Abstract
The intricate and dynamic tryptophan (Trp) metabolic pathway in both the microbiome and host cells highlights its profound implications for health and disease. This pathway involves complex interactions between host cellular and bacteria processes, producing bioactive compounds such as 5-hydroxytryptamine (5-HT) and kynurenine derivatives. Immune responses to Trp metabolites through specific receptors have been explored, highlighting the role of the aryl hydrocarbon receptor in inflammation modulation. Dysregulation of this pathway is implicated in various diseases, such as Alzheimer’s and Parkinson’s diseases, mood disorders, neuronal diseases, autoimmune diseases such as multiple sclerosis (MS), and cancer. In this article, we describe the impact of the 5-HT, Trp, indole, and Trp metabolites on health and disease. Furthermore, we review the impact of microbiome-derived Trp metabolites that affect immune responses and contribute to maintaining homeostasis, especially in an experimental autoimmune encephalitis model of MS.
Keywords: brain disease, GPR35, immune cell, kynurenic acid
Tryptophan and its metabolites in immunity
Graphical Abstract
Graphical Abstract.
Introduction
The tryptophan (Trp) metabolic pathway within the microbiome and host cells constitutes a complex and dynamic system with profound implications for health and disease (1–6). This pathway involves an intricate interplay between host cellular and bacteria processes involving Trp, which is an essential amino acid. In health, the Trp metabolic pathway plays a pivotal role in maintaining homeostasis and supporting physiological functions. Interactions between the microbiome and host cells contribute to the production of bioactive compounds, including 5-hydroxytryptamine (5-HT) and kynurenine (Kyn) derivatives, exerting far-reaching effects on both local and systemic processes (7–9).
Therefore, dysregulation of the microbiome–host cells Trp metabolic pathway has been implicated in the pathogenesis of various diseases (10–16). This review provides a comprehensive overview of the Kyn and 5-HT pathways and their functional implications in neuronal diseases, autoimmunity, and cancer, incorporating recent research findings. Both pathways commence with the utilization of Trp. Trp plays a pivotal role in mammalian physiology and exerts diverse effects on various aspects of human health. First, we describe the tryptophan metabolic pathway and discuss its role in homeostasis.
The roles of Trp
Trp is one of the 20 standard amino acids that are building blocks of proteins and are incorporated into polypeptide chains during protein synthesis, thereby contributing to protein structure and function (17–20). Trp is also a precursor for the synthesis of 5-HT (21, 22), a neurotransmitter that plays a crucial role in mood regulation, sleep–wake cycles, and appetite (23, 24). Trp is also a precursor for the synthesis of melatonin, a hormone that regulates the sleep–wake cycle (25, 26). In the pineal gland, Trp is converted to 5-HT, and then to melatonin, through a series of enzymatic reactions. Moreover, Trp serves as a precursor for the synthesis of niacin (vitamin B3) (27), which is essential for various physiological processes including energy metabolism (28, 29), DNA repair (30), and cell signaling (31–33). Trp can be metabolized to produce nitric oxide (34), a signaling molecule with various physiological functions, including regulation of blood vessel dilation and immune responses (11).
Trp also has antioxidant properties that contribute to host cells’ defense against oxidative stress (35). It participates in the synthesis of molecules with antioxidant activity, helping neutralize free radicals. Trp is involved in the synthesis of collagen (36), a structural protein that provides strength and support to tissues, such as the skin, bones, and cartilage. Notably, Trp availability can be influenced by dietary factors, and a balanced diet that includes sufficient protein sources is crucial to meet the body’s Trp requirements. In addition, certain medical conditions or medications may affect Trp metabolism (37). The major Trp metabolic pathways are (i) the 5-HT pathway, (ii) the indole pathway, and (iii) the Kyn pathway. As well as a detailed description of these pathways, we will mention which pathways are dominant in various host cells and bacteria.
Trp pathway in the host cells and bacteria
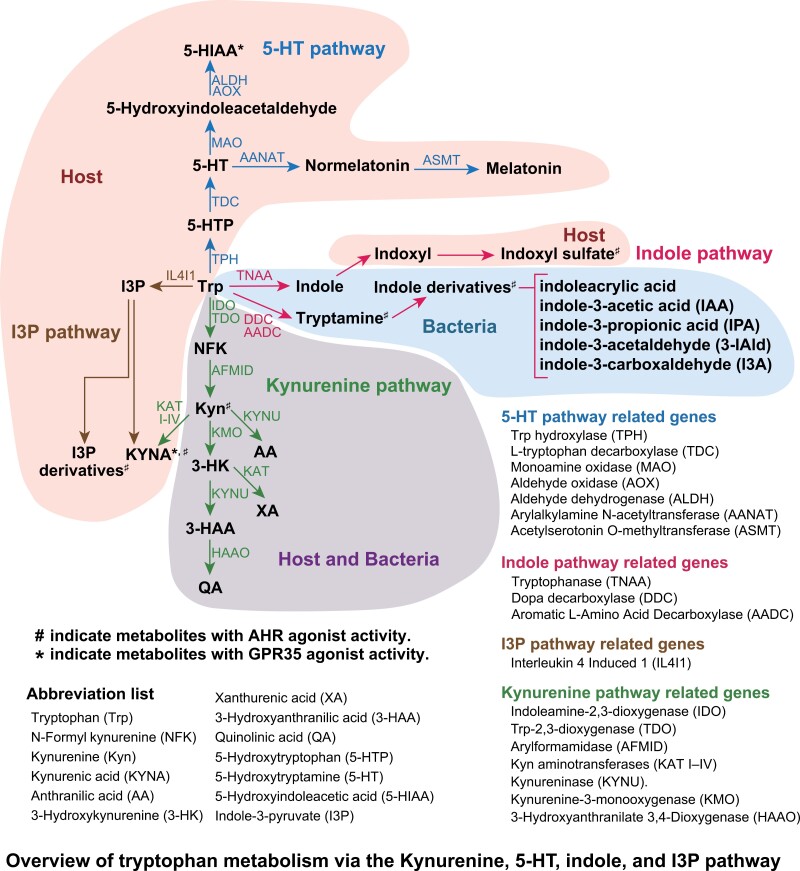
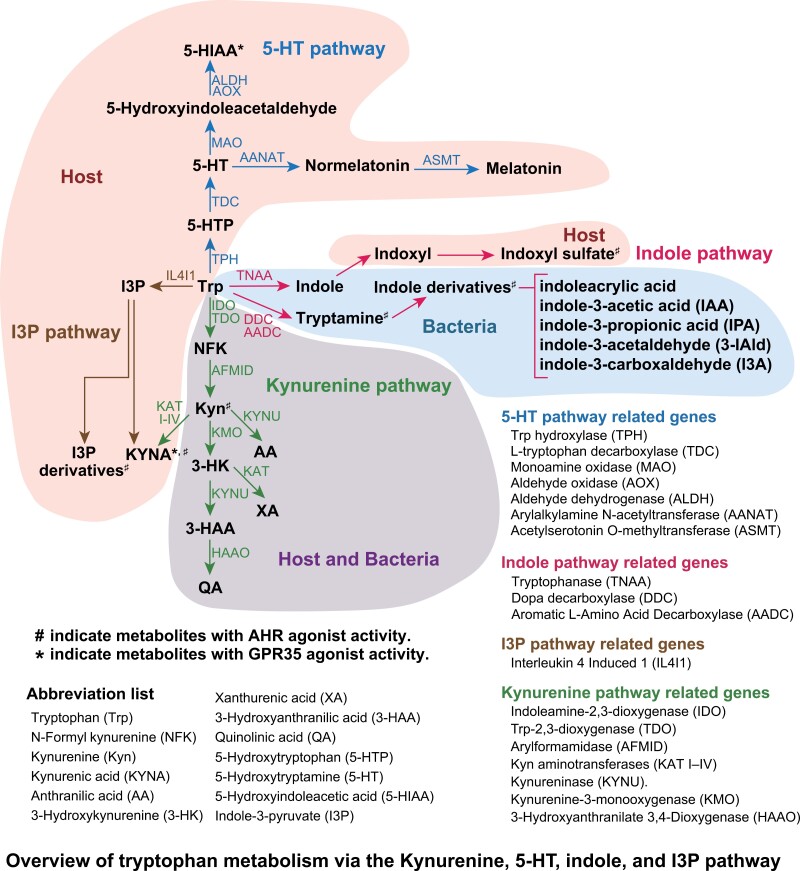
Most tryptophan metabolites are produced by both the host cells and bacteria (1, 38–40). However, it remains unclear whether these metabolites are synthesized in vivo, as the data indicating their production capacity were obtained from gene sets with the potential to convert tryptophan metabolites (41). In this study, bacterial genes were identified using AnnoTree (version 2.0.0) (default parameters) (42) searches targeting K numbers associated with tryptophan metabolism (map00380) in the KEGG pathway (43) (Supplementary Figure 1). Tryptophan metabolites, such as N-formyl kynurenine (NFK) and Kyn, can be digested by both the host cells and bacteria. Indole and indole-3-acetamide are only produced by the microbiome, whereas indole-3-pyruvate and 5-HT are produced by the host cells. Based on their genes, the predominant metabolic pathway differs between the host cells and bacteria (Fig. 1).
Figure 1.
Overview of tryptophan metabolism via the kynurenine, 5-HT, indole, and I3P pathway.
The Trp digestive pathway differs from that of the host cells and bacteria. Many bacteria possess enzymes that can break down tryptophan to indole and indole-3-carboxaldehyde (I3A) in the indole pathway. On the other hand, host cells have enzymes involved in the Kyn pathway with continuous expression of indoleamine 2,3-dioxygenase (IDO) (44–46). Immune cells, macrophages, and dendritic cells, in which the Kyn pathway mainly dominates, upregulate IDO in response to stimuli (47, 48). The Kyn pathway is dominant in astrocytes and microglia in the brain, whereas the 5-HT pathway is dominant in serotonergic neurons in the central nervous system (CNS) (49–51). Other cells in the peripheral tissues such as hepatocytes express tryptophan 2,3-dioxygenase (TDO) (52). Therefore, the Kyn pathway is thought to dominate in these cells.
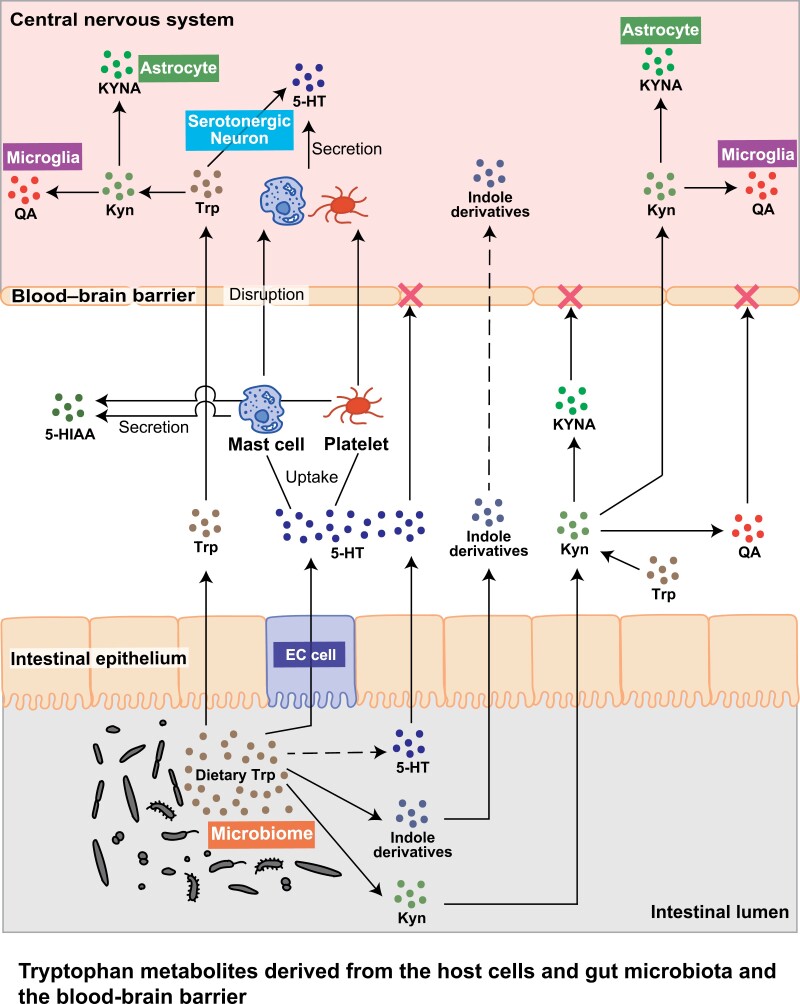
The 5-HT pathway is primarily associated with serotonergic neurons in the CNS that produce and release 5-HT (51, 53). The 5-HT pathway is dominant in enterochromaffin (EC) cells in the gastrointestinal tract, resulting in the synthesis and release of 5-HT (54–57) (Fig. 2). Platelets and mast cells do not synthesize 5-HT but they can store and release it after they are activated (51, 58, 59). Platelets can enter the blood–brain barrier (BBB) and are a major source of 5-HT. Trp and Kyn can pass BBB, on the other hand, other metabolites, 5-HT, kynurenic acid (KYNA), and quinolinic acid (QA), cannot pass the BBB, which means these metabolites in the brain are derived from the neurons, astrocytes, and microglia or from the platelets that carry 5-HT (60–66).
Figure 2.
Tryptophan metabolites derived from the host cells and gut microbiota and the blood–brain barrier.
The 5-HT pathway
The 5-HT metabolic pathway involves the conversion of Trp into various important molecules including 5-HT and melatonin (Fig. 1). This pathway is essential for the synthesis of neurotransmitters and plays a crucial role in regulating mood, sleep–wake cycles, and other physiological functions (67–69). 5-HT is stored in vesicles within nerve terminals (70, 71). Upon neuronal stimulation, 5-HT is released into the synaptic cleft, where it binds to receptors on the postsynaptic neurons and transmits signals (72–74).
The first step in the 5-HT pathway involves the enzymatic conversion of Trp to 5-hydroxytryptophan (5-HTP), catalyzed by tryptophan hydroxylase (TPH), wherein tetrahydrobiopterin (BH4) plays a crucial role (75). Subsequently, l-tryptophan decarboxylase (TDC) decarboxylates 5-HTP to produce 5-HT (21). 5-HT can be metabolized by monoamine oxidase to form 5-hydroxyindoleacetic acid (5-HIAA), which is excreted in urine. This step is crucial for terminating the expression of 5-HT in the synaptic cleft (23, 76). 5-HT can also be metabolized to form melatonin in the pineal gland. This pathway is important for the regulation of circadian rhythms and sleep–wake cycles. The 5-HT pathway is not only important for the synthesis of 5-HT and melatonin but also contributes to the production of various biologically active compounds (77, 78).
As 5-HT is a key neurotransmitter involved in mood regulation, disturbances in this pathway can have implications for mental health. Selective 5-HT reuptake inhibitors are commonly used to treat conditions, such as depression, by modulating 5-HT levels in the brain (79). Furthermore, intestinal neurons sense 5-HT and regulate their movement rhythms (80). As previously mentioned, 5-HT in serum does not cross the BBB. 5-HT in the brain are mostly derived from platelets. EC cells produce 5-HT and release it into the serum (Fig. 2). In addition, serum 5-HT concentrations in germ-free (GF) mice were reduced when juxtaposed with specific pathogen-free (SPF) mice, providing evidence for the impact of gut bacteria on 5-HT levels (81, 82).
The indole pathway
The gut microbiota converts Trp into indole and its derivatives such as indoleacrylic acid, indole-3-acetic acid (IAA), indole-3-propionic acid (IPA), indole-3 acetaldehyde (3-IAld), and tryptamine (83) (Fig. 1). The fecal indole level in GF mice was lower than that in SPF mice (84). Anerostipes, Bacteroides, Clostridium, Bifidobacterium, and Lactobacillus spp. catabolize Trp into its indole derivatives (85). Lactobacillus spp. metabolizes Trp to I3A; Clostridium sporogenes and Ruminococci convert Trp to tryptamine; and Staphylococcus, Providencia, and Pseudomonas convert Trp to IAA (41, 86). Furthermore, tryptamine induces 5-HT in EC cells (39). A recent study reported that supplementation with 3-IAld elicited antidepressant effects in mice subjected to stress (87). The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor activated by the indole pathway derivatives Kyn and KYNA (88–91). The BBB exhibits increased permeability in adult GF mice and monocolonization with Bacteroides thetaiotaomicron and Clostridium tyrobutyricum with sodium butyrate decreases the permeability of the BBB (92). These data suggest that microbiota-induced metabolites affect the permeability of BBB, and one of the candidate metabolites is the AHR ligand (93–95) (Fig. 2).
The Kyn pathway
Trp is metabolized to Kyn via a series of enzymatic reactions (Fig. 1). Kyn can be further metabolized, leading to the synthesis of various neuroactive compounds, including KYNA and QA. The initial and rate-limiting steps of the Kyn pathway involve the enzymatic conversion of Trp to Kyn catalyzed by TDO or IDO, depending on the tissue, such as the brain, lung, liver, small intestine, and colon (78, 96–100). Kyn can be metabolized further, producing KYNA, a neuroactive compound (101). This conversion is catalyzed by kynurenine aminotransferases (97). Kyn can also be converted to QA. Kyn is first transformed to 3-hydroxykynurenine (3-HK), which is catalyzed by kynurenine 3-monooxygenase (KMO). Next, 3-HK is transformed into 3-hydroxyanthranilic acid (3-HAA) catalyzed by KYNU. Finally, 3-HAA is converted to QA by 3-hydroxyanthranilate 3,4-dioxygenase. QA is converted to nicotinamide adenine dinucleotide (NAD+), an important coenzyme in energy production, cell division, and mitochondrial function, by QA phosphoribosyltransferase and NAD synthase (102).
Involvement of the Kyn and 5-HT pathways in immune diseases
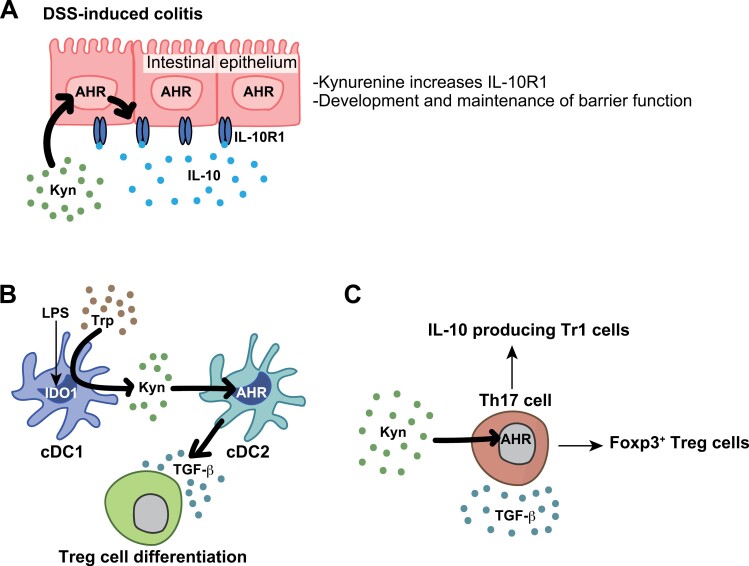
Trp metabolites can transmit signals through cellular receptors that exhibit tissue-specific expression and are regulated by their circumstances. These receptors include AHR, alpha 7 nicotinic acetylcholine receptor (α7nAChR), and G-protein-coupled receptor (GPR) 35. IDO1 and TDO, which are involved in the initial steps of Trp metabolism, are constitutively expressed in tumors. However, the expression of these genes is induced in immune and epithelial cells by inflammatory signals. Local and systemic inflammation induce the initial expression of IDO1 in epithelial and myeloid cells (103, 104). Kyn amplifies the IDO1–Kyn–AHR loop to suppress inflammatory mediators through AHR signaling in immune and epithelial cells (105) (Fig. 3A).
Figure 3.
The role of the Kyn pathway in immune regulation. (A) In DSS-induced colitis, kynurenine via the intestinal epithelial AHR leads to an increase in IL-10 receptor-1 (IL-10R1) expression. This consequently exerts an anti-inflammatory effect through IL-10 signaling. (B) The IDO1-AHR axis in the induction of infection resistance upregulates TGF-β and induces Tregs. (C) The collaborative interaction between TGF-β and AHR plays a pivotal role in the transdifferentiation process of Th17 cells, leading to the generation of IL-10-producing Tr1 cells and Foxp3+ Treg cells.
In the initial steps of the immune response, antigen-presenting cells and dendritic cells (DCs) play a crucial role in both the initiation and maintenance of immune responses. IDO1 expression in DCs is increased by lipopolysaccharide (LPS), extracellular and intracellular DNA, and type l and type II interferons (106). Kyn, which is initially released by IDO1-expressing type 1 conventional DCs (cDC1s), recruits AHR-expressing cDC2s. cDC2s produce transforming growth factor-β (TGF-β), which induces anti-inflammatory forkhead box protein 3+ (Foxp3+) regulatory T cells (Tregs) (Fig. 3B). The TGF-β–IDO1–AHR loop is crucial for the generation of tolerogenic DCs, resulting in self-tolerance and LPS tolerance. However, its direct effect on tolerogenic DCs differentiation remains unclear.
T helper (Th)17 cells and Tregs play a central role in immune function during colitis and cancer progression (107–113). AHR plays a role in the induction of the effector cytokine interleukin (IL-)17A and is expressed in both Th17 cells and Tregs (114). AHR expression in Tregs enhances their immunosuppressive function (115). TGF-β and AHR promote Th17 cell differentiation into IL-10-producing type 1 regulatory T (Tr1) cells especially in the resolution phase of intestinal inflammation (116) (Fig. 3C).
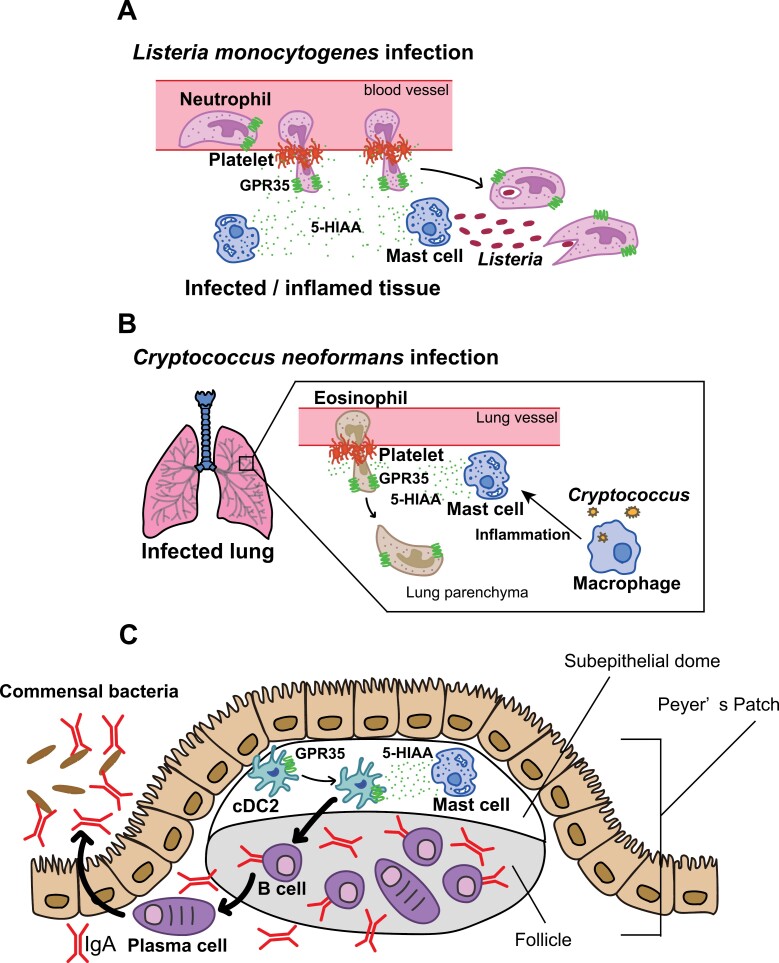
Tumour-associated macrophages (TAMs) play a crucial role in tumor progression. Tumour cells elicit AHR expression and activation in TAMs by releasing IL-1β/IL-6 and Kyn (117). In addition, AHR-enhanced macrophages have the potential to differentiate into TAMs, suppressing the antitumour activity of CD8+ T cells. Higher levels of IDO1 and TDO2 are associated with the immunosuppressive function of Tregs in tumors. Overexpression of IDO1/TDO2 in tumor cells can enhance tumor progression by suppressing the function of Tregs and M2-TAMs (118). Additionally, KYNA exerts an anti-inflammatory role in human invariant natural killer (iNK) cells through activation of GPR35 (119–121). GPR35-mediated KYNA sensing plays a crucial role in preserving the integrity of the intestinal barrier against damage in dextran sulfate sodium (DSS)-induced enteritis (122). Conversely, 5-HIAA released by platelets and mast cells recruits pathogenic neutrophils and eosinophils to induce inflammation (123, 124) (Fig. 4A and B). Moreover, mast cells in the subepithelial dome secrete 5-HIAA to attract GPR35+ cDC2s. This sequential cascade of events leads to the augmented synthesis of immunoglobulin A (IgA) by plasma cells (125) (Fig. 4C).
Figure 4.
The 5-HIAA-GPR35 axis is implicated in the recruitment of immune cells (A) platelet- and mast cell-derived, a metabolite of serotonin, 5-HIAA serves as a ligand for the chemoattractant receptor GPR35, facilitating GPR35+ neutrophil transendothelial migration and their recruitment to inflammatory tissue during Listeria monocytogenes infections. (B) When Cryptococcus neoformans infects the lungs, it produces 5-HIAA derived from platelets and mast cells through macrophage-mediated inflammation. This process promotes the recruitment of GPR35+ eosinophils to the infected lung, leading to the exacerbation of the disease. (C) Mast cells located in the subepithelial dome produce 5-HIAA to recruit GPR35+ cDC2s. This consecutive series of events results in an increased synthesis of immunoglobulin A (IgA) by plasma-cells.
Furthermore, individuals with inflammatory bowel disease (IBD), multiple sclerosis (MS), or chronic kidney disease exhibited elevated concentrations of serum KYNA (126–131). Meanwhile, serum metabolomic analysis in patients with coronavirus disease 2019 (COVID-19) showed elevated KYNA levels and an increased KYNA:Kyn ratio in male patients. The clinical prognosis of COVID-19 is less favorable in males than in females, and this sex-based disparity is attributed to immune responses. These metabolite alterations are positively associated with age, as well as with inflammatory cytokines and chemokines (132).
In summary, Trp plays a crucial role in modifying the response of immune cells, particularly in reducing inflammation. However, the specific roles of other Trp metabolites, such as 5-HIAA, remain unclear.
Involvement of the Kyn and 5-HT pathways in CNS diseases
The Kyn and 5-HT pathways are interconnected and play crucial roles in maintaining normal brain function. Dysregulation of these pathways has been implicated in the development and progression of various brain diseases, including neurodegenerative diseases, mood disorders, and autoimmune conditions.
Neurodegenerative diseases
Individuals with Alzheimer’s disease had increased brain levels of Kyn and its metabolites, such as QA (133, 134). These metabolites may contribute to neuroinflammation and neurotoxicity. Meanwhile, an altered Trp metabolism has been observed in Parkinson’s disease, leading to changes in the Kyn pathway (135). Imbalances in the Kyn pathway may contribute to oxidative stress and brain inflammation.
Mood disorders
Dysregulation of the Kyn pathway has been implicated in the pathophysiology of depression. Increased levels of Kyn and its metabolites, along with reduced 5-HT levels, may contribute to depressive symptoms (136). Furthermore, abnormalities in Trp metabolism are associated with schizophrenia, and changes in Kyn pathway metabolites may contribute to the cognitive and neuroinflammatory aspects of this disorder (137, 138).
Autoimmune conditions
The Kyn pathway has been implicated in the pathogenesis of MS (128, 139–141). Imbalances in Trp metabolism may contribute to neuroinflammation and CNS demyelination.
Connection between KYNA/QA and neurons
KYNA also exhibits neuroprotective properties (142–145). High levels of KYNA competitively inhibit ionotropic glutamate receptors (146, 147). Moreover, it selectively decreases the activity of the glycine co-agonist side of the N-methyl-D-aspartate (NMDA) receptor, which is involved in excitatory neurotransmission (148–150). Administration of low KYNA concentrations reduces glutamate levels by 30%–40% (146). KYNA also putatively acts as a negative allosteric modulator at the α7nAChR (151–154). KYNA also acts as an agonist at GPR35, which was thought to be an ‘orphan’ receptor, modulating cAMP production and inhibiting the N-type Ca2+ channels of sympathetic neurons and astrocytes, causing suppression of many inflammatory pathways (120, 155). By blocking this receptor, KYNA regulates the balance of neurotransmitters and prevents excessive excitotoxicity (156, 157).
In contrast, abnormal QA levels are implicated in neurodegenerative disorders. QA is an NMDA receptor agonist that exhibits neurotoxic effects (158–161), inhibits glutamate reuptake by astrocytes, and contributes to excitotoxicity when present in excessive amounts. QA generates reactive oxygen species (ROS), promotes tau phosphorylation, and disrupts the BBB. In addition, QA acts on astrocytes to produce inflammatory mediators (162). A balance between the production of neuroprotective KYNA and neurotoxic QA is crucial in maintaining normal brain function.
Trp metabolites and the microbiome
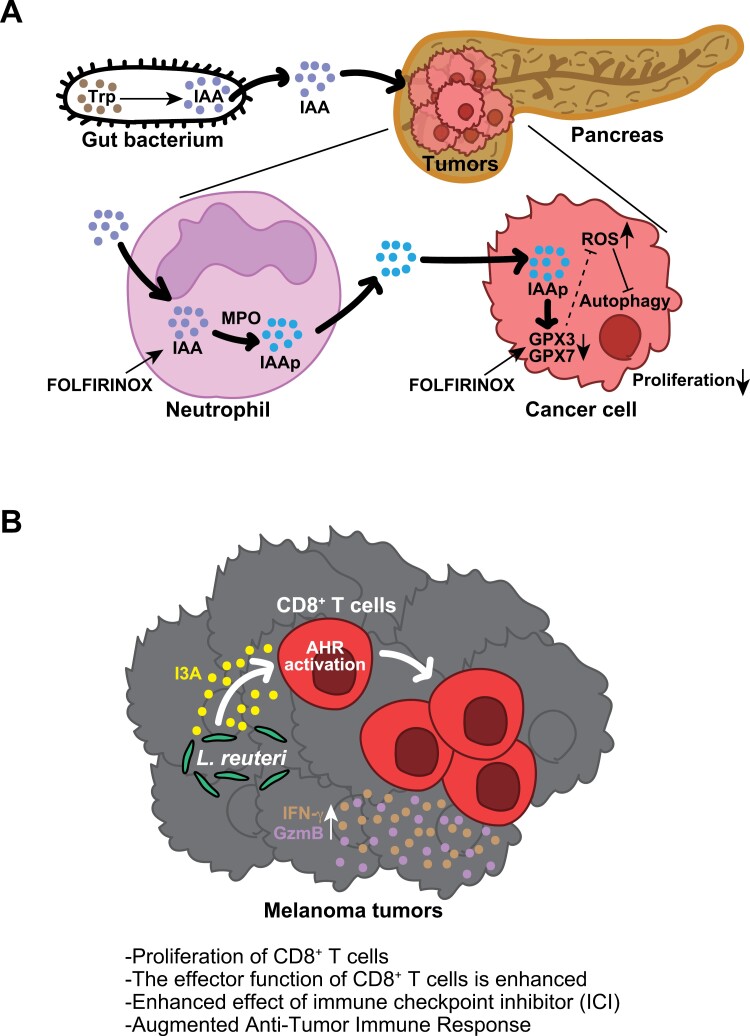
The microbiome has been shown to affect the immune system. Intestinal bacteria are involved in the induction of specific immune cells as well as activating immune cells as antigens. For example, Lactobacillus spp. digest dietary Trp and produce the AHR ligand indole-3-propionic acid (I3P) (Fig. 1), and these metabolite polarizations of tumor-promoting TAMs and other Limosilactobacillus (Lactobacillus) reuteri induce intraepithelial lymphocytes (IELs) in the small intestine (163, 164). Recently, in two distinct cohorts of pancreatic ductal adenocarcinoma (PDAC), a noteworthy correlation was observed between the therapeutic response and levels of IAA, a Trp metabolite derived from the microbiota that serves as an AHR ligand (165, 166) (Fig. 5A). Indole derivatives produced by L. reuteri have shown anticancer properties (167, 168) (Fig. 5B).
Figure 5.
Indole derivatives derived from bacteria serve as facilitators for augmenting the efficacy of chemotherapy and ICI in cancer. (A) Trp metabolites originating from the gut microbiome accelerate the chemotherapy response in pancreatic cancer. Intestinal bacteria generate IAA from absorbed dietary Trp. IAA is transported to PDAC through the bloodstream, where it may undergo oxidation to produce toxic molecules (IAAp) facilitated by myeloperoxidase (MPO) and cytotoxic anticancer drugs such as 5-fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) within intratumoural neutrophils. Subsequently, IAAp and FOLFIRINOX jointly contribute to the downregulation of GPX3/7, enzymes responsible for degrading ROS, leading to the accumulation of ROS within cancer cells. Ultimately, elevated ROS levels inhibit the autophagy pathway, a crucial process in cancer cell proliferation. (B) L. reuteri translocates to, colonizes, and persists within melanoma, where, through the release of its dietary tryptophan catabolite I3A, it locally enhances the generation of IFN-γ-producing CD8+ T cells, thereby augmenting the efficacy of ICI. Furthermore, I3A was found to be both necessary and sufficient to stimulate antitumour immunity, and the loss of AHR signaling within CD8+ T cells abolished antitumour effects.
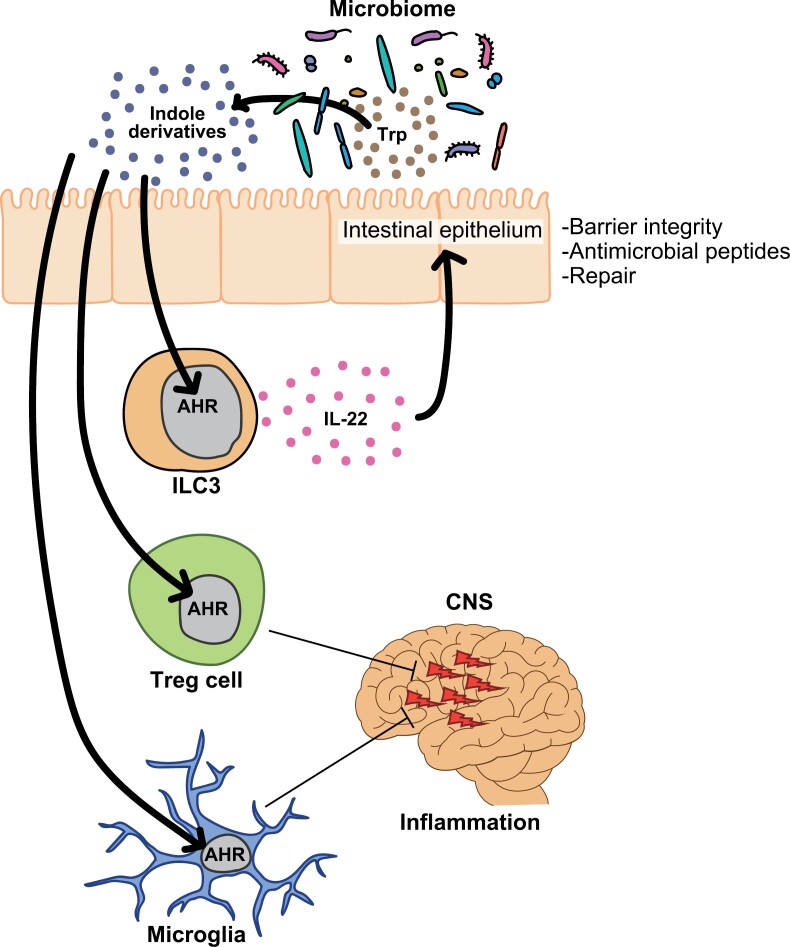
Trp metabolites at the interface between the microbiota and host cells are important for maintaining body homeostasis. Organs utilize Trp metabolites produced by bacteria under inflammatory conditions to induce AHR upregulation in epithelial and immune cells. Indole derivatives from intestinal bacteria enhance the intestinal barrier function by promoting the production of IL-22 through AHR expressed on innate lymphoid cells (ILCs) 3 (169–174) (Fig. 6).
Figure 6.
Indole derivatives originating from the gut microbiota exert anti-inflammatory effects through the AHR. Indole derivatives, generated by the microbial conversion of dietary Trp, can activate AHR in Group 3 innate lymphoid cells (ILC3s), thereby promoting IL-22-mediated tissue protection. Indole derivatives can activate AHR in T cells, leading to the generation of Tregs and subsequent reduction in inflammation, resulting in improved disease outcomes in EAE. Additionally, AHR in microglia contributes to the suppression of inflammation in EAE.
AHR activated by indole derivatives can stimulate the expansion of Tregs and concurrently suppress experimental autoimmune encephalitis (EAE) (175). AHR signaling in microglia, which is mediated by indole derivatives, induces alterations in immune signaling within astrocytes, leading to a reduction in disease severity in EAE (176, 177). Trp-deficient and Trp metabolite-deficient diets induce chronic tissue inflammation, as IELs, Th17 cells, Tregs, and ILC3s express AHR, which is involved in mucosal homeostasis in the gut. In contrast, host cells affect the composition of the microbiota. Deletion of CARD9, an IBD-related gene, leads to exacerbated colitis owing to a reduction in Trp metabolites in Lactobacillus spp (178). These results imply that intestinal bacteria and immune cells live in symbiosis with Trp.
Bacteria-mediated Trp metabolite, KYNA induces EAE
EAE is used as an animal model for MS in humans. Trp metabolites act on AHR in astrocytes, reducing the inflammation of encephalitis (Fig. 6) (177). Moreover, these astrocytes are controlled by AHR in microglial cells via TGF-α expression (176). Trp-deficient diets exacerbate EAE because of the reduced stimulation of microglial and astrocyte AHR, and I3S supplementation ameliorates EAE. On the basis of this evidence, Trp metabolites are beneficial neuroprotective metabolites.
The microbiome was shown to have the potential to induce EAE, as GF mice did not develop EAE (179). The involvement of the microbiota in EAE and MS remains unclear; however, studies on humans have implied that the composition of the microbiota in MS differed from that in other populations (180–183). In addition, L. reuteri enhances the disease score of EAE because it possesses a peptide that mimics myelin oligodendrocyte glycoprotein (MOG). Moreover, Erysipelotrichaceae bacteria (EB) act as an adjuvant to enhance Th17 cell responses in the small intestine (184, 185). Although L. reuteri and EB enhance the disease activity of EAE with the accumulation of the Th17 cells in the spinal cord (SC), the proportion of Th17 cells in the small intestine did not increase. Furthermore, the levels of the Th17 cell driver serum amyloid A were not increased in the small intestine. It is unclear whether T cells course through the small intestine and SC in EAE.
Recently, blocking the pathway that involves α4β7-integrin and its ligand mucosal addressin cell adhesion molecule-1 (MAdCAM-1), which mediates T cell migration to the intestine, was found to ameliorate encephalitis (186). Moreover, Schnell et al. and Miyamoto et al. showed that Th17 cells in the small-intestinal circuit directly enter the neural circuit to induce encephalitis in photoconversion ‘Kaede mice’ (187, 188). Kaede mice emit green fluorescence constitutively in all the cells. After irradiation with violet light to the mesenteric lymph nodes draining from the small intestine, green-to-red photoconversion occurs only in the exposure site, to enable us to track the cells and monitor precise cellular movement in vivo (189). We observed the red cells in the SC of the EAE mice. Taken together, these findings suggest that certain T cells originating from the small-intestinal population may translocate to the SC, playing a role in the induction of myelitis.
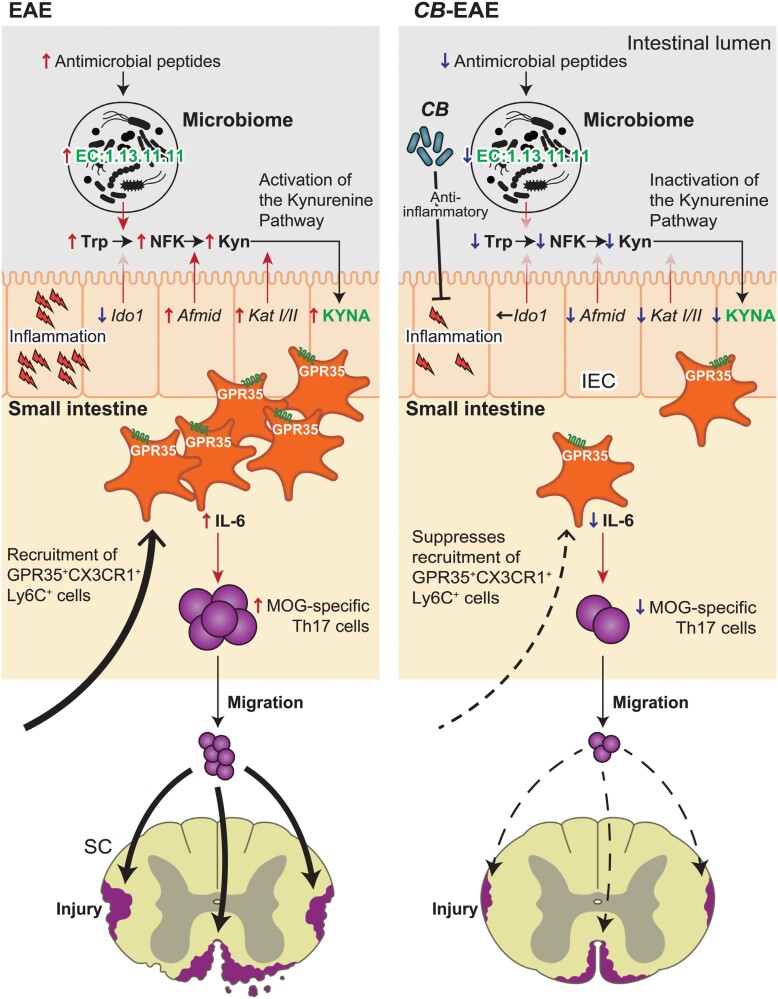
Intestinal Th17 cells are induced by antigen-presenting cells. Miyamoto et al. showed that CX3CR1+ Ly6C+ GPR35+ macrophages potentially induce the accumulation of Th17 cells in the small intestine of EAE mice (188). The major GPR35 ligands are cGMP, lysophosphatidic acid, 5-HIAA, and KYNA (131, 190). The concentration of KYNA increased in the small intestine of EAE mice, whereas the others did not increase, compared with non-EAE mice. Furthermore, the expression levels of afmid, kat1, and kat2 (see Fig. 1) were higher in EAE mice than in non-EAE mice. Notably, the expression levels of Ido1 did not increase in EAE mice (Fig. 7).
Figure 7.
The gut microbiota-induced KYNA recruits GPR35+ macrophages to promote experimental encephalitis. (Left) Inflammation was initiated in the small intestine prior to the manifestation of the phenotype in the EAE model of MS. Inflammation elevated antimicrobial peptides and modified the microbiome. The intestinal epithelium cells (IECs) and microbiome collaborated in the production of KYNA. GPR35+ CX3CR1+ Ly6C+ cells utilizing KYNA as a chemokine ligand were recruited to the small intestine. GPR35+ CX3CR1+ Ly6C+ cells exhibit high levels of IL-6 expression and an expanded population of pathogenic myelin-responsive Th17 cells. Pathogenic myelin-responsive Th17 cells migrated to the SC, triggering inflammation. (Right) The administration of CB led to the suppression of inflammation in the small intestine. CB altered the microbiome and gene expression in the IECs, leading to the inactivation of the Kyn pathway. The diminished KYNA levels resulted in a reduced recruitment of GPR35+ CX3CR1+ Ly6C+ cells. The number of pathogenic myelin-responsive Th17 cells induced by GPR35+ CX3CR1+ Ly6C+ cells was decreased. Inflammation was attenuated due to a decrease in the number of pathogenic myelin-responsive Th17 cells migrating to the SC. The potential preventive effect of CB on MS was suggested.
Interestingly, the microbiome potentially harbors a Kyn pathway that digests Trp to form NFK and Kyn. Intestinal bacteria possess the enzyme groups EC:1.13.11.11, which converts Trp to NFK, and EC:3.5.1.9, which converts NFK to Kyn. The expression of EC:1.13.11.11, but not of EC:3.5.1.9, increased in fecal bacteria in both EAE mice and patients with MS (180). Previous reports have shown that EB are increased in EAE mice (188), but EB do not possess EC1.13.11.11. Sporosarcina pasteurii (SP), Staphylococcus lentus, Pseudoxanthomonas mexicana, and Sphingomonas are potential possessors of the EC:1.13.11.11 gene. Notably, the abundance of SP was higher in fecal samples of EAE mice than those of non-EAE mice. Miyamoto et al. inserted the EC:1.13.11.11 gene into the Escherichia coli JCM1649 (ECWT) and generated a strain that can convert Trp into NFK (ECKynA) (188). Mice mono-associated with ECKynA exhibited significantly higher EAE scores than mice mono-associated with ECWT following MOG induction.
Overall, these results indicate that KYNA in the small intestine plays an inflammatory role in EAE, whereas KYNA has a neuroprotective role. In addition, the Trp metabolic pathway and microbiome harbor both the indole and Kyn pathways, especially the initial step of Trp conversion to Kyn. Further investigations focusing on Trp metabolites and their pathways are required to understand their action on neurons and immune cells. Inhibitors of rate-limiting enzymes that play crucial roles in Trp metabolism, such as IDO/TDO, KMO, and TPH, are candidates for modulating Trp metabolites in neuronal diseases or tumors. Modulation of the gut microbiome may also regulate Trp metabolites. The specific mechanism remains unknown; however, controlling Trp metabolites by modulating the composition of microbiota is a safe and promising method in discovering therapeutic options. Clostridium butyricum MIYAIRI 588 (CB) is known to reduce the disease activity of EAE following a reduction in Trp metabolites (Fig. 7).
Conclusion
Trp metabolites play key roles in immune function, neuronal excretion, and energy metabolism. An imbalance in these metabolites induces neuropsychiatric disorders and inflammation. These pathways are complicated because (i) they interact with each other and are not independent; (ii) Trp metabolites are generated not only by vertebrates but also by bacteria; and (iii) Trp metabolites act differently on each cell. For example, KYNA has a neuroprotective role in astrocytes but is pathogenic to intestinal macrophages in the EAE model.
Supplementary data
Supplementary data are available at International Immunology online.
Acknowledgements
We thank S. Suzuki, Y. Yoshimatsu, and T. Miyamoto for their help in preparing this manuscript. We thank Editage for editing a draft of this manuscript.
Contributor Information
Kentaro Miyamoto, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan; Miyarisan Pharmaceutical Co., Research Laboratory, Tokyo, Japan.
Tomohisa Sujino, Center for Diagnostic and Therapeutic Endoscopy, Keio University School of Medicine, Tokyo, Japan; Keio Global Research Institute, Keio University, Tokyo, Japan.
Takanori Kanai, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan.
Conflict of interest statement. K.M. is an employee of Miyarisan Pharmaceutical.
Funding
This work was supported by The Japan Science and Technology Agency Fusion Oriented Research for Disruptive Science and Technology (FOREST) (JPMJFR210P to T.S.), Grants-in-Aid from the Japanese Society for the Promotion of Science (20H00536 to T.K., 23H02899, 21K18272, 21H02905 to T.S.), the Japan Agency for Medical Research and Development (CREST 21gm1510002h0001 to T.K.), and Miyarisan Pharmaceutical (T.K.).
References
- 1. Agus A, Planchais J, Sokol H.. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018;23:716–24. https://doi.org/ 10.1016/j.chom.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 2. Liu M, Nieuwdorp M, de Vos WM, et al. Microbial tryptophan metabolism tunes host immunity, metabolism, and extraintestinal disorders. Metabolites 2022;12:834. https://doi.org/ 10.3390/metabo12090834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hou Y, Li J, Ying S.. Tryptophan metabolism and gut microbiota: a novel regulatory axis integrating the microbiome, immunity, and cancer. Metabolites 2023;13:1166. https://doi.org/ 10.3390/metabo13111166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palego L, Betti L, Rossi A, et al. Tryptophan biochemistry: structural, nutritional, metabolic, and medical aspects in humans. J Amino Acids 2016;2016:8952520. https://doi.org/ 10.1155/2016/8952520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arifuzzaman M, Collins N, Guo CJ, et al. Nutritional regulation of microbiota-derived metabolites: implications for immunity and inflammation. Immunity 2024;57:14–27. https://doi.org/ 10.1016/j.immuni.2023.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davidson M, Rashidi N, Nurgali K, et al. The role of tryptophan metabolites in neuropsychiatric disorders. Int J Mol Sci 2022;23:9968. https://doi.org/ 10.3390/ijms23179968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jamshed L, Debnath A, Jamshed S, et al. An emerging cross-species marker for organismal health: tryptophan-kynurenine pathway. Int J Mol Sci 2022;23:6300. https://doi.org/ 10.3390/ijms23116300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moulin D, Millard M, Taïeb M, et al. Counteracting tryptophan metabolism alterations as a new therapeutic strategy for rheumatoid arthritis. Ann Rheum Dis 2023;83:312–23. https://doi.org/ 10.1136/ard-2023-224014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lukić I, Ivković S, Mitić M, et al. Tryptophan metabolites in depression: modulation by gut microbiota. Front Behav Neurosci 2022;16:987697. https://doi.org/ 10.3389/fnbeh.2022.987697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao K, Mu CL, Farzi A, et al. Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr 2020;11:709–23. https://doi.org/ 10.1093/advances/nmz127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xue C, Li G, Zheng Q, et al. Tryptophan metabolism in health and disease. Cell Metab 2023;35:1304–26. https://doi.org/ 10.1016/j.cmet.2023.06.004 [DOI] [PubMed] [Google Scholar]
- 12. Saito T, Iwata N, Tsubuki S, et al. Somatostatin regulates brain amyloid beta peptide Abeta42 through modulation of proteolytic degradation. Nat Med 2005;11:434–9. https://doi.org/ 10.1038/nm1206 [DOI] [PubMed] [Google Scholar]
- 13. Fila M, Chojnacki J, Pawlowska E, et al. Kynurenine pathway of tryptophan metabolism in migraine and functional gastrointestinal disorders. Int J Mol Sci 2021;22:10134. https://doi.org/ 10.3390/ijms221810134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng Y, Zhou M, Wang J, et al. Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021;13:1–16. https://doi.org/ 10.1080/19490976.2020.1869501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mor A, Tankiewicz-Kwedlo A, Krupa A, et al. Role of kynurenine pathway in oxidative stress during neurodegenerative disorders. Cells 2021;10:1603. https://doi.org/ 10.3390/cells10071603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCann JR, Rawls JF.. Essential amino acid metabolites as chemical mediators of host-microbe interaction in the gut. Annu Rev Microbiol 2023;77:479–97. https://doi.org/ 10.1146/annurev-micro-032421-111819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Umeda S, Sujino T, Miyamoto K, et al. D-amino acids ameliorate experimental colitis and cholangitis by inhibiting growth of proteobacteria: potential therapeutic role in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 2023;16:1011–31. https://doi.org/ 10.1016/j.jcmgh.2023.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bongioanni A, Bueno MS, Mezzano BA, et al. Amino acids and its pharmaceutical applications: a mini review. Int J Pharm 2022;613:121375. https://doi.org/ 10.1016/j.ijpharm.2021.121375 [DOI] [PubMed] [Google Scholar]
- 19. Iacone R, Scanzano C, Santarpia L, et al. Macronutrients in parenteral nutrition: amino acids. Nutrients 2020;12:772. https://doi.org/ 10.3390/nu12030772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delompré T, Guichard E, Briand L, et al. Taste perception of nutrients found in nutritional supplements: a review. Nutrients 2019;11:2050. https://doi.org/ 10.3390/nu11092050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu N, Sun S, Wang P, et al. The mechanism of secretion and metabolism of gut-derived 5-Hydroxytryptamine. Int J Mol Sci 2021;22:7931. https://doi.org/ 10.3390/ijms22157931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Höglund E, Øverli O, Winberg S.. Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Front Endocrinol (Lausanne) 2019;10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pourhamzeh M, Moravej FG, Arabi M, et al. The roles of serotonin in neuropsychiatric disorders. Cell Mol Neurobiol 2022;42:1671–92. https://doi.org/ 10.1007/s10571-021-01064-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu J, Tan Y, Cheng H, et al. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis 2022;13:1106–26. https://doi.org/ 10.14336/AD.2022.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gillette MU, Wang TA.. Brain circadian oscillators and redox regulation in mammals. Antioxid Redox Signal 2014;20:2955–65. https://doi.org/ 10.1089/ars.2013.5598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poza JJ, Pujol M, Ortega-Albás JJ, et al. Melatonin in sleep disorders. Neurologia (Engl Ed) 2022;37:575–85. https://doi.org/ 10.1016/j.nrleng.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 27. Fukuwatari T, Shibata K.. Nutritional aspect of tryptophan metabolism. Int J Tryptophan Res 2013;6:3–8. https://doi.org/ 10.4137/IJTR.S11588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palzer L, Bader JJ, Angel F, et al. Alpha-amino-beta-carboxy-muconate-semialdehyde decarboxylase controls dietary niacin requirements for NAD(+) synthesis. Cell Rep 2018;25:1359–70.e4. https://doi.org/ 10.1016/j.celrep.2018.09.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wirthgen E, Hoeflich A, Rebl A, et al. Kynurenic acid: the Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front Immunol 2017;8:1957. https://doi.org/ 10.3389/fimmu.2017.01957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Surjana D, Halliday GM, Damian DL.. Role of nicotinamide in DNA damage, mutagenesis, and DNA repair. J Nucleic Acids 2010;2010:157591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ansarey SH. Inflammation and JNK’s role in Niacin-GPR109A diminished flushed effect in microglial and neuronal cells with relevance to schizophrenia. Front Psychiatry 2021;12:771144. https://doi.org/ 10.3389/fpsyt.2021.771144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Curran CS, Kopp JB.. The complexity of nicotinamide adenine dinucleotide (NAD), hypoxic, and aryl hydrocarbon receptor cell signaling in chronic kidney disease. J Transl Med 2023;21:706. https://doi.org/ 10.1186/s12967-023-04584-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wuerch E, Urgoiti GR, Yong VW.. The promise of niacin in neurology. Neurotherapeutics 2023;20:1037–54. https://doi.org/ 10.1007/s13311-023-01376-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Talari NK, Panigrahi M, Madigubba S, et al. Altered tryptophan metabolism in human meningioma. J Neurooncol 2016;130:69–77. https://doi.org/ 10.1007/s11060-016-2225-7 [DOI] [PubMed] [Google Scholar]
- 35. Xu K, Liu G, Fu C.. The tryptophan pathway targeting antioxidant capacity in the placenta. Oxid Med Cell Longev 2018;2018:1054797. https://doi.org/ 10.1155/2018/1054797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stuart, PS, Bell, SJ, Molnar J.. Use of tryptophan-fortified hydrolyzed collagen for nutritional support. J Diet Suppl 2008;5:383. [DOI] [PubMed] [Google Scholar]
- 37. Li D, Yu S, Long Y, et al. Tryptophan metabolism: mechanism-oriented therapy for neurological and psychiatric disorders. Front Immunol 2022;13:985378. https://doi.org/ 10.3389/fimmu.2022.985378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gupta SK, Vyavahare S, Duchesne Blanes IL, et al. Microbiota-derived tryptophan metabolism: impacts on health, aging, and disease. Exp Gerontol 2023;183:112319. https://doi.org/ 10.1016/j.exger.2023.112319 [DOI] [PubMed] [Google Scholar]
- 39. Benech N, Rolhion N, Sokol H.. Tryptophan metabolites get the gut moving. Cell Host Microbe 2021;29:145–7. https://doi.org/ 10.1016/j.chom.2021.01.009 [DOI] [PubMed] [Google Scholar]
- 40. Li Y, Liu N, Ge Y, et al. Tryptophan and the innate intestinal immunity: crosstalk between metabolites, host innate immune cells, and microbiota. Eur J Immunol 2022;52:856–68. https://doi.org/ 10.1002/eji.202149401 [DOI] [PubMed] [Google Scholar]
- 41. Kaur H, Bose C, Mande SS.. Tryptophan metabolism by gut microbiome and gut-brain-axis: an in silico analysis. Front Neurosci 2019;13:1365. https://doi.org/ 10.3389/fnins.2019.01365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mendler K, Chen H, Parks DH, et al. AnnoTree: visualization and exploration of a functionally annotated microbial tree of life. Nucleic Acids Res 2019;47:4442–8. https://doi.org/ 10.1093/nar/gkz246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kanehisa M, Goto S.. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 2000;28:27–30. https://doi.org/ 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abd El-Fattah EE. IDO/kynurenine pathway in cancer: possible therapeutic approaches. J Transl Med 2022;20:347. https://doi.org/ 10.1186/s12967-022-03554-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stone TW, Williams RO.. Interactions of IDO and the kynurenine pathway with cell transduction systems and metabolism at the inflammation-cancer interface. Cancers (Basel) 2023;15:2895. https://doi.org/ 10.3390/cancers15112895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lashgari NA, Roudsari NM, Shayan M, et al. IDO/Kynurenine; novel insight for treatment of inflammatory diseases. Cytokine 2023;166:156206. https://doi.org/ 10.1016/j.cyto.2023.156206 [DOI] [PubMed] [Google Scholar]
- 47. Krupa A, Kowalska I.. The kynurenine pathway-new linkage between innate and adaptive immunity in autoimmune endocrinopathies. Int J Mol Sci 2021;22:9879. https://doi.org/ 10.3390/ijms22189879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krupa A, Krupa MM, Pawlak K.. Kynurenine pathway-an underestimated factor modulating innate immunity in sepsis-induced acute kidney injury? Cells 2022;11:2604. https://doi.org/ 10.3390/cells11162604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haroon E, Raison CL, Miller AH.. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012;37:137–62. https://doi.org/ 10.1038/npp.2011.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stone TW, Clanchy FIL, Huang YS, et al. An integrated cytokine and kynurenine network as the basis of neuroimmune communication. Front Neurosci 2022;16:1002004. https://doi.org/ 10.3389/fnins.2022.1002004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Imamdin A, van der Vorst EPC.. Exploring the role of serotonin as an immune modulatory component in cardiovascular diseases. Int J Mol Sci 2023;24:1549. https://doi.org/ 10.3390/ijms24021549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu L, Ling J, Su C, et al. Emerging roles on immunological effect of indoleamine 2,3-dioxygenase in liver injuries. Front Med (Lausanne) 2021;8:756435. https://doi.org/ 10.3389/fmed.2021.756435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Layunta E, Buey B, Mesonero JE, et al. Crosstalk between intestinal serotonergic system and pattern recognition receptors on the microbiota-gut-brain axis. Front Endocrinol (Lausanne) 2021;12:748254. https://doi.org/ 10.3389/fendo.2021.748254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rezzani R, Franco C, Franceschetti L, et al. A focus on enterochromaffin cells among the enteroendocrine cells: localization, morphology, and role. Int J Mol Sci 2022;23:3758. https://doi.org/ 10.3390/ijms23073758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 2003;111:931–43. https://doi.org/ 10.1172/JCI18326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu X, Chen R, Zhan G, et al. Enterochromaffin cells: sentinels to gut microbiota in hyperalgesia? Front Cell Infect Microbiol 2021;11:760076. https://doi.org/ 10.3389/fcimb.2021.760076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Linan-Rico A, Ochoa-Cortes F, Beyder A, et al. Mechanosensory signaling in enterochromaffin cells and 5-HT release: potential implications for gut inflammation. Front Neurosci 2016;10:564. https://doi.org/ 10.3389/fnins.2016.00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kopeikina E, Ponomarev ED.. The role of platelets in the stimulation of neuronal synaptic plasticity, electric activity, and oxidative phosphorylation: possibilities for new therapy of neurodegenerative diseases. Front Cell Neurosci 2021;15:680126. https://doi.org/ 10.3389/fncel.2021.680126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rieder M, Gauchel N, Bode C, et al. Serotonin: a platelet hormone modulating cardiovascular disease. J Thromb Thrombolysis 2021;52:42–7. https://doi.org/ 10.1007/s11239-020-02331-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Skorobogatov K, De Picker L, Verkerk R, et al. Brain versus blood: a systematic review on the concordance between peripheral and central kynurenine pathway measures in psychiatric disorders. Front Immunol 2021;12:716980. https://doi.org/ 10.3389/fimmu.2021.716980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Allison DJ, Ditor DS.. The common inflammatory etiology of depression and cognitive impairment: a therapeutic target. J Neuroinflammation 2014;11:151. https://doi.org/ 10.1186/s12974-014-0151-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pathak S, Nadar R, Kim S, et al. The influence of kynurenine metabolites on neurodegenerative pathologies. Int J Mol Sci 2024;25:853. https://doi.org/ 10.3390/ijms25020853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aaldijk E, Vermeiren Y.. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: a narrative review. Ageing Res Rev 2022;75:101556. https://doi.org/ 10.1016/j.arr.2021.101556 [DOI] [PubMed] [Google Scholar]
- 64. O’Reilly K, O’Farrell K, Midttun O, et al. Kynurenic acid protects against reactive glial-associated reductions in the complexity of primary cortical neurons. J Neuroimmune Pharmacol 2021;16:679–92. https://doi.org/ 10.1007/s11481-020-09976-x [DOI] [PubMed] [Google Scholar]
- 65. Guillemin GJ, Meininger V, Brew BJ.. Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodegener Dis 2005;2:166–76. https://doi.org/ 10.1159/000089622 [DOI] [PubMed] [Google Scholar]
- 66. Santoro A, Ostan R, Candela M, et al. Gut microbiota changes in the extreme decades of human life: a focus on centenarians. Cell Mol Life Sci 2018;75:129–48. https://doi.org/ 10.1007/s00018-017-2674-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Monti JM. Serotonin control of sleep-wake behavior. Sleep Med Rev 2011;15:269–81. https://doi.org/ 10.1016/j.smrv.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 68. Nakamaru-Ogiso E, Miyamoto H, Hamada K, et al. Novel biochemical manipulation of brain serotonin reveals a role of serotonin in the circadian rhythm of sleep-wake cycles. Eur J Neurosci 2012;35:1762–70. https://doi.org/ 10.1111/j.1460-9568.2012.08077.x [DOI] [PubMed] [Google Scholar]
- 69. Miyamoto H, Nakamaru-Ogiso E, Hamada K, et al. Serotonergic integration of circadian clock and ultradian sleep-wake cycles. J Neurosci 2012;32:14794–803. https://doi.org/ 10.1523/JNEUROSCI.0793-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cortes-Altamirano JL, Olmos-Hernandez A, Jaime HB, et al. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 receptors and their role in the modulation of pain response in the central nervous system. Curr Neuropharmacol 2018;16:210–21. https://doi.org/ 10.2174/1570159X15666170911121027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kulikov AV, Gainetdinov RR, Ponimaskin E, et al. Interplay between the key proteins of serotonin system in SSRI antidepressants efficacy. Expert Opin Ther Targets 2018;22:319–30. https://doi.org/ 10.1080/14728222.2018.1452912 [DOI] [PubMed] [Google Scholar]
- 72. Lovinger DM. Communication networks in the brain: neurons, receptors, neurotransmitters, and alcohol. Alcohol Res Health 2008;31:196–214. [PMC free article] [PubMed] [Google Scholar]
- 73. Andrews PW, Bosyj C, Brenton L, et al. All the brain’s a stage for serotonin: the forgotten story of serotonin diffusion across cell membranes. Proc Biol Sci 2022;289:20221565. https://doi.org/ 10.1098/rspb.2022.1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huang Y, Thathiah A.. Regulation of neuronal communication by G protein-coupled receptors. FEBS Lett 2015;589:1607–19. https://doi.org/ 10.1016/j.febslet.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 75. Schott DA, Nicolai J, de Vries JE, et al. Disorder in the serotonergic system due to tryptophan hydroxylation impairment: a cause of hypothalamic syndrome? Horm Res Paediatr 2010;73:68–73. https://doi.org/ 10.1159/000271918 [DOI] [PubMed] [Google Scholar]
- 76. Bunin MA, Wightman RM.. Paracrine neurotransmission in the CNS: involvement of 5-HT. Trends Neurosci 1999;22:377–82. https://doi.org/ 10.1016/s0166-2236(99)01410-1 [DOI] [PubMed] [Google Scholar]
- 77. Fanciulli G, Ruggeri RM, Grossrubatscher E, et al. Serotonin pathway in carcinoid syndrome: clinical, diagnostic, prognostic and therapeutic implications. Rev Endocr Metab Disord 2020;21:599–612. https://doi.org/ 10.1007/s11154-020-09547-8 [DOI] [PubMed] [Google Scholar]
- 78. Melhem NJ, Taleb S.. Tryptophan: from diet to cardiovascular diseases. Int J Mol Sci 2021;22:9904. https://doi.org/ 10.3390/ijms22189904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fakhoury M. Revisiting the serotonin hypothesis: implications for major depressive disorders. Mol Neurobiol 2016;53:2778–86. https://doi.org/ 10.1007/s12035-015-9152-z [DOI] [PubMed] [Google Scholar]
- 80. Jordan LM, Sławińska U.. Modulation of rhythmic movement: control of coordination. Prog Brain Res 2011;188:181–95. https://doi.org/ 10.1016/B978-0-444-53825-3.00017-6 [DOI] [PubMed] [Google Scholar]
- 81. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. https://doi.org/ 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sanidad KZ, Rager SL, Carrow HC, et al. Gut bacteria-derived serotonin promotes immune tolerance in early life. Sci Immunol 2024;9:eadj4775. https://doi.org/ 10.1126/sciimmunol.adj4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wei GZ, Martin KA, Xing PY, et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A 2021;118:e2021091118. https://doi.org/ 10.1073/pnas.2021091118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shimada Y, Kinoshita M, Harada K, et al. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS One 2013;8:e80604. https://doi.org/ 10.1371/journal.pone.0080604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Roager HM, Licht TR.. Microbial tryptophan catabolites in health and disease. Nat Commun 2018;9:3294. https://doi.org/ 10.1038/s41467-018-05470-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li S. Modulation of immunity by tryptophan microbial metabolites. Front Nutr 2023;10:1209613. https://doi.org/ 10.3389/fnut.2023.1209613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cheng L, Wu H, Cai X, et al. A Gpr35-tuned gut microbe-brain metabolic axis regulates depressive-like behavior. Cell Host Microbe 2024;32:227–243.e6.e6. https://doi.org/ 10.1016/j.chom.2023.12.009 [DOI] [PubMed] [Google Scholar]
- 88. Salminen A. Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res Rev 2022;75:101573. https://doi.org/ 10.1016/j.arr.2022.101573 [DOI] [PubMed] [Google Scholar]
- 89. Modoux M, Rolhion N, Mani S, et al. Tryptophan metabolism as a pharmacological target. Trends Pharmacol Sci 2021;42:60–73. https://doi.org/ 10.1016/j.tips.2020.11.006 [DOI] [PubMed] [Google Scholar]
- 90. Liu JR, Miao H, Deng DQ, et al. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol Life Sci 2021;78:909–22. https://doi.org/ 10.1007/s00018-020-03645-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stone TW, Williams RO.. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends Pharmacol Sci 2023;44:442–56. https://doi.org/ 10.1016/j.tips.2023.04.006 [DOI] [PubMed] [Google Scholar]
- 92. Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 2014;6:263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang X, Hawkins BT, Miller DS.. Activating PKC-β1 at the blood-brain barrier reverses induction of P-glycoprotein activity by dioxin and restores drug delivery to the CNS. J Cereb Blood Flow Metab 2011;31:1371–5. https://doi.org/ 10.1038/jcbfm.2011.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang X, Hawkins BT, Miller DS.. Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. FASEB J 2011;25:644–52. https://doi.org/ 10.1096/fj.10-169227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chang CC, Lee PS, Chou Y, et al. Mediating effects of aryl-hydrocarbon receptor and RhoA in altering brain vascular integrity: the therapeutic potential of statins. Am J Pathol 2012;181:211–21. https://doi.org/ 10.1016/j.ajpath.2012.03.032 [DOI] [PubMed] [Google Scholar]
- 96. Teunis C, Nieuwdorp M, Hanssen N.. Interactions between tryptophan metabolism, the gut microbiome and the immune system as potential drivers of non-alcoholic fatty liver disease (NAFLD) and metabolic diseases. Metabolites 2022;12:514. https://doi.org/ 10.3390/metabo12060514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ye Z, Yue L, Shi J, et al. Role of IDO and TDO in cancers and related diseases and the therapeutic implications. J Cancer 2019;10:2771–82. https://doi.org/ 10.7150/jca.31727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li C, Zhao H.. Tryptophan and its metabolites in lung cancer: basic functions and clinical significance. Front Oncol 2021;11:707277. https://doi.org/ 10.3389/fonc.2021.707277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu M, Wang X, Wang L, et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol 2018;11:100. https://doi.org/ 10.1186/s13045-018-0644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Grifka-Walk HM, Jenkins BR, Kominsky DJ.. Amino acid TRP: the far out impacts of host and commensal tryptophan metabolism. Front Immunol 2021;12:653208. https://doi.org/ 10.3389/fimmu.2021.653208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Török N, Tanaka M, Vécsei L.. Searching for peripheral biomarkers in neurodegenerative diseases: the tryptophan-kynurenine metabolic pathway. Int J Mol Sci 2020;21:9338. https://doi.org/ 10.3390/ijms21249338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jones SP, Guillemin GJ, Brew BJ.. The kynurenine pathway in stem cell biology. Int J Tryptophan Res 2013;6:57–66. https://doi.org/ 10.4137/IJTR.S12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pallotta MT, Rossini S, Suvieri C, et al. Indoleamine 2,3-dioxygenase 1 (IDO1): an up-to-date overview of an eclectic immunoregulatory enzyme. FEBS J 2022;289:6099–118. https://doi.org/ 10.1111/febs.16086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Campbell BM, Charych E, Lee AW, et al. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci 2014;8:12. https://doi.org/ 10.3389/fnins.2014.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lanis JM, Alexeev EE, Curtis VF, et al. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol 2017;10:1133–44. https://doi.org/ 10.1038/mi.2016.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gargaro M, Scalisi G, Manni G, et al. Indoleamine 2,3-dioxygenase 1 activation in mature cDC1 promotes tolerogenic education of inflammatory cDC2 via metabolic communication. Immunity 2022;55:1032–50.e14. https://doi.org/ 10.1016/j.immuni.2022.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sujino T, Kanai T, Ono Y, et al. Regulatory T cells suppress development of colitis, blocking differentiation of T-helper 17 into alternative T-helper 1 cells. Gastroenterology 2011;141:1014–23. https://doi.org/ 10.1053/j.gastro.2011.05.052 [DOI] [PubMed] [Google Scholar]
- 108. Sujino T, London M, Hoytema van Konijnenburg DP, et al. Tissue adaptation of regulatory and intraepithelial CD4⁺ T cells controls gut inflammation. Science 2016;352:1581–6. https://doi.org/ 10.1126/science.aaf3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tanemoto S, Sujino T, Miyamoto K, et al. Single-cell transcriptomics of human gut T cells identifies cytotoxic CD4(+)CD8A(+) T cells related to mouse CD4 cytotoxic T cells. Front Immunol 2022;13:977117. https://doi.org/ 10.3389/fimmu.2022.977117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yoshimatsu Y, Sujino T, Miyamoto K, et al. Aryl hydrocarbon receptor signals in epithelial cells govern the recruitment and location of Helios(+) Tregs in the gut. Cell Rep 2022;39:110773. https://doi.org/ 10.1016/j.celrep.2022.110773 [DOI] [PubMed] [Google Scholar]
- 111. Martin F, Apetoh L, Ghiringhelli F.. Controversies on the role of Th17 in cancer: a TGF-β-dependent immunosuppressive activity? Trends Mol Med 2012;18:742–9. https://doi.org/ 10.1016/j.molmed.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 112. Nanki K, Fujii M, Shimokawa M, et al. Somatic inflammatory gene mutations in human ulcerative colitis epithelium. Nature 2020;577:254–9. https://doi.org/ 10.1038/s41586-019-1844-5 [DOI] [PubMed] [Google Scholar]
- 113. Zou W, Restifo NP.. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol 2010;10:248–56. https://doi.org/ 10.1038/nri2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Esser C, Rannug A, Stockinger B.. The aryl hydrocarbon receptor in immunity. Trends Immunol 2009;30:447–54. https://doi.org/ 10.1016/j.it.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 115. Ye J, Qiu J, Bostick JW, et al. The aryl hydrocarbon receptor preferentially marks and promotes gut regulatory T cells. Cell Rep 2017;21:2277–90. https://doi.org/ 10.1016/j.celrep.2017.10.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gagliani N, Amezcua Vesely MC, Iseppon A, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 2015;523:221–5. https://doi.org/ 10.1038/nature14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Takenaka MC, Gabriely G, Rothhammer V, et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci 2019;22:729–40. https://doi.org/ 10.1038/s41593-019-0370-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Campesato LF, Budhu S, Tchaicha J, et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-kynurenine. Nat Commun 2020;11:4011. https://doi.org/ 10.1038/s41467-020-17750-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang J, Simonavicius N, Wu X, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem 2006;281:22021–8. https://doi.org/ 10.1074/jbc.M603503200 [DOI] [PubMed] [Google Scholar]
- 120. Fallarini S, Magliulo L, Paoletti T, et al. Expression of functional GPR35 in human iNKT cells. Biochem Biophys Res Commun 2010;398:420–5. https://doi.org/ 10.1016/j.bbrc.2010.06.091 [DOI] [PubMed] [Google Scholar]
- 121. Sun T, Xie R, He H, et al. Kynurenic acid ameliorates NLRP3 inflammasome activation by blocking calcium mobilization via GPR35. Front Immunol 2022;13:1019365. https://doi.org/ 10.3389/fimmu.2022.1019365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang D, Wang W, Bing X, et al. GPR35-mediated kynurenic acid sensing contributes to maintenance of gut microbiota homeostasis in ulcerative colitis. FEBS Open Bio 2023;13:1415–33. https://doi.org/ 10.1002/2211-5463.13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. De Giovanni M, Tam H, Valet C, et al. GPR35 promotes neutrophil recruitment in response to serotonin metabolite 5-HIAA. Cell 2022;185:815–30.e19. https://doi.org/ 10.1016/j.cell.2022.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. De Giovanni M, Dang EV, Chen KY, et al. Platelets and mast cells promote pathogenic eosinophil recruitment during invasive fungal infection via the 5-HIAA-GPR35 ligand-receptor system. Immunity 2023;56:1548–60.e5. https://doi.org/ 10.1016/j.immuni.2023.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. De Giovanni M, Vykunta VS, Biram A, et al. Mast cells help organize the Peyer’s patch niche for induction of IgA responses. Sci Immunol 2024;9:eadj7363. https://doi.org/ 10.1126/sciimmunol.adj7363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Forrest CM, Youd P, Kennedy A, et al. Purine, kynurenine, neopterin and lipid peroxidation levels in inflammatory bowel disease. J Biomed Sci 2002;9:436–42. https://doi.org/ 10.1007/BF02256538 [DOI] [PubMed] [Google Scholar]
- 127. Forrest CM, Gould SR, Darlington LG, et al. Levels of purine, kynurenine and lipid peroxidation products in patients with inflammatory bowel disease. Adv Exp Med Biol 2003;527:395–400. https://doi.org/ 10.1007/978-1-4615-0135-0_46 [DOI] [PubMed] [Google Scholar]
- 128. Hartai Z, Klivenyi P, Janaky T, et al. Kynurenine metabolism in multiple sclerosis. Acta Neurol Scand 2005;112:93–6. https://doi.org/ 10.1111/j.1600-0404.2005.00442.x [DOI] [PubMed] [Google Scholar]
- 129. Oxenkrug GF. Increased plasma levels of xanthurenic and kynurenic acids in type 2 diabetes. Mol Neurobiol 2015;52:805–10. https://doi.org/ 10.1007/s12035-015-9232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schefold JC, Zeden JP, Fotopoulou C, et al. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant 2009;24:1901–8. https://doi.org/ 10.1093/ndt/gfn739 [DOI] [PubMed] [Google Scholar]
- 131. Kaya B, Melhem H, Niess JH.. GPR35 in intestinal diseases: from risk gene to function. Front Immunol 2021;12:717392. https://doi.org/ 10.3389/fimmu.2021.717392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cai Y, Kim DJ, Takahashi T, et al. Kynurenic acid may underlie sex-specific immune responses to COVID-19. Sci Signal 2021;14:eabf8483. https://doi.org/ 10.1126/scisignal.abf8483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zádori D, Veres G, Szalárdy L, et al. Alzheimer’s disease: recent concepts on the relation of mitochondrial disturbances, excitotoxicity, neuroinflammation, and kynurenines. J Alzheimers Dis 2018;62:523–47. https://doi.org/ 10.3233/JAD-170929 [DOI] [PubMed] [Google Scholar]
- 134. Lovelace MD, Varney B, Sundaram G, et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017;112:373–88. https://doi.org/ 10.1016/j.neuropharm.2016.03.024 [DOI] [PubMed] [Google Scholar]
- 135. Lim CK, Fernández-Gomez FJ, Braidy N, et al. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog Neurobiol 2017;155:76–95. https://doi.org/ 10.1016/j.pneurobio.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 136. Correia AS, Vale N.. Tryptophan metabolism in depression: a narrative review with a focus on serotonin and kynurenine pathways. Int J Mol Sci 2022;23:8493. https://doi.org/ 10.3390/ijms23158493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Tufvesson-Alm M, Schwieler L, Schwarcz R, et al. Importance of kynurenine 3-monooxygenase for spontaneous firing and pharmacological responses of midbrain dopamine neurons: relevance for schizophrenia. Neuropharmacology 2018;138:130–9. https://doi.org/ 10.1016/j.neuropharm.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Erhardt S, Schwieler L, Imbeault S, et al. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 2017;112:297–306. https://doi.org/ 10.1016/j.neuropharm.2016.05.020 [DOI] [PubMed] [Google Scholar]
- 139. Fathi M, Vakili K, Yaghoobpoor S, et al. Dynamic changes in kynurenine pathway metabolites in multiple sclerosis: a systematic review. Front Immunol 2022;13:1013784. https://doi.org/ 10.3389/fimmu.2022.1013784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pukoli D, Polyák H, Rajda C, et al. Kynurenines and neurofilament light chain in multiple sclerosis. Front Neurosci 2021;15:658202. https://doi.org/ 10.3389/fnins.2021.658202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yadav SK, Ito K, Dhib-Jalbut S.. Interaction of the gut microbiome and immunity in multiple sclerosis: impact of diet and immune therapy. Int J Mol Sci 2023;24:14756. https://doi.org/ 10.3390/ijms241914756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Martos D, Tuka B, Tanaka M, et al. Memory enhancement with kynurenic acid and its mechanisms in neurotransmission. Biomedicines 2022;10:849. https://doi.org/ 10.3390/biomedicines10040849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Szalardy L, Zadori D, Toldi J, et al. Manipulating kynurenic acid levels in the brain - on the edge between neuroprotection and cognitive dysfunction. Curr Top Med Chem 2012;12:1797–806. [PubMed] [Google Scholar]
- 144. Guo S, Vecsei L, Ashina M.. The L-kynurenine signalling pathway in trigeminal pain processing: a potential therapeutic target in migraine? Cephalalgia 2011;31:1029–38. https://doi.org/ 10.1177/0333102411404717 [DOI] [PubMed] [Google Scholar]
- 145. Büki A, Kekesi G, Horvath G, et al. A potential interface between the kynurenine pathway and autonomic imbalance in schizophrenia. Int J Mol Sci 2021;22:10016. https://doi.org/ 10.3390/ijms221810016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ruddick JP, Evans AK, Nutt DJ, et al. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med 2006;8:1–27. https://doi.org/ 10.1017/S1462399406000068 [DOI] [PubMed] [Google Scholar]
- 147. Nagy-Grócz G, Spekker E, Vécsei L.. Kynurenines, neuronal excitotoxicity, and mitochondrial oxidative stress: role of the intestinal Flora. Int J Mol Sci 2024;25:1698. https://doi.org/ 10.3390/ijms25031698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Stone TW. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J Neurochem 2020;152:627–49. https://doi.org/ 10.1111/jnc.14907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bauminger H, Gaisler-Salomon I.. Beyond NMDA receptors: homeostasis at the glutamate tripartite synapse and its contributions to cognitive dysfunction in schizophrenia. Int J Mol Sci 2022;23:8617. https://doi.org/ 10.3390/ijms23158617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tsuji A, Ikeda Y, Yoshikawa S, et al. The tryptophan and kynurenine pathway involved in the development of immune-related diseases. Int J Mol Sci 2023;24:5742. https://doi.org/ 10.3390/ijms24065742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Anderson G, Maes M.. Interactions of tryptophan and its catabolites with melatonin and the Alpha 7 nicotinic receptor in central nervous system and psychiatric disorders: role of the aryl hydrocarbon receptor and direct mitochondria regulation. Int J Tryptophan Res 2017;10:1178646917691738. https://doi.org/ 10.1177/1178646917691738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Majláth Z, Török N, Toldi J, et al. Memantine and kynurenic acid: current neuropharmacological aspects. Curr Neuropharmacol 2016;14:200–9. https://doi.org/ 10.2174/1570159x14666151113123221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hilmas C, Pereira EF, Alkondon M, et al. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci 2001;21:7463–73. https://doi.org/ 10.1523/JNEUROSCI.21-19-07463.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Schwarcz R, Bruno JP, Muchowski PJ, et al. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 2012;13:465–77. https://doi.org/ 10.1038/nrn3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wu Y, Zhang P, Fan H, et al. GPR35 acts a dual role and therapeutic target in inflammation. Front Immunol 2023;14:1254446. https://doi.org/ 10.3389/fimmu.2023.1254446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Otkur W, Liu X, Chen H, et al. GPR35 antagonist CID-2745687 attenuates anchorage-independent cell growth by inhibiting YAP/TAZ activity in colorectal cancer cells. Front Pharmacol 2023;14:1126119. https://doi.org/ 10.3389/fphar.2023.1126119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Otkur W, Wang J, Hou T, et al. Aminosalicylates target GPR35, partly contributing to the prevention of DSS-induced colitis. Eur J Pharmacol 2023;949:175719. https://doi.org/ 10.1016/j.ejphar.2023.175719 [DOI] [PubMed] [Google Scholar]
- 158. Behl T, Kaur I, Sehgal A, et al. The footprint of kynurenine pathway in neurodegeneration: janus-faced role in Parkinson’s disorder and therapeutic implications. Int J Mol Sci 2021;22:6737. https://doi.org/ 10.3390/ijms22136737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Parrott JM, Redus L, Santana-Coelho D, et al. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry 2016;6:e918. https://doi.org/ 10.1038/tp.2016.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J 2012;279:1356–65. https://doi.org/ 10.1111/j.1742-4658.2012.08485.x [DOI] [PubMed] [Google Scholar]
- 161. Colpo GD, Venna VR, McCullough LD, et al. Systematic review on the involvement of the kynurenine pathway in stroke: pre-clinical and clinical evidence. Front Neurol 2019;10:778. https://doi.org/ 10.3389/fneur.2019.00778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Pierozan P, Pessoa-Pureur R.. Cytoskeleton as a target of quinolinic acid neurotoxicity: insight from animal models. Mol Neurobiol 2018;55:4362–72. https://doi.org/ 10.1007/s12035-017-0654-8 [DOI] [PubMed] [Google Scholar]
- 163. Hezaveh K, Shinde RS, Klötgen A, et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 2022;55:324–40.e8. https://doi.org/ 10.1016/j.immuni.2022.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Cervantes-Barragan L, Chai JN, Tianero MD, et al. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8αα(+) T cells. Science 2017;357:806–10. https://doi.org/ 10.1126/science.aah5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tintelnot J, Xu Y, Lesker TR, et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 2023;615:168–74. https://doi.org/ 10.1038/s41586-023-05728-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Seo YD, Wargo JA.. From bugs to drugs: bacterial 3-IAA enhances efficacy of chemotherapy in pancreatic cancer. Cell Rep Med 2023;4:101039. https://doi.org/ 10.1016/j.xcrm.2023.101039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bender MJ, McPherson AC, Phelps CM, et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 2023;186:1846–62.e26. https://doi.org/ 10.1016/j.cell.2023.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Han JX, Tao ZH, Wang JL, et al. Microbiota-derived tryptophan catabolites mediate the chemopreventive effects of statins on colorectal cancer. Nat Microbiol 2023;8:919–33. https://doi.org/ 10.1038/s41564-023-01363-5 [DOI] [PubMed] [Google Scholar]
- 169. Lee JS, Cella M, McDonald KG, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol 2011;13:144–51. https://doi.org/ 10.1038/ni.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Li S, Bostick JW, Zhou L.. Regulation of innate lymphoid cells by aryl hydrocarbon receptor. Front Immunol 2017;8:1909. https://doi.org/ 10.3389/fimmu.2017.01909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Stockinger B, Shah K, Wincent E.. AHR in the intestinal microenvironment: safeguarding barrier function. Nat Rev Gastroenterol Hepatol 2021;18:559–70. https://doi.org/ 10.1038/s41575-021-00430-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kiss EA, Vonarbourg C, Kopfmann S, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011;334:1561–5. https://doi.org/ 10.1126/science.1214914 [DOI] [PubMed] [Google Scholar]
- 173. Qiu J, Guo X, Chen ZM, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity 2013;39:386–99. https://doi.org/ 10.1016/j.immuni.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Qiu J, Heller JJ, Guo X, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 2012;36:92–104. https://doi.org/ 10.1016/j.immuni.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008;453:65–71. https://doi.org/ 10.1038/nature06880 [DOI] [PubMed] [Google Scholar]
- 176. Rothhammer V, Borucki DM, Tjon EC, et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018;557:724–8. https://doi.org/ 10.1038/s41586-018-0119-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Rothhammer V, Mascanfroni ID, Bunse L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 2016;22:586–97. https://doi.org/ 10.1038/nm.4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 2016;22:598–605. https://doi.org/ 10.1038/nm.4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011;479:538–41. https://doi.org/ 10.1038/nature10554 [DOI] [PubMed] [Google Scholar]
- 180. Miyake S, Kim S, Suda W, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One 2015;10:e0137429. https://doi.org/ 10.1371/journal.pone.0137429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 2016;7:12015. https://doi.org/ 10.1038/ncomms12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Chen J, Chia N, Kalari KR, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 2016;6:28484. https://doi.org/ 10.1038/srep28484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Noto D, Miyake S.. Gut dysbiosis and multiple sclerosis. Clin Immunol 2022;235:108380. https://doi.org/ 10.1016/j.clim.2020.108380 [DOI] [PubMed] [Google Scholar]
- 184. Miyauchi E, Kim SW, Suda W, et al. Gut microorganisms act together to exacerbate inflammation in spinal cords. Nature 2020;585:102–6. https://doi.org/ 10.1038/s41586-020-2634-9 [DOI] [PubMed] [Google Scholar]
- 185. Miyauchi E, Shimokawa C, Steimle A, et al. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat Rev Immunol 2023;23:9–23. https://doi.org/ 10.1038/s41577-022-00727-y [DOI] [PubMed] [Google Scholar]
- 186. Duc D, Vigne S, Bernier-Latmani J, et al. Disrupting myelin-specific Th17 cell gut homing confers protection in an adoptive transfer experimental autoimmune encephalomyelitis. Cell Rep 2019;29:378–90.e4. https://doi.org/ 10.1016/j.celrep.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 187. Schnell A, Huang L, Singer M, et al. Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity. Cell 2021;184:6281–98.e23. https://doi.org/ 10.1016/j.cell.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Miyamoto K, Sujino T, Harada Y, et al. The gut microbiota-induced kynurenic acid recruits GPR35-positive macrophages to promote experimental encephalitis. Cell Rep 2023;42:113005. https://doi.org/ 10.1016/j.celrep.2023.113005 [DOI] [PubMed] [Google Scholar]
- 189. Tomura M, Yoshida N, Tanaka J, et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc Natl Acad Sci U S A 2008;105:10871–6. https://doi.org/ 10.1073/pnas.0802278105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kaya B, Doñas C, Wuggenig P, et al. Lysophosphatidic acid-mediated GPR35 signaling in CX3CR1(+) macrophages regulates intestinal homeostasis. Cell Rep 2020;32:107979. https://doi.org/ 10.1016/j.celrep.2020.107979 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.