Abstract
Background
Sleep and immune function are interconnected aspects of health that mutually impact each other in disease development and inflammatory homeostasis. Different aspects of immunology are regulated by different sleep characteristics, impacting on specific aspects of immune function including cytokine production and T-cell activity. Ongoing disruptions of sleep have been linked to heightened inflammation and are suspected in the pathogenesis and disease course of a range of life-style-related illnesses, including diabetes and neurodegenerative diseases.
Summary
This review provides a comprehensive overview of knowledge on the interaction of sleep with the immune system, its modulation of inflammatory balance, and the pathogenesis of many diseases. It emphasizes how sleep deficiency compromises immune function by means of a systemic, low-grade inflammatory response, while adequate sleep promotes intense immune responses and thus enables efficient pathogen clearance and the maintenance of immune memory. The mutual influence of sleep on the immune system underlines its critical involvement in health preservation and the course of disease.
Key Message
Sleep plays an indispensable role in immune health, mediating the efficiency of immune responses and the course of the regulation of inflammation. Chronic sleep deprivation can result in a low-grade inflammation that substantially contributes to the onset and exacerbation of metabolic and neurodegenerative disorders. The intimate linkage between sleep and immune function can be one strategic approach to therapy, improving health outcomes by leveraging this sleep-immune connection.
Keywords: Cytokines, inflammation, sleep-wake cycle, immune system, antigenic challengers
Introduction
The interactions of our sleep and the immune system can be observed in everyday life. The amount of sleep increases to improve the recovery rate whenever an individual is infected or catches up viral. Good sleep is significant in boosting the strength and immunity of an individual.
The daily bodily needs are modulated with the help of time perception, an essential aspect of the body mechanisms to work. To maintain these body requirements, various cycles oscillate hormones as in the neuroendocrine system, temperature, and sleep-wakefulness as observed in the circadian rhythm. These biological cycles, when disrupted, can lead to health problems. When the sleep-wakefulness cycle is disrupted, it can be a potential risk factor for metabolic diseases like diabetes, cancer, and cardiovascular anomalies. 1 During sleep, it is seen that there is functional activation of particular brain regions, which is a result of the inert withdrawal of all afferent stimuli. Sleep is, therefore, not a simple process; it modulates memory consolidation, mood, concentration, and temperature, along with modulating the immunological response and immunity. Hence immunity is affected due to lack of sleep which leads to more probability of getting or acquiring a disease. Various field studies have observed changes in sleep patterns due to chronic immune system activation. 2
According to many studies, disturbed or improper sleep (short duration) increases the chances of viral infections (common cold). 3 Mental health and everyday behaviour are negatively altered due to lack of sleep. 4 Healing is known to be modulated by sleep. Scientific evidence from the last 1.5 decades of neuroimmunology research suggests that immunity and immunological defence are increased with the help of sleep. Many particular physio-anatomical conditions are the basis of such neuro-immunological defence.
These conditions include:
Blood pressure, blood flow, and lymph flow are affected indirectly, while hormones are affected directly when the autonomic nervous system (ANS) and the neuroendocrine system regulate the immune system. 5
Peptidergic, sympathetic and sensory nerve fibres innervate the thymus and bone marrow and mucosa-associated lymphatic tissue, lymph nodes, and spleen. 6
Chemokine and cytokines, hormones, neuromodulators, and neurotransmitters are the intercellular signals which are commonly shared by glial cells, immune cells, neurons, and receptors are expressed for such signals. 7
These intercellular signals have the ability to cross the blood-brain barrier (BBB) from either direction. 8
Brain parenchyma, nerve endings, and meningeal borders all over the body have traffic of immune cells. 9
This review discusses the involvement of several inflammatory mediators in natural, normal sleep in the presence of viral inflammatory conditions. Research focuses on both sudden and long-term immune responses to infections and inflammation. Recent studies have elucidated critical connections between sleep and immune system function. Research indicates that sleep enhances the efficiency of immune responses by promoting the production of cytokines and supporting T-cell activity.10, 11 Chronic sleep deprivation has been linked to increased inflammation and impaired immune responses, contributing to conditions such as diabetes and neurodegenerative diseases.11–13 Additionally, studies have highlighted the role of sleep in modulating the gut microbiome, which in turn influences immune homeostasis.14, 15 These findings underscore the bidirectional relationship between sleep and immune function, emphasising the importance of adequate sleep for maintaining health and preventing disease. With this review we aim to synthesise current knowledge on the interplay between sleep and immune function, highlighting recent advancements and identifying gaps in the literature. As there is a growing evidence of sleep’s crucial role in immune regulation and its impact on inflammatory diseases, a comprehensive review is essential. By consolidating these findings, we seek to provide a clearer understanding of the mechanisms involved and to underscore the importance of adequate sleep for maintaining immune health and preventing disease. This synthesis will aid researchers and clinicians in developing targeted interventions and inform future research directions.
Methodology
Search Strategy
We conducted a comprehensive literature search using databases such as PubMed, Google Scholar, and Scopus. Keywords included ‘sleep’, ‘immune system’, ‘cytokines’, ‘inflammation’ and ‘immune regulation’.
Inclusion Criteria
We included peer-reviewed articles published in the last 10 years that focused on the interactions between sleep and the immune system. Both human and animal studies were considered.
Analysis Methods
Selected studies were analysed for relevance, methodological rigour, and findings. Data were synthesised to highlight key mechanisms and the impact of sleep on immune responses and inflammatory homeostasis.
Stages of Sleep and Assessment Methods
Sleep comprises two phases in general, that is, rapid eye movement (REM) stage and non-REM (NREM) sleep. NREM further consists of three stages, the N1, N2 and N3, also called as slow wave sleep (SWS). Whereas REM sleep includes REM sleep amount, REM latency, REM density, and REM duration. REM sleep is characterised by the presence of REM and low muscle tone. 16 Total sleep time and sleep efficiency, waking up after sleep onset, and total sleep latency are measures of total sleep continuity. Imaging techniques like electroencephalography (EEG), electromyography (EMG), and electrooculography (EOG) are used to understand the substrates that make the sleep cycle complete. These methods allow the observer to note different stages of sleep leading to REM sleep. In addition, the multiple electrodes of the EEG or EMG will enable one to know the stage of sleep or wakefulness. EOG accesses the random eye movements and EMG the muscle tone. 17
Spectral analysis is the mathematical tool used to study the EEG waveforms to further understand sleep components. They are used to deconstruct complex EEG waveforms into their constituent frequency waveforms to study the graph. In sleep medicine and preclinical trials, an algorithm-based method called Fast Fourier transformation is usually employed. For select frequency bands, Fast Fourier transformation of the EEG yields units of power. Frequency bands that are reported in sleep studies include slow frequency observed as less than 5Hz known (delta frequency), faster bands, that is, theta 6–9 Hz or beta 12–14 Hz and spindle frequency activity. 17
The frequency of these bands is associated with NREM sleep (spindle frequency and delta frequency bands). REM sleep has theta frequency bands. From wakefulness to NREM sleep, the brain waveforms transition from higher to lower frequency waveforms, and the amplitude increases the delta waveform. In contrast to this, during the transition from NREM to REM sleep, the amplitude of the EEG waveform decreases, and the low frequency of the wave is exchanged with high-frequency theta waves and spindle activity. During REM sleep, the EMG drops to the lowest level, depicting low striated muscle activity levels and general activity absences. 17 The sensitivity of spectral analysis takes into account factors such as age, insomnia complaints, and medication, which can further delineate discrepancies between subjective and objective measures of sleep. 17
Borbély and Achermann 17 stated that the longer one stays awake, the larger the homeostatic drive to sleep, that is, the sleepier one becomes. When wakefulness is prolonged, humans and animals sleep longer during the recovery night yet do not fully recover all the lost sleep. Delta waves characterise the recovery night, deep sleep, and EEG waves are slow activity. Sleep loss during prolonged wakefulness may partly be recovered by sleeping for a longer duration with higher intensity, but certain population groups like adults and patients with depression find it hard to recover NREM sleep, whereas REM sleep is retrieved more than what was lost by sleeping for longer. 17
Immune System Cells in Host Defence
Our body protects itself due to the presence of a defence system present within called the immune system. It serves the function of detecting foreign particles and eliminating the threat in order to prevent any disease or infections in the body. Various molecules and cells are located in specific regions, such as white blood cells (WBCs) or macrophages which act as the barrier to any molecular invasion. The body’s outer surface comprises physical obstacles like mucosa or epithelium layer to prevent bacterial invasion. Macrophages break down the pathogens crossing the physical barrier layer through specific receptors. These pattern recognition receptors (PRPs) induce activating signals to inflammatory mediators to generate a signalling cascade. 2
WBCs also known as leukocytes are of different forms depending on their maturation site as in bone marrow for B cells and thymus for T cells, antigen specificity as in antigen-specific T and B cells or plasmacytoid dendritic cells (pDC) and natural killer cells (NK), cytokine profile like Th1, Th2, Th17 development as in adaptive or innate, the primary site of action, function as in T helper (Th) cells, regulatory T cells, memory cells, cytotoxic T lymphocytes (CTLs), antigen-presenting cells (APCs), NK cells and haematopoietic lineage as in lymphoid or myeloid lineage (Figure 1). Cytokines and other molecules act as mediators between immune and non-immune cells. 2
Figure 1. Host Defence System.
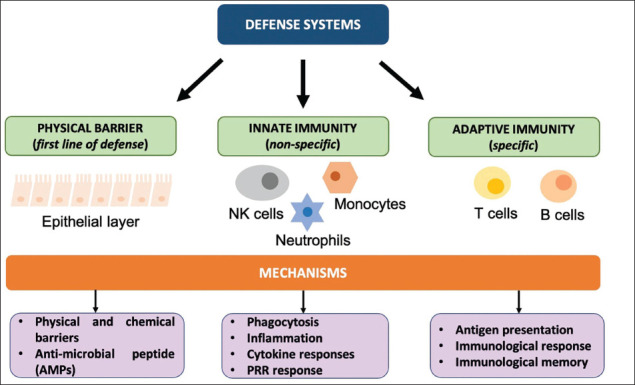
Notes: Types of defence systems found in immunological response. The first encounter is the physical layer covered in the form of a mucosal or epithelial layer throughout the gastrointestinal tract. The second line of defence is innate immunity which constitutes various glial cells in the CNS. Adaptive immunity is carried by T cell and B lymphocyte cells which are involved in antigen presentation of cells. Abbreviations: NK cells, natural killer cells; PRR, pattern recognition receptors.
An abnormal state of the body due to infectious agents causes central nervous system (CNS) responses such as fever 18 and other symptoms, including fatigue, sleepiness, social withdrawal, negative mood (depression, anxiety), pain hypersensitivity, and decreased appetite. 19 These symptoms are considered to aid in the recovery of the body from the abnormal state and can be considered adaptive responses carried out by the CNS. For example, fever promotes the body’s action against the infectious agent by trying to weaken it or eliminate it. It has been established that fever and sickness are responses to infection and inflammatory diseases which are marked and mediated by inflammatory mediators, including cytokines and prostaglandins (PGs) that signal to the brain.19, 20 On the other hand, it has been observed that ageing and physiological conditions negatively affect neurological and immunological health.21–25 In this article, the importance of these mediators will be reviewed in the context of spontaneous, physiological sleep in the case of infectious inflammatory changes. We studied acute changes and also chronic activation for infectious and inflammatory changes.
Immunological Response and Memory Consolidation in Effect
All the altered self-antigens and the foreign antigens represented by cells or molecules are first detected and then eliminated by all the immune defence cells.26, 27 When a foreign antigen invades, adaptive immune system is activated. APCs like dendritic cells (DC) B cells, and macrophages bind to the foreign invading antigen. APCs present the foreign antigen to the T cell receptor and an immunological synapse is formed with the Th cells after APCs are migrated via afferent lymphatics to draining lymph nodes. 28 Because of antigen presentation, Th cell’s progeny is produced as Th cells activate, then proliferate, and finally differentiate into two subcategories Th1 or Th2 cells. This helps in the antibody production (from the B cells) which supports the CTL and macrophages after leaving the lymph nodes, which then eliminates the foreign antigen/pathogen. Immunological memory is formed when B cells, Th cells, and CTL which are antigen-specific survive. This immunological memory helps in a faster immune system response when the same foreign particle invades. Major histocompatibility complexes keep control of the immune system activation, and this is done by counter-regulatory and co-stimulatory signals as in the anti-inflammatory and pro-inflammatory signalling. When such signalling is altered, it results in many different conditions like allergies, immunodeficiencies, autoimmunity, and septic shock. In the case of memory, both the CNS and the immune system share one aspect which is memory. During this memory consolidation in both systems synapses are formed and include cell-to-cell contacts. 29 The synapse which is involved in the immune system is called the immunological synapse, and it is formed between T cells and APCs.28, 30 There are different stages of immunological memory, including the encoding phase, as in the antigen presentation, the consolidation phase, and the recall phase, which helps mediate the immune response when the antigen is re-encountered. These two systems are different, but the formation of neurobiological memory is regulated by sleep. 31 This suggests that immune response is also regulated with the help of sleep and can be inferred as immunological memory formation is regulated and enhanced with sleep.
Immune System and Sleep-wake Cycle
The bodily functions and behaviour are regulated with the help of circadian rhythm which is controlled by the suprachiasmatic nuclei (SCN) situated in the hypothalamus and is also known as the ‘hypothalamic pacemaker’. Circadian rhythm determines the sleep-wake cycle regulated by different clock genes controlling sleep and circadian rhythm. Cytokine production and leukocyte proliferation are the immune functions that are also regulated with the help of the sleep-wake cycle besides the cardiovascular function, mental or physical activity, and temperature regulation which coincide.
Sleep deprivation directly influences inflammatory markers and cytokine levels, primarily affecting cortisol production. SCN controls the behaviour of cortisol secretion on a daily basis which can be altered due to complications in circadian rhythm via the hypothalamic pituitary adrenal (HPA) axis and sleep-wake cycle. Changes in both pro-inflammatory proteins (TNF- α) and anti-inflammatory proteins were also recorded. 32 Tumour necrosis factor (TNF)-α and interleukin (IL) are key factors to induce alterations in NREM sleep period and sleep-wake cycle (Figure 2). Studies have concluded that anti-TNF-based therapy has shown positive results in terms of REM sleep enhancement with increased higher efficiency of sleep. 2
Figure 2. Associated Pathways with the Sleep-wake Cycle.
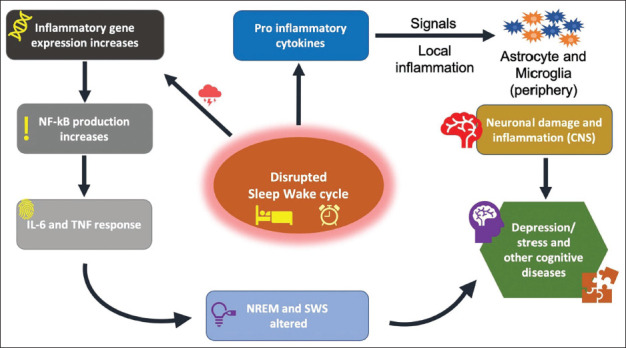
Notes: An inflammatory response signal is generated due to a disturbed sleep cycle for a varied time period. This follows into two pathways; first, gene expression activity increases, resulting in TNF – α and IL response. NREM sleep patterns are altered due to this response. Second, pro-inflammatory cytokine induced inflammatory response in glial cells alongside neuron damage in CNS, contributing in diseases.
Abbreviations: TNF-α, tumour necrosis factor-alpha(α); IL, interleukin; NREM, non-rapid eye movement; SWS, slow wave sleep; CNS, central nervous system.
A Pro-inflammatory State is seen During the Resting Period
It is well reported that in humans during normal sleep there are decreased levels of norepinephrine, cortisol, and epinephrine in the blood, increased levels of melatonin, prolactin, GH (growth hormone) released from the pituitary, and along with this SNS (sympathetic nervous system) and HPA axis are also down-regulated.33–35 The adipocytes inhibit hunger with enhanced secretion of leptin during sleep. 36 All these factors interdependently act on sleep and the immune system. The pro-inflammatory cytokines are produced when the immune cells activate, proliferate, and differentiate in the presence of pro-inflammatory signals. 37 These pro-inflammatory cytokines include tumour necrosis factor (TNF)-α, interleukin (IL)-12, IL-1, and interferon (IFN)-γ.38–42 Anti-inflammatory signals such as catecholamines and cortisol inhibit immune function.43, 44 Hence there, it was inferred that during sleep (nocturnal) inflammatory peak is reached, which is regulated by close coordination of the immune system and the endocrine system suggesting that the pro-inflammatory signals increase, and along with this, during wakefulness, anti-inflammatory signalling activity is enhanced.45, 46
During the SWS phase, it is seen that cytokines, as well as pro-inflammatory signals, are enhanced on the level of protein and mRNA in plasma,47–50 peripheral blood cells,51–57 splenic macrophages,58, 59 lymph nodes, 40 brain 49 and adipose tissue. 49 The pro-inflammatory signals help in regulating the adaptive immune responses as they act to eliminate the endogenous danger signals like metabolism, cell injury, physical activity, synaptic transmission, ATP (nucleotides), ROS (reactive oxygen species), and HSP (heat shock proteins) and the exogenous danger signals like LPS (lipopolysaccharide), toll-like receptor ligands, which produced pro-inflammatory signals with the help of APCs.38–42,60
In vitro, the production of pro-inflammatory cytokines is regulated by their cellular clocks (intrinsic) which include peripheral Th cells splenic macrophages and peritoneal macrophages.59, 60
Immune activation is enhanced with the help of intrinsic clock gene activity along with the exogenous danger signal and the endogenous danger signal. Besides this, the neuroendocrine system enhances immune activation during the early SWS period, which decreases the release of anti-inflammatory hormones and promotes the release of pro-inflammatory hormones. At the time of vaccination, this function of sleep as in the pro-inflammatory function of sleep, helps in achieving adaptive immunity. 59
The Peak of Immune Cell Differentiation During the Sleep Phase in Lymph Nodes and Peripheral Blood
New immune cells are continuously released in the blood circulation from the bone marrow, conducting the migration of immune cells. Neutrophils, macrophages, and DC extravasate to the periphery, whereas B cells and T cells are readily present as they circulate in the blood and start acting the immune response when any antigen is encountered in the lymphatic tissue (secondary lymphatic tissue). To reach the lymph nodes and later return to circulation through the thoracic duct and efferent lymphatics, CD62L-expressing T cells exit the blood with the help of HEVs (high endothelial venules) in humans. 61 Circadian rhythms in these cells are visible in peripheral blood and during the early rest period of sleep, and equilibrium is maintained between the lymphatic system and the blood as these immune cells show a peak, and this rhythm is seen in Th cells and T cells in the lymph nodes.62, 63 Along with these, studies have shown that sleep seems to be related to lymphocyte accumulation/collection in lymph nodes.64, 65 Rhythms of cortisol hormone in blood also affect the rhythms of T cells inversely as the peak of cortisol in blood inhibits the elevation of T cells rhythm, and with the help of increased expression of CXCR4, this cortisol-mediated redirection of T cells is mediated. 63 CXCL12 is the ligand expressed by the bone marrow which is produced throughout the active period of sleep and reaches its peak. The SNS and the clock genes regulate these rhythms. 66 Haematopoietic stem cells also express CXCR4, which the excessively into the peripheral bloodstream and includes memory T cells and naive T cells. 67 T cells expressing CXCR4 are known as the CXCR4+ T cells and are released from the bone marrow during the resting period of sleep while the HPA axis and cortisol are inhibited. These CXCR4+ T cells have the purpose of getting distributed to other areas where immune action is needed. As T cells extravasation is modulated with the help of low levels of cortisol and the extravasated to the lymph nodes from the blood. It is seen that migration of T lymphocytes from the HEV is obstructed due to glucocorticoids. 68 The lymph node rhythm of immune cells is regulated with different species-specific regulators in various animals, as seen in many animal studies. 69 Throughout early sleep, it is seen that lymph nodes have accumulated lymphocytes due to inhibited activity of SNS and enhanced GH levels.70–72
All the studies inferred that APCs, pro-inflammatory signals, central memory T cells, and naive T cells in lymph nodes are activated and proliferated during the resting period of sleep. It is still unclear that the immune rhythms are time-regulated. Pain, fatigue, immobility, malaise, mental illness, and cognitive impairment are common symptoms of inflammation during the awake stage. 73 The neuroendocrine modulations while sleeping regulate the insulin-dependent tissues, allowing the distribution of energy consumed by the proliferation of cells, resulting in immune activation.34, 47 The oxidative stress and cell damage caused by inflammation may be successfully controlled by melatonin, which is regulated by the haematopoietic stem cells and the free radicals that eventually provide cellular energy.
The association of pro-inflammatory cytokines and initiation of adaptive immunity are regulated with the reasons listed above, supported by the circadian rhythm’s particular time.
Sleep is Regulated by Inflammatory Mediators which Include
PGs: they have been shown to have sleep-regulatory effects. PGs are lipid mediators which are made from omega-6 fatty arachidonic acid, enzymes cyclooxygenase COX-1 and COX-2 and specific synthesis act on mega six fatty acid arachidonic acid de novo Cardinal symptoms of inflammation such as fever and pain are regulated by PG. Non-steroidal anti-inflammatory drugs (NSAIDs) like, ibuprofen act against the synthesis of PGs by inhibiting COX enzymes, 74 thus showing the involvement of PGs in cardinal information symptoms. According to the literature75, 76 the dysregulation of PG contributes to chronic low-grade inflammation or unresolved inflammation, thereby providing evidence of its role in inflammation regulation. Research by Hagaishi and colleagues in identifying PG2 elucidated that the most abundant prostanoid in rodents is a potent sleep-inducing substance.77, 78 Injecting PGD2 in the intra cerebro-ventricular space (subarachnoid space) in rats significantly increases NREM sleep.79, 80 Upon inhibition of PG production by COX-2, the painters reduced TNF-induced and spontaneous NREM keep an animal model81, 82; it was further observed that moderate sleep deprivation induces an increase in PGD-2, E-2, and F2A in rats. 82 This can help the hypothesis that sleep may target Cox enzymes as pressure for all functional PGs, rather than specific ones. In humans, that assessment in the blood is complex due to PG’s short half-life and rapid metabolism. 83 However, their precursors or metabolic products are relatively stable and thus are used as a viable method of PG production estimation. In healthy participants, lipocalin-type PG synthase, which catalyses the conversion from PGH 2 to PGD 2, has been reported to vary throughout the day, with the highest levels at night and the lowest in the afternoon. The increase in daytime levels and reduction in nighttime levels due to total sleep deprivation 84 suggest a role of PGD 2 in human sleep physiology. With the use of a model sleep restriction of 10 days produces results in the increase of PGD 2 and PG’s 2%~20%, which is statically insignificant, while a total sleep deprivation of 3 days results in a 30% increase of PG’s 2(85). A few articles also discuss the role of NSAIDs and the inhibition of PG production. Proper aspirin administration at the recommended daily dose in healthy participants causes disruption of sleep (decreased sleep efficiency, quality, and intensity).86, 87 Acute administration of ibuprofen led to delayed SWS 86 and sleep-disrupting effects. These mechanisms provide evidence for the involvement of PGs in sleep Physiology, but the mechanism of action and PG involvement remains unexplored. Since the effects of chronic administration of NSAIDs are unknown future studies may address the long-term effects on sleep physiology because most of the population consumes them regularly. Furthermore, the PG system has a role in promoting and regulating inflammation. 75 Blocking the PG system by NSAID may contribute to unresolved inflammation in response to sleep deprivation as such further research is needed to elucidate the importance of PG system mechanisms and align the association between sleep deprivation and inflammation in humans (low-grade).
Cytokines: The existence of a substance that generally induces sleep was first discussed by Ishimori in Japan and Piéron in France in the early 1900s. The first shreds of evidence were obtained by inducing sleep in well-rested dogs by injecting them with the CSF of sleep-deprived dogs. The follow-up experiments using EEG quantified the theory of sleep in various animals. 88 At the same time, researchers were trying to find the responsible material for inducing sleep, in the brain and other body fluids. Pappenheimer 1975 was able to isolate the substance for the CSF and the neural tissue of the sleep-deprived animals and named the substance ‘factor S’ (S stands for sleep-promoting). 89 Thereafter muramyl peptide was identified as factor S [84], which is produced during phagocytosis of bacteria by macrophages. 90 It was known that muramyl peptides function in cytokine production in macrophages. The role of cytokines (in particular IL-1 and TNF) in maintaining homeostatic NREM sleep in animals has been studied intensively. For a substance to come under the category of sleep-regulating substance, its administration should induce sleep or increase sleep time. In contrast, its antagonists or biological reduction of the production of the said substance should cause a decrease in sleep amount and the diurnal variations should match the sleep-wake behaviour. 91 To check the properties of the cytokines, their antagonists [e.g., IL-1 receptor antagonist (IL-1ra) or anti-IL-1 antibody] are implied to inhibit their biological action. Findings suggest that the use of antagonists to limit the action of cytokines IL-1 and TNF resulted in the reduction of the NREM sleep or NREM sleep rebound after sleep deprivation. Conversely, increasing the availability of the cytokines promotes NREM sleep, both amount and intensity and represses REM sleep. This evidence suggests that cytokines are substances essential for the regulation of sleep. In regards to this, we cannot surely ignore the fact that the amount of REM sleep (here decreased) and the increased and intensified non-REM sleep depends on several factors such as the time of day, dosage, and routine (e.g., in rats NREM sleep increasing effects if IL-1 occurs only in a small dose window).92–95
Discussion
The complex interaction between sleep and the immune system has a major impact on both the aetiology of disease and general health. The current review synthesises recent knowledge on how sleep modulates immune functions, emphasising the roles of cytokines, hormones, and neurotransmitters in maintaining inflammatory homeostasis. By integrating the findings on common mechanisms, our review highlights the complex yet essential role of sleep in immune regulation and its broader implications for health and disease management. This comprehensive synthesis underscores the critical need for further research to explore therapeutic interventions that leverage the sleep-immune connection to improve health outcomes.
We discussed how different cytokines play a crucial role in the bidirectional relationship between sleep and the immune system. Pro-inflammatory cytokines such as IL-1β and TNF-α promote sleep, particularly NREM sleep, by acting on specific brain regions like the hypothalamus. These cytokines are typically elevated during infections and inflammatory responses, leading to increased sleepiness, which is thought to facilitate recovery. Conversely, chronic sleep deprivation has been shown to increase the production of these cytokines, contributing to a state of low-grade inflammation that can exacerbate various health conditions, including metabolic and cardiovascular diseases.
Importantly, hormones such as cortisol and melatonin are integral to the regulation of sleep and immune functions. Cortisol, a glucocorticoid hormone released by the adrenal cortex, follows a circadian rhythm, peaking in the early morning and decreasing throughout the day. It has immunosuppressive effects, reducing inflammation and modulating immune responses. Prolonged sleep deprivation disrupts cortisol rhythms, potentially leading to immune dysregulation and increased susceptibility to infections. Melatonin, produced by the pineal gland during darkness, supports immune function by enhancing the activity of NKs and T lymphocytes. It also possesses antioxidant properties that protect immune cells from oxidative stress.
Nevertheless, neurotransmitters, like serotonin and gamma-aminobutyric acid (GABA), are pivotal in the sleep-immune interaction. Serotonin, primarily known for its role in mood regulation, also influences immune function by modulating the activity of various immune cells, such as T cells and macrophages. It helps regulate sleep by converting to melatonin in the pineal gland. GABA, the primary inhibitory neurotransmitter in the CNS, promotes sleep by reducing neuronal excitability. It also affects immune function by inhibiting the release of pro-inflammatory cytokines. Alterations in GABAergic activity due to sleep disturbances can therefore impact both sleep quality and immune responses.
As thoroughly discussed in the article, the interplay between sleep and the immune system is inherently bidirectional. Immune activation, through cytokine release, can alter sleep patterns, increasing the need for restorative sleep during illness. Conversely, adequate sleep enhances immune surveillance and response, facilitating efficient pathogen clearance and immune memory consolidation. This dynamic relationship underscores the importance of maintaining healthy sleep patterns for optimal immune function. The common mechanisms discussed have significant implications for understanding the pathogenesis of various diseases. For instance, chronic sleep deprivation-induced inflammation is linked to the development and progression of metabolic disorders, cardiovascular diseases, and neurodegenerative conditions. Understanding these mechanisms provides insights into potential therapeutic targets, such as cytokine modulation, hormone regulation, and neurotransmitter balance, to mitigate the adverse effects of sleep disturbances on health.
Strengths and Weaknesses of this Review
One of the key strengths of our review is the comprehensive synthesis of recent findings on the interplay between sleep and the immune system. By integrating a wide range of studies, we provide a detailed overview of the roles of cytokines, hormones, and neurotransmitters in this interaction. Our systematic approach to literature selection ensures that the most relevant and up-to-date research is included, enhancing the robustness of our conclusions.
However, there are also some limitations. Despite our efforts to be thorough, some relevant studies may have been inadvertently excluded due to search strategy constraints or publication bias. Additionally, the inherent variability in study designs, populations, and methodologies among the reviewed studies presents challenges in drawing definitive conclusions. Potential biases in the selected studies, such as small sample sizes or lack of longitudinal data, may also influence our findings. Future research with standardised methodologies and larger, more diverse populations will be crucial to further validate and expand upon our conclusions.
Conclusion and Future Directions
In the immunological processes, maintaining sleep cycle patterns, circadian rhythm plays a major role. It also plays an essential role in determining the bidirectional communication established between the immune system and the CNS via shared signals (such as neurotransmitters, hormones, and cytokines) and by the ANS in the form of direct interventions. Many immune functions work in tandem with the 24-hour circadian rhythm and sleep, thus showing a relation between the sleep and sleep-wake cycle with the immune system. The immediate effector cells of the immune system, such as cytotoxic NK cells and terminally differentiated CTL are most active during the awake period of circadian rhythm to allow an immediate response to any pathogen which can be virulent or any other form of antigen. This also allows for faster repair of the damaged tissue due to the activity during the wake cycle. Adaptive immunity is generally more active during the night as the undifferentiated or less differentiated cells like naïve, and memory T cells peak during the night. During the nighttime sleep and especially the SWS phase of sleep, the anti-inflammatory action of cortisol and catecholamines are at the lowest level. In contrast, nighttime sleep promotes the release of GH and prolactin. The endocrine milieu during early sleep critically supports the enhanced production of IL-12 and increases the interaction between APC and T cells as shown by the evidence. The cytokine balance shifts towards Th1 Cytokines. Th1 cell proliferation is increased. There’s a possibility that it might also facilitate the migration of naive T cells to lymph nodes. The endocrine milieu thereby supports longer-lasting immune memory responses during early sleep stages (because Th1 endorses the formation of long immunological memories). Chronic lack of sleep leads to stress and causes the release of persistent unspecific pro-inflammatory substances, which can best be described as chronic low-grade inflammation and produces immunodeficiency, which is detrimental to health.
Although the exact relationship between the activation of adaptive and innate immunity with the circadian rhythm is unknown, we know that innate immunity is active during the day and adaptive immunity is active during the night. The exact mechanism of interaction with the immune system, endocrine system, and CNS has to be uncovered so that we can understand and develop medicines that act with best efficiency according to the circadian rhythm.
This review uniquely consolidates recent findings on the intricate crosstalk between sleep and the immune system, providing a holistic overview of how sleep influences immune function and inflammatory homeostasis. Our synthesis highlights novel insights into the role of sleep in modulating specific cytokines, immune cell activity, and the gut microbiome. Additionally, we explore the bidirectional nature of sleep and immune interactions, emphasising the implications for lifestyle-related diseases. By integrating these diverse aspects, our review offers new perspectives on potential therapeutic approaches and underscores the critical importance of sleep in maintaining immune health and preventing chronic diseases.
Abbreviations
APCs, antigen-presenting cells; ANS, autonomic nervous system; REM, rapid eye movement; NREM, non-rapid eye movement; SWS, slow wave sleep; EEG, electroencephalography; EMG, electromyography; EOG, electrooculography; WBCs, white blood cells; PRPs, pattern recognition receptors; pDC, plasmacytoid dendritic cells; NK, natural killer cells; CTLs, cytotoxic T lymphocytes; CNS, central nervous system; PGs, prostaglandins; DC, dendritic cells; SCN, suprachiasmatic nuclei; HPA, hypothalamic-pituitary-adrenal; TNF, tumor necrosis factor; SNS, sympathetic nervous system; IL, interleukin; GH, growth hormone; ROS, reactive oxygen species; LPS, lipopolysaccharide; HEVs, high endothelial venules; NSAIDs, non-steroidal anti-inflammatory drugs.
Acknowledgement
JKS and SG acknowledge the research support from International Brain Research Organisation (IBRO).
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Krishna Kumar Singh  https://orcid.org/0000-0003-3849-5945
https://orcid.org/0000-0003-3849-5945
Prashant Verma  https://orcid.org/0000-0001-5948-6170
https://orcid.org/0000-0001-5948-6170
Authors Contributions
The conceptualization was done by KKS, SG, AB and JKS. The initial draft was written by AB, KKS and SG. The later version and diagrams were compiled and completed in its final form by all the authors.
ICMJE Statement
All authors have contributed significantly in manuscript preparation and submission.
Statement of Ethics
Not applicable.
References
- 1.Farhud D and Aryan Z.. Circadian rhythm, lifestyle and health: a narrative review. Iran J Public Health 2018; 47(8): 1068–1076. [PMC free article] [PubMed] [Google Scholar]
- 2.Besedovsky L, Lange T and Haack M.. The sleep-immune crosstalk in health and disease. Physiol Rev 2019; 99(3): 1325–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prather AA, Janicki-Deverts D, Hall MH, et al. Behaviorally assessed sleep and susceptibility to the common cold. Sleep 2015; 38(9): 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tempesta D, Socci V, De Gennaro L, et al. Sleep and emotional processing. Sleep Med Rev 2018; 40: 183–195. [DOI] [PubMed] [Google Scholar]
- 5.Maestroni GJ. Neural regulation of dendritic cell function. Adv Exp Med Biol 2001; 495: 111–119. [DOI] [PubMed] [Google Scholar]
- 6.Nance DM, Sanders VM.. Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav Immun 2007; 21(6): 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davanger S. Colocalization of amino acid signal molecules in neurons and endocrine cells. Anat Embryol (Berl) 1996; 194(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 8.Capuron L and Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 2011; 130(2): 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ron-Harel N, Cardon M and Schwartz M.. Brain homeostasis is maintained by “danger” signals stimulating a supportive immune response within the brain’s borders. Brain Behav Immun 2011; 25(5): 1036–1043. [DOI] [PubMed] [Google Scholar]
- 10.Sang D, Lin K, Yang Y, et al. Prolonged sleep deprivation induces a cytokine-storm-like syndrome in mammals. Cell 2023; 186(25): 5500–5516.e21. [DOI] [PubMed] [Google Scholar]
- 11.Ince LM, Barnoud C, Lutes LK, et al. Influence of circadian clocks on adaptive immunity and vaccination responses. Nat Commun 2023; 14(1): 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Z, Yang X and Huang S.. Sleep deprivation: a risk factor for the pathogenesis and progression of Alzheimer’s disease. Heliyon 2024; 10(7): e28819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh S, Vashisth K, Ghosh S, et al. From sleep to cancer to neurodegenerative disease: the crucial role of Hsp70 in maintaining cellular homeostasis and potential therapeutic implications. J Biomol Struct Dyn 2023; 1–12. [DOI] [PubMed] [Google Scholar]
- 14.Sharma SA, Oladejo SO and Kuang Z.. Chemical interplay between gut microbiota and epigenetics: implications in circadian biology. Cell Chem Biol 2024. S2451-9456(24)00178-8. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S, Dhungel S, Shaikh MF, et al. Editorial: world digestive health day: investigating the link between neurodegenerative disease and gut microbiota. Front Aging Neurosci 2023; 15: 1351855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha JK, Vashisth K and Ghosh S.. The importance of sleep studies in improving the health indices of a nation. Sleep Med X 2022; 4: 100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borbély AA and Achermann P.. Sleep homeostasis and models of sleep regulation. J Biol Rhythms 1999; 14(6): 557–568. [DOI] [PubMed] [Google Scholar]
- 18.Harden LM, Kent S, Pittman QJ, et al. Fever and sickness behavior: friend or foe. Brain Behav Immun 2015; 50: 322–333. [DOI] [PubMed] [Google Scholar]
- 19.Dantzer R and Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 2007; 21(2): 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saper CB, Romanovsky AA and Scammell TE.. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci 2012; 15(8): 1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha JK and Ghosh S.. Scoring more than ten plus century - antiquity in gerontology? Indian J Med Res 2010; 131: 586–587. [PubMed] [Google Scholar]
- 22.Mishra P, Mittal AK, Rajput SK, et al. Cognition and memory impairment attenuation via reduction of oxidative stress in acute and chronic mice models of epilepsy using antiepileptogenic Nux vomica. J Ethnopharmacol 2021; 267: 113509. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh S, Sinha JK, Khandelwal N, et al. Increased stress and altered expression of histone modifying enzymes in brain are associated with aberrant behaviour in vitamin B12 deficient female mice. Nutr Neurosci 2020; 23(9): 714–723. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh S, Sinha JK, Muralikrishna B, et al. Chronic transgenerational vitamin B12 deficiency of severe and moderate magnitudes modulates adiposity-probable underlying mechanisms. Biofactors 2017; 43(3): 400–414. [DOI] [PubMed] [Google Scholar]
- 25.Sinha JK, Trisal A, Ghosh S, et al. Psychedelics for Alzheimer’s disease-related dementia: unveiling therapeutic possibilities and pathways. Ageing Res Rev 2024; 96: 102211. [DOI] [PubMed] [Google Scholar]
- 26.Grossman Z. Recognition of self and regulation of specificity at the level of cell populations. Immunol Rev 1984; 79: 119–138. [DOI] [PubMed] [Google Scholar]
- 27.Gallucci S and Matzinger P.. Danger signals: SOS to the immune system. Curr Opin Immunol 2001; 13(1): 114–119. [DOI] [PubMed] [Google Scholar]
- 28.Grakoui A, Bromley SK, Sumen C, et al. The immunological synapse: a molecular machine controlling T cell activation. Science 1999; 285(5425): 221–227. [DOI] [PubMed] [Google Scholar]
- 29.Kioussis D and Pachnis V.. Immune and nervous systems: more than just a superficial similarity? Immunity 2009; 31(5): 705–710. [DOI] [PubMed] [Google Scholar]
- 30.Dustin ML and Colman DR.. Neural and immunological synaptic relations. Science 2002; 298(5594): 785–789. [DOI] [PubMed] [Google Scholar]
- 31.Diekelmann S and Born J.. The memory function of sleep. Nat Rev Neurosci 2010; 11(2): 114–126. [DOI] [PubMed] [Google Scholar]
- 32.Wright KP, Lowry CA and Lebourgeois MK.. Circadian and wakefulness-sleep modulation of cognition in humans. Front Mol Neurosci 2012; 5: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Born J and Fehm HL. Hypothalamus-pituitary-adrenal activity during human sleep: a coordinating role for the limbic hippocampal system. Exp Clin Endocrinol Diabetes 1998; 106(3): 153–163. [DOI] [PubMed] [Google Scholar]
- 34.Haus E. Chronobiology in the endocrine system. Adv Drug Deliv Rev 2007; 59(9–10): 985–1014. [DOI] [PubMed] [Google Scholar]
- 35.Reis ES, Lange T, Köhl G, et al. Sleep and circadian rhythm regulate circulating complement factors and immunoregulatory properties of C5a. Brain Behav Immun 2011; 25(7): 1416–1426. [DOI] [PubMed] [Google Scholar]
- 36.Simon C, Gronfier C, Schlienger JL, et al. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J Clin Endocrinol Metab 1998; 83(6): 1893–1899. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh S, Sinha JK and “Obesageing”, Raghunath M.: linking obesity & ageing. Indian J Med Res 2019; 149(5): 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev 2009; 13(4): 257–264. [DOI] [PubMed] [Google Scholar]
- 39.Drazen DL, Bilu D, Bilbo SD, et al. Melatonin enhancement of splenocyte proliferation is attenuated by luzindole, a melatonin receptor antagonist. Am J Physiol Regul Integr Comp Physiol . 2001; 280(5): R1476–R1482. [DOI] [PubMed] [Google Scholar]
- 40.Esquifino AI, Alvarez MP, Cano P, et al. 24-hour pattern of circulating prolactin and growth hormone levels and submaxillary lymph node immune responses in growing male rats subjected to social isolation. Endocrine 2004; 25(1): 41–48. [DOI] [PubMed] [Google Scholar]
- 41.Hattori N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm IGF Res 2009; 19(3): 187–197. [DOI] [PubMed] [Google Scholar]
- 42.Kelley KW, Weigent DA and Kooijman R.. Protein hormones and immunity. Brain Behav Immun 2007; 21(4): 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Besedovsky HO and del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev 1996; 17(1): 64–102. [DOI] [PubMed] [Google Scholar]
- 44.Elenkov IJ, Kvetnansky R, Hashiramoto A, et al. Low- versus high-baseline epinephrine output shapes opposite innate cytokine profiles: presence of Lewis- and Fischer-like neurohormonal immune phenotypes in humans. J Immunol 2008; 181(3): 1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lange T, Dimitrov S and Born J.. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci 2010; 1193: 48–59. [DOI] [PubMed] [Google Scholar]
- 46.Scheff JD, Calvano SE, Lowry SF, et al. Modeling the influence of circadian rhythms on the acute inflammatory response. J Theor Biol 2010; 264(3): 1068–1076. [DOI] [PubMed] [Google Scholar]
- 47.Straub RH and Cutolo M.. Circadian rhythms in rheumatoid arthritis: Implications for pathophysiology and therapeutic management. Arthritis Rheum 2007; 56(2): 399–408. [DOI] [PubMed] [Google Scholar]
- 48.Cano P, Cardinali DP, Ríos-Lugo MJ, et al. Effect of a high-fat diet on 24-hour pattern of circulating adipocytokines in rats. Obesity (Silver Spring) 2009; 17(10): 1866–1871. [DOI] [PubMed] [Google Scholar]
- 49.Guan Z, Vgontzas AN, Omori T, et al. Interleukin-6 levels fluctuate with the light-dark cycle in the brain and peripheral tissues in rats. Brain Behav Immun 2005; 19(6): 526–529. [DOI] [PubMed] [Google Scholar]
- 50.Luna-Moreno D, Aguilar-Roblero R and Díaz-Muñoz M.. Restricted feeding entrains rhythms of inflammation-related factors without promoting an acute-phase response. Chronobiol Int 2009; 26(7): 1409–1429. [DOI] [PubMed] [Google Scholar]
- 51.Bollinger T, Bollinger A, Skrum L, et al. Sleep-dependent activity of T cells and regulatory T cells. Clin Exp Immunol 2009; 155(2): 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bollinger T, Bollinger A, Naujoks J, et al. The influence of regulatory T cells and diurnal hormone rhythms on T helper cell activity. Immunology 2010; 131(4): 488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Born J, Lange T, Hansen K, et al. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol 1997; 158(9): 4454–4464. [PubMed] [Google Scholar]
- 54.Dimitrov S, Lange T, Tieken S, et al. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immun 2004; 18(4): 341–348. [DOI] [PubMed] [Google Scholar]
- 55.Dimitrov S, Lange T, Benedict C, et al. Sleep enhances IL-6 trans-signaling in humans. FASEB J 2006; 20(12): 2174–2176. [DOI] [PubMed] [Google Scholar]
- 56.Dimitrov S, Lange T, Nohroudi K, et al. Number and function of circulating human antigen presenting cells regulated by sleep. Sleep 2007; 30(4): 401–411. [DOI] [PubMed] [Google Scholar]
- 57.Lange T, Dimitrov S, Fehm HL, et al. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med 2006; 166(16): 1695–1700. [DOI] [PubMed] [Google Scholar]
- 58.Hayashi M, Shimba S and Tezuka M.. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull 2007; 30(4): 621–626. [DOI] [PubMed] [Google Scholar]
- 59.Keller M, Mazuch J, Abraham U, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A 2009; 106(50): 21407–21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh S, Sinha JK, Khan T, et al. Pharmacological and therapeutic approaches in the treatment of epilepsy. Biomedicines 2021; 9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westermann J and Pabst R.. Lymphocyte subsets in the blood: a diagnostic window on the lymphoid system? Immunol Today 1990; 11(11): 406–410. [DOI] [PubMed] [Google Scholar]
- 62.Bonacho MG, Cardinali DP, Castrillón P, et al. Aging-induced changes in 24-h rhythms of mitogenic responses, lymphocyte subset populations and neurotransmitter and amino acid content in rat submaxillary lymph nodes during Freund’s adjuvant arthritis. Exp Gerontol 2001; 36(2): 267–282. [DOI] [PubMed] [Google Scholar]
- 63.Dimitrov S, Benedict C, Heutling D, et al. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood 2009; 113(21): 5134–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dickstein JB, Hay JB, Lue FA, et al. The relationship of lymphocytes in blood and in lymph to sleep/wake states in sheep. Sleep 2000; 23(2): 185–190. [PubMed] [Google Scholar]
- 65.Zager A, Andersen ML, Ruiz FS, et al. Effects of acute and chronic sleep loss on immune modulation of rats. Am J Physiol Regul Integr Comp Physiol 2007; 293(1): R504–R509. [DOI] [PubMed] [Google Scholar]
- 66.Méndez-Ferrer S, Lucas D, Battista M, et al. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008; 452(7186): 442–447. [DOI] [PubMed] [Google Scholar]
- 67.Lucas D, Battista M, Shi PA, et al. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell 2008; 3(4): 364–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cox JH and Ford WL.. The migration of lymphocytes across specialized vascular endothelium. IV. Prednisolone acts at several points on the recirculation pathways of lymphocytes. Cell Immunol 1982; 66(2): 407–422. [DOI] [PubMed] [Google Scholar]
- 69.Griffin AC and Whitacre CC.. Sex and strain differences in the circadian rhythm fluctuation of endocrine and immune function in the rat: implications for rodent models of autoimmune disease. J Neuroimmunol 1991; 35(1–3): 53–64. [DOI] [PubMed] [Google Scholar]
- 70.Ottaway CA and Husband AJ.. Central nervous system influences on lymphocyte migration. Brain Behav Immun 1992; 6(2): 97–116. [DOI] [PubMed] [Google Scholar]
- 71.Smaniotto S, Ribeiro-Carvalho MM, Dardenne M, et al. Growth hormone stimulates the selective trafficking of thymic CD4+CD8- emigrants to peripheral lymphoid organs. Neuroimmunomodulation 2004; 11(5): 299–306. [DOI] [PubMed] [Google Scholar]
- 72.Taub DD, Tsarfaty G, Lloyd AR, et al. Growth hormone promotes human T cell adhesion and migration to both human and murine matrix proteins in vitro and directly promotes xenogeneic engraftment. J Clin Invest 1994; 94(1): 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petrovsky N. Towards a unified model of neuroendocrine-immune interaction. Immunol Cell Biol 2001; 79(4): 350–357. [DOI] [PubMed] [Google Scholar]
- 74.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971; 231(25): 232–235. [DOI] [PubMed] [Google Scholar]
- 75.Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J 2017; 31(4): 1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sugimoto MA, Sousa LP, Pinho V, et al. Resolution of inflammation: What controls its onset. Front Immunol 2016; 7: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang ZL, Urade Y and Hayaishi O.. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Curr Opin Pharmacol 2007; 7(1): 33–38. [DOI] [PubMed] [Google Scholar]
- 78.Urade Y and Hayaishi O.. Prostaglandin D2 and sleep/wake regulation. Sleep Med Rev 2011; 15(6): 411–418. [DOI] [PubMed] [Google Scholar]
- 79.Inoué S, Honda K, Komoda Y, et al. Differential sleep-promoting effects of five sleep substances nocturnally infused in unrestrained rats. Proc Natl Acad Sci U S A 1984; 81(19): 6240–6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsumura H, Nakajima T, Osaka T, et al. Prostaglandin D2-sensitive, sleep-promoting zone defined in the ventral surface of the rostral basal forebrain. Proc Natl Acad Sci U S A 1994; 91(25): 11998–12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Terao A, Matsumura H, Yoneda H, et al. Enhancement of slow-wave sleep by tumor necrosis factor-alpha is mediated by cyclooxygenase-2 in rats. Neuroreport 1998; 9(17): 3791–3796. [DOI] [PubMed] [Google Scholar]
- 82.Ram A, Pandey HP, Matsumura H, et al. CSF levels of prostaglandins, especially the level of prostaglandin D2, are correlated with increasing propensity towards sleep in rats. Brain Res 1997; 751(1): 81–89. [DOI] [PubMed] [Google Scholar]
- 83.Schuligoi R, Schmidt R, Geisslinger G, et al. PGD2 metabolism in plasma: kinetics and relationship with bioactivity on DP1 and CRTH2 receptors. Biochem Pharmacol 2007; 74(1): 107–117. [DOI] [PubMed] [Google Scholar]
- 84.Jordan W, Tumani H, Cohrs S, et al. Prostaglandin D synthase (beta-trace) in healthy human sleep. Sleep 2004; 27(5): 867–874. [DOI] [PubMed] [Google Scholar]
- 85.Haack M, Lee E, Cohen DA, et al. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain 2009; 145(1–2): 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murphy PJ, Badia P, Myers BL, et al. Nonsteroidal anti-inflammatory drugs affect normal sleep patterns in humans. Physiol Behav 1994; 55(6): 1063–1066. [DOI] [PubMed] [Google Scholar]
- 87.Horne JA, Percival JE and Traynor JR.. Aspirin and human sleep. Electroencephalogr Clin Neurophysiol 1980; 49(3–4): 409–413. [DOI] [PubMed] [Google Scholar]
- 88.Borbély AA and Tobler I.. Endogenous sleep-promoting substances and sleep regulation. Physiol Rev 1989; 69(2): 605–670. [DOI] [PubMed] [Google Scholar]
- 89.Pappenheimer JR, Koski G, Fencl V, et al. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol 1975; 38(6): 1299–1311. [DOI] [PubMed] [Google Scholar]
- 90.Johannsen L, Wecke J, Obál F, et al. Macrophages produce somnogenic and pyrogenic muramyl peptides during digestion of staphylococci. Am J Physiol 1991; 260(1 Pt 2): R126–R133. [DOI] [PubMed] [Google Scholar]
- 91.Clinton JM, Davis CJ, Zielinski MR, et al. Biochemical regulation of sleep and sleep biomarkers. J Clin Sleep Med 2011; 7(5 Suppl): S38–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krueger JM and Majde JA.. Cytokines and sleep. Int Arch Allergy Immunol 1995; 106(2): 97–100. [DOI] [PubMed] [Google Scholar]
- 93.Opp MR. Cytokines and sleep. Sleep Med Rev 2005; 9(5): 355–364. [DOI] [PubMed] [Google Scholar]
- 94.Ghosh S, Manchala S, Raghunath M, et al. Role of phytomolecules in the treatment of obesity: targets, mechanisms and limitations. Curr Top Med Chem 2021. b; 21(10): 863–877. [DOI] [PubMed] [Google Scholar]
- 95.Ghosh S, Sinha JK and Raghunath M.. Epigenomic maintenance through dietary intervention can facilitate DNA repair process to slow down the progress of premature aging. IUBMB Life 2016; 68(9): 717–721. [DOI] [PubMed] [Google Scholar]


