Abstract
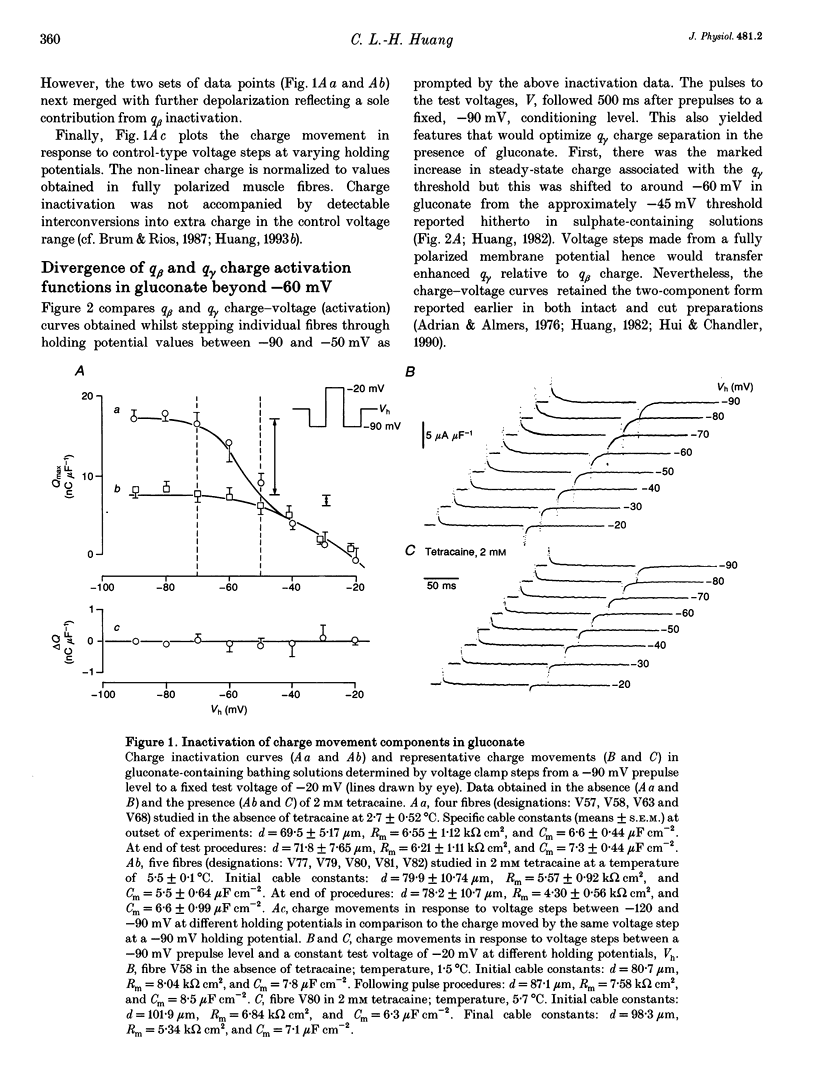
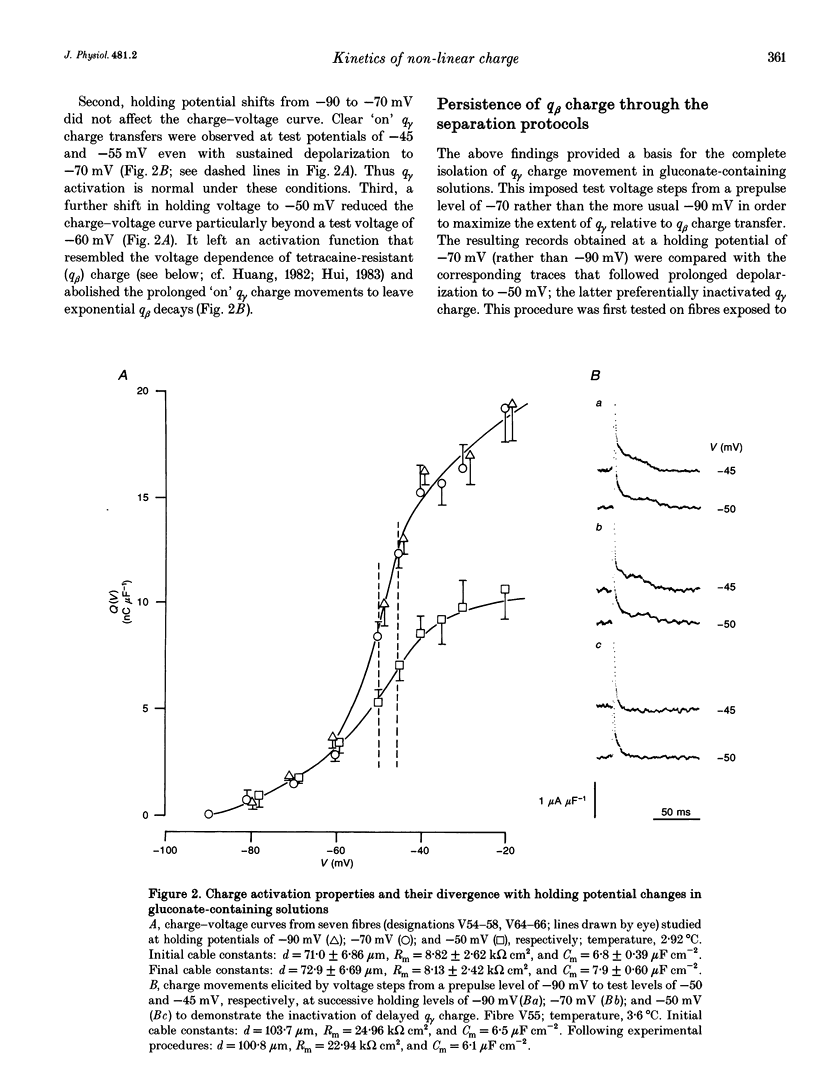
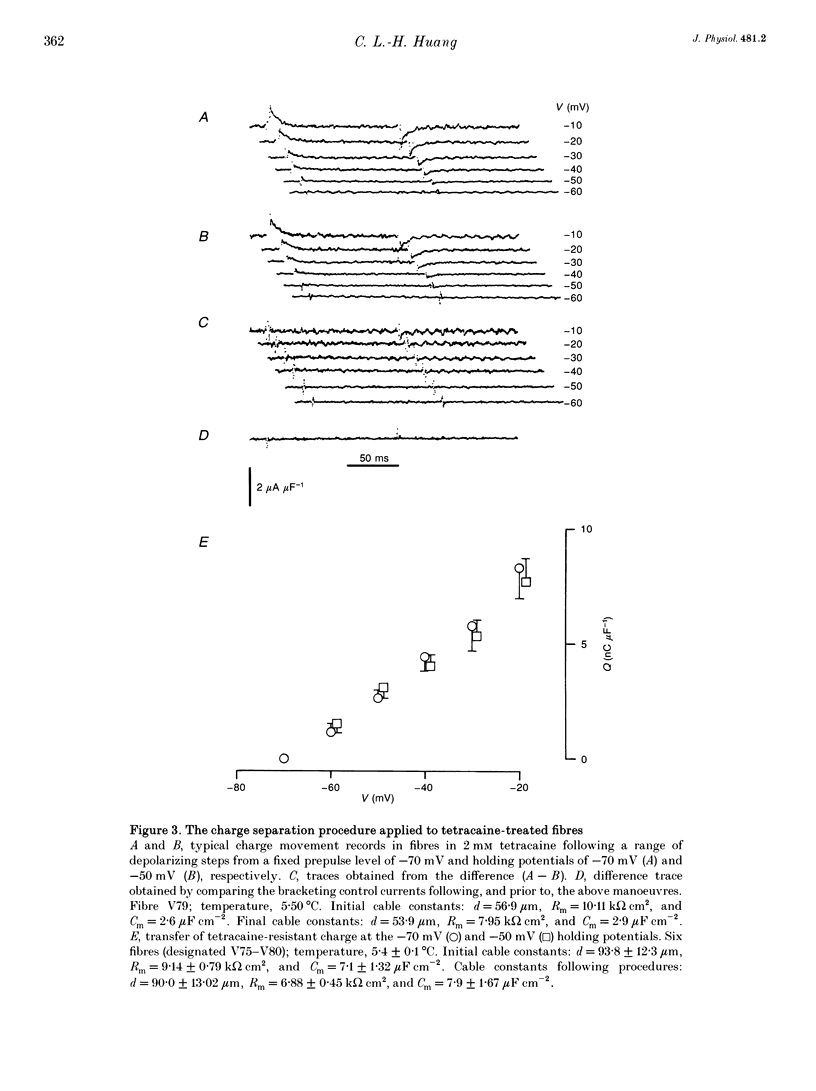
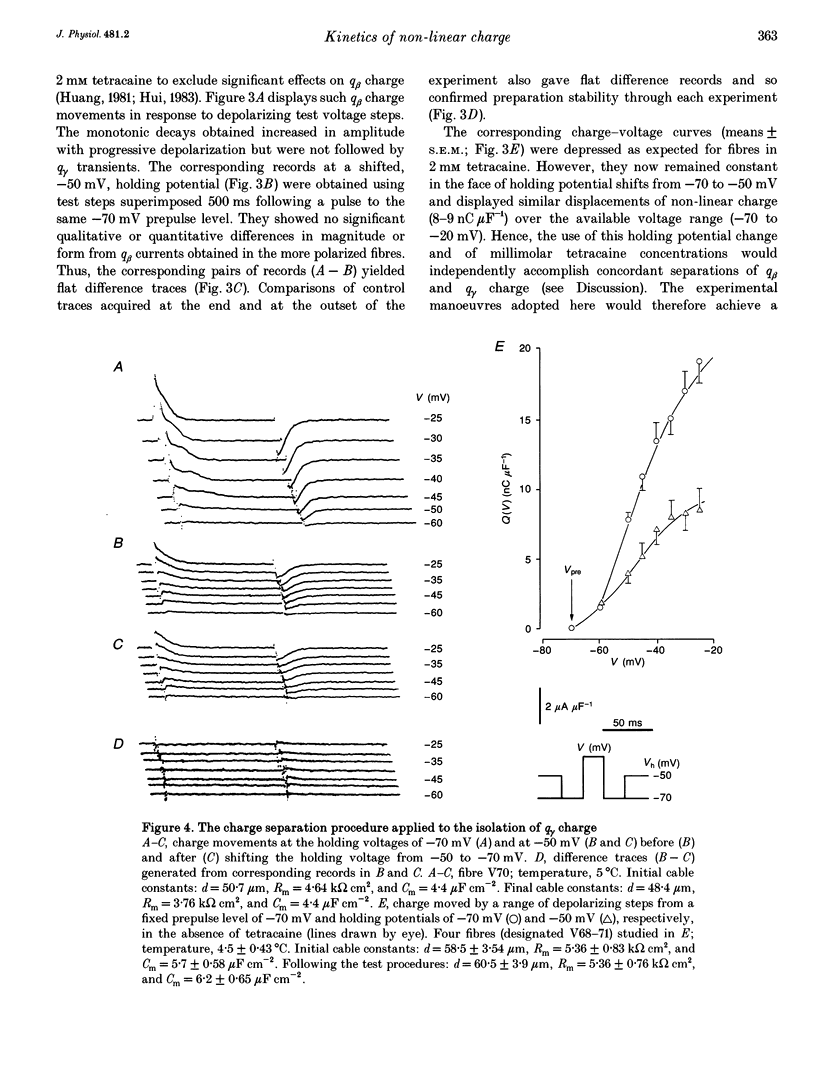
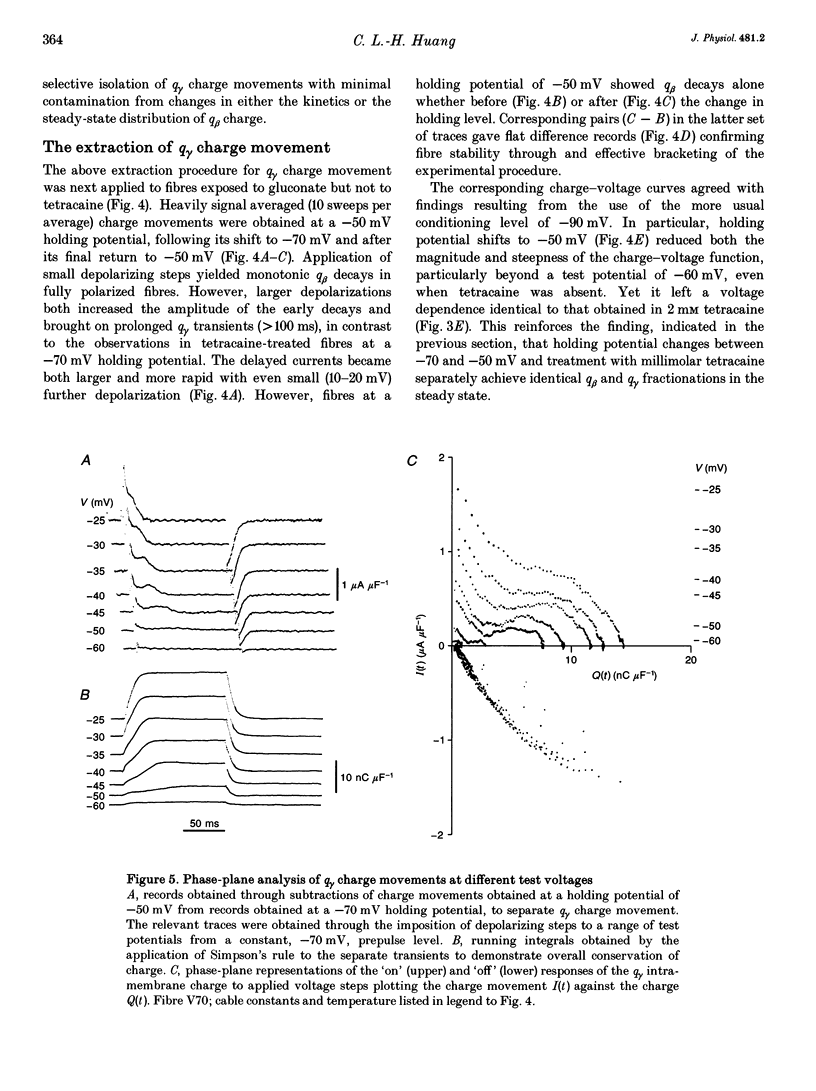
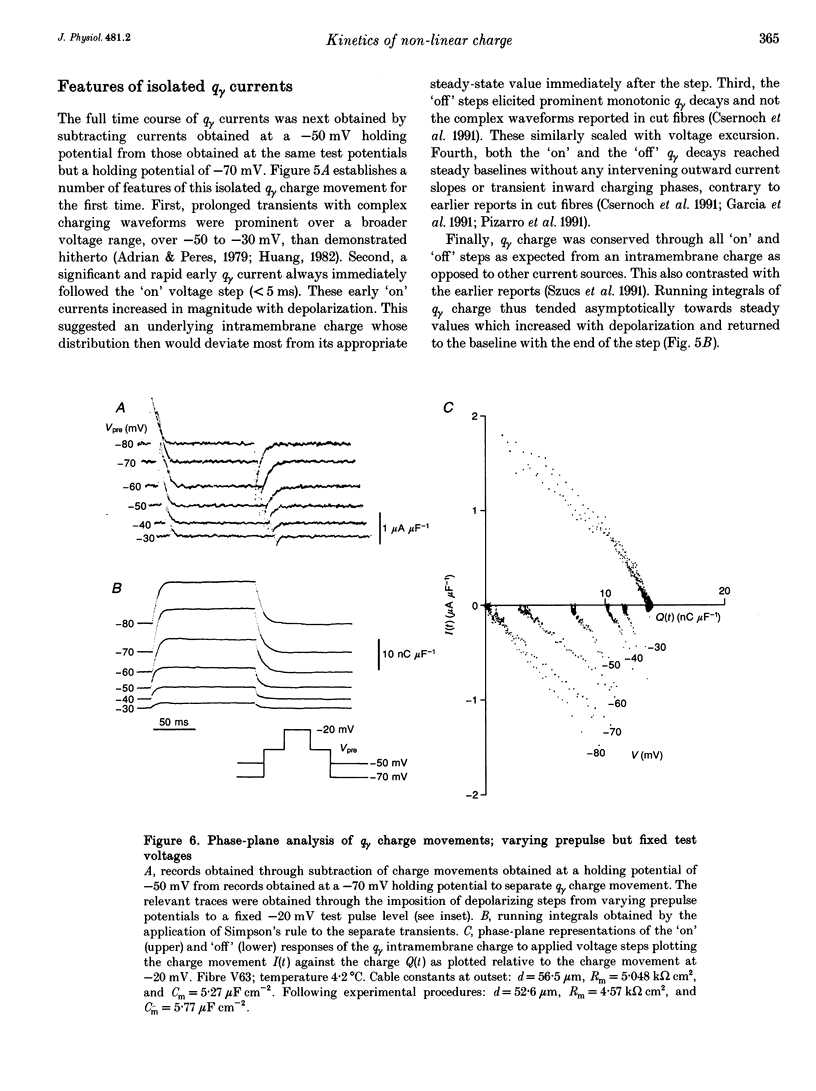
1. Procedures for a complete charge movement separation employed a combination of its steady-state inactivation and activation properties in intact frog skeletal muscle fibres in gluconate-containing solutions. 2. Holding potential shifts from -70 to -50 mV reduced the total charge available between -90 and -20 mV from 16.76 +/- 1.70 nC microF-1 (mean +/- S.E.M.; n = 4 fibres) to 9.25 +/- 1.43 nC microF-1 without significant loss of tetracaine-resistant charge (q beta). 3. The steady-state and kinetic properties of tetracaine-sensitive charge (q gamma) persisted through holding potential changes from -90 to -70 mV in the presence of gluconate and generally resembled activation properties established hitherto in sulphate-containing solutions. 4. Further holding potential displacement to -50 mV abolished q gamma charge movements and depressed the charge-voltage curve. 5. Test voltage steps applied from a -70 mV prepulse level gave rapid monotonic q beta decays and similarly depressed activation functions in 2 mM tetracaine unchanged by holding potential shifts between -70 and -50 mV. 6. The isolated 'on' q gamma charge movements, I(t), always included early transients that preceded any prolonged charging phases and which increased with depolarization. They decayed to stable baselines in the absence of prolonged time-dependent or inward-current phases and yielded integrals, Q(t), that monotonically increased with test voltage. 7. 'Off' steps always elicited rapid monotonic q gamma decays that fully returned the 'on' charge. 8. 'On' and 'off' q gamma currents, I(t), following voltage steps from fixed conditioning to varying test levels mapped onto topologically distinct higher-order phase-plane trajectories, I(Q), that steeply varied with test voltage. 9. In contrast, voltage steps to fixed test potentials of either -70 or -20 mV elicited identical q gamma phase-plane trajectories independent of prepulse history. 10. The q gamma current thus reflects an independent, capacitative process driven uniquely by higher-order dependences upon charge distribution, Q(t), and test voltage, V(t), autonomous of prepulse history or time, t.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. Charge movement in the membrane of striated muscle. J Physiol. 1976 Jan;254(2):339–360. doi: 10.1113/jphysiol.1976.sp011235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Almers W. Membrane capacity measurements on frog skeletal muscle in media of low ion content. J Physiol. 1974 Mar;237(3):573–605. doi: 10.1113/jphysiol.1974.sp010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H. Charge movement in the membrane of striated muscle. Annu Rev Biophys Bioeng. 1978;7:85–112. doi: 10.1146/annurev.bb.07.060178.000505. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Huang C. L. Charge movements near the mechanical threshold in skeletal muscle of Rana temporaria. J Physiol. 1984 Apr;349:483–500. doi: 10.1113/jphysiol.1984.sp015169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peres A. Charge movement and membrane capacity in frog muscle. J Physiol. 1979 Apr;289:83–97. doi: 10.1113/jphysiol.1979.sp012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Rios E. Intramembrane charge movement in frog skeletal muscle fibres. Properties of charge 2. J Physiol. 1987 Jun;387:489–517. doi: 10.1113/jphysiol.1987.sp016586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hui C. S. Membrane capacitance in frog cut twitch fibers mounted in a double vaseline-gap chamber. J Gen Physiol. 1990 Aug;96(2):225–256. doi: 10.1085/jgp.96.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L., Pizarro G., Uribe I., Rodríguez M., Ríos E. Interfering with calcium release suppresses I gamma, the "hump" component of intramembranous charge movement in skeletal muscle. J Gen Physiol. 1991 May;97(5):845–884. doi: 10.1085/jgp.97.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García J., Pizarro G., Ríos E., Stefani E. Effect of the calcium buffer EGTA on the "hump" component of charge movement in skeletal muscle. J Gen Physiol. 1991 May;97(5):885–896. doi: 10.1085/jgp.97.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Analysis of 'off' tails of intramembrane charge movements in skeletal muscle of Rana temporaria. J Physiol. 1984 Nov;356:375–390. doi: 10.1113/jphysiol.1984.sp015471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Charge conservation in intact frog skeletal muscle fibres in gluconate-containing solutions. J Physiol. 1994 Jan 1;474(1):161–171. doi: 10.1113/jphysiol.1994.sp020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Charge inactivation in the membrane of intact frog striated muscle fibers. J Physiol. 1993 Aug;468:107–124. doi: 10.1113/jphysiol.1993.sp019762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Dielectric components of charge movements in skeletal muscle. J Physiol. 1981;313:187–205. doi: 10.1113/jphysiol.1981.sp013658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L., Peachey L. D. Anatomical distribution of voltage-dependent membrane capacitance in frog skeletal muscle fibers. J Gen Physiol. 1989 Mar;93(3):565–584. doi: 10.1085/jgp.93.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Pharmacological separation of charge movement components in frog skeletal muscle. J Physiol. 1982 Mar;324:375–387. doi: 10.1113/jphysiol.1982.sp014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Separation of intramembrane charging components in low-calcium solutions in frog skeletal muscle. J Gen Physiol. 1991 Aug;98(2):249–263. doi: 10.1085/jgp.98.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Time domain spectroscopy of the membrane capacitance in frog skeletal muscle. J Physiol. 1983 Aug;341:1–24. doi: 10.1113/jphysiol.1983.sp014789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Voltage-dependent block of charge movement components by nifedipine in frog skeletal muscle. J Gen Physiol. 1990 Sep;96(3):535–557. doi: 10.1085/jgp.96.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S., Chandler W. K. Intramembranous charge movement in frog cut twitch fibers mounted in a double vaseline-gap chamber. J Gen Physiol. 1990 Aug;96(2):257–297. doi: 10.1085/jgp.96.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S., Chandler W. K. Q beta and Q gamma components of intramembranous charge movement in frog cut twitch fibers. J Gen Physiol. 1991 Sep;98(3):429–464. doi: 10.1085/jgp.98.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S., Chen W. Separation of Q beta and Q gamma charge components in frog cut twitch fibers with tetracaine. Critical comparison with other methods. J Gen Physiol. 1992 Jun;99(6):985–1016. doi: 10.1085/jgp.99.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S. Differential properties of two charge components in frog skeletal muscle. J Physiol. 1983 Apr;337:531–552. doi: 10.1113/jphysiol.1983.sp014640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S. Factors affecting the appearance of the hump charge movement component in frog cut twitch fibers. J Gen Physiol. 1991 Aug;98(2):315–347. doi: 10.1085/jgp.98.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Intrinsic optical and passive electrical properties of cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):1–40. doi: 10.1085/jgp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Simultaneous monitoring of changes in magnesium and calcium concentrations in frog cut twitch fibers containing antipyrylazo III. J Gen Physiol. 1989 Apr;93(4):585–608. doi: 10.1085/jgp.93.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Melzer W., Schneider M. F., Simon B. J., Szucs G. Intramembrane charge movement and calcium release in frog skeletal muscle. J Physiol. 1986 Apr;373:481–511. doi: 10.1113/jphysiol.1986.sp016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro G., Csernoch L., Uribe I., Rodríguez M., Ríos E. The relationship between Q gamma and Ca release from the sarcoplasmic reticulum in skeletal muscle. J Gen Physiol. 1991 May;97(5):913–947. doi: 10.1085/jgp.97.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Karhanek M., Ma J., González A. An allosteric model of the molecular interactions of excitation-contraction coupling in skeletal muscle. J Gen Physiol. 1993 Sep;102(3):449–481. doi: 10.1085/jgp.102.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szücs G., Csernoch L., Magyar J., Kovács L. Contraction threshold and the "hump" component of charge movement in frog skeletal muscle. J Gen Physiol. 1991 May;97(5):897–911. doi: 10.1085/jgp.97.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]


