Abstract
Adoptive T cell therapy is a pivotal strategy in cancer immunotherapy, demonstrating potent clinical efficacy. However, its limited durability often results in primary resistance. High-throughput screening technologies, which include both genetic and non-genetic approaches, facilitate the optimization of adoptive T cell therapies by enabling the selection of biologically significant targets or substances from extensive libraries. In this review, we examine advancements in high-throughput screening technologies and their applications in adoptive T cell therapies. We highlight the use of genetic screening for T cells, tumor cells, and other promising combination strategies, and elucidate the role of non-genetic screening in identifying small molecules and targeted delivery systems relevant to adoptive T cell therapies, providing guidance for future research and clinical applications.
Keywords: High-throughput screening, Adoptive T cell therapies, Genetic screening, Non-genetic screening, CRISPR screening, CAR-T cells, TCR-T cells
Introduction
Cancer immunotherapy has advanced rapidly in recent years, with the interactions between immune cells and tumor cells playing a crucial role in its effectiveness [1]. Adoptive cell transfer (ACT) involves collecting immune cells from either the patient or a donor, culturing or modifying them ex vivo, and then reintroducing them into the patient to enhance their ability to eliminate tumor cells. Particularly noteworthy in this area are T cell-based ACTs, such as Tumor Infiltrating Lymphocytes (TILs), engineered T Cell Receptor (TCR) T cell therapy, and Chimeric Antigen Receptor (CAR) T cell therapy [2]. These approaches have shown significant clinical effectiveness in treating hematologic malignancies [3]. However, adoptive T cell therapy is not always effective against certain hematologic malignancies and most solid tumors, often leading to the development of primary resistance. Possible mechanisms for this resistance include impaired T cell proliferation and differentiation, reduced cytotoxicity, the emergence of exhausted T cell phenotypes [4, 5], as well as factors such as downregulation of antigens and internal inhibitory pathways in tumor cells [6]. Consequently, understanding the positive and negative signals that govern the interaction between T cells and tumor cells, and thereby enhancing T cell effector functions, is a critical research focus in cancer immunotherapy.
High-throughput screening technology offers an unbiased platform for the large-scale identification of optimal genetic targets and other substances, encompassing both genetic and non-genetic screening approaches. This technology significantly enhances the efficiency and scope of research. Genetic screening involves designing and introducing extensive gene editing libraries into cell pools to identify biologically significant sites for further validation through functional experiments. This approach overcomes the limitations of editing single gene sites and substantially advances our understanding of cancer genomics [7]. The protein engineering of conventional zinc finger nucleases and transcription activator-like effector nucleases requires a complex design and construction process to determine the amino acid substitutions needed for selective binding to target genomic sequences. In contrast, the CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated 9) system, known for its flexibility and editing efficiency, has emerged as the leading high-throughput genetic screening method currently in use [8]. As CRISPR technology continues to evolve, new methods such as base editors (BEs) and epigenetic editors—techniques that do not rely on double-strand breaks—offer viable solutions to technological challenges. These advancements have found extensive applications in adoptive T cell therapy and related strategies [9]. By integrating genetic and non-genetic screening approaches, researchers can deepen their understanding of drug combination therapies and targeted delivery systems. This review highlights the latest technologies in high-throughput genetic and non-genetic screening and discusses recent developments in applying these technologies to T cells, tumor cells under T cell pressure, and other promising combination strategies. It aims to lay the groundwork for future clinical advancements.
Current adoptive T cell therapies’ applications and limitations
Adoptive T cell therapy primarily includes three modalities: TILs, TCR-T, and CAR-T therapies. TIL therapy involves the ex vivo expansion of tumor-reactive T cells isolated after tumor resection, enhancing T cell infiltration and cytotoxicity against solid tumors with a generally favorable safety profile. However, its highly personalized nature results in elevated production costs, and clinical studies have largely focused on melanoma, where objective response rates are generally lower compared to CAR-T and TCR-T therapies [10]. A clinical trial (NCT03215810) involving non-small cell lung cancer patients demonstrated promising results: among 13 evaluable patients, 3 exhibited confirmed responses, and 11 experienced tumor burden reduction, with a median best change of 35%, indicating notable clinical efficacy [11]. To mitigate the limitations imposed by the external microenvironment and internal inhibitory signals, genetic ablation of RASA2 in T cells has been shown to enhance their antigen sensitivity and persistence [12].
CAR-T therapy has shown significant potential in treating various hematologic malignancies and select solid tumors, particularly in B-cell malignancies. To date, six CAR-T therapeutic products have been approved by the U.S. Food and Drug Administration (FDA), demonstrating remarkable clinical efficacy and safety profiles [13]. However, CAR-T therapy also faces challenges such as severe cytokine release syndrome and neurotoxicity, which require ongoing management. TCR-T therapy, on the other hand, can recognize both cell surface antigens and intracellular tumor antigens presented by peptide-major histocompatibility complexes, making it particularly suitable for targeting solid tumors. The FDA has approved Kimmtrak for treating patients with metastatic uveal melanoma, highlighting the clinical potential of this approach [14]. Nevertheless, TCR-T therapy faces challenges such as potential off-target effects and the need for precise antigen targeting. Gene editing technologies have become crucial in addressing the limitations of both CAR-T and TCR-T therapies. For example, CRISPR/Cas9-mediated knockout of programmed cell death 1 (PD-1) in mouse models is a prevalent method to enhance anti-tumor responses and reduce CAR-T cell exhaustion. Additionally, CAR-T cells with the CAR construct integrated into the PD-1 locus have demonstrated promising efficacy in both preclinical and clinical studies [15]. In TCR-T cell therapy, CRISPR/Cas9 technology has been used to simultaneously ablate endogenous TCR genes while introducing neoantigen-specific TCR genes. Clinical trials (NCT03970382) have shown that some patients exhibit disease remission with this approach [16]. Overall, while current adoptive T cell therapies offer substantial promise, they also face specific challenges and limitations. Advances in gene editing and continued research are essential for optimizing these therapies and addressing their current constraints.
Despite the impressive preclinical anti-tumor efficacy and clinical successes of adoptive T cell therapies, several significant challenges persist. The most prevalent issues include the development of resistance or post-treatment relapse, which limits therapeutic efficacy. These outcomes are primarily due to the functional decline of persistently infiltrating T cells and mechanisms of antigen escape or downregulation [17]. Antigen-positive relapse or resistance is often associated with T cell exhaustion, suboptimal CAR potency, and reduced tumor responsiveness to CAR-T cells. For example, studies have shown that CARs with 4-1BB costimulatory domains exhibit enhanced persistence compared to those with CD28 costimulatory domains [18]. Conversely, antigen-negative relapse or recurrence may be caused by genetic mutations or trogocytosis-mediated antigen loss [19]. To address antigen-positive relapse or resistance, strategies such as T cell functional screening, CAR structural optimization, and intratumoral functional assessment are promising. Additionally, investigating antigen presentation mechanisms and other relevant intracellular pathways may offer strategies to mitigate antigen-negative relapse or resistance. Furthermore, adverse events such as cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, and off-target tumor toxicity raise safety concerns regarding cellular therapies [20]. In conclusion, employing high-throughput screening methodologies to identify viable gene targets for promotion or inhibition, as well as potential drugs or delivery vectors, is a crucial direction for advancing CAR-T cell therapy. These efforts aim to enhance the efficacy and safety of CAR-T treatments, improve their accessibility, and ultimately broaden the therapeutic landscape of this promising approach.
Advanced technologies of high-throughput screening
Genetic screening
Mechanism and features
Genetic screening, an effective tool for identifying unknown targets and mechanisms, is distinguished by its high throughput and efficiency, encompassing small interfering RNA (siRNA), short hairpin RNA (shRNA), and, most prominently, CRISPR screening. siRNAs are synthetically produced short RNA fragments typically employed in independent phenotypic screenings using 96-well or 384-well culture plates. Alternatively, commercially available siRNA libraries, where each siRNA possesses a unique barcode, can be utilized. The efficacy of specific siRNAs is determined by quantifying their respective barcodes. However, this approach is limited in scope, operationally complex, and cost-prohibitive for large-scale experiments [21]. shRNAs offer a more streamlined and efficient approach through the construction of mixed libraries using viral vectors, with data acquisition via deep sequencing. However, RNAi techniques, including shRNAs, face limitations in high-throughput applications due to incomplete gene expression suppression, resulting in unstable phenotypes. Additionally, high off-target effects compromise the accuracy of these methods, rendering their practical application suboptimal [22]. In contrast, the currently predominant CRISPR screening methodology offers versatile applications including knockout (KO), knock-in (KI), activation, and inhibition, providing diverse and robust modes of action. Moreover, the design and construction of single guide RNA (sgRNA) libraries for CRISPR screening are relatively straightforward. Furthermore, CRISPR screening extends beyond targeting gene coding regions, enabling the interrogation of non-coding sequences such as promoters, enhancers, and long non-coding RNAs (lncRNAs), thereby elucidating their roles in gene expression regulation [23]. Additionally, the development of multiplex CRISPR libraries utilizing 4gRNA-combo (combinations of four gRNAs) enables simultaneous targeting of multiple gene pairs within a single cell, thereby enhancing the capacity to explore combinatorial effects of gene interactions [24]. However, the accessibility of CRISPR screening applications remains limited, with constraints on delivery and expression in certain cell types or organisms. For instance, large-scale expression in primary tumor cells and primary T cells continues to pose significant challenges, thereby restricting its application in adoptive T cell therapies for cancer treatment [25]. A comparative analysis of these three genetic screening methodologies is presented in Table 1.
Table 1.
Comparisons of different screening methods
| Screening methods | Advantages | Disadvantages | Research purpose |
|---|---|---|---|
| siRNA screening |
1. Easy to construct with established technology 2. Rapid onset of action |
1. Limited to independent phenotypic screening on culture plates or the use of commercial libraries, resulting in a narrow scope and high costs 2. Lower stability and higher off-target effects 3. Limited to targeting mRNA molecules 4. The interference effects of different genes vary across different cell types |
1. Ideal for short-term gene silencing experiments 2. Quick validation of gene function |
| shRNA screening |
1. Suitable for mixed library screening 2. Enhanced stability 3. Extended duration of action |
1. Limited to gene silencing; cannot fully suppress gene expression to achieve a stable phenotype during high-throughput screening 2. Some off-target effects still exist 3. Prolonged expression may trigger non-specific immunity, resulting in cytotoxicity 4. Limited to targeting mRNA molecules 5. The interference effects of different genes vary across different cell types |
1. Ideal for long-term gene silencing experiments 2. Enables controlled or reversible silencing experiments of gene expression |
| CRISPR screening |
1. High gene editing efficiency and low off-target effects 2. Cost-effective and suitable for large-scale gene screening 3. Supports multiple editing forms, including KO, KI, activation, and inhibition 4. Capable of targeting non-coding sequences 5. Possesses potential for multiplex gene editing |
1. CRISPR delivery and expression are restricted in certain cells or organisms 2. Requires a high level of technical skill 3. Unable to dynamically regulate gene expression 4. Chromosomal toxicity may arise following gene editing 5. Off-target effects continue to be a concern |
1. Applications for precise and permanent gene alteration 2. Suitable for screening non-coding sequences 3. Multiplex gene pair screening, expanding the ability to explore combinations of gene interactions and behaviors |
Libraries for whole-genome or large-scale functionally relevant gene edits are created and transduced into target cell pools, notably T cells and tumor cells. Screening conditions are applied, such as co-culturing tumor cells with T cells, continuous antibody or therapeutic drug stimulation, or in vivo screening in immune-competent or immune-deficient NSG mice, with blank or conditional controls, or occasionally using survival pressure for comparison. In the event of altered phenotypes due to gene editing, cells can be categorized using Fluorescence-Activated Cell Sorting (FACS) based on varying surface markers or cytokines produced. Following that, high-throughput sequencing technology counts sgRNAs or other gene editing components in each group, reads barcodes after Polymerase Chain Reaction (PCR) amplification, and analyzes the depletion or enrichment of sgRNAs in gene-edited cells. This process helps uncover genes linked to T cell proliferation, activation, differentiation, and cytotoxicity in tumor immunity, as well as mechanisms behind tumor resistance to adoptive T cell therapy [7].
Genetic screening is categorized into loss-of-function (LOF) and gain-of-function (GOF) screening. Traditional siRNA, shRNA, and CRISPR/Cas9 systems typically silence or knock out genes within cells, aiming to identify genes in tumor cells sensitive to resistance or negative regulators of anti-tumor functions in other immune cells. Although LOF screening enhances cell therapy, the translational potential of knocking in large gene cassettes to rewrite cellular programming is more significant. GOF screening chiefly utilizes CRISPR-mediated gene activation (CRISPRa) methods for temporary gene transcription activation. Nevertheless, the large size and immunogenicity of many Cas9 transcription activator fusion proteins restrict their use in screenings, necessitating a robust CRISPR KI system for GOF screening. Roth et al. developed a parallel targeting library of KI protein constructs at the same site, utilizing barcodes and tracking of large non-viral DNA templates. This identified genes enhancing T cell adaptability and allowed for efficient and precise GOF pooled screening [26]. Employing a lentiviral library with barcoded human open reading frames (ORFs) enables genome-scale GOF screening in primary human CD4+ and CD8+ T cells. A novel single-cell sequencing method, integrated with direct ORF capture, has been developed, broadening the scale and scope of GOF applications [27]. The ongoing advancements in genetic screening technology have been instrumental in laying the groundwork for the evolution of tumor immunotherapy techniques.
Downstream analysis, a vital step post-target identification, typically uses gene set enrichment analysis (GSEA) to identify functional pathways in T cell-mediated tumor cell cytotoxicity and validate key phenotypes of related functions. For instance, employing CRISPR/Cas9 for cellular engineering or small molecule drugs to modulate targets can confirm their effects on positive aspects like T cell activation, differentiation, and tumor cytotoxicity, as well as on negative states like exhaustion. Additionally, these findings are implemented in adoptive T cell therapies or other combination strategies, aiming to enhance the efficacy of adoptive T cell therapy. The advancement of high-throughput sequencing and multi-omics technologies has propelled the development of screening precision and research depth [28]. Most CRISPR screening methods depend on bulk sgRNA readouts from selected cell phenotypes, thus often neglecting the heterogeneity among cells. Contrastingly, single-cell CRISPR (scCRISPR) screening methods combine gene perturbations with single-cell RNA sequencing (scRNA-seq), categorizing into four main types based on different single-cell technologies: scCRISPR with RNA-seq, scCRISPR with Assay for Targeting Accessible-Chromatin with high-throughput sequencing, scCRISPR with proteomics, and imaging-based scCRISPR [29]. These methods enable transcriptomic analysis of individual genetic perturbations within complex cell pools, offering detailed molecular insights into targeted disruptions at the single-cell level, and have achieved notable progress in T cell research [25, 30–34] (Fig. 1).
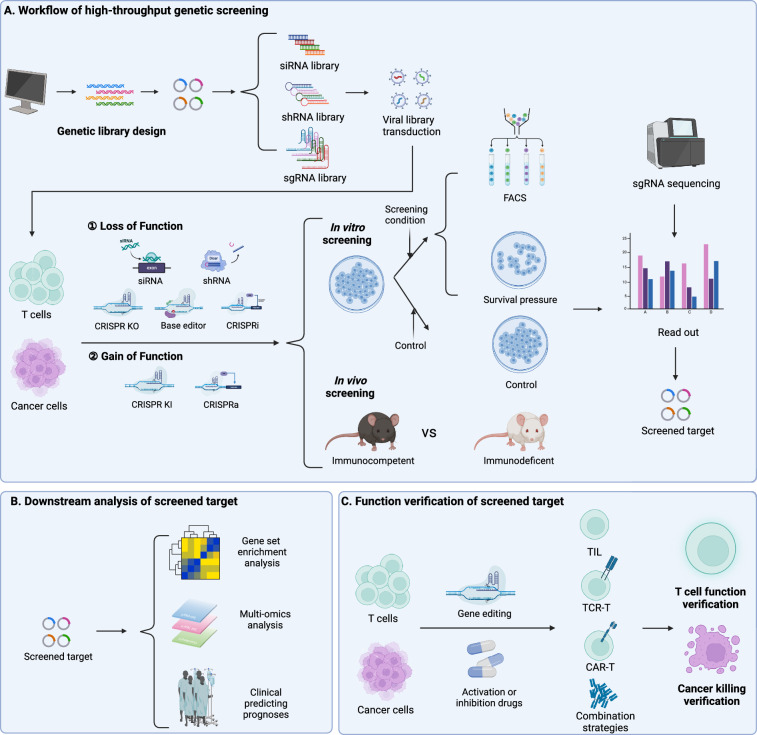
Fig. 1.
Advanced technologies of high-throughput genetic screening. A Libraries of siRNA, shRNA, and sgRNA were designed and constructed for delivery into tumor cells or T cells using viral vectors. The screening was categorized into LOF and GOF approaches, utilizing siRNA, shRNA, CRISPR KO, base editing, or CRISPR interference (CRISPRi) for the former, and CRISPR KI and CRISPRa for the latter. In vitro screening involved comparing experimental groups under specific conditions with control groups or sorting based on surface markers or other phenotypes using FACS. For in vivo screening, comparisons were made between immunocompetent and immunodeficient groups, with the identification of screened targets achieved through sgRNA sequencing. B Downstream analysis of the screened targets primarily encompassed GSEA, multi-omics analysis, and clinical prognosis prediction. C In T cells and tumor cells, genetic editing on screened targets or the application of activating or inhibitory drugs aims to enhance adoptive T cell therapy and other promising combination strategies, affirming T cell functionality and tumoricidal efficacy
Moreover, enhancing the sensitivity and efficiency of high-throughput screening technologies while minimizing potential biases remains a critical technical challenge. The large-scale nature of these technologies, combined with their high result specificity and diverse screening targets and applications, means that even minor sample variations can significantly influence result analysis. Consequently, these technologies face challenges such as reduced measurability due to high interference characteristics and limited reproducibility stemming from false-positive signals. The velocity and volume of data generation in high-throughput methodologies significantly surpass those of traditional experimental techniques. Therefore, standardization and reproducibility are paramount for ensuring data reliability. As screening throughput expands, maintaining sample integrity and minimizing interference from extraneous factors require substantial technical discourse.
Emerging CRISPR screening tools
Traditional CRISPR/Cas9-mediated genome editing induces targeted DNA double-strand breaks (DSBs), resulting in numerous indel edits and genetic instability. Deactivated Cas9 (dCas9) or single-strand cutting nickase Cas9 (nCas9) can prevent DSBs, thus minimizing genetic mutation risks. DNA BEs are precise technologies for substituting single nucleotides, by fusing dCas9 or nCas9 with deaminases targeting single-strand DNA. Anchored by sgRNA to target sites, they induce GC-to-TA or AT-to-GC mutations for accurate nucleotide replacement in genes [35]. CRISPR screening uncovers genes impacting immune cell functions, but different alleles within the same gene can influence protein structure and function differently. Thus, base editing screening provides high-resolution analysis of individual genes' key nucleotides, facilitating precise modulation of protein function [36–38]. Additionally, CRISPRi incorporates the Kruppel-associated box transcription repression domain into dCas9, directing the complex to the transcription start site (TSS) via sgRNA, thereby reducing the expression of target genes. Conversely, CRISPRa employs transcriptional activators like VP64 at the TSS, with dCas9 engineered to fuse with various transcriptional activation domains such as RTA, VP64, HSF1, and p65. This fusion strategy enables the upregulation of specific genes across different cell types, selectively activating endogenous gene transcription mechanisms and enhancing target region expression [39]. Moreover, the fusion of dCas9 with epigenetic modifiers like DNA methyltransferases and ten-eleven translocation enzymes facilitates the development of epigenetic editing systems. This non-DNA-cutting method is reversible and avoids permanent genomic alterations. Unlike traditional gene knockout techniques, it minimizes severe extracellular effects and intrinsic phenotypic ambiguity. This approach can also identify genes that may be inactive under test conditions but influence phenotypic expression. Combining CRISPRa and CRISPRi screening can complement each other to reveal both positive and negative regulatory factors within cells [25, 40, 41].
Analogous to Cas9, Cas12a induces DSBs for gene editing but exhibits a more relaxed binding affinity to the target genome, demonstrating enhanced reversibility in enzyme-target sequence interactions. Cas12a requires only CRISPR RNA (crRNA) for its expression, eliminating the need for tracrRNA and reducing system complexity. This simplification results in lower cloning costs and mitigates delivery challenges. Consequently, CRISPR-Cas12a has emerged as a potent and promising gene editing tool. Additionally, the recently discovered Cas13d demonstrates precise message RNA (mRNA) targeting and degradation capabilities. Its high efficiency in gene expression interference positions Cas13d as a potential successor to traditional RNA interference technologies. Through the construction of multi-crRNA expression arrays, Cas13d exhibits the capacity for multi-gene expression regulation, offering an expansive editing scope. As a compact RNA editing system, Cas13d offers significant advantages in vector delivery [42].
Non-genetic screening
In adoptive T cell therapy, the integration of genetic and non-genetic screening underpins combination therapy and affinity research. Large-scale drug sensitivity screening reveals the mechanisms of drug therapy combined with tumor immunotherapy at the genomic level. Tumor cells labeled with luciferase (Luc), T cells, and a drug library are co-cultured in plates like 96-well or 384-well. By measuring Luc activity, drugs that enhance adoptive T cell therapy and improve tumor cell killing are selected. CRISPR screening then identifies specific mechanisms, uncovering the immunomodulatory traits of cancer drugs.
Additionally, non-genetic screening of chemical non-viral delivery systems is performed using synthetic chemical combinatorial libraries. Initially, physicochemical properties are characterized and screened in vitro using techniques such as dynamic light scattering, transmission electron microscopy, and chromatography. These methods assess properties like lipid nanoparticle (LNP) size, zeta potential, and pKa. Following this, a combination of in vitro and in vivo experiments monitors protein expression in external cells and the system’s delivery targeting to internal organs and cells. This approach helps identify the optimal structure of innovative non-viral delivery systems, enhancing targeting capabilities and providing a platform for thorough characterization and screening of the delivery system’s specificity and efficiency.
Genetic screening applied for T cells
T cell screening with cytotoxic functions
Cytotoxic CD8+ T cells are crucial in tumor immunity, integrating both positive and negative signals to facilitate functions such as proliferation, activation, infiltration, and targeted cytotoxicity [43]. High-throughput genetic screening of T cells is conducted through TCR stimulation with anti-CD3/CD28 and IL-2, co-culturing with tumor cells (in vitro), or implantation in tumor-bearing mice (in vivo). Phenotypes are sorted using FACS, or cell groups under various screening conditions are sequenced and compared to identify genes regulating specific functional phenotypes. Single gene editing has been employed to enhance TIL and CAR-T therapies, demonstrating increased success rates in treating tumors with adoptive T cell therapy, as validated in both in vitro and in vivo models (Table 2 and Fig. 2).
Table 2.
Representative Studies of Genetic Screening for T cell screening with cytotoxic functions
| Screening methods | Screening cells | Screening model | Library design | Screening strategies | Comparison and readout | Screened targets | Function of Screened target | Refs. |
|---|---|---|---|---|---|---|---|---|
| CRISPR KO | OT-I T cells | in vivo | Activates differentially expressed gene library between CD8 T cells and naïve CD8 T cells | T cells were injected into B16-OVA mice, and the distribution of T cells was observed | Compare the lung/liver group, the stay in the circulation group, and the untreated group | St3gal1 | St3gal1 regulates LFA-1 mediated CAR-T cell migration | [45] |
| CRISPR KO | OT-I T cells | in vitro | Gene library of PI3K-related pathways | TCR and scICAM were stimulated, FACS sorts ICAM expression | Compare ICAMpos group and ICAMneg group | RASA3 | RASA3 is a negative regulator of LFA-1-mediated adhesion in T cells | [46] |
| CRISPR KO | OT-I T cells | in vitro | Genome-wide library | TCR stimulation, FACS sorts CFSE expression | Compare dividing T cells and non-dividing T cells | RASA2 | RASA2 ablation in T cells boosts antigen sensitivity and long-term function | [12] |
| CRISPRi | Jurkat and primary human T cells | in vitro | Genome-wide library | TCR stimulation, FACS sorts GFP expression | Compare GFPhigh group and GFPlow group | NAMPT | NAMPT is required for T cell activation | [47] |
| CRISPRa | Primary human CD4+ and CD8+ T cells | in vitro | Human ORF Library | Pre-stimulation, FACS sorts CFSE expression | Compare dividing T cells and non-dividing T cells | LTBR | LTBR increase T-cell effector functions, and resistance to exhaustion in chronic stimulation | [27] |
| CRISPR KO | OT-I T cells | in vitro | Kinase-related gene library | TCR stimulation, FACS sorting cell proliferation, CD62L, ROS, γH2AX expression | Compare the four phenotypic high-expression and low-expression groups | P38 | p38 kinase is a central regulator of all four phenotypes, including cell expansion, differentiation, oxidative stress, and genomic stress | [48] |
| CRISPRi | Jurkat t cells | in vitro | Genome-wide libraries | TCR stimulation, FACS sorting CD69 | Compare CD69high group and CD69lowgroup | FAM49B | FAM49B is a key regulator of actin dynamics and T cell activation | [49] |
| Base editing | Primary human CD8+ T cells | in vitro | T cell activity-related gene libraries | TCR stimulation, FACS sorting TNF, IFN-γ, PD-1, CD25 expression | Compare the production of TNF and IFN-γ, the high and low surface expressions of CD25 and PD-1 | PIK3CD, VAV1, LCP2, PLCG1,DGKZ | Reduce T cell cytotoxic function | [36] |
| CRISPR KO | OT-I T cells |
in vivo in vitro |
Genome-wide libraries |
T cells are injected into Rag1- mice bearing E0771-mCh-OVA to observe T cell killing T cells were co-cultured with SIINFEKL-pulsed E0771 cells, and FACS sorted CD107a expression |
Direct readout to survival T cells in vivo Compare CD107ahigh group and CD107alow group |
DHX37 | DHX37 modulates NF-kB to suppress effector functions, cytokine production, and T cell activation | [50] |
| CRISPRa | OT-I T cells | in vitro | Genome-wide libraries | T cells were co-cultured with SIINFEKL-pulsed E0771 cells, and FACS sorted CD107a expression | Compare CD107ahigh group and CD107alow group | PRODH2 | PRODH2 enhances the metabolic and immune functions of CAR-T cells against cancer | [41] |
| CRISPR KO | Primary human CD4+ T cells | in vitro | GPCR’s gene library | TCR activation and sorting of CD69, IL-2 and IFN-γ expression by FACS | Compare the the high and low surface expressions of CD69, the production of IL-2 and IFN-γ | S1PR1, GPR183 | Contribute to T cell activation | [51] |
| CRISPR KO | Jurkat T cells | in vitro | Genome-wide libraries | FASL stimulation | Compare the control group and the FASL stimulation group | EBF4 | EBF4 modulates FAS-mediated apoptosis and promotes cytotoxic function | [52] |
| shRNA | CD8+ T cells | in vitro | shRNA libraries associated with chromatin | FACS sorts CD4 expression | Compare CD4+ group and CD4− group | CAF-1 | CAF-1 stably repress expression of Cd4 gene | [53] |
| CRISPRa and CRISPRi | Primary human CD8+ T cell | in vitro | A gene library of epigenetic modifiers associated with T cell status | FACS sorts CCR7 expression | Compare CCR7high group and CCR7low group | BATF3 | BATF3 promotes specific features of memory T cells | [54] |
| CRISPR KO | OT-I T cells | In vivo | Metabolism-related gene libraries | Injected into mice, FACS sorts KLRG1−CD127+ (MP) and KLRG1+CD127− (TE) phenotypes | Compare the MP group, TE group and non-injected cell group | Slc7a1, Slc38a2 | Slc7a1 and Slc38a2 dampene the magnitude of TMEM differentiation through modulating mTORC1 signaling | [57] |
| CRISPR KO | OT-I T cells | in vivo | A library that targets proteins involved in epigenetic modifications | Injected into mice, FACS sorts TE, MP, and normal phenotypes | Compare the MP group, TE group and non-injected cell group | cBAF | cBAF inhibit Tmem cells formation | [56] |
| CRISPR KO | OT-I T cells | in vivo | Genome-wide libraries | TCR was repeatedly stimulated to observe T cell changes | Compare the IL-2 stimulation alone group and the α-CD3 and IL-2 stimulation group | INO80, Arid1a | INO80 and BAF chromatin inhibit remodeling complexes improved T cell persistence | [59] |
| Single-cell CRISPR KO | OT-I T cells | in vivo | Transcription factor gene library | B16-OVA mice were injected to observe T cell survival | Direct sequencing reads after 7 days | IKAROS ETS1 | The IKAROS–TCF-1 axis in Tpex cell quiescence exit and the ETS1–BATF axis limits Tex cell generation | [30] |
| CRISPR KO | CD8+ P14 cells | in vitro | TF library | Co-cultured with CD11C+DC cells for acute and chronic GP33 and IL-2 stimulation | Compare T cells on the second day versus the seventh day | BHLHE40 | BHLHE40 regulates T cell exhaustion | [60] |
| CRISPR KO | Primary human CD8+ T cell | in vitro | Epigenetic Regulator Library | Acute and chronic TCR stimulation was performed in vitro, and FACS sorted PD1+/TIM3+ expression | Compare Acute and chronic stimulation groups | mSWI/SNF | mSWI/SNF family chromatin remodeling complexes direct T cell activation and exhaustion | [61] |
| CRISPR KO | 68–41 T cells | in vitro | Genome-wide libraries | After in vitro culture, FACS sorts PD-1 expression | Compare PD-1high group and PD-1low group | Fut8 | Fut8 induce cell-surface expression of PD-1 and T cell exhaustion | [62] |
St3gal1 ST3 β-galactoside α-2,3-sialyltransferase 1, RASA3 Ras gtpase activating proteins3, RASA2 Ras gtpase activating proteins2, NAMPT nicotinamide phosphoribosyltransferase, LTBR lymphotoxin-β receptor, P38 a class of mitogen-activated protein kinases, FAM49B family with sequence similarity 49 member B, PIK3CD phosphoinositide-3-kinase catalytic subunit delta, DHX37 DEAH-Box Helicase 37, PRODH2 Proline Dehydrogenase 2, S1PR1 sphingosine-1-phosphate receptor, GPR183 G Protein-Coupled Receptor 183, EBF4 early B cell factor 4, CAF-1 chromatin assembly factor, BATF3 Basic leucine zipper transcription factor ATF-like 3, Slc7a1 and Slc38a2 amino acid transporters, cBAF mammalian canonical Brg1/Brg-associated factor, INO80 INO80 Complex ATPase Subunit, Arid1a AT-Rich Interaction Domain 1A, IKAROS Family Zinc Finger Protein 2, ETS1 E-twenty six1, BHLHE40 Basic Helix-Loop-Helix Family Member E40, mSWI/SNF mammalian SWItch/Sucrose Non-Fermentable, Fut8 Fucosyltransferase 8
Fig. 2.
T cell screening with cytotoxic functions. After introducing the viral library, T cells with gene edits are obtained. These edited T cells can be stimulated via TCR activation using α-CD3/CD28 and IL-2 or co-cultured directly with tumor cells under specific conditions. For evaluating T cell homing, screening for ICAM-1 or analyzing in vivo distribution is performed. Cell proliferation and activation are assessed using the CFSE method and CD69 expression, while CD107a and IFN-γ serve as classic phenotypic markers of T cell cytotoxicity. The Fas pathway also plays a crucial role in cellular apoptosis. CD8+ T cells TEFF and TMEM, with CCR7 and CD62L aiding in identifying regulators of TMEM differentiation and maintenance. TEFF cells can be further divided into short-lived effector cells (TE; KLRG1+ CD127−) and memory precursor cells (MP; KLRG1− CD127+), with MPs evolving into TMEM to provide durable protective tumor immunity. Continuous in vitro assays and temporal comparisons help identify enduring targets in T cell therapy, particularly focusing on exhausted cells that express inhibitory factors like PD-1
Due to challenges like insufficient targeted delivery efficiency, solid tumor barriers, and organ aggregation, intravenously administered adoptive T cells often fail to fully target tumor cells, leading to reduced therapeutic effects and acute toxicity [44]. For example, Hong and colleagues found that CD8+ T cells with an sgRNA library, when implanted into mice, exhibited enhanced homing to tumor tissues with the overexpression of St3gal1 and βII-spectrin [45]. Additionally, integrin lymphocyte function-associated antigen 1 (LFA-1) facilitates T cell migration and adhesion by binding to intercellular adhesion molecule 1 (ICAM-1). Screening for ICAM-1pos and ICAM-1neg cells identified RASA3 as a gene that enhances the migratory efficiency of adoptive T cell therapy [46]. T cell proliferation is indicated by carboxyfluorescein succinimidyl amino ester (CFSE) dye activity. Selecting T cells with high proliferation rates and validating them in CAR-T cells allows for the identification of active positive regulatory genes [12, 27, 47, 48]. For example, CD19 CAR-T cells with RASA2 knockout demonstrated enhanced adaptability and effector functions in a B-cell acute lymphoblastic leukemia (B-ALL) mouse model [12]. CD69, an early-expressed molecule in lymphocyte activation, helps identify modulators of T cell activity. Sorting CD69high and CD69low cell groups via FACS revealed that FAM49B inhibits T cell activation by modulating Rac activity and cytoskeletal reorganization [49].
Functional activation sites in T cells can be identified by targeting cytokines such as interferon-γ (IFN-γ) or CD8+CD107a+ T cells [36, 41, 50, 51]. For example, Ye et al. identified GOF targets like Proline Dehydrogenase 2 (PRODH2) in CD8+CD107a+ T cells through CRISPRa screening. Integrating PRODH2 into primary or CAR-T cells reshapes metabolic pathways, enhancing outcomes across diverse tumor types [41]. Advanced base editing screening has also revealed alleles, such as those encoding the phosphoinositide-3-kinase catalytic subunit delta (PIK3CD) protein, that modulate T cell cytotoxic functions in both positive and negative ways [36]. In T cell apoptosis, Fas-FasL (Factor-related Apoptosis ligand) signaling is significant. Continuous FasL stimulation compared to normal controls showed that Early B cell factor 4 (EBF4) regulates the degradation of anti-apoptotic cellular FLICE-inhibitory protein (c-FLIP), inducing the dissolution of cytotoxic T cells [52]. Various gene expression programs facilitate the induction and maintenance of diverse T cell subsets in adaptive tumor immunity. CD4+ and CD8+ single-positive cells require several rounds of selective differentiation. For instance, shRNA screening targeting CD4 expression has shown that chromatin assembly factor (CAF-1) and DNA methyltransferase collaborate to silence the CD4 gene, maintaining the CD8+ T cell phenotype [53]. Furthermore, studies indicate that CD8+ T cell immunity depends on differentiation into effector (TEFF) and memory (TMEM) cells. C–C motif chemokine receptor (CCR7) and CD62L are recognized TMEM surface markers, aiding in identifying regulators of TMEM differentiation and maintenance [48, 54]. For example, CRISPRa and CRISPRi screening of CCR7 expression revealed that Basic Leucine Zipper ATF-Like Transcription Factor 3 (BATF3) overexpression boosts specific TMEM traits, enhancing HER2+ CAR-T efficacy against breast cancer [54]. Short-term effector cells (TE; KLRG1+ CD127−) and memory precursor cells (MP; KLRG1− CD127+) are distinguishable, with TEs perishing within days to weeks, while MPs evolve into TMEMs and provide prolonged protective tumor immune responses [55]. Comparing MP/TE post-cultivation in mice identifies regulatory factors associated with a more memory-like phenotype, shaping the destiny of CD8+ T cells [56, 57].
Many patients struggle with long-term responses to adoptive T cell immunotherapy, often facing drug resistance or relapse post-treatment. Identifying factors influencing T cell functional persistence is critical. T cell exhaustion, characterized by diminished effector capacity and heightened immune response suppressor (IRS) expression, is a key factor [58]. High-throughput genetic screening can discern persistence factors by repeatedly stimulating T cells with anti-CD3/CD28 and IL-2 in vitro, or by implanting them into tumor-bearing mice and comparing chronically stimulated T cells with unstimulated counterparts [30, 59–61]. Examining regulators of exhaustion, such as characteristic IRS like PD-1, involves screening, reading, and validation processes. Techniques such as gene ablation or pharmacological inhibition of core fucosylation (Fuco) to reduce PD-1 expression can enhance the anti-tumor effectiveness of adoptive T cell therapy [62].
TCR-T and CAR-T cell directly screening
Direct screening within TCR-T and CAR-T cells is an emerging area of research with limited exploration to date. Typically, engineered TCR or CAR sequences are introduced into activated ex vivo primary T cells using retrovirus or lentivirus vectors. Similar to primary T cell screening, sgRNA or other gene-editing libraries are introduced into TCR-T or CAR-T cell pools. The cytotoxicity of adoptive T cells is assessed by co-culturing with tumor cells, followed by direct sequencing of groups with high or low functional cytokine expression or PD-1+ and PD-1− cell groups to identify factors affecting TCR-T and CAR-T cell cytotoxic function or persistence. Researchers employ FACS or scRNA-seq to isolate T cell subpopulations and identify functional genes through sequencing. FACS uses fluorescent labeling and voltage adjustments to isolate cells based on specific markers like functional cytokines and PD-1, followed by conventional sequencing to pinpoint functional genes. scRNA-seq enables direct isolation and high-throughput sequencing of individual T cells, providing more accurate identification of functional genes across diverse T cell populations.
Researchers focus on enhancing the expression of cytotoxicity-associated molecules while suppressing exhaustion-related factors in T cells to amplify T cell activation signals and overall functionality. For instance, Freitas et al. isolated GD-2 CAR-T cells co-cultured with tumor cells based on cytotoxic cytokine expression (TNF and IL-2) and identified MED12 and CCNC, components of the mediator cell cycle protein-dependent kinase module, as targets that enhanced anti-tumor immunity through transcriptional and epigenetic changes [63]. Furthermore, the longevity of T cells can be extended by isolating cell populations exhibiting downregulation of T cell exhaustion-associated proteins. Wang and team used CRISPR screening of PD1+ HER2+ CAR-T cells and glioblastoma stem cells to find that knocking out Transducin-Like Enhancer of split 4 (TLE4) reduced apoptotic traits, sustaining CAR-T cell function, while ablation of Ikaros family zinc finger protein 2 (IKZF2) improved CAR-T anti-tumor efficacy by regulating NFAT signaling [64]. NY-ESO-1 TCR-T cells generated from CD8+ T cells revealed that Subsequent investigations revealed that sorting nexin 9 (SNX9) regulates both T cell exhaustion (PD1+) and cytotoxic function (CD107a+), with SNX9 knockout enhancing T cell activation signals and anti-tumor potential [65]. Direct screening within TCR-T and CAR-T cells not only intuitively discovers adoptive T cell therapy’s regulatory mechanisms but also utilizes engineered TCR and CAR molecules in T cells for heightened tumor-targeting cytotoxicity. This method improves the efficacy of tumor cell co-culture screening and more readily establishes continuous tumor antigen stimulation models, advancing adoptive T cell therapy.
Direct screening in TCR-T and CAR-T cells offers intuitive insights into adoptive T cell therapy’s regulatory mechanisms, improving tumor-targeting cytotoxicity by utilizing engineered TCR and CAR molecules. However, challenges include reduced efficiency of subsequent viral transductions for gene editing due to pre-transduction of engineered sequences, which can disrupt target genes and complicate data interpretation [9]. To address these issues, Dai et al. developed the CLASH system using AAV-KIKO and CRISPR/Cas12a, where Cas12a mRNA is introduced into human T cells, and a crRNA library targets both CAR sequences and the T Cell Receptor Alpha Constant (TRAC) locus. This approach enables high-throughput CRISPR KO screening and CAR knock-in at the TRAC locus, overcoming the limitations of traditional CAR gene transduction methods. Utilizing CLASH, they identified the exon3 skip mutant of PR/SET Domain 1(PRDM1) as significantly boosting anti-tumor effects in CD22 CAR-T cells [66]. Similarly, Blaeschke et al. introduced the Modular Pooled Knock-In (ModPoKI) approach, attaching functional modular libraries to sgRNA for TCR-T and CAR-T production at the TRAC locus, revealing that overexpression of BATF and transcription factor AP4 (TFAP4) jointly influences TCR/CAR gene expression and T cell functionality in anti-tumor immunity [67] (Table 3 and Fig. 3A).
Table 3.
Representative Studies of Genetic Screening for TCR-T and CAR-T cells
| Screening methods | Screening cells | Screening model | Construction of TCR-T or CAR-T | Library design | Screening strategies | Comparison and readout | Screened targets | Function of Screened target | Ref |
|---|---|---|---|---|---|---|---|---|---|
| CRISPR KO | NY-ESO-1 TCR-T cells | in vitro | Lentivirus transduction | Cytotoxic T cell gene pool | TCR stimulation, FACS sorting CD107a expression | Compare CD107a+ group and CD107a− group | SNX9 | Deletion of SNX9 alleviates CD8 T cell exhaustion | [65] |
| CRISPR KO | HER2 CAR-T cells | in vitro | Lentivirus transduction | Genome-wide libraries | Co-cultured with Glioblastoma stem cells, FACS sorts PD1 expression | Compare of PD1+ and PD1− groups | TLE4, IKZF2 | TLE4 and IKZF2 induce exhaustion responses through transcriptional process | [64] |
| CRISPR KO | GD2 CAR-T cells | in vitro | Retrovirus transduction | Genome-wide libraries | Co-cultured with GD2+ tumor cells, FACS sorted TNF and IL-2 expression | Compare TNF± with IL-2± groups | MED12 | MED12 deletion enhanced antitumor activity and sustained the effector phenotype in CAR- T cells | [63] |
| CRISPR KO | CD22 CAR-T cells |
in vitro in vivo |
CLASH platform | Descartes library |
Co-cultured with the NALM6 cell line NALM6-GL mice were injected into the body, and the changes of T cells over time were observed |
Compare T cells over three time periods | PRDM1 | The PRDM1 Δexon3 mutant improves the persistence of CAR-T cell | [66] |
| CRISPR KI | NY-ESO-1 TCR-T cells, CD19 CAR-T cells | in vitro | ModPoKI platform | TF library and surface receptor library | TCR was repeatedly stimulated to observe T cell changes | Compare T cells over different time periods | BATF, TFAP4 | Overexpressed BATF and TFAP4 improves the persistence of T cells | [67] |
SNX9 sorting nexin-9, TLE4 Transducin Like Enhancer of Split 4, IKZF2 Ikaros Family Zinc Finger Protein 2, MED12 Mediator Complex Subunit 12, PRDM1 PR/SET Domain 1, BATF Basic Leucine Zipper ATF-Like Transcription Factor, TFAP4 Transcription Factor AP-4
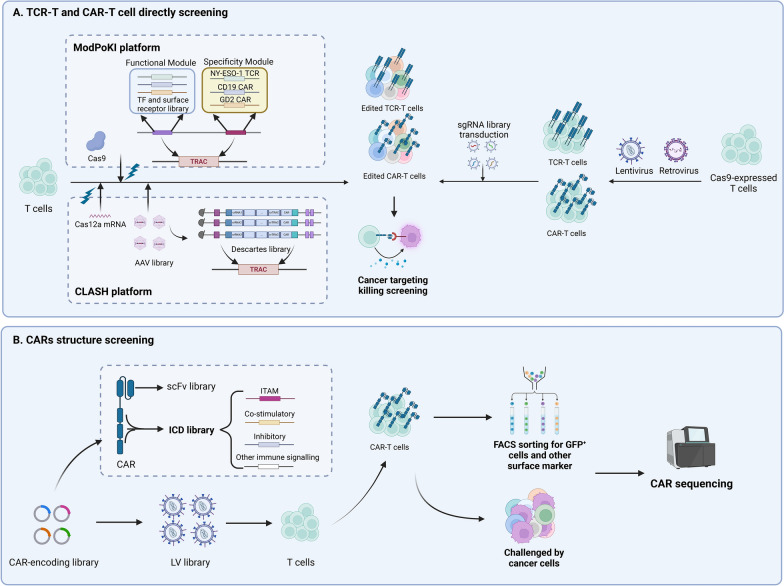
Fig. 3.
Adoptive T cell directly screening and CARs structure screening. A In the context of TCR-T and CAR-T cell therapies, introducing TCR and CAR constructs into T cells via lentiviruses and leveraging the ModPoKI platform enables the co-targeting of sgRNA and Cas9, equipped with Functional and Specificity Modules, to the TRAC locus through electroporation. This approach facilitates the generation of TCR-T and CAR-T knock-in libraries. Concurrently, the CLASH platform’s CRISPR KI/KO AAV library allows for simultaneous CAR integration and genetic modification at the TRAC locus in CAR-T cells, minimizing potential inaccuracies caused by the use of dual viral vectors. The subsequent co-culture of these genetically engineered cells with tumor cells enables the direct selection of effective TCR-T and CAR-T candidates based on their tumoricidal activity. B The architecture of CARs principally comprises the scFv and the ICD. Constructing an ICD library incorporating motifs such as ITAMs, co-stimulatory, and inhibitory domains, followed by lentiviral transduction into T cells, allows for the selection of CAR-T cells based on criteria including proliferation, differentiation, cytotoxicity, and durability. Sequencing of CARs then facilitates the identification of the most effective CAR-T configurations
CARs structure screening
The specificity of CAR-T and TCR-T therapies is derived from the targeted recognition between tumor cell surface antigens and the engineered antibodies or TCRs on T cells. Identifying suitable target antigens remains a significant challenge in engineered adoptive T cell therapy. The tumor microenvironment (TME) often inhibits T cell infiltration and may lack tumor-specific antigens, leading CAR-T therapies to target tumor-associated antigens (TAAs). However, TAAs can also be present in normal tissues, which may result in unpredictable on-target, off-tumor (OTOT) effects [68]. Consequently, optimizing CAR structures to enhance affinity is a promising strategy. By utilizing CAR structure screening, researchers can identify constructs with improved specificity and affinity for target antigens, thereby gaining deeper insights into the functional and mechanistic differences among CAR structures. By modulating CAR affinity, CAR-T cells can be engineered to better differentiate between normal cells with low antigen density and tumor cells with higher antigen density. This approach may enhance CAR-T cell functionality while minimizing off-target effects, toxicity, and side effects, ultimately improving CAR-T cell durability. Researchers can also optimize CARs with varying molecular backgrounds by screening different modules of CAR structures to meet diverse clinical needs, establishing a foundation for the discovery of next-generation CARs [69–72].
CARs are composed of three main components: the extracellular single-chain variable fragment (scFv), the hinge and transmembrane domain, and the intracellular signaling domain. The scFv component, which targets the antigen, is introduced into CAR structures via lentivirus, with extensive scFv production achieved through “light chain shuffling.” Methods of affinity screening and efficacy assessment evaluate the affinity, proliferation, differentiation, and tumor-killing potential of CD38 CAR-T cells. It was found that reducing the affinity of CD38-CAR T cells by approximately 1,000-fold was most effective at lysing CD38+ multiple myeloma (MM) cells [72]. Additionally, a Combinatorial Cellular Library of CD38 CARs enables the enrichment of specific CD38 subgroups through negative screening with normal tissue cells and positive screening with tumor cells, revealing the highest affinity CAR structure designs [73]. Moreover, modifying the complementary determining region of HER2+ CAR-T’s variable heavy chain, while monitoring CAR expression, antigen recognition, and signal transduction, demonstrates the importance of CAR structural design in affecting affinity and cytotoxicity, thereby reducing OTOT effects [74].
Intracellular domains (ICDs) are composed of various signaling modules that convert antigen recognition into downstream anti-tumor actions. These domains are crucial in orchestrating CAR-T cell behavior and primarily include basic modules of CD3ζ’s immunoreceptor tyrosine-based activation motif (ITAM), co-stimulatory domains like CD28 and 4-1BB, as well as other activating or inhibitory signals [75]. Different combinations of these signaling domains can significantly influence T cell therapeutic efficacy. However, designing and testing each CAR-T construct individually is labor-intensive. To address this, researchers introduce an orthogonally-designed CAR library—which includes diverse ITAMs, co-stimulatory domains, inhibitory signals, and other immune signals—into T cells via lentivirus at a consistent locus, creating a large CAR-T cell pool. Assessment and selection are based on cell proliferation, differentiation, cytotoxicity, and persistence. Sequencing these CAR constructs helps confirm the optimal functionality of the CAR-T cells [71, 76–78]. Gorden and his team employed the CARPOOL strategy to swiftly enrich and identify novel CD19 CARs with clinically valuable phenotypes. They found that the Var1 CAR outperformed the widely-used BBζ CAR in vitro, although both exhibited comparable efficacy in vivo [78]. Additionally, the speedingCARs platform was developed, combining HER2+ CAR signal domain recombination with collective functional screening. This approach revealed the impacts of CAR variations through scRNA-seq and single-cell CAR sequencing [76]. Establishing similar platforms can help overcome the limitations of CAR-T therapy and be extended to include TCR-T and other cellular therapies, such as NK cells and macrophages (Fig. 3B).
Current CAR structure screening primarily relies on immortalized cell lines, which display altered baseline signaling and metabolism. As a result, certain T cell functions, such as anti-exhaustion and effective tumor killing, may not be accurately reflected. Furthermore, there is significant heterogeneity in CAR structure screening, with in vivo improvements often being less pronounced than those observed in vitro. In vitro models may amplify differences in antigen exposure abundance and kinetics, overlooking the impact of the immune microenvironment. Additionally, the scope of current CAR structure screening is narrow, and the efficacy of screened CAR structures across different tumors is not yet clear, with insufficient exploration of their associated safety. Significant challenges remain in translating novel CAR structures identified through CAR structure screening into clinical applications.
Genetic screening applied for tumor cells under T cell pressure
Tumor cells commonly evade adoptive T cell immunotherapy due to various factors such as antigen downregulation, reduced cytokine sensitivity, and metabolic alterations, which contribute to resistance and relapse in T cell-focused treatments [79]. High-throughput genetic screening serves as an impartial platform for target discovery, offering a thorough method to characterize tumor cell and T cell interactions. Applying T cell pressure to tumors can mimic adoptive T cell immunotherapy and is crucial for identifying new therapeutic targets or potential regulatory mechanisms to improve efficacy. Similar to T cell screening, tumor cells, after introducing sgRNA libraries and other gene-editing tools, are co-cultured in vitro with T cells or CAR-T cells. These are compared to cells without survival stress, or direct data readouts from surviving tumor cells are analyzed to identify genes linked to drug resistance. In in vivo screening, tumor cells can be transplanted into both immunosuppressed and immunocompetent mice, facilitating a deeper analysis of key regulatory mechanisms in tumor immune infiltration. This process allows for the validation and enhancement of adoptive T cell therapies using small molecule drugs (Table 4).
Table 4.
Representative Studies of Genetic Screening for tumor cells under T cell pressure
| Screening methods | Screening cells | Screening model | Library design | Screening strategies | Comparison and readout | Screened targets | Function of Screened target | Ref |
|---|---|---|---|---|---|---|---|---|
| CRISPR KO |
MHC I Negative K-562 cells |
in vitro | 220 k sgRNA library |
Three rounds of FACS Sorting MHC I expression |
Compare the MHC Ipos group and MHC Ineg group | PRC2 | PRC2 silences the MHC I antigen presentation pathway and enables immune evasion | [80] |
| CRISPR KO |
MHC II Negative MOLM13 and OCI-AML3 cells |
in vitro | 15.3 k or 220 k sgRNA library |
Three rounds of FACS Sorting MHC II expression |
Compare the MHC IIpos group and MHC IIneg group |
CtBP, FBXO11 |
Inhibition of the CtBP complex and FBXO11 enhances MHC class II expression and anti-cancer immune responses | [81] |
| CRISPR KO | A357 cells | in vitro | Genome-wide libraries | CD58 staining, FACS sorting CD58 expression | Compare CD58mi group and CD58lo group | CMTM6 | CMTM6 is critical for CD58 stability and upregulation of PD-L1 upon CD58 loss | [89] |
| CRISPR KO | BCP-ALL cells | in vitro | Genome-wide libraries | After in vitro culture, FACS sorting CD19 expression | Compare the CD19high group and CD19low group | ZNF143, NUDT21 | ZNF143 promotes CD19 activation and NUDT21 limits expression of CD19 | [87] |
| CRISPRa and CRISPRi | AMO1 cells | in vitro | Genome-wide libraries | Anti-BCMA antibody stimulation, FACS sorting BCMA expression | Compare the BCMAhigh group and BCMAlow group | HDAC, Sec61 | Inhibition of HDAC7 and the Sec61 complex increased cell surface BCMA | [86] |
| CRISPR KO | Daudi cells | in vitro | Genome-wide libraries | Co-cultured with Vγ9Vδ2 T cells to observe cancer killing | Compare the killed cells and survival cells | AMPK | Activation of AMPK led to increased expression of the BTN2A1– BTN3A complex and increased Vγ9Vδ2 T cell receptor-mediated killing | [85] |
| CRISPR KO | Colorectal cancer cells | in vitro | Genome-wide libraries | IFN-γ stimulation, FACS sorting MHC-I and PD-L1 expression | Compare the stimulation group and the untreated group | JAK1 | Mutations in JAK1 enhanced or reduced sensitivity to autologous tumor-reactive T cells | [38] |
| CRISPR KO | B16-F10 cells | in vitro | Genome-wide libraries |
IFN-γ and TNF overnight culture to observe cancer cell changes |
Compare cancer cells over different time periods | HOIP | Inhibition of HOIP promote tumor cell sensitivity to TNF and IFN-γ | [92] |
| CRISPR KO | Melanoma cells | in vitro | Genome-wide libraries |
Antibody Staining, FACS sorting IFN-γR1 expression |
Compare IFN-γR1high group, IFN-γR1low group, and the untreated group | STUB1 | STUB1 inactivation amplifies IFN-γ signaling, sensitizing tumor cells to cytotoxic T cells | [94] |
| CRISPR KO | KPC3-OVA cancer cells | in vitro | Genome-wide libraries | Co-cultured with OT-I T cells to observe cancer killing | Compare Epi cancer cells and Mes cancer cells | Egfr, Mfge8 | Egfr and Mfge8 are significantly higher expressed in Mes cancer cells, and their depletion sensitized Mes cancer cells to CTL-mediated killing | [118] |
| CRISPR KO | AML3 cells | in vitro | Epi-drug library | Co-cultured with DNT cells, FACS sorting Annexin V | Compare killed cells and survival cells | CD64 | The level of CD64 expression correlated strongly with the sensitivity of AML cells to DNT treatment | [119] |
KO Knock out, MHC Major histocompatibility complex, FACS Fluorescence activated Cell Sorting, PRC2 polycomb repressive complex 2, CtBP C-terminal binding protein, CMTM6 CKLF-like MARVEL transmembrane domain-containing protein 6, ZNF 143 Zinc Finger Protein 143, NUDT21 Nudix Hydrolase 21, BCMA B-cell maturation antigen, HDAC Histone deacetylase, AMPK AMP-activated protein kinase, BTN2A1 Butyrophilin 2A1, IFN-γ Interferon-γ, TNF Tumor necrosis factor, Epi Epithelial–like, Mes Mesenchymal-like, Egfr epidermal growth factor receptor, Mfge8 Milk Fat Globule-EGF Factor 8 Protein, DNT Double Negative T
Targeted antigen presentation
In adoptive cell therapies, particularly engineered TCR-T and CAR-T therapies, specific recognition between tumor cell surface antigens and engineered TCRs or antibodies on T cells is essential. Enhancing antigen presentation and preventing antigen downregulation are vital strategies to mitigate resistance. After Major Histocompatibility Complex (MHC) antibody stimulation or co-culturing, direct screening based on the expression of MHC molecules or other TAAs is performed. Sequencing following FACS sorting reveals regulatory factors linked to antigen expression, both positive and negative [80–87].
Researchers conducted gene editing on MHC-Ineg cells using an sgRNA library. After three rounds of FACS selection for MHC-Ipos cells, they identified PRC2-mediated transcriptional silencing in the MHC-I antigen processing pathway (MHC-I APP). PRC2-mediated silencing of MHC-I drives resistance of small cell lung cancer to T cell-mediated killing and promotes the spread of non-tissue-compatible receptors, thereby facilitating T cell immune evasion. Genetic disruption or pharmacological inhibition of PRC2 can restore tumor MHC-I antigen presentation, enabling effective targeting by CD8+ T cells and enhancing tumor immunity. While this study's data primarily highlight the role of PRC2 in MHC-I antigen presentation, other candidate genes identified through screening remain. Future research should explore the roles of additional transcription factors in regulating antigen presentation [80]. Similarly, MHC-IIpos screening revealed that the CtBP complex transcriptionally represses the MHC II presentation pathway. Targeting these inhibitory mechanisms can selectively induce the upregulation of MHC class II in various AML cell lines, thereby stimulating antigen-dependent CD4+ T cell activation and promoting an effective anti-tumor immune response [81]. Additionally, the tumor cell surface protein CD58 binds to CD2 receptors on T cells. Post-TCR and MHC antigen presentation, this binding delivers co-stimulatory signals. CD58 serves as an adhesion molecule, enhancing the initial binding of effector T cells [88]. Sorting CD58high and CD58low tumor cells revealed that CKLF-like MARVEL Transmembrane Domain Containing 6 (CMTM6) synergistically regulates co-stimulatory (CD58) and co-inhibitory (PD-L1) signals in cancer cells. Both CD58 and PD-L1 may need to bind directly to the extracellular loop of CMTM6. In the absence of CD58 expression, additional PD-L1 protein binds to the released CMTM6 and stabilizes it on the cell surface by enhancing circulation and reducing lysosomal degradation, potentially decreasing resistance to immune checkpoint blockade (ICB) therapy. CMTM6 serves as a crucial regulator of co-stimulatory and co-inhibitory signals in cancer cells. However, further research is necessary to elucidate the structure of CMTM6 and determine its precise interactions with CD58 and PD-L1, providing better insights for therapeutic design [89].
In CAR T-cell therapy, CD19 is a prevalent target for blood cancer treatment. Screening CD19+ expression in B cell precursor acute lymphoblastic leukemia (BCP-ALL) revealed the contrasting functions of Zinc Finger Protein 143 and Nudix Hydrolase 21 (NUDT21). The absence of NUDT21 in BCP-ALL cells enhances CD19 expression, increasing susceptibility to CD19-specific CAR-T [87]. B cell maturation antigen (BCMA) CAR-T therapy for MM also employs CRISPRa screening to pinpoint regulators of antigen presentation and loss [86]. For particular lymphocytes like γδ T cells, which mostly function in innate immunity without needing MHC pathway activation, Vγ9Vδ2 T cells are the predominant subgroup. They recognize complexes with butyrophilin Subfamily 2 Member A1 (BTN2A1) and butyrophilin Subfamily 3 Member A1. Enhanced BTN3A expression on tumor cells can activate γδ T cell cytotoxicity, providing a novel method to boost γδ T cell anti-cancer activity and to develop γδ T cell-based therapies [85].
Intrinsic mechanisms of cytokine resistance
Beyond antigen downregulation, tumor cells exhibit various other immune escape and resistance mechanisms. A prevalent mechanism is their inherent insensitivity to cytokines secreted by cytotoxic T cells, such as IFN-γ and TNF. IFN-γ enhances immune infiltration by secreting chemokines that attract lymphocytes, aiding in tumor antigen presentation and processing, and increasing tumor cell sensitivity to destruction. Consequently, abnormalities in the IFN-γ-JAK-STAT pathway can lead to resistance [90]. TNF-mediated nuclear factor kappa-B signaling exerts inhibitory effects on tumor cells or enhances the migration and infiltration of activated immune cells, working alongside IFN-γ to achieve anti-tumor effects [91]. Continuous stimulation of sgRNA-transduced tumor cells with IFN-γ or TNF allows for the identification of regulatory factors that are sensitive or resistant to these cytokine pathways, based on the survival of the tumor cells [38, 92, 93]. Coelho et al. used base editing screening to identify mediators of sensitivity or resistance to IFN-γ in colorectal cancer, revealing hundreds of missense mutations that can predict alterations in IFN-γ pathway activity [38]. Freeman et al. found that the LUBAC catalytic subunit HOIP inhibits both intrinsic and extrinsic apoptotic pathways, thereby diminishing tumor immunity, through screening with combined stimulation of IFN-γ and TNF [92]. Additionally, Apriamashvili et al. identified that the E3 ubiquitin ligase STUB1, which negatively regulates IFN-γ signaling, is a mechanism of tumor cell resistance. This was achieved by sorting the top 10% and bottom 10% of IFN-γ receptor 1 (IFN-γR1) melanoma cells using FACS, broadening research on the IFN-γ pathway from the receptor level [94].
Comparing tumor and T cell co-cultures in vitro and in vivo, analyzing the effects of treatment on gene expression, or contrasting situations in immunocompetent or deficient mice, researchers use GSEA or functional verification experiments to search for or validate upstream factors of targeted antigen presentation and intrinsic mechanisms of resistance. Identified targets reflect tumor cell sensitivity or resistance under T cell pressure, typically affecting antigen presentation, IFN and TNF signaling, and cytotoxic T cell killing functions. This common research model has achieved results in primary T cells [95–112] and CAR-T cells [113–116]. For example, the creation of the immune escape cancer cell line KPIE2, in co-culture with gp100 TCR-T, involved CRISPRa screening of melanoma cells' lncRNAs. GSEA analysis identified IL10RB Divergent Transcript (IL10RB-DT) as an inhibitor of antigen presentation and IFN-γ-JAK-STAT1 signaling [95]. In a lung cancer mouse model, introducing sgRNA alongside ACT revealed the testicular cancer antigen ADAM Metallopeptidase Domain 2 (ADAM2) as an immune modulator. ADAM2 reduces tumor antigen expression and impedes the Type I and II IFN and TNFα pathways, contributing to the establishment of a cold TME. However, it suppresses immune checkpoint molecules PD-L1, Lymphocyte Activating 3, and T Cell Immunoreceptor With Ig And ITIM Domains, thereby enhancing the efficacy of adoptively transferred cytotoxic T cells in tumors overexpressing ADAM2 [100]. Co-culturing Epidermal Growth Factor Receptor (EGFR) CAR-T cells with glioblastoma cells transfected with sgRNA for CRISPR screening demonstrated that the IFN-γ-R signaling pathway confers greater resistance to CAR-T cell killing in glioblastoma and other solid tumors, but not in leukemia and lymphoma, indicating distinct effects between solid and hematologic malignancies [113].
Other mechanisms and strategies for tumor cells
The epithelial-mesenchymal transition (EMT) represents a novel mechanism of tumor escape, characterized by epithelial (Epi) cancer cells losing intercellular adhesion and transforming into mesenchymal-like (Mes) cancer cells. This change facilitates migration and invasion, thereby enhancing resistance to immunotherapies [117]. In vitro, the EMT system, induced by transforming growth factor-β and Doxycycline, yields tumor cells with distinct Epi or Mes characteristics. Upon sgRNA transfection and co-culturing with CD8+ T cells, EGFR and Milk Fat Globule-EGF Factor (Mfge8) emerged as Mes-specific cytotoxic regulators that facilitate immune escape in CD8+ T cells, highlighting compelling pharmacological targets [118].
DNTs (TCR+CD3+CD4−CD8−) are peripheral mature T cells that do not provoke host anti-graft or graft-versus-host reactions, making allogeneic DNTs a promising candidate for "off-the-shelf" adoptive T cell therapy. Co-culture screening identified that components of the SPT-ADA-GCN5 acetyltransferase deubiquitination complex and CD64, respectively, downregulate and upregulate DNT cytotoxicity against AML, thereby improving the efficacy of DNT therapy [119].
Genetic screening applied for promising combination strategies
Immune checkpoint blockade
ICB therapy inhibits the IRS, leading to the reversal of T cell exhaustion and the restoration of T cell infiltration into tumors. It also blocks checkpoint ligands between tumor cells and other antigen-presenting cells (APCs), thereby facilitating T cell signal transduction and cytotoxicity. Clinical evidence has shown that blockade of the PD-1/PD-L1 pathway and Cytotoxic T-Lymphocyte-Associated protein 4 (CTLA-4) leads to sustained anti-tumor responses, significantly altering treatment outcomes in advanced and metastatic cancers. This signifies a conventional T-cell mediated approach in tumor immunotherapy [120]. However, the majority of patients exhibit limited response to checkpoint immunotherapy and develop resistance following treatment, making it crucial to thoroughly investigate the mechanisms regulating ICB efficacy [121]. In high-throughput genetic screening, the ICB treatment process in cancer patients is simulated by implanting gene-edited tumor cells in mice and administering anti-PD-1 or anti-CTLA-4 intravenously. This method, using sgRNA readouts, identifies genes that act as drivers or inhibitors in ICB treatment [122–126]. For instance, introducing an sgRNA library into various tumor cells and categorizing them into in vitro cultures, immunodeficient NSG mice, wild-type mice, and ICB treatment groups, reveals that IFN-induced non-classical MHC class I molecules, Qa-1b/HLA-E, can impede ICB treatment [122]. Additionally, intrinsic Anti-Silencing Function 1A Histone Chaperone (ASF1A) deficiency in tumor cells can enhance anti-PD-1 therapy efficacy by upregulating Granulocyte–Macrophage Colony-Stimulating Factor (GM-CSF) expression [126]. For instance, introducing an sgRNA library into various tumor cells and categorizing them into in vitro culture, immunodeficient NSG mice, wild-type mice, and ICB treatment groups, reveals that IFN-induced non-classical MHC class I molecules, Qa-1b/HLA-E, can impede ICB treatment [122]. Additionally, intrinsic ASF1A deficiency in tumor cells can enhance anti-PD-1 therapy efficacy by upregulating GM-CSF expression [126].
Upregulation of PD-L1 in tumor cells, which facilitates escape from T-cell-based immunotherapy, is a potential mechanism behind resistance to adoptive cell therapy and ICB treatment [121]. Consequently, PD-L1 expression regulators identified via genetic screening represent potential pharmacological targets for ICB combination therapies [84, 89, 93, 127–129]. In a comprehensive genomic CRISPR/Cas9 screening within gastric cancer cells, identifying PD-L1pos and PD-L1neg sgRNA readouts via FACS revealed that Tripartite motif-containing 28 (TRIM28) is a crucial activator of PD-L1. Combining TANK Binding Kinase 1 inhibitors with anti-CTLA-4 demonstrated significant therapeutic efficacy in gastric cancer [128]. However, research suggests that increased PD-L1 expression in tumors may correlate with improved responses to anti-PD-1/PD-L1 therapies when combined with ICB [130]. Therefore, inhibiting the identified negative regulator of PD-L1, SET domain bifurcated histone lysine methyltransferase 1 (SETDB1), during ICB treatment has been found to simultaneously upregulate PD-L1 and enhance T-cell infiltration, resulting in a dual therapeutic effect [131].
ICB is a T-cell-based immunotherapy, and the factors affecting its efficacy are complex. Thus, the positive and negative regulatory factors found in in vitro and in vivo co-cultures of T cells and tumor cells can be modulated using engineered cells or small molecule inhibitors, with subsequent ICB application to validate efficacy and indirectly identify targets to enhance ICB therapy [104, 132–134]. Whole-genome CRISPR screening has revealed that Protein Arginine Methyltransferase 1 and Receptor Interacting Serine/Threonine Kinase 1 regulate cytotoxicity. Inhibiting these regulators can significantly boost the efficacy of anti-PD-1/TNF Receptor Superfamily Member 4 therapy in mice [132]. Consequently, the combined use of adoptive T cell therapy and ICB is a promising combination strategy, with gene screening directly or indirectly paving the way for these two types of immunotherapy.
Bispecific T-cell engagers therapy
Bispecific T-cell engagers (BiTEs) have emerged as a significant immunotherapy strategy for cancer treatment in recent years. This approach involves concurrently binding the CD3 subunit of the TCR complex on cytotoxic T cells and TAAs on tumor cells. By redirecting T cells to target tumor cells, BiTEs increase recruitment and immune infiltration, leading to notable clinical outcomes. Resistance mechanisms to BiTE therapy are similar to those observed in adoptive T cell therapies, particularly CAR-T cells, and primarily involve the loss of tumor target antigens and other intrinsic tumor mechanisms [135]. High-throughput genetic screening, which involves transferring an sgRNA library into tumor cells and co-culturing them with CD8+ T cells and BiTEs, helps identify resistance mechanisms and targets in BiTE therapy. This approach provides rationales for patient selection and combination strategies with adoptive T cell therapies. Blinatumomab, an anti-CD19 BiTE, is the only clinically approved BiTE for treating B-ALL. Research by Li et al. revealed that loss of CD58, mediated by mutations in Paired Box 5, negates T cell activation induced by blinatumomab, highlighting its epigenetic mechanism of resistance[136].
In addition to anti-CD19 BiTEs, other BiTEs targeting various antigens in both hematological and solid tumors have been developed. The genetic resistance mechanisms of these BiTEs can be explored through high-throughput genetic screening [137–140]. For example, Flotetuzumab has shown therapeutic promise in relapsed/refractory AML. Whole-genome CRISPR screening identified a deficit in IFN-γ signaling as a key factor in tumor resistance [138]. Similarly, in vitro CRISPR KO and CRISPRa screening on tumor cells treated with Mesothelin and CD70 BiTEs identified the absence of factors such as Caspase-8, FADD-like apoptosis regulator, BCL2 Like 1, and BH3 interacting domain death agonist as key modulators of BiTE treatment sensitivity [140].
Modulating different immune cell types in TME
The TME plays a crucial role in tumor growth, metastasis, and prognosis, featuring immune cells with diverse functions that exert dual effects in adoptive T cell therapy. On one hand, the TME includes immune-activating cells like APCs and T follicular helper cells (Tfh), which can trigger potent immune cytotoxicity beneficial for tumor treatment. On the other hand, inhibitory cells in the TME, such as regulatory T cells (Tregs) and their secreted soluble factors, create an immunosuppressive environment that impedes T cell efficacy [141]. High-throughput genetic screening to identify targets in macrophages, dendritic cells, CD4+ T cells, and Treg cells within the TME can indirectly enhance the tumor-killing efficacy of adoptive T cell therapy (Fig. 4).
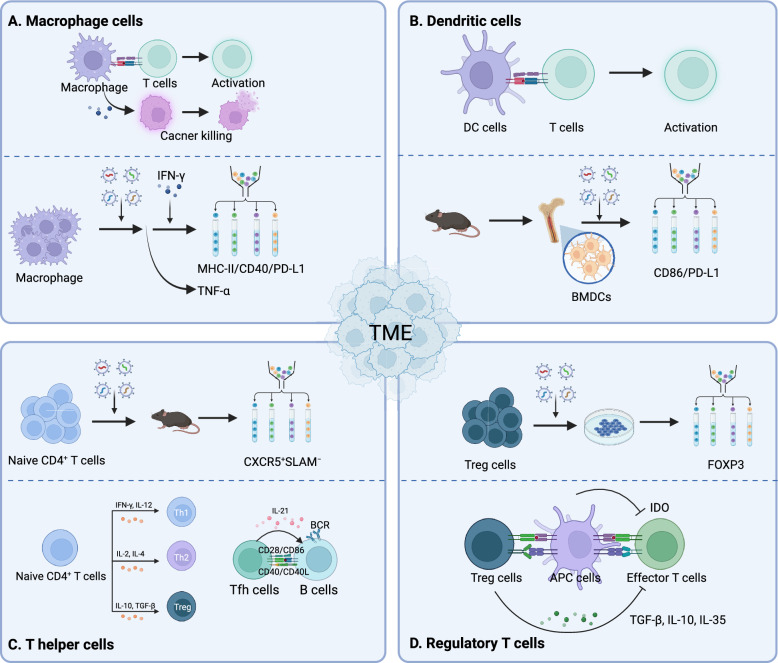
Fig. 4.
Genetic screening for modulating the TME. A Macrophages can either activate T cell functions or directly exert cytotoxic effects on tumors. Screening for macrophages involves isolating cells based on MHC-II, CD40, and PD-L1 expression under IFN-γ stimulation to evaluate their antigen-presenting capabilities, or assessing TNF-α levels to determine their cytotoxic potential. B DCs are the most important antigen-presenting cells, activating T cell functions. BMDCs are extracted from mice, and screening for CD86 and PD-L1 demonstrates their antigen-presenting capability. C CD4+ cells can differentiate into TH1, TH2, and Treg cells under the influence of various cytokines. CD4+ Tfh cells primarily aid in B cell proliferation and antibody production. In vivo screening of naïve CD4+ cells can identify Tfh cells through CXCR5+SLAM−. D Treg cells negatively impact T cell cytotoxicity, primarily through mediators such as TGF-β, IL-10, and IL-35. Regulators of Treg cells can be identified through screening for Foxp3
Immune-enhancing cells
M1-type macrophages are crucial in shaping and maintaining the TME. They exhibit innate immunity and the capability to phagocytize tumor cells, contributing to direct tumor elimination, and serve as APCs to activate cytotoxic T cells, thereby greatly advancing adoptive T cell therapy. Enhancing macrophage innate immunity through co-culturing with tumor cells [142, 143] and large-scale genetic editing screening, utilizing TNF-α to validate innate immune functions [144], is critical. Following IFN-γ stimulation, the assessment of antigen-presenting capacity via MHC-II, CD40, and PD-L1 promotes T cell activation [145, 146], offering novel targets for enhancing tumor immunity. For instance, glycogen synthase kinase 3β and mediator complex subunit 16, identified through screening, can regulate MHC-II expression influenced by IFN-γ [146].
Additionally, dendritic cells (DCs) are pivotal APCs within the body, central to initiating, regulating, and maintaining immune responses. Through whole-genome screening of bone marrow-derived DCs (BMDCs), the functional impact of CD86 and its regulators was uncovered. Co-culturing these genetically modified DCs with OT-I CD8 T cells, both in vitro and in vivo, confirms their antigen-presenting capacity, leading to T cell activation and cytotoxicity [147].
CD4+ helper T cells (Th), including Th1, Th2, Th17, and Tregs, exhibit unique functions and molecular features. Cytokine-mediated signaling often dictates their differentiation. High-throughput screening using FACS to sort cytokines, differentiation-related transcription factors, and specific surface receptors in activated CD4+ T cells helps to decipher key regulators of cell fate, a technique effectively utilized in Th1 and Th2 cells [148, 149]. Specifically, CD4+ Tfh cells, chiefly located in germinal centers and instrumental in their formation and upkeep, play a critical role in assisting B cell proliferation and antibody production, thereby contributing to humoral immunity. This understanding offers a fresh perspective in optimizing adoptive T cell therapy strategies [150]. By transferring CD4+ cells into mice bearing inflammation or tumors and analyzing sgRNA expression differences between Tfh (CXCR5+SLAM−) and Th1 (CXCR5−SLAM+) cells, researchers can identify the regulatory factors that influence Tfh differentiation [151, 152]. As an illustration, Huang and colleagues identified the inhibitory role of hypoxia-inducible factor-1 in Tfh, establishing a foundation for further modulation of the Th cell lineage within tumor immunology [152].
Immunosuppressive cells
Tregs, a distinct subset of CD4+ T cells, play a key role in modulating the body's immune response negatively through cell–cell interactions and the secretion of inhibitory cytokines. In the TME, Tregs often impede the effectiveness of adoptive T cell therapy, thereby contributing to tumor immune escape [153]. The central transcription factor Forkhead box P3 (Foxp3) is crucial for driving the Treg phenotype and maintaining Treg cell differentiation. Notably, Tregs that lack Foxp3 expression can regain the ability to produce pro-inflammatory cytokines, demonstrating the plasticity of Treg-mediated immune suppression [154]. High-throughput genetic screening, which involves introducing a comprehensive gene editing library into polarized Treg cell lines, has been instrumental in identifying regulatory targets and mechanisms affecting Foxp3 expression. By using FACS to sort Foxp3high and Foxp3low Treg populations and performing deep sequencing, researchers have uncovered both positive and negative regulators of Tregs [155–158]. For instance, Pinioti et al. discovered through CRISPR KO screening that the GDP-fucose transporter Slc35c1, along with broader fucose-related factors, positively regulates Foxp3. Knocking out these factors improved the immune functions of both CD4+ and CD8+ cells and significantly reduced colorectal cancer growth [157]. Similarly, ubiquitin-specific peptidase 22 (Usp22) has been shown to regulate Foxp3 expression at the transcriptional level, with Usp22 knockout demonstrating beneficial outcomes in several mouse tumor models [155]. In vivo screenings involving gene-edited MHC-II CD4+ cell subsets transplanted into mice with inflammation or tumors have further elucidated genes linked to immune responses. Validation studies revealed that methylenetetrahydrofolate dehydrogenase-2 deficiency could promote Treg cell differentiation, highlighting its potential as a target in tumor immunotherapy [159]. Additionally, mammalian target of rapamycin complex 1 (mTORC1) signaling is crucial for nutrient-driven cell metabolism, which impacts immune functions and plays a vital role in Tregs [160]. Long et al. performed α-CD3 continuous stimulation in nutrient-rich Treg polarized cell populations, analyzing sequencing data for mTORC1-dependent phosphorylation of S6 levels. Their protein–protein interaction network analysis identified positive and negative regulators of mTORC1 signaling. For instance, the Coiled-Coil Domain Containing 101-associated coactivator complex can restrict nutrient transport, and its specific depletion might enhance tumor immunity [161].
Non-genetic screening applications for adoptive T cell therapies
By utilizing genetic screening, researchers can identify malfunctioning molecules within the metabolic pathways of tumors and cancers and apply existing technologies to target these molecules, thereby inhibiting or destroying tumors. Conversely, non-genetic screening can help clarify the genetic composition of drug-sensitive tumors, facilitating the development of targeted therapies or specific drugs. Additionally, it can identify the most suitable delivery vehicles to maximize drug transport to target sites. Integrating both approaches allows researchers to address dysfunctional molecules in the tumor signaling pathway by screening for appropriate therapeutic agents and combining drug therapy with cell therapy to enhance tumor-killing efficacy. Alternatively, researchers can identify abnormal molecular targets through genetic screening and subsequently find delivery vehicles with the highest affinity and specificity for delivering the corresponding drugs and cells, thereby improving the effectiveness of tumor treatment.
Small-molecule compound screening
Small-molecule drugs have the potential to enhance adoptive T cell therapy by unexpectedly modulating immune pathways. Large-scale drug sensitivity screenings using co-cultures of tumor cells and T cells can identify optimal candidate drugs for combination tumor immunotherapy. When combined with genetic screening, this approach clarifies the mechanisms of action of small-molecule drugs, leading to improvements in the efficacy and safety of combination therapies. For instance, CD19+ CAR-T cells and NALM6 cell lines were co-cultured in multi-well plates with 526 different drugs, including chemotherapy agents, kinase inhibitors, epigenetic modulators, and non-cancer drugs. Evaluating cell viability revealed how these drugs affected CAR-T cytotoxicity. SMAC mimetics or inhibitors of apoptosis proteins antagonists, such as birinapant, Xevinapant, and LCL-161, were found to significantly enhance anti-tumor efficacy. Integrating this with CRISPR screening uncovered the sensitization mechanisms of SMAC mimetics and their association with death receptor expression [162]. Similarly, erlotinib, an EGFR inhibitor, emerged as a preferred compound for boosting CD8+ T cell-mediated cytotoxicity in ovarian cancer cells [163].
Immune and cancer cells share numerous essential metabolic pathways, leading to intense nutrient competition within the TME. Metabolic reprogramming in both tumor cells and T cells plays a crucial role in tumor immunity, particularly in pancreatic ductal adenocarcinoma. Genetic screening is employed to explore fundamental metabolic pathways during pancreatic tumor progression and TME-induced dependencies, identifying potential therapeutic targets [164, 165]. To pinpoint the metabolic pathways critical for T cell activation, a screening of a metabolite inhibitor library was conducted on sgRNA-modified Jurkat T cells and primary human T cells. By tracking T cell activation markers (CD69, ICOS7, and CD25), nicotinamide phosphoribosyltransferase (NAMPT), an essential enzyme in NAD+ biosynthesis, was identified as crucial for T cell activation. This suggests that NAD+ supplements could enhance T cell-based immunotherapy [47].
Currently, small-molecule drug screening faces challenges, including uncertainty about the specific mechanisms of action of the screened drugs and their effects being limited to a small number of genes. Moreover, translating these findings into clinical applications requires careful consideration of drug toxicity and potential impacts on normal bodily functions, which may worsen the condition or cause severe adverse reactions. Addressing these challenges is essential. In the future, integrating drug screening with immunotherapy and gene therapy may offer a multi-faceted approach to targeting tumors, thereby inflicting damage and enhancing therapeutic efficacy.
Targeted delivery system screening
TCR-T and CAR-T cells utilize lentiviral or retroviral vectors to randomly integrate plasmids encoding the TCR α and β chain genes from effector T cells, which are induced by tumor antigens, or scFvs that specifically recognize tumor antigens, into the genome of peripheral blood T cells. This integration ensures stable, continuous expression through cell division. However, this method carries risks, such as potential tumor formation due to insertional mutagenesis. Additionally, the complexity of producing clinical-grade viral vectors leads to high costs and extended production times, which can reduce clinical accessibility and increase the risk of T cell exhaustion following prolonged activation [166]. As a result, mRNA-mediated adoptive cell infusion therapies have emerged as a promising alternative. These therapies benefit from non-integrative expression and simplified non-viral delivery systems, which enhance control over therapeutic pharmacokinetics. This method improves the efficacy, safety, and accessibility of adoptive T cell therapies [167]. In this approach, transiently expressed CAR-T cells are generated by introducing mRNA encoding the CAR sequence into autologous CD8+ T cells ex vivo, using techniques such as electroporation or LNP delivery. The anti-tumor efficacy and safety of this method have been demonstrated in various in vitro and in vivo tumor models [168–172]. Similarly, introducing mRNA for TCR sequences into T cells ex vivo to create TCR-T cells has been shown to induce strong anti-tumor immunity [170] (Fig. 5A).
Fig. 5.
Non-genetic screening applications for adoptive T cell therapies. A To identify the optimal combination of drugs that enhance the tumoricidal effect of CAR-T cells, a drug library is added to tumor cells and CAR-T cells within 96-well or 384-well plates. The tumor-killing effect is screened using Luc assays to identify drugs that either potentiate or inhibit the efficacy of CAR-T therapy. The identified drugs are then co-cultured with tumor cells engineered with an sgRNA library, with or without concurrent CAR-T treatment. This co-culture helps to uncover the molecular mechanisms underlying any observed drug synergy through combined genetic screening. B For transient CAR-T cells using mRNA, LNPs consist mainly of four components: auxiliary lipid, cholesterol, ionizable lipid, and mRNA. Orthogonal library design is used to optimize the first three components. Transduction efficiency and cell targeting of LNPs are assessed through material characterization and Jurkat T cell line experiments, with the CAR-T cells constructed in primary T cells to verify their tumor-killing function
LNPs are commonly used for mRNA delivery but often accumulate in the liver, lacking specificity for T cells. High-throughput screening of various LNP formulations for mRNA delivery in Jurkat cell lines is employed to identify those with enhanced affinity and efficacy for T cells. This screening involves assessing protein expression, cell function, and tumor cell co-culture [173–175], thereby laying the groundwork for non-viral adoptive T cell therapies. For instance, Billingsley et al. conducted high-throughput screening in Jurkat cell lines and primary human T cells, identifying C14-4 as the most effective ionizable lipid for LNPs in T cells. They validated its effectiveness in CAR-T cell engineering by expressing the CD19 CAR gene and demonstrated its efficacy in targeting ALL tumor cells [175] (Fig. 5B).
In carrier screening, specificity remains a critical limitation. Researchers must focus on identifying the most suitable components for targeting specific organs through screening. Additionally, screening the structures of delivered mRNA is essential for comparing different mRNAs, as well as their effects on stability and expression efficiency. This enhances targeting design and helps identify optimization patterns. Furthermore, screening improves the controllability of targeted delivery and allows a more comprehensive evaluation of safety, toxicity, and stability in clinical applications. The stability of the carrier is vital for enhancing delivery efficiency, as stable carriers can transport the maximum amount of drug to target organs and cells. Future efforts should leverage carrier screening to select suitable carriers, enabling effective drug delivery to various target cells and maximizing therapeutic efficacy [176].
Conclusion and future perspective
Adoptive T cell therapy represents a significant advancement in cancer immunotherapy research. Emerging high-throughput screening technologies provide an unbiased means to uncover resistance mechanisms and propose optimization strategies. This article focuses on CRISPR screening within high-throughput gene screening as an advanced tool, highlighting its superior flexibility and editing efficiency compared to siRNA and shRNA methods. CRISPR-based screening enhances accuracy and safety, establishing it as a leading method in current gene screening practices. The article also emphasizes specific applications of gene screening technologies in optimizing adoptive cell therapy, including T cell screening, tumor cell screening under T cell pressure, and various combination strategy screenings. The advancement of high-throughput technologies reflects the trend towards precision and specificity in molecular biology, especially in light of detailed research. The rapid development of biotechnologies and their complementary advantages set the stage for enhancing T cell-related therapies, including adoptive cell therapy and ICB therapies.
Despite the potential of gene-editing technologies like siRNA, shRNA, and CRISPR for cancer therapy, several limitations and challenges hinder their widespread clinical translation. A primary concern is off-target effects, leading to unintended gene silencing, which impacts both therapeutic efficacy and safety. Efficient delivery, especially for systemic administration, remains a significant obstacle, including challenges such as cellular uptake, endosomal escape, and targeted delivery to tumor cells. The high cost of development and manufacturing, particularly for personalized CRISPR therapies, poses a significant barrier, especially when considering large-scale clinical implementation and the complexities of delivering these gene-editing tools. Additionally, tumor heterogeneity and the potential for acquired resistance underscore the need for combinatorial therapies and personalized treatment strategies. Future research should prioritize enhancing target specificity, developing more efficient and biocompatible delivery systems, and reducing manufacturing costs. Addressing these challenges is crucial for translating promising preclinical findings into effective and accessible cancer therapies, ultimately revolutionizing cancer treatment. Developing robust preclinical models that accurately recapitulate human tumor biology and enable rigorous evaluation of efficacy and safety prior to clinical translation is essential. This advancement will support the development of next-generation RNAi and CRISPR-based therapies capable of targeting a broad spectrum of cancer types and achieving durable clinical responses.
The establishment and transduction of large-scale screening libraries enable researchers to identify new targets and uncover the mechanisms of interaction between tumor cells and adoptive T cell therapy, with validation through computational and experimental approaches. Nonetheless, enhancing the sensitivity and efficiency of high-throughput screening technologies in tumor immunology research is a critical challenge [177]. Firstly, the use of CRISPR variants has consistently improved precision, efficiency, and safety, as well as expanded the scope of target identification. However, it also faces challenges related to unpredictable off-target risks [178]. High-throughput sequencing technologies can be used for sensitive and efficient detection of off-target effects. Integrating these technologies with machine learning models to predict gene editing efficiency and off-target probabilities, and capitalizing on large-scale sample analysis, can provide more objective insights into screening outcomes [179]. Secondly, high-throughput data generation exceeds traditional experimental methods in speed and volume. The standardization and reproducibility of these high-throughput techniques are vital for data reliability. Technical discussions are needed to maintain sample purity and minimize irrelevant factor interference while increasing screening throughput [180]. With the gradual commercialization of microfluidics and droplet sorting technologies, we anticipate the development of more sensitive high-throughput systems, subject to stricter standardized operating procedures and quality control measures [181]. High-throughput screening technologies, with their precision and specificity, can enhance the controllability of T cell therapies [182]. They offer comprehensive means to assess safety, toxicity, and stability in clinical applications. Combined with other therapeutic approaches, these technologies facilitate synergistic effects, enhancing the likelihood of clinical translation.
Effective delivery of CRISPR screening components into cell pools is essential for successful screening. Delivery systems primarily involve viral vectors and electroporation [183]. Non-viral carriers such as liposomes, polymers, and LNPs are increasingly used for their simplicity, scalability, and controllable targeting. These non-viral systems, with their larger packaging capacity and reduced immunogenicity, offer significant potential, particularly in improving target specificity in high-throughput screening and facilitating in vivo applications [184]. CRISPR screening primarily involves sgRNA and Cas9 protein. Beyond delivery methods, optimizing these components can significantly improve their functional efficiency [185]. For instance, stabilizing sgRNA signals and extending their detection without signal decay present challenges. Utilizing high-fidelity engineered nucleases, such as Cas-CLOVER, HF1, and HiFi (High-precision, long-read genome sequencing technology), enhances DNA cutting accuracy, thus minimizing off-target effects associated with Cas proteins [186].
In summary, this review explores the potential of advanced high-throughput screening in adoptive T cell therapies and combination strategies for malignant tumors. By leveraging continuous improvements in high-throughput screening technologies and tumor immunotherapy, targets and downstream pathways can be optimized through engineering techniques or small molecule medications. This progress is poised to energize the future landscape, potentially revolutionizing disease diagnosis, treatment, and personalized medicine.
Abbreviations
- AAV
Adeno-associated virus
- ADAM2
ADAM Metallopeptidase Domain 2
- ALL
Acute lymphoeytic leukemia
- AML
Acute myeloid leukemia
- AP4
Transcription factor
- APCs
Antigen-presenting cells
- Arid1a
AT-Rich Interaction Domain 1A
- ASF1A
Anti-Silencing Function 1A Histone Chaperone
- ATAC-seq
Assay for Targeting Accessible-Chromatin with high-throughput sequencing
- B-ALL
B cell-acute lymphoblastic leukemia
- BAF
B cell-activating factor
- BATF
Basic Leucine Zipper ATF-Like Transcription Factor
- BCL
B cell lymphoma
- BCL2L1
BCL2 Like 1
- BCMA
B cell maturation antigen
- BCP-ALL
B cell precursor acute lymphoblastic leukemia
- BE
Base editor
- BHLHE40
Basic Helix-Loop-Helix Family Member E40
- BID
BH3 interacting-domain death agonist
- BiTE
Bispecific T-cell engagers
- BMDCs
Bone marrow-derived DCs
- BTN2A1
Butyrophilin Subfamily 2 Member A1
- BTN3A
Butyrophilin Subfamily 3 Member A
- CAF-1
Chromatin assembly factor
- CAR
Chimeric antigen receptor
- Cas12a
CRISPR-associated protein 12a
- Cas9
CRISPR-associated protein 9
- CASTOR1
Cytosolic Arginine Sensor For MTORC1 Subunit 1
- cBAF
Canonical Brgl/Brg-associated factor
- CCC
Combinatorial Cellular Library
- CCDC101
Coiled-Coil Domain Containing 101
- CCNC
Cyclin C
- CCR7
C–C motif chemokine receptor
- CD
Cluster of differentiation
- CDK
Cycle-dependent kinase
- CDRH3
Complementary determining region
- CFLAR
Caspase-8, FADD-like apoptosis regulator
- CFSE
Carboxyfluorescein succinimidyl amino ester
- c-FLIP
Cellular FLICE-inhibitory protein
- CLASH
CRISPR-based library-scale AAV perturbation with simultaneous HDR knock-in
- CMTM6
CKLF Like MARVEL Transmembrane Domain Containing 6
- CRISPR
Clustered Regularly Interspaced Short Palindromic Repeats
- CRISPR KI
CRISPR Knock in
- CRISPR KO
CRISPR Knock out
- CRISPRa
CRISPR action
- CRISPRi
CRISPR interfrence
- crRNA
CRISPR RNA
- CTL
Cytotoxic T cells
- CTLA-4
Cytotoxic T-Lymphocyte-Associated protein 4
- DC
Dendritic cell
- dCas9
Deactivated Cas9
- DHX37
DEAH-Box Helicase 37
- DISC
Death-inducing signaling complex
- DLS
Dynamic light scattering
- DNTs
Double-negative T cells
- DSB
DNA double-strand breaks
- EBF4
Early B cell factor 4
- EGFR
Epidermal Growth Factor Receptor
- EMT
Epithelial-mesenchymal transition
- Epi
Epithelial
- ETS1
ETS Proto-Oncogene 1
- FACS
Fluorescence-activated cell sorting
- FAM49B
Family with sequence similarity 49 member B
- FASL
Factor-related Apoptosis ligand
- Foxp3
Forkhead box P3
- Fuco
Fucosylation
- Fut8
Fucosyltransferase 8
- GCs
Germinal centers
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- GOF
Gain-of-function
- GPR183
G Protein-Coupled Receptor 183
- GSEA
Gene set enrichment analysis
- GSK3β
Glycogen Synthase Kinase 3 β
- HDR
Homology-directed repair
- HF1
H Factor 1
- HIF-1α
Hypoxia-inducible factor-1
- HiFi
High-precision, long-read genome sequencing technology
- IAP
Inhibitors of apoptosis proteins
- ICAM-1
Intercellular adhesion molecule 1
- ICB
Immune checkpoint blocks
- ICDs
Intracellular domains
- IFN-γ
Interferon-γ
- IKZF2
Ikaros family zinc finger protein 2
- IL10RB-DT
IL10RB Divergent Transcript
- INO80
INO80 Complex ATPase Subunit
- IRS
Immune response suppressors
- ITAM
Immunoreceptor tyrosine-based activation motif
- JAK
Janus Kinase
- KRAB
Kruppel-associated box
- LFA-1
Lymphocyte function-associated antigen 1
- lncRNA
Long non-coding RNA
- LNP
Lipid nanoparticles
- LOF
Loss-of-function
- LTBR
Lymphotoxin-β receptor
- LUBAC
Linear ubiquitin chain assembly complex
- Luc
Luciferase
- MED16
Mediator complex subunit 16
- Mes
Mesenchymal-like
- Mfge8
Milk Fat Globule-EGF Factor
- MHC
Major histocompatibility complex
- MHC-I APP
MHC-I antigen processing pathway
- MM
Multiple myeloma
- ModPoKI
Modular Pooled Knockin
- MP
Memory precursor cells
- mRNA
Messenger RNA
- MSLN
Mesothelin
- mSWI/SNF
Mammalian SWItch/Sucrose Non-Fermentable
- MTHFD2
Methylenetetrahydrofolate dehydrogenase-2
- mTORC1
Mechanistic target of rapamycin complex 1
- NAMPT
Nicotinamide phosphoribosyltransferase
- nCas9
Nickase Cas9
- NFATC2
Nuclear Factor of Activated T Cells 2
- NF-κb
Nuclear factor kappa-B
- NK
Natural killer
- NR4A1/3
Nuclear receptor subfamily 4 group A member 1/3
- NUDT21
Nudix Hydrolase 21
- ORF
Open reading frame
- OTOT
On-target, off-tumor
- OX40
TNF Receptor Superfamily Member 4
- P38
A class of mitogen-activated protein kinases
- PAX5
Paired Box 5
- PCR
Polymerase chain reaction
- PDAC
Pancreatic ductal adenocarcinoma
- PD-1
Programmed cell death 1
- PD-L1
Programmed Death Ligand 1
- PIK3CD
Phosphoinositide-3-kinase catalytic subunit delta
- PLCγ-1
Phospholipase C γ 1
- PPI
Protein–protein interaction
- PRDM1
PR/SET Domain 1
- PRMT1
Protein Arginine Methyltransferase 1
- PRODH2
Proline Dehydrogenase 2
- p-S6
Phosphorylation of S6
- RASA
RAS P21 Protein Activator
- RIPK1
Receptor Interacting Serine/Threonine Kinase 1
- RNAi
RNA interference
- S1PR1
Sphingosine-1-phosphate receptor
- SAGA
Spt-Ada-Gcn5-acetyltransferase
- SATB1
Special AT-Rich Sequence Binding Protein 1
- scCAR-seq
Single-cell CAR sequencing
- scCRISPR
Single-cell CRISPR
- scFv
Single-chain variable fragment
- scRNA-seq
Single-cell RNA sequencing
- SET
SET Nuclear Proto-Oncogene
- SETDB1
SET domain bifurcated histone lysine methyltransferase 1
- sgRNA
Single guide RNA
- Slc35c1
Solute Carrier Family 35 Member C1
- SNX9
Sorting nexin 9
- St3gal1
ST3 β-galactoside α-2,3-sialyltransferase 1
- STAT1
Signal Transducer and Activator of Transcription 1
- STUB1
STIP1 Homology And U-Box Containing Protein 1
- TAA
Tumor-associated antigens
- TCR
T cell receptor
- TE
Term effector cells
- TEFF
T effector cell
- TFAP4
Transcription Factor AP-4
- Tfh
T follicular helper cells
- Th
CD4+ helper T cells
- TILs
Tumor-infiltrating lymphocytes
- TLE4
Transducin-like enhancer of split 4
- TM
Transmembrane domain
- TME
Tumor micro-environment
- TMEM
T memory cell
- TNF
Tumor necrosis factor
- TRAC
T Cell Receptor Alpha Constant
- TRAIL
TNF-related apoptosis-inducing ligand
- Treg
Regulatory T cell
- TRIM28
Tripartite motif-containing 28
- TSA
Tumor-specific antigens
- TSS
Transcription start site
- Usp22
Ubiquitin Specific Peptidase 22
- VP64
Virus protein 64
Author contributions
YZ, XX and ML contributed to conception and manuscript design. YZ, QX and ZG drafted the manuscript. YZ, QX, ZG and HZ prepared the tables and figures. YZ, QX and ZG collected the related references. XX, ML, YZ, QX, ZG and HZ participated in the revision of the manuscript. XX and ML were involved in funding acquisition. All authors read and approved the final manuscript.
Funding
This work was supported by Guangdong Basic and Applied Basic Research Foundation (2021A1515110974), the National Natural Science Foundation of China (82270233, U2001224), the Science and Technology Program of Guangzhou (202201011041).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuchen Zhang, Qinglong Xu and Zhifei Gao have contributed equally to this work.
Contributor Information
Xiaoling Xie, Email: xxl413@smu.edu.cn.
Meifang Li, Email: lmf442887107@126.com.
References
- 1.Stribbling SM, Beach C, Ryan AJ. Orthotopic and metastatic tumour models in preclinical cancer research. Pharmacol Ther. 2024;257:108631. [DOI] [PubMed] [Google Scholar]
- 2.Jeffreys N, Brockman JM, Zhai Y, Ingber DE, Mooney DJ. Mechanical forces amplify TCR mechanotransduction in T cell activation and function. Appl Phys Rev. 2024;11:011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez M, Moon EK. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019;10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishna S, Lowery FJ, Copeland AR, Bahadiroglu E, Mukherjee R, Jia L, et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science. 2020;370:1328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baulu E, Gardet C, Chuvin N, Depil S. TCR-engineered T cell therapy in solid tumors: state of the art and perspectives. Sci Adv. 2023;9:eadf3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Q, Zhong X, Li J, Hu R, Yi J, Sun J, et al. Exosomal ncRNAs: multifunctional contributors to the immunosuppressive tumor microenvironment of hepatocellular carcinoma. Biomed Pharmacother. 2024;173:116409. [DOI] [PubMed] [Google Scholar]
- 7.Dong MB, Tang K, Zhou X, Zhou JJ, Chen S. Tumor immunology CRISPR screening: present, past, and future. Trends Cancer. 2022;8:210–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintero-Ruiz N, de Oliveira W, L, Esteca MV, Granato DC, Simabuco FM. Uncovering the bookshelves of CRISPR-based libraries: advances and applications in cancer studies. Crit Rev Oncol Hematol. 2024;196:104287. [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Zhao X, Tang A, Xu X, Liu S, Zha L, et al. CRISPR screen in mechanism and target discovery for cancer immunotherapy. Biochim Biophys Acta Rev Cancer. 2020;1874:188378. [DOI] [PubMed] [Google Scholar]
- 10.Reardon S. First cell therapy for solid tumours heads to the clinic: what it means for cancer treatment. Nature. 2024. 10.1038/d41586-024-00673-w. [DOI] [PubMed] [Google Scholar]
- 11.Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021;27:1410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carnevale J, Shifrut E, Kale N, Nyberg WA, Blaeschke F, Chen YY, et al. RASA2 ablation in T cells boosts antigen sensitivity and long-term function. Nature. 2022;609:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan K, Farrukh H, Chittepu VCSR, Xu H, Pan C-X, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. 2022;41:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan P, Hassel JC, Rutkowski P, Baurain J-F, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385:1196–206. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Hu Y, Yang J, Li W, Zhang M, Wang Q, et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature. 2022;609:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foy SP, Jacoby K, Bota DA, Hunter T, Pan Z, Stawiski E, et al. Non-viral precision T cell receptor replacement for personalized cell therapy. Nature. 2023;615:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, Sun Q, Liang X, Chen Z, Zhang X, Zhou X, et al. Mechanisms of relapse after CD19 CAR T-cell therapy for acute lymphoblastic leukemia and its prevention and treatment strategies. Front Immunol. 2019;10:2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28:415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamieh M, Dobrin A, Cabriolu A, van der Stegen SJC, Giavridis T, Mansilla-Soto J, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao R, Han X, Bai X, Yu J, Ma Y, Chen W, et al. Revolutionizing cancer treatment: enhancing CAR-T cell therapy with CRISPR/Cas9 gene editing technology. Front Immunol. 2024;15:1354825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alshaer W, Zureigat H, Al Karaki A, Al-Kadash A, Gharaibeh L, Hatmal MM, et al. siRNA: mechanism of action, challenges, and therapeutic approaches. Eur J Pharmacol. 2021;905:174178. [DOI] [PubMed] [Google Scholar]
- 22.Rao DD, Vorhies JS, Senzer N, Nemunaitis J. siRNA vs. shRNA: similarities and differences. Adv Drug Deliv Rev. 2009;61:746–59. [DOI] [PubMed] [Google Scholar]
- 23.Chan Y-T, Lu Y, Wu J, Zhang C, Tan H-Y, Bian Z-X, et al. CRISPR-Cas9 library screening approach for anti-cancer drug discovery: overview and perspectives. Theranostics. 2022;12:3329–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Z, Ni K, Wang Y, Zhou Y, Li Y, Yan J, et al. An in-library ligation strategy and its application in CRISPR/Cas9 screening of high-order gRNA combinations. Nucleic Acids Res. 2022;50:6575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt R, Steinhart Z, Layeghi M, Freimer JW, Bueno R, Nguyen VQ, et al. CRISPR activation and interference screens decode stimulation responses in primary human T cells. Science. 2022;375:eabj4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth TL, Li PJ, Blaeschke F, Nies JF, Apathy R, Mowery C, et al. Pooled knockin targeting for genome engineering of cellular immunotherapies. Cell. 2020;181:728-744.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Legut M, Gajic Z, Guarino M, Daniloski Z, Rahman JA, Xue X, et al. A genome-scale screen for synthetic drivers of T cell proliferation. Nature. 2022;603:728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng J, Lin G, Wang T, Wang Y, Guo W, Liao J, et al. Massively parallel CRISPR-based genetic perturbation screening at single-cell resolution. Adv Sci. 2023;10: e2204484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyers S, Demeyer S, Cools J. CRISPR screening in hematology research: from bulk to single-cell level. J Hematol Oncol. 2023;16:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou P, Shi H, Huang H, Sun X, Yuan S, Chapman NM, et al. Single-cell CRISPR screens in vivo map T cell fate regulomes in cancer. Nature. 2023;624:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mimitou EP, Cheng A, Montalbano A, Hao S, Stoeckius M, Legut M, et al. Multiplexed detection of proteins, transcriptomes, clonotypes and CRISPR perturbations in single cells. Nat Methods. 2019;16:409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Datlinger P, Rendeiro AF, Boenke T, Senekowitsch M, Krausgruber T, Barreca D, et al. Ultra-high-throughput single-cell RNA sequencing and perturbation screening with combinatorial fluidic indexing. Nat Methods. 2021;18:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan B, Zhou C, Zhu C, Yu Y, Li G, Zhang S, et al. Model-based understanding of single-cell CRISPR screening. Nat Commun. 2019;10:2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Luo K, Liang L, Chen M, He X. A new Bayesian factor analysis method improves detection of genes and biological processes affected by perturbations in single-cell CRISPR screening. Nat Methods. 2023;20:1693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porto EM, Komor AC, Slaymaker IM, Yeo GW. Base editing: advances and therapeutic opportunities. Nat Rev Drug Discov. 2020;19:839–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt R, Ward CC, Dajani R, Armour-Garb Z, Ota M, Allain V, et al. Base-editing mutagenesis maps alleles to tune human T cell functions. Nature. 2024;625:805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanna RE, Hegde M, Fagre CR, DeWeirdt PC, Sangree AK, Szegletes Z, et al. Massively parallel assessment of human variants with base editor screens. Cell. 2021;184:1064-1080.e20. [DOI] [PubMed] [Google Scholar]
- 38.Coelho MA, Cooper S, Strauss ME, Karakoc E, Bhosle S, Gonçalves E, et al. Base editing screens map mutations affecting interferon-γ signaling in cancer. Cancer Cell. 2023;41:288-303.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bendixen L, Jensen TI, Bak RO. CRISPR-Cas-mediated transcriptional modulation: the therapeutic promises of CRISPRa and CRISPRi. Mol Ther. 2023;31:1920–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu B, Jing Z, Zhang X, Chen Y, Mao S, Kaundal R, et al. Large-scale multiplexed mosaic CRISPR perturbation in the whole organism. Cell. 2022;185:3008-3024.e16. [DOI] [PubMed] [Google Scholar]
- 41.Ye L, Park JJ, Peng L, Yang Q, Chow RD, Dong MB, et al. A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy. Cell Metab. 2022;34:595-614.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konermann S, Lotfy P, Brideau NJ, Oki J, Shokhirev MN, Hsu PD. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell. 2018;173:665-676.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weng X, Zheng M, Liu Y, Lou G. The role of Bach2 in regulating CD8 + T cell development and function. Cell Commun Signal. 2024;22:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albelda SM. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat Rev Clin Oncol. 2024;21:47–66. [DOI] [PubMed] [Google Scholar]
- 45.Hong Y, Walling BL, Kim H-R, Serratelli WS, Lozada JR, Sailer CJ, et al. ST3GAL1 and βII-spectrin pathways control CAR T cell migration to target tumors. Nat Immunol. 2023;24:1007–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansen KH, Golec DP, Huang B, Park C, Thomsen JH, Preite S, et al. A CRISPR screen targeting PI3K effectors identifies RASA3 as a negative regulator of LFA-1-mediated adhesion in T cells. Sci Signal. 2022;15:eabl9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Wang F, Wang L, Qiu S, Yao Y, Yan C, et al. NAD+ supplement potentiates tumor-killing function by rescuing defective TUB-mediated NAMPT transcription in tumor-infiltrated T cells. Cell Rep. 2021;36:109516. [DOI] [PubMed] [Google Scholar]
- 48.Gurusamy D, Henning AN, Yamamoto TN, Yu Z, Zacharakis N, Krishna S, et al. Multi-phenotype CRISPR-Cas9 screen identifies p38 kinase as a target for adoptive immunotherapies. Cancer Cell. 2020;37:818-833.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shang W, Jiang Y, Boettcher M, Ding K, Mollenauer M, Liu Z, et al. Genome-wide CRISPR screen identifies FAM49B as a key regulator of actin dynamics and T cell activation. Proc Natl Acad Sci U S A. 2018;115:E4051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong MB, Wang G, Chow RD, Ye L, Zhu L, Dai X, et al. Systematic immunotherapy target discovery using genome-scale in vivo CRISPR screens in CD8 T cells. Cell. 2019;178:1189-1204.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Felce JH, Parolini L, Sezgin E, Céspedes PF, Korobchevskaya K, Jones M, et al. Single-molecule, super-resolution, and functional analysis of G protein-coupled receptor behavior within the T cell immunological synapse. Front Cell Dev Biol. 2020;8:608484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubo S, Kataria R, Yao Y, Gabrielski JQ, Zheng L, Markowitz TE, et al. Early B cell factor 4 modulates FAS-mediated apoptosis and promotes cytotoxic function in human immune cells. Proc Natl Acad Sci U S A. 2022;119: e2208522119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng C, Aichinger M, Nguyen T, Au C, Najar T, Wu L, et al. The histone chaperone CAF-1 cooperates with the DNA methyltransferases to maintain Cd4 silencing in cytotoxic T cells. Genes Dev. 2019;33:669–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCutcheon SR, Swartz AM, Brown MC, Barrera A, McRoberts Amador C, Siklenka K, et al. Transcriptional and epigenetic regulators of human CD8+ T cell function identified through orthogonal CRISPR screens. Nat Genet. 2023;571:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koga T. Understanding the pathogenic significance of altered calcium-calmodulin signaling in T cells in autoimmune diseases. Clin Immunol. 2024;262:110177. [DOI] [PubMed] [Google Scholar]
- 56.Guo A, Huang H, Zhu Z, Chen MJ, Shi H, Yuan S, et al. cBAF complex components and MYC cooperate early in CD8+ T cell fate. Nature. 2022;607:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang H, Zhou P, Wei J, Long L, Shi H, Dhungana Y, et al. In vivo CRISPR screening reveals nutrient signaling processes underpinning CD8+ T cell fate decisions. Cell. 2021;184:1245-1261.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J, Zhang B, Zhang G, Shang D. Reprogramming of regulatory T cells in inflammatory tumor microenvironment: can it become immunotherapy turning point? Front Immunol. 2024;15:1345838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belk JA, Yao W, Ly N, Freitas KA, Chen Y-T, Shi Q, et al. Genome-wide CRISPR screens of T cell exhaustion identify chromatin remodeling factors that limit T cell persistence. Cancer Cell. 2022;40:768-786.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu JE, Manne S, Ngiow SF, Baxter AE, Huang H, Freilich E, et al. In vitro modeling of CD8+ T cell exhaustion enables CRISPR screening to reveal a role for BHLHE40. Sci Immunol. 2023;8:eade3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Battistello E, Hixon KA, Comstock DE, Collings CK, Chen X, Rodriguez Hernaez J, et al. Stepwise activities of mSWI/SNF family chromatin remodeling complexes direct T cell activation and exhaustion. Mol Cell. 2023;83:1216-1236.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okada M, Chikuma S, Kondo T, Hibino S, Machiyama H, Yokosuka T, et al. Blockage of core fucosylation reduces cell-surface expression of PD-1 and promotes anti-tumor immune responses of T cells. Cell Rep. 2017;20:1017–28. [DOI] [PubMed] [Google Scholar]
- 63.Freitas KA, Belk JA, Sotillo E, Quinn PJ, Ramello MC, Malipatlolla M, et al. Enhanced T cell effector activity by targeting the Mediator kinase module. Science. 2022;378:eabn5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang D, Prager BC, Gimple RC, Aguilar B, Alizadeh D, Tang H, et al. CRISPR screening of CAR T cells and cancer stem cells reveals critical dependencies for cell-based therapies. Cancer Discov. 2021;11:1192–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trefny MP, Kirchhammer N, Auf der Maur P, Natoli M, Schmid D, Germann M, et al. Deletion of SNX9 alleviates CD8 T cell exhaustion for effective cellular cancer immunotherapy. Nat Commun. 2023;14:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dai X, Park JJ, Du Y, Na Z, Lam SZ, Chow RD, et al. Massively parallel knock-in engineering of human T cells. Nat Biotechnol. 2023;41:1239–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blaeschke F, Chen YY, Apathy R, Daniel B, Chen AY, Chen PA, et al. Modular pooled discovery of synthetic knockin sequences to program durable cell therapies. Cell. 2023;186:4216-4234.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flugel CL, Majzner RG, Krenciute G, Dotti G, Riddell SR, Wagner DL, et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat Rev Clin Oncol. 2023;20:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Z, Li D, Yun H, Liu W, Chai K, Tong J, et al. CAR-T cells in the treatment of urologic neoplasms: present and future. Front Oncol. 2022;12:915171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shen J, Lyu S, Xu Y, Zhang S, Li L, Li J, et al. Activating innate immune responses repolarizes hPSC-derived CAR macrophages to improve anti-tumor activity. Cell Stem Cell. 2024;31:1003-1019.e9. [DOI] [PubMed] [Google Scholar]
- 71.Si W, Fan Y-Y, Qiu S-Z, Li X, Wu E-Y, Ju J-Q, et al. Design of diversified chimeric antigen receptors through rational module recombination. iScience. 2023;26:106529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drent E, Themeli M, Poels R, de Jong-Korlaar R, Yuan H, de Bruijn J, et al. A rational strategy for reducing on-target off-tumor effects of CD38-chimeric antigen receptors by affinity optimization. Mol Ther. 2017;25:1946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma P, Ren P, Zhang C, Tang J, Yu Z, Zhu X, et al. Avidity-based selection of tissue-specific CAR-T cells from a combinatorial cellular library of CARs. Adv Sci. 2021;8:2003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Roberto RB, Castellanos-Rueda R, Frey S, Egli D, Vazquez-Lombardi R, Kapetanovic E, et al. A functional screening strategy for engineering chimeric antigen receptors with reduced on-target. Off-Tumor Activation Mol Ther. 2020;28:2564–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smirnov S, Mateikovich P, Samochernykh K, Shlyakhto E. Recent advances on CAR-T signaling pave the way for prolonged persistence and new modalities in clinic. Front Immunol. 2024;15:1335424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Castellanos-Rueda R, Di Roberto RB, Bieberich F, Schlatter FS, Palianina D, Nguyen OTP, et al. speedingCARs: accelerating the engineering of CAR T cells by signaling domain shuffling and single-cell sequencing. Nat Commun. 2022;13:6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goodman DB, Azimi CS, Kearns K, Talbot A, Garakani K, Garcia J, et al. Pooled screening of CAR T cells identifies diverse immune signaling domains for next-generation immunotherapies. Sci Transl Med. 2022;14:eabm1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gordon KS, Kyung T, Perez CR, Holec PV, Ramos A, Zhang AQ, et al. Screening for CD19-specific chimaeric antigen receptors with enhanced signalling via a barcoded library of intracellular domains. Nat Biomed Eng. 2022;6:855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He R, Huang S, Lu J, Su L, Gao X, Chi H. Unveiling the immune symphony: decoding colorectal cancer metastasis through immune interactions. Front Immunol. 2024;15:1362709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burr ML, Sparbier CE, Chan KL, Chan Y-C, Kersbergen A, Lam EYN, et al. An evolutionarily conserved function of polycomb silences the MHC class I antigen presentation pathway and enables immune evasion in cancer. Cancer Cell. 2019;36:385-401.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chan KL, Gomez J, Cardinez C, Kumari N, Sparbier CE, Lam EYN, et al. Inhibition of the CtBP complex and FBXO11 enhances MHC class II expression and anti-cancer immune responses. Cancer Cell. 2022;40:1190-1206.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen X, Lu Q, Zhou H, Liu J, Nadorp B, Lasry A, et al. A membrane-associated MHC-I inhibitory axis for cancer immune evasion. Cell. 2023;186:3903-3920.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spel L, Nieuwenhuis J, Haarsma R, Stickel E, Bleijerveld OB, Altelaar M, et al. Nedd4-binding protein 1 and TNFAIP3-interacting protein 1 control MHC-1 display in neuroblastoma. Cancer Res. 2018;78:6621–31. [DOI] [PubMed] [Google Scholar]
- 84.Gu SS, Zhang W, Wang X, Jiang P, Traugh N, Li Z, et al. Therapeutically increasing MHC-I expression potentiates immune checkpoint blockade. Cancer Discov. 2021;11:1524–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mamedov MR, Vedova S, Freimer JW, Sahu AD, Ramesh A, Arce MM, et al. CRISPR screens decode cancer cell pathways that trigger γδ T cell detection. Nature. 2023;621:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramkumar P, Abarientos AB, Tian R, Seyler M, Leong JT, Chen M, et al. CRISPR-based screens uncover determinants of immunotherapy response in multiple myeloma. Blood Adv. 2020;4:2899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Witkowski MT, Lee S, Wang E, Lee AK, Talbot A, Ma C, et al. NUDT21 limits CD19 levels through alternative mRNA polyadenylation in B cell acute lymphoblastic leukemia. Nat Immunol. 2022;23:1424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Liu Q, Yang S, Liao Q. CD58 Immunobiology at a Glance. Front Immunol. 2021;12:705260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ho P, Melms JC, Rogava M, Frangieh CJ, Poźniak J, Shah SB, et al. The CD58-CD2 axis is co-regulated with PD-L1 via CMTM6 and shapes anti-tumor immunity. Cancer Cell. 2023;41:1207-1221.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oftedal BE, Sjøgren T, Wolff ASB. Interferon autoantibodies as signals of a sick thymus. Front Immunol. 2024;15:1327784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luciano N, Barone E, Timilsina S, Gershwin ME, Selmi C. Tumor necrosis factor alpha inhibitors and cardiovascular risk in rheumatoid arthritis. Clin Rev Allergy Immunol. 2023;65:403–19. [DOI] [PubMed] [Google Scholar]
- 92.Freeman AJ, Vervoort SJ, Michie J, Ramsbottom KM, Silke J, Kearney CJ, et al. HOIP limits anti-tumor immunity by protecting against combined TNF and IFN-gamma-induced apoptosis. EMBO Rep. 2021;22: e53391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sreevalsan S, Döring M, Paszkowski-Rogacz M, Brux M, Blanck C, Meyer M, et al. MLLT6 maintains PD-L1 expression and mediates tumor immune resistance. EMBO Rep. 2020;21: e50155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Apriamashvili G, Vredevoogd DW, Krijgsman O, Bleijerveld OB, Ligtenberg MA, de Bruijn B, et al. Ubiquitin ligase STUB1 destabilizes IFNγ-receptor complex to suppress tumor IFNγ signaling. Nat Commun. 2022;13:1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crowther MD, Dolton G, Legut M, Caillaud ME, Lloyd A, Attaf M, et al. Genome-wide CRISPR-Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat Immunol. 2020;21:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deseke M, Rampoldi F, Sandrock I, Borst E, Böning H, Ssebyatika GL, et al. A CMV-induced adaptive human Vδ1+ γδ T cell clone recognizes HLA-DR. J Exp Med. 2022;219: e20212525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kishton RJ, Patel SJ, Decker AE, Vodnala SK, Cam M, Yamamoto TN, et al. Cancer genes disfavoring T cell immunity identified via integrated systems approach. Cell Rep. 2022;40:111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y, Zhao Y, Guo W, Yadav GS, Bhaskarla C, Wang Z, et al. Genome-wide gain-of-function screening characterized lncRNA regulators for tumor immune response. Sci Adv. 2022;8:eadd0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sang W, Zhou Y, Chen H, Yu C, Dai L, Liu Z, et al. Receptor-interacting protein kinase 2 is an immunotherapy target in pancreatic cancer. Cancer Discov. 2023;14:326–47. [DOI] [PubMed] [Google Scholar]
- 100.Dervovic D, Malik AA, Chen ELY, Narimatsu M, Adler N, Afiuni-Zadeh S, et al. In vivo CRISPR screens reveal Serpinb9 and Adam2 as regulators of immune therapy response in lung cancer. Nat Commun. 2023;14:3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Han P, Dai Q, Fan L, Lin H, Zhang X, Li F, et al. Genome-wide CRISPR screening identifies JAK1 deficiency as a mechanism of T-cell resistance. Front Immunol. 2019;10:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kearney CJ, Vervoort SJ, Hogg SJ, Ramsbottom KM, Freeman AJ, Lalaoui N, et al. Tumor immune evasion arises through loss of TNF sensitivity. Sci Immunol. 2018;3:eaar3451. [DOI] [PubMed] [Google Scholar]
- 103.Park JJ, Codina A, Ye L, Lam S, Guo J, Clark P, et al. Double knockout CRISPR screen for cancer resistance to T cell cytotoxicity. J Hematol Oncol. 2022;15:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williams JB, Li S, Higgs EF, Cabanov A, Wang X, Huang H, et al. Tumor heterogeneity and clonal cooperation influence the immune selection of IFN-γ-signaling mutant cancer cells. Nat Commun. 2020;11:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li M, Xu Y, Liang J, Lin H, Qi X, Li F, et al. USP22 deficiency in melanoma mediates resistance to T cells through IFNγ-JAK1-STAT1 signal axis. Mol Ther. 2021;29:2108–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Z, Kong X, Ligtenberg MA, van Hal-van Veen SE, Visser NL, de Bruijn B, et al. RNF31 inhibition sensitizes tumors to bystander killing by innate and adaptive immune cells. Cell Rep Med. 2022;3:100655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, et al. Identification of essential genes for cancer immunotherapy. Nature. 2017;548:537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herzfeldt A-K, Gamez MP, Martin E, Boryn LM, Baskaran P, Huber HJ, et al. Complementary CRISPR screen highlights the contrasting role of membrane-bound and soluble ICAM-1 in regulating antigen-specific tumor cell killing by cytotoxic T cells. Elife. 2023;12: e84314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cui Y, Miao Y, Cao L, Guo L, Cui Y, Yan C, et al. Activation of melanocortin-1 receptor signaling in melanoma cells impairs T cell infiltration to dampen antitumor immunity. Nat Commun. 2023;14:5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Frey N, Tortola L, Egli D, Janjuha S, Rothgangl T, Marquart KF, et al. Loss of Rnf31 and Vps4b sensitizes pancreatic cancer to T cell-mediated killing. Nat Commun. 2022;13:1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Joung J, Kirchgatterer PC, Singh A, Cho JH, Nety SP, Larson RC, et al. CRISPR activation screen identifies BCL-2 proteins and B3GNT2 as drivers of cancer resistance to T cell-mediated cytotoxicity. Nat Commun. 2022;13:1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li J, Yuan S, Norgard RJ, Yan F, Sun YH, Kim I-K, et al. Epigenetic and transcriptional control of the epidermal growth factor receptor regulates the tumor immune microenvironment in pancreatic cancer. Cancer Discov. 2021;11:736–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Larson RC, Kann MC, Bailey SR, Haradhvala NJ, Llopis PM, Bouffard AA, et al. CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours. Nature. 2022;604:563–70. [DOI] [PubMed] [Google Scholar]
- 114.Yan X, Chen D, Ma X, Wang Y, Guo Y, Wei J, et al. CD58 loss in tumor cells confers functional impairment of CAR T cells. Blood Adv. 2022;6:5844–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yan X, Chen D, Wang Y, Guo Y, Tong C, Wei J, et al. Identification of NOXA as a pivotal regulator of resistance to CAR T-cell therapy in B-cell malignancies. Signal Transduct Target Ther. 2022;7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hagel KR, Arafeh R, Gang S, Arnoff TE, Larson RC, Doench JG, et al. Systematic interrogation of tumor cell resistance to chimeric antigen receptor T-cell therapy in pancreatic cancer. Cancer Res. 2023;83:613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jabbarzadeh Kaboli P, Chen H-F, Babaeizad A, Roustai Geraylow K, Yamaguchi H, Hung M-C. Unlocking c-MET: a comprehensive journey into targeted therapies for breast cancer. Cancer Lett. 2024;588:216780. [DOI] [PubMed] [Google Scholar]
- 118.Gu Y, Zhang Z, Camps MGM, Ossendorp F, Wijdeven RH, Ten Dijke P. Genome-wide CRISPR screens define determinants of epithelial-mesenchymal transition mediated immune evasion by pancreatic cancer cells. Sci Adv. 2023;9:eadf9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soares F, Chen B, Lee JB, Ahmed M, Ly D, Tin E, et al. CRISPR screen identifies genes that sensitize AML cells to double-negative T-cell therapy. Blood. 2021;137:2171–81. [DOI] [PubMed] [Google Scholar]
- 120.Zhou P, Gao Y, Kong Z, Wang J, Si S, Han W, et al. Immune checkpoint inhibitors and acute kidney injury. Front Immunol. 2024;15:1353339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bell HN, Zou W. Beyond the barrier: unraveling the mechanisms of immunotherapy resistance. Annu Rev Immunol. 2024;624:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dubrot J, Du PP, Lane-Reticker SK, Kessler EA, Muscato AJ, Mehta A, et al. In vivo CRISPR screens reveal the landscape of immune evasion pathways across cancer. Nat Immunol. 2022;23:1495–506. [DOI] [PubMed] [Google Scholar]
- 123.Huang Q, Li F, Hu H, Fang Z, Gao Z, Xia G, et al. Loss of TSC1/TSC2 sensitizes immune checkpoint blockade in non-small cell lung cancer. Sci Adv. 2022;8:eabi9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Manguso RT, Pope HW, Zimmer MD, Brown FD, Yates KB, Miller BC, et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547:413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang G, Chow RD, Zhu L, Bai Z, Ye L, Zhang F, et al. CRISPR-GEMM pooled mutagenic screening identifies KMT2D as a major modulator of immune checkpoint blockade. Cancer Discov. 2020;10:1912–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li F, Huang Q, Luster TA, Hu H, Zhang H, Ng W-L, et al. In vivo epigenetic CRISPR screen identifies Asf1a as an immunotherapeutic target in Kras-Mutant lung adenocarcinoma. Cancer Discov. 2020;10:270–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burr ML, Sparbier CE, Chan Y-C, Williamson JC, Woods K, Beavis PA, et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature. 2017;549:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma X, Jia S, Wang G, Liang M, Guo T, Du H, et al. TRIM28 promotes the escape of gastric cancer cells from immune surveillance by increasing PD-L1 abundance. Signal Transduct Target Ther. 2023;8:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun G, Zhao S, Fan Z, Wang Y, Liu H, Cao H, et al. CHSY1 promotes CD8+ T cell exhaustion through activation of succinate metabolism pathway leading to colorectal cancer liver metastasis based on CRISPR/Cas9 screening. J Exp Clin Cancer Res. 2023;42:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cao J, Yan Q. Cancer epigenetics, tumor immunity, and immunotherapy. Trends Cancer. 2020;6:580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin J, Guo D, Liu H, Zhou W, Wang C, Müller I, et al. The SETDB1-TRIM28 complex suppresses antitumor immunity. Cancer Immunol Res. 2021;9:1413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hou J, Wang Y, Shi L, Chen Y, Xu C, Saeedi A, et al. Integrating genome-wide CRISPR immune screen with multi-omic clinical data reveals distinct classes of tumor intrinsic immune regulators. J Immunother Cancer. 2021;9: e001819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pan D, Kobayashi A, Jiang P, Ferrari de Andrade L, Tay RE, Luoma AM, et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science. 2018;359:770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dmello C, Zhao J, Chen L, Gould A, Castro B, Arrieta VA, et al. Checkpoint kinase 1/2 inhibition potentiates anti-tumoral immune response and sensitizes gliomas to immune checkpoint blockade. Nat Commun. 2023;14:1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zarezadeh Mehrabadi A, Tat M, Ghorbani Alvanegh A, Roozbahani F, Esmaeili Gouvarchin Ghaleh H. Revolutionizing cancer treatment: the power of bi- and tri-specific T-cell engagers in oncolytic virotherapy. Front Immunol. 2024;15:1343378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Y, Moriyama T, Yoshimura S, Zhao X, Li Z, Yang X, et al. PAX5 epigenetically orchestrates CD58 transcription and modulates blinatumomab response in acute lymphoblastic leukemia. Sci Adv. 2022;8:eadd6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Decker CE, Young T, Pasnikowski E, Chiu J, Song H, Wei Y, et al. Genome-scale CRISPR activation screen uncovers tumor-intrinsic modulators of CD3 bispecific antibody efficacy. Sci Rep. 2019;9:20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu S-Q, Grantham A, Landry C, Granda B, Chopra R, Chakravarthy S, et al. A CRISPR screen reveals resistance mechanisms to CD3-bispecific antibody therapy. Cancer Immunol Res. 2021;9:34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Molony RD, Funk T, Trabucco G, Corcoran E, Ruddy D, Varadarajan M, et al. CRISPR screening identifies T cell-intrinsic regulators of CD3-bispecific antibody responses. Front Immunol. 2022;13:909979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shen Y, Eng JS, Fajardo F, Liang L, Li C, Collins P, et al. Cancer cell-intrinsic resistance to BiTE therapy is mediated by loss of CD58 costimulation and modulation of the extrinsic apoptotic pathway. J Immunother Cancer. 2022;10: e004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moinuddin A, Poznanski SM, Portillo AL, Monteiro JK, Ashkar AA. Metabolic adaptations determine whether natural killer cells fail or thrive within the tumor microenvironment. Immunol Rev. 2024. 10.1111/imr.13316. [DOI] [PubMed] [Google Scholar]
- 142.Wang X, Tokheim C, Gu SS, Wang B, Tang Q, Li Y, et al. In vivo CRISPR screens identify the E3 ligase Cop1 as a modulator of macrophage infiltration and cancer immunotherapy target. Cell. 2021;184:5357-5374.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kamber RA, Nishiga Y, Morton B, Banuelos AM, Barkal AA, Vences-Catalán F, et al. Inter-cellular CRISPR screens reveal regulators of cancer cell phagocytosis. Nature. 2021;597:549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tong J, Wang X, Liu Y, Ren X, Wang A, Chen Z, et al. Pooled CRISPR screening identifies m6A as a positive regulator of macrophage activation. Sci Adv. 2021;7:4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kiritsy MC, McCann K, Mott D, Holland SM, Behar SM, Sassetti CM, et al. Mitochondrial respiration contributes to the interferon gamma response in antigen-presenting cells. Elife. 2021;10: e65109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kiritsy MC, Ankley LM, Trombley J, Huizinga GP, Lord AE, Orning P, et al. A genetic screen in macrophages identifies new regulators of IFNγ-inducible MHCII that contribute to T cell activation. Elife. 2021;10: e65110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xia L, Komissarova A, Jacover A, Shovman Y, Arcila-Barrera S, Tornovsky-Babeay S, et al. Systematic identification of gene combinations to target in innate immune cells to enhance T cell activation. Nat Commun. 2023;14:6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sutra Del Galy A, Menegatti S, Fuentealba J, Lucibello F, Perrin L, Helft J, et al. In vivo genome-wide CRISPR screens identify SOCS1 as intrinsic checkpoint of CD4+ TH1 cell response. Sci Immunol. 2021;6:eabe8219. [DOI] [PubMed] [Google Scholar]
- 149.Henriksson J, Chen X, Gomes T, Ullah U, Meyer KB, Miragaia R, et al. Genome-wide CRISPR screens in T Helper cells reveal pervasive crosstalk between activation and differentiation. Cell. 2019;176:882-896.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Song W, Craft J. T follicular helper cell heterogeneity. Annu Rev Immunol. 2023;42:127–52. [DOI] [PubMed] [Google Scholar]
- 151.Fu G, Guy CS, Chapman NM, Palacios G, Wei J, Zhou P, et al. Metabolic control of TFH cells and humoral immunity by phosphatidylethanolamine. Nature. 2021;595:724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang B, Phelan JD, Preite S, Gomez-Rodriguez J, Johansen KH, Shibata H, et al. In vivo CRISPR screens reveal a HIF-1α-mTOR-network regulates T follicular helper versus Th1 cells. Nat Commun. 2022;13:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kumagai S, Itahashi K, Nishikawa H. Regulatory T cell-mediated immunosuppression orchestrated by cancer: towards an immuno-genomic paradigm for precision medicine. Nat Rev Clin Oncol. 2024;21:337–53. [DOI] [PubMed] [Google Scholar]
- 154.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cortez JT, Montauti E, Shifrut E, Gatchalian J, Zhang Y, Shaked O, et al. CRISPR screen in regulatory T cells reveals modulators of Foxp3. Nature. 2020;582:416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Loo C-S, Gatchalian J, Liang Y, Leblanc M, Xie M, Ho J, et al. A genome-wide CRISPR screen reveals a role for the non-canonical nucleosome-remodeling BAF complex in Foxp3 expression and regulatory T cell function. Immunity. 2020;53:143-157.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pinioti S, Sharma H, Flerin NC, Yu Q, Tzoumpa A, Trusso Cafarello S, et al. A metabolic gene survey pinpoints fucosylation as a key pathway underlying the suppressive function of regulatory T cells in cancer. Cancer Immunol Res. 2023;11:1611–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schumann K, Raju SS, Lauber M, Kolb S, Shifrut E, Cortez JT, et al. Functional CRISPR dissection of gene networks controlling human regulatory T cell identity. Nat Immunol. 2020;21:1456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sugiura A, Andrejeva G, Voss K, Heintzman DR, Xu X, Madden MZ, et al. MTHFD2 is a metabolic checkpoint controlling effector and regulatory T cell fate and function. Immunity. 2022;55:65-81.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wilfahrt D, Delgoffe GM. Metabolic waypoints during T cell differentiation. Nat Immunol. 2024;25:206–17. [DOI] [PubMed] [Google Scholar]
- 161.Long L, Wei J, Lim SA, Raynor JL, Shi H, Connelly JP, et al. CRISPR screens unveil signal hubs for nutrient licensing of T cell immunity. Nature. 2021;600:308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dufva O, Koski J, Maliniemi P, Ianevski A, Klievink J, Leitner J, et al. Integrated drug profiling and CRISPR screening identify essential pathways for CAR T-cell cytotoxicity. Blood. 2020;135:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lizotte PH, Hong R-L, Luster TA, Cavanaugh ME, Taus LJ, Wang S, et al. A High-throughput immune-oncology screen identifies EGFR inhibitors as potent enhancers of antigen-specific cytotoxic T-lymphocyte tumor cell killing. Cancer Immunol Res. 2018;6:1511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhu XG, Chudnovskiy A, Baudrier L, Prizer B, Liu Y, Ostendorf BN, et al. Functional genomics in vivo reveal metabolic dependencies of pancreatic cancer cells. Cell Metab. 2021;33:211-221.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu L, Jin Y, Zhao X, Tang K, Zhao Y, Tong L, et al. Tumor aerobic glycolysis confers immune evasion through modulating sensitivity to T cell-mediated bystander killing via TNF-α. Cell Metab. 2023;35:1580-1596.e9. [DOI] [PubMed] [Google Scholar]
- 166.Irvine DJ, Maus MV, Mooney DJ, Wong WW. The future of engineered immune cell therapies. Science. 2022;378:853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Han G, Noh D, Lee H, Lee S, Kim S, Yoon HY, et al. Advances in mRNA therapeutics for cancer immunotherapy: from modification to delivery. Adv Drug Deliv Rev. 2023;199:114973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Foster JB, Griffin C, Rokita JL, Stern A, Brimley C, Rathi K, et al. Development of GPC2-directed chimeric antigen receptors using mRNA for pediatric brain tumors. J Immunother Cancer. 2022;10: e004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lin L, Cho S-F, Xing L, Wen K, Li Y, Yu T, et al. Preclinical evaluation of CD8+ anti-BCMA mRNA CAR T-cells for treatment of multiple myeloma. Leukemia. 2021;35:752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Parayath NN, Stephan SB, Koehne AL, Nelson PS, Stephan MT. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat Commun. 2020;11:6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Si K, Dai Z, Li Z, Ye Z, Ding B, Feng S, et al. Engineered exosome-mediated messenger RNA and single-chain variable fragment delivery for human chimeric antigen receptor T-cell engineering. Cytotherapy. 2023;25:615–24. [DOI] [PubMed] [Google Scholar]
- 172.Ye Z, Chen J, Zhao X, Li Y, Harmon J, Huang C, et al. In vitro engineering chimeric antigen receptor macrophages and T cells by lipid nanoparticle-mediated mRNA delivery. ACS Biomater Sci Eng. 2022;8:722–33. [DOI] [PubMed] [Google Scholar]
- 173.Billingsley MM, Hamilton AG, Mai D, Patel SK, Swingle KL, Sheppard NC, et al. Orthogonal design of experiments for optimization of lipid nanoparticles for mRNA engineering of CAR T cells. Nano Lett. 2022;22:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Patel SK, Billingsley MM, Frazee C, Han X, Swingle KL, Qin J, et al. Hydroxycholesterol substitution in ionizable lipid nanoparticles for mRNA delivery to T cells. J Control Release. 2022;347:521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Billingsley MM, Singh N, Ravikumar P, Zhang R, June CH, Mitchell MJ. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20:1578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang Y, Gao Z, Yang X, Xu Q, Lu Y. Leveraging high-throughput screening technologies in targeted mRNA delivery. Mater Today Bio. 2024;26:101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhan T, Rindtorff N, Betge J, Ebert MP, Boutros M. CRISPR/Cas9 for cancer research and therapy. Semin Cancer Biol. 2019;55:106–19. [DOI] [PubMed] [Google Scholar]
- 178.Li J, Hong S, Chen W, Zuo E, Yang H. Advances in detecting and reducing off-target effects generated by CRISPR-mediated genome editing. J Genet Genom. 2019;46:513–21. [DOI] [PubMed] [Google Scholar]
- 179.Yamankurt G, Berns EJ, Xue A, Lee A, Bagheri N, Mrksich M, et al. Exploration of the nanomedicine-design space with high-throughput screening and machine learning. Nat Biomed Eng. 2019;3:318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wójcik M, Telzerow A, Quax WJ, Boersma YL. High-throughput screening in protein engineering: recent advances and future perspectives. Int J Mol Sci. 2015;16:24918–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mazutis L, Gilbert J, Ung WL, Weitz DA, Griffiths AD, Heyman JA. Single-cell analysis and sorting using droplet-based microfluidics. Nat Protoc. 2013;8:870–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yin Q, Tang J, Zhu X. Next-generation sequencing technologies accelerate advances in T-cell therapy for cancer. Brief Funct Genom. 2019;18:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li T, Yang Y, Qi H, Cui W, Zhang L, Fu X, et al. CRISPR/Cas9 therapeutics: progress and prospects. Signal Transduct Target Ther. 2023;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mashel TV, Tarakanchikova YV, Muslimov AR, Zyuzin MV, Timin AS, Lepik KV, et al. Overcoming the delivery problem for therapeutic genome editing: current status and perspective of non-viral methods. Biomaterials. 2020;258:120282. [DOI] [PubMed] [Google Scholar]
- 185.Kazemian P, Yu S-Y, Thomson SB, Birkenshaw A, Leavitt BR, Ross CJD. Lipid-nanoparticle-based delivery of CRISPR/Cas9 genome-editing components. Mol Pharm. 2022;19:1669–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen Y, Hu Y, Wang X, Luo S, Yang N, Chen Y, et al. Synergistic engineering of CRISPR-Cas nucleases enables robust mammalian genome editing. Innovation. 2022;3:100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.