI] [PubMed] [Google Scholar]
- 187.Malavera A, et al. Prognostic significance of early pyrexia in acute intracerebral haemorrhage: the INTERACT2 study. J Neurol Sci. 2021;423:117364. 10.1016/j.jns.2021.117364. [DOI] [PubMed] [Google Scholar]
- 188.Matsukawa H, Shinoda M, Fujii M, Takahashi O, Murakata A. Risk factors for mortality in patients with non-traumatic pontine hemorrhage. Acta Neurol Scand. 2015;131:240–5. 10.1111/ane.12312. [DOI] [PubMed] [Google Scholar]
- 189.Matsuzono K, et al. Real-time data on the prognosis of acute ischemic stroke patients in the Tochigi Clinical ObservatioNal registry for 1-year mortality of aCute ischEmic stRoke patieNt (T-CONCERN) study. Neurol Sci. 2022;43:6855–64. 10.1007/s10072-022-06377-1. [DOI] [PubMed] [Google Scholar]
- 190.Matuja SS, et al. Predictors of 30-day mortality among patients with stroke admitted at a tertiary teaching hospital in Northwestern Tanzania: a prospective cohort study. Front Neurol. 2022;13:1100477. 10.3389/fneur.2022.1100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Melmed KR, et al. Systemic inflammatory response syndrome is associated with hematoma expansion in intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2021;30:105870. 10.1016/j.jstrokecerebrovasdis.2021.105870. [DOI] [PubMed] [Google Scholar]
- 192.Middleton S, et al. Vital sign monitoring following stroke associated with 90-day independence: a secondary analysis of the QASC cluster randomized trial. Int J Nurs Stud. 2019;89:72–9. 10.1016/j.ijnurstu.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 193.Millan M, et al. Body temperature and response to thrombolytic therapy in acute ischaemic stroke. Eur J Neurol. 2008;15:1384–9. 10.1111/j.1468-1331.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 194.Mohamed WS, Kamel AE, Abdelwahab AH, Mahdy ME. High neutrophil-to-lymphocyte ratio predicts early neurological deterioration in spontaneous intracerebral hemorrhage patients. Egypt J Neurol Psychiatry Neurosurg. 2021;57:29. 10.1186/s41983-020-00267-z. [Google Scholar]
- 195.Muehlschlegel S, et al. Frequency and impact of intensive care unit complications on moderate-severe traumatic brain injury: early results of the Outcome Prognostication in Traumatic Brain Injury (OPTIMISM) Study. Neurocrit Care. 2013;18:318–31. 10.1007/s12028-013-9817-2. [DOI] [PubMed] [Google Scholar]
- 196.Muscari A, et al. Prognostic significance of diabetes and stress hyperglycemia in acute stroke patients. Diabetol Metab Syndr. 2022;14:126. 10.1186/s13098-022-00896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Naess H, et al. Inverse relationship of baseline body temperature and outcome between ischemic stroke patients treated and not treated with thrombolysis: the Bergen stroke study. Acta Neurol Scand. 2010;122:414–7. 10.1111/j.1600-0404.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 198.Naidech AM, et al. Fever burden and functional recovery after subarachnoid hemorrhage. Neurosurgery. 2008;63:212–7. 10.1227/01.NEU.0000320453.61270.0F. [DOI] [PubMed] [Google Scholar]
- 199.Nutakki A, et al. Predictors of in-hospital and 90-day post-discharge stroke mortality in Lusaka. Zambia J Neurol Sci. 2022;437:120249. 10.1016/j.jns.2022.120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Oh HS, Jeong HS, Seo WS. Non-infectious hyperthermia in acute brain injury patients: relationships to mortality, blood pressure, intracranial pressure and cerebral perfusion pressure. Int J Nurs Pract. 2012;18:295–302. 10.1111/j.1440-172X.2012.02039.x. [DOI] [PubMed] [Google Scholar]
- 201.Alessandro O, et al. C-reactive protein elevation predicts in-hospital deterioration after aneurysmal subarachnoid hemorrhage: a retrospective observational study. Acta Neurochir (Wien). 2022;164:1805–14. 10.1007/s00701-022-05256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Park YK, et al. Predictive factors of fever after aneurysmal subarachnoid hemorrhage and its impact on delayed cerebral ischemia and clinical outcomes. World Neurosurg. 2018;114:e524–31. 10.1016/j.wneu.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 203.Pegoli M, Mandrekar J, Rabinstein AA, Lanzino G. Predictors of excellent functional outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2015;122:414–8. 10.3171/2014.10.JNS14290. [DOI] [PubMed] [Google Scholar]
- 204.Phipps MS, Desai RA, Wira C, Bravata DM. Epidemiology and outcomes of fever burden among patients with acute ischemic stroke. Stroke. 2011;42:3357–62. 10.1161/STROKEAHA.111.621425. [DOI] [PubMed] [Google Scholar]
- 205.Reith J, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet. 1996;347:422–5. 10.1016/s0140-6736(96)90008-2. [DOI] [PubMed] [Google Scholar]
- 206.Rincon F, Lyden P, Mayer SA. Relationship between temperature, hematoma growth, and functional outcome after intracerebral hemorrhage. Neurocrit Care. 2013;18:45–53. 10.1007/s12028-012-9779-9. [DOI] [PubMed] [Google Scholar]
- 207.Rincon F, Hunter K, Schorr C, Dellinger RP, Zanotti-Cavazzoni S. The epidemiology of spontaneous fever and hypothermia on admission of brain injury patients to intensive care units: a multicenter cohort study. J Neurosurg. 2014;121:950–60. 10.3171/2014.7.JNS132470. [DOI] [PubMed] [Google Scholar]
- 208.Rordorf G, Koroshetz W, Efird JT, Cramer SC. Predictors of mortality in stroke patients admitted to an intensive care unit. Crit Care Med. 2000;28:1301–5. 10.1097/00003246-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 209.Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315–21. 10.1161/STROKEAHA.107.484360. [DOI] [PubMed] [Google Scholar]
- 210.Roy MK, Ray A. Effect of body temperature on mortality of acute stroke. J Assoc Physicians India. 2004;52:959–61. [PubMed] [Google Scholar]
- 211.Ryttlefors M, Howells T, Nilsson P, Ronne-Engstrom E, Enblad P. Secondary insults in subarachnoid hemorrhage: occurrence and impact on outcome and clinical deterioration. Neurosurgery. 2007;61:704–14. 10.1227/01.NEU.0000298898.38979.E3. [DOI] [PubMed] [Google Scholar]
- 212.Saini M, et al. Effect of hyperthermia on prognosis after acute ischemic stroke. Stroke. 2009;40:3051–9. 10.1161/STROKEAHA.109.556134. [DOI] [PubMed] [Google Scholar]
- 213.Saripalli M, Tan D, Chandra RV, Lai LT. Predictive relevance of early temperature elevation on the risk of delayed cerebral ischemia development following aneurysmal subarachnoid hemorrhage. World Neurosurg. 2021;150:e474–81. 10.1016/j.wneu.2021.03.031. [DOI] [PubMed] [Google Scholar]
- 214.Schirmer-Mikalsen K, Moen KG, Skandsen T, Vik A, Klepstad P. Intensive care and traumatic brain injury after the introduction of a treatment protocol: a prospective study. Acta Anaesthesiol Scand. 2013;57:46–55. 10.1111/j.1399-6576.2012.02785.x. [DOI] [PubMed] [Google Scholar]
- 215.Schwarz S, Hafner K, Aschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54:354–61. 10.1212/wnl.54.2.354. [DOI] [PubMed] [Google Scholar]
- 216.Seo WK, Yu SW, Kim JH, Park KW, Koh SB. The impact of hyperthermia and infection on acute ischemic stroke patients in the intensive care unit. Neurocrit Care. 2008;9:183–8. 10.1007/s12028-008-9056-0. [DOI] [PubMed] [Google Scholar]
- 217.Shin H, et al. Effect of hypothermia and hyperthermia on all-cause in-hospital mortality in emergencies: a comprehensive nationwide analysis from the Republic of Korea. Signa Vitae. 2023;19:136–42. 10.22514/sv.2022.056. [Google Scholar]
- 218.Song J, et al. Prediction of mortality among patients with isolated traumatic brain injury using machine learning models in asian countries: an international multi-center cohort study. J Neurotrauma. 2023;40:1376–87. 10.1089/neu.2022.0280. [DOI] [PubMed] [Google Scholar]
- 219.Springer MV, et al. Predictors of global cognitive impairment 1 year after subarachnoid hemorrhage. Neurosurgery. 2009;65:1043–50. 10.1227/01.NEU.0000359317.15269.20. [DOI] [PubMed] [Google Scholar]
- 220.Stosser S, et al. Severe dysphagia predicts poststroke fever. Stroke. 2021;52:2284–91. 10.1161/STROKEAHA.120.033396. [DOI] [PubMed] [Google Scholar]
- 221.Suehiro E, et al. Importance of early postoperative body temperature management for treatment of subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2016;25:1482–8. 10.1016/j.jstrokecerebrovasdis.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 222.Suzuki S, et al. Acute leukocyte and temperature response in hypertensive intracerebral hemorrhage. Stroke. 1995;26:1020–3. 10.1161/01.str.26.6.1020. [DOI] [PubMed] [Google Scholar]
- 223.Swor DE, et al. Admission heart rate variability is associated with fever development in patients with intracerebral hemorrhage. Neurocrit Care. 2019;30:244–50. 10.1007/s12028-019-00684-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Szczudlik A, Turaj W, Slowik A, Strojny J. Hyperthermia is not an independent predictor of greater mortality in patients with primary intracerebral hemorrhage. Med Sci Monit. 2002;8:CR702-707. [PubMed] [Google Scholar]
- 225.Szczudlik A, Turaj W, Slowik A, Strojny J. Microalbuminuria and hyperthermia independently predict long-term mortality in acute ischemic stroke patients. Acta Neurol Scand. 2003;107:96–101. 10.1034/j.1600-0404.2003.01363.x. [DOI] [PubMed] [Google Scholar]
- 226.Tanaka C, et al. Intracranial pressure management and neurological outcome for patients with mild traumatic brain injury who required neurosurgical intervention: a Japanese database study. Brain Inj. 2019;33:869–74. 10.1080/02699052.2019.1614667. [DOI] [PubMed] [Google Scholar]
- 227.Tegegne NG, Fentie DY, Tegegne BA, Admassie BM. Incidence and predictors of mortality among patients with traumatic brain injury at university of gondar comprehensive specialized hospital, Northwest Ethiopia: a retrospective follow-up study. Patient Relat Outcome Meas. 2023;14:73–85. 10.2147/PROM.S399603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tiainen M, et al. Body temperature, blood infection parameters, and outcome of thrombolysis-treated ischemic stroke patients. Int J Stroke. 2013;8:632–8. 10.1111/ijs.12039. [DOI] [PubMed] [Google Scholar]
- 229.Todd MM, et al. Perioperative fever and outcome in surgical patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2009;64:897–908. 10.1227/01.NEU.0000341903.11527.2F. [DOI] [PubMed] [Google Scholar]
- 230.Tseng WC, Chiu YH, Chen YC, Chen HS, Hsiao MY. Early fever in patients with primary intracerebral hemorrhage is associated with worse long-term functional outcomes: a prospective study. BMC Neurol. 2023;23:375. 10.1186/s12883-023-03426-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ueno T, et al. Association of survival and hyperthermia after rt-PA for ischemic stroke. Acta Neurol Scand. 2018;138:574–8. 10.1111/ane.13011. [DOI] [PubMed] [Google Scholar]
- 232.Vallee F, et al. The ICEBERG: a score and visual representation to track the severity of traumatic brain injury: design principles and preliminary results. J Trauma Acute Care Surg. 2022;93:229–37. 10.1097/TA.0000000000003515. [DOI] [PubMed] [Google Scholar]
- 233.Vapalahti M, Troupp H. Prognosis for patients with severe brain injuries. Br Med J. 1971;3:404–7. 10.1136/bmj.3.5771.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Volbers B, et al. Peak perihemorrhagic edema correlates with functional outcome in intracerebral hemorrhage. Neurology. 2018;90:e1005–12. 10.1212/WNL.0000000000005167. [DOI] [PubMed] [Google Scholar]
- 235.Walelgn N, Abyu GY, Seyoum Y, Habtegiorgis SD, Birhanu MY. The survival status and predictors of mortality among stroke patients at North West Ethiopia. Risk Manag Healthc Policy. 2021;14:2983–94. 10.2147/RMHP.S322001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wang Y, Lim LL, Levi C, Heller RF, Fisher J. Influence of admission body temperature on stroke mortality. Stroke. 2000;31:404–9. 10.1161/01.str.31.2.404. [DOI] [PubMed] [Google Scholar]
- 237.Wang R, et al. Prediction of mortality in geriatric traumatic brain injury patients using machine learning algorithms. Brain Sci. 2023. 10.3390/brainsci13010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang Y, et al. A comparison of random survival forest and Cox regression for prediction of mortality in patients with hemorrhagic stroke. BMC Med Inform Decis Mak. 2023;23:215. 10.1186/s12911-023-02293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wartenberg KE, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–23. 10.1097/01.ccm.0000201903.46435.35. [DOI] [PubMed] [Google Scholar]
- 240.Weimer JM, Gordon E, Frontera JA. Predictors of functional outcome after subdural hematoma: a prospective study. Neurocrit Care. 2017;26:70–9. 10.1007/s12028-016-0279-1. [DOI] [PubMed] [Google Scholar]
- 241.Svedung Wettervik T, Hanell A, Ronne-Engstrom E, Lewen A, Enblad P. Temperature changes in poor-grade aneurysmal subarachnoid hemorrhage: relation to injury pattern, intracranial pressure dynamics, cerebral energy metabolism, and clinical outcome. Neurocrit Care. 2023. 10.1007/s12028-023-01699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wijdicks EF, St Louis E. Clinical profiles predictive of outcome in pontine hemorrhage. Neurology. 1997;49:1342–6. 10.1212/wnl.49.5.1342. [DOI] [PubMed] [Google Scholar]
- 243.Wu F, et al. Fever burden within 24 h after hematoma evacuation predicts early neurological deterioration in patients with intracerebral hemorrhage: a retrospective analysis. Front Neurol. 2023;14:1205031. 10.3389/fneur.2023.1205031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wu F, et al. Prediction of death in intracerebral hemorrhage patients after minimally invasive surgery by vital signs and blood glucose. World Neurosurg. 2024;184:e84–94. 10.1016/j.wneu.2024.01.061. [DOI] [PubMed] [Google Scholar]
- 245.Yamamoto T, Mori K, Maeda M. Assessment of prognostic factors in severe traumatic brain injury patients treated by mild therapeutic cerebral hypothermia therapy. Neurol Res. 2002;24:789–95. 10.1179/016164102101200906. [DOI] [PubMed] [Google Scholar]
- 246.Yang Z, et al. The impact of heart rate circadian rhythm on in-hospital mortality in patients with stroke and critically ill: insights from the eICU collaborative research database. Heart Rhythm. 2022;19:1325–33. 10.1016/j.hrthm.2022.03.1230. [DOI] [PubMed] [Google Scholar]
- 247.Yokobori S, et al. Treatment of geriatric traumatic brain injury: a nationwide cohort study. J Nippon Med Sch. 2021;88:194–203. 10.1272/jnms.JNMS.2021_88-404. [DOI] [PubMed] [Google Scholar]
- 248.Zhang G, Zhang JH, Qin X. Fever increased in-hospital mortality after subarachnoid hemorrhage. Acta Neurochir Suppl. 2011;110:239–43. 10.1007/978-3-7091-0353-1_42. [DOI] [PubMed] [Google Scholar]
- 249.Zhang W, et al. Impact of body temperature in patients with acute basilar artery occlusion: analysis of the BASILAR database. Front Neurol. 2022;13:907410. 10.3389/fneur.2022.907410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhang GJ, Zhao JY, Zhang T, You C, Wang XY. Construction of a nomogram to reveal the prognostic benefit of spontaneous intracranial hemorrhage among Chinese adults: a population-based study. Neurol Sci. 2022;43:2449–60. 10.1007/s10072-021-05684-3. [DOI] [PubMed] [Google Scholar]
- 251.Zhao J, Zhang S, Ma J, Shi G, Zhou J. Admission rate-pressure product as an early predictor for in-hospital mortality after aneurysmal subarachnoid hemorrhage. Neurosurg Rev. 2022;45:2811–22. 10.1007/s10143-022-01795-3. [DOI] [PubMed] [Google Scholar]
- 252.Zhou Z, et al. A nomogram for predicting the risk of poor prognosis in patients with poor-grade aneurysmal subarachnoid hemorrhage following microsurgical clipping. Front Neurol. 2023;14:1146106. 10.3389/fneur.2023.1146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zou J, et al. Development and validation of a nomogram to predict the 30-day mortality risk of patients with intracerebral hemorrhage. Front Neurosci. 2022;16:942100. 10.3389/fnins.2022.942100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.