Abstract
Background
Little research is available to provide practical guidance to health care providers for exercise preparticipation screening and referral of patients with interstitial lung diseases (ILDs), including lymphangioleiomyomatosis (LAM), to participate in remote, unsupervised exercise programs.
Research Question
What exercise preparticipation screening steps are essential to determine whether a patient with LAM is medically appropriate to participate in a remote, unsupervised exercise program?
Study Design and Methods
Sixteen experts in LAM and ILD participated in a two-round modified Delphi study, ranking their level of agreement for 10 statements related to unsupervised exercise training in LAM, with an a priori definition of consensus. Additionally, 60 patients with LAM completed a survey of the perceived risks and benefits of remote exercise training in LAM.
Results
Seven of the 10 statements reached consensus among experts. Experts agreed that an in-person clinical exercise test is indicated to screen for exercise-induced hypoxemia and prescribe supplemental oxygen therapy as indicated prior to initiating a remote exercise program. Patients with recent pneumothorax should wait to start an exercise program for at least 4 weeks until after resolution of pneumothorax and clearance by a physician. Patients with high cardiovascular risk for event during exercise, severe resting pulmonary hypertension, or risk for falls may be more appropriate for referral to a rehabilitation center. A LAM-specific remote exercise preparticipation screening tool was developed from the consensus statements and agreed upon by the panelists.
Interpretation
A modified Delphi study approach was useful to develop disease-specific recommendations for safety and preparticipation screening prior to unsupervised, remotely administered exercise in LAM. The primary product of this study is a clinical decision aid for providers to use when medically screening patients prior to participation in the newly launched LAMFit remote exercise program.
Key Words: Delphi study, digital health, exercise risk stratification, exercise training, interstitial lung disease, LAM, lymphangioleiomyomatosis, preparticipation screening, remote monitoring
Executive Summary
Patients with interstitial lung diseases (ILDs), including lymphangioleiomyomatosis (LAM), have unique risks for adverse health events during exercise training outside of a medically supervised setting. Traditionally, patients with ILD have been referred to supervised, center-based pulmonary rehabilitation programs for availability of supplemental oxygen, assistance with oxygen titration, and direct supervision during exercise. However, center-based programs may not be as accessible as home-based programs and may not be preferred by all patients with LAM. Consensus recommendations were developed as guidance for exercise preparticipation screening in LAM to determine medical appropriateness to participate in a remote, unsupervised exercise program.
A multidisciplinary panel of experts in LAM and ILD participated in a two-round modified Delphi study and ranked their level of agreement for statements related to safety and risk mitigation during exercise training in LAM.
Ten statements were formulated and modified based on expert survey results and discussion at an in-person panel meeting. Seven of the 10 final statements reached consensus among experts. A LAM-specific remote exercise preparticipation screening tool was developed from the consensus statements and agreed upon by the full panel.
The panel provides a list of recommendations and a consensus LAM-specific exercise preparticipation screening tool for provider use prior to patient enrollment in the LAMFit remote exercise program.
Summary of Recommendations
• An in-person clinical exercise test is indicated to screen for exercise-induced hypoxemia and prescribe supplemental oxygen therapy as needed prior to initiating a remote exercise program.
• Patients with recent pneumothorax should wait to start an exercise program for at least 4 weeks until after resolution of pneumothorax and clearance by a physician.
• Patients with high cardiovascular risk for event during exercise, severe resting pulmonary hypertension, or risk for falls may be more appropriate for referral to exercise under supervision in a rehabilitation center.
Background
With the rapid uptake and growing availability of new models of exercise programming and telerehabilitation in chronic respiratory diseases, the exercise setting is an important new variable to consider in exercise risk stratification. In settings where exercise training occurs without direct supervision, health care providers have a reduced capacity to respond to an adverse event or medical emergency. However, a hybrid or remote exercise or pulmonary rehabilitation (PR) delivery model reduces barriers to referral, attendance, and affordability.1, 2, 3 The 2023 American Thoracic Society (ATS) clinical practice guideline “Pulmonary Rehabilitation for Adults with Chronic Respiratory Disease” strongly recommends offering a choice between telerehabilitation and center-based PR for stable patients with chronic respiratory disease but acknowledges that the majority of telerehabilitation studies to date are in COPD.4 Compared with COPD, less evidence is available to support the development of guidelines for exercise programming in ILD.3
ILD comprises a heterogeneous group of disorders that share some, but not all, similarities in risks for events during exercise. Individual disease pathology, sequelae, and other system comorbidities can contribute additional risks for exertional complications. Thus, a patient-centered and disease-specific approach to exercise preparticipation risk screening is preferred for risk mitigation. Patients with the rare, female-predominant ILD LAM commonly experience exertional hypoxemia as the disease progresses,5,6 similar to other ILDs.7 Pulmonary manifestations of LAM include cystic lung destruction,8 slow annual FEV1 decline, and progressive gas exchange impairment.9 Patients with LAM are generally younger than patients with other chronic respiratory diseases, with an average age of onset of symptoms of 38.9 ± 0.73 years.10 Many patients are still heavily involved in career and family responsibilities.11
Traditionally, patients with ILD, including LAM, have been referred to supervised, center-based PR programs for availability of supplemental oxygen, assistance with oxygen titration, and direct supervision during exercise. However, center-based PR programs may not be as accessible as home-based programs and may not be preferred by patients with LAM. The recent 2023 ATS guideline authors suggest that exercise-based PR for adults with ILD should be delivered in a setting in which supplemental oxygen can be administered during training, given the primary risk for exertional desaturation.4 If sufficient supplemental oxygen is available to support exercise training, a home-based approach removes many of the participation barriers encountered by patients with LAM and ILD.12 Most adults with LAM are young to middle-aged at the time of diagnosis,11 and thus are less likely to have age-related challenges to digital health literacy and barriers to use of wearable fitness trackers,13 both important factors to the success of a digitally enabled care approach in chronic respiratory disease.14,15 Our research team recently demonstrated that a remote, asynchronously monitored home exercise program is safe in LAM, with no study-related adverse events and high participant satisfaction, and improves symptoms of dyspnea, fatigue, and exercise intolerance.5 In community-academic partnership16 with The LAM Foundation, we will now implement “LAMFit,” a program to expand access to the health benefits of exercise for more individuals with LAM.
Current population-based models for exercise preparticipation screening, including the 2015 American College of Sports Medicine Exercise Preparticipation Health Screening Tool17 and the 2018 Physical Activity Guidelines for Americans,18 are based on an individual’s current physical activity levels, the presence of signs or symptoms and/or known cardiovascular, metabolic, or renal disease, and planned exercise intensity, all of which are risk modulators of exercise-related cardiovascular events.19 Notably, both of these resources do not advise on risk factors related to chronic respiratory diseases. There is a gap in the literature of available screening tools to assist providers when prescreening patients with LAM and other chronic respiratory diseases who want to initiate an unsupervised home exercise program.
The purpose of this study was to develop an expert consensus exercise preparticipation screening tool specifically for patients with LAM, to be used by LAM providers when determining medical appropriateness to participate in the remote, unsupervised LAMFit exercise program.
Study Design and Methods
To synthesize the opinions of health care providers, patients, and other stakeholders in the LAM community related to screening for medical appropriateness for unsupervised exercise in LAM, we conducted a two-round modified Delphi study consisting of a first-round online survey and a second round in-person meeting for panel discussion. A Delphi study methodology is an acceptable method for generating consensus when there is a paucity of available literature and an urgent need to develop guidelines.20,21 A modified RAND/UCLA Appropriateness Method22 (Fig 1) was used to structure each round, as it is a rigorous Delphi-style method with extensive use cases in health care for generating consensus about the appropriateness of procedures and the prospective development of clinical decision aids.23, 24, 25, 26, 27 Key components and methodological priorities of the RAND/UCLA Appropriateness Method are provided in e-Table 1. Study procedures were approved by the University of Washington Institutional Review Board (#STUDY00017539).
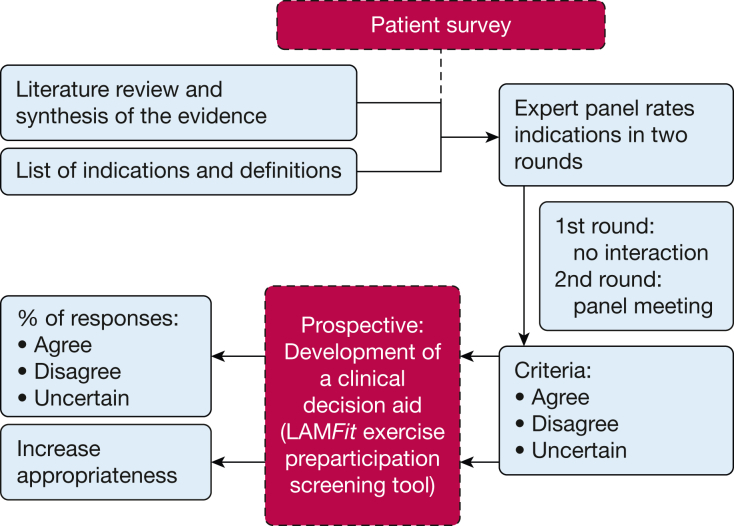
Figure 1.
An overview of the Delphi study method, adapted from the RAND/UCLA Appropriateness Method22 for the prospective development of a clinical decision aid by the LAMFit Delphi panel in this study. The RAND/UCLA Appropriateness Method user manual22 provides guidance for structuring expert panel processes to measure and increase the appropriateness of a clinical tool or set of guidelines. Investigators in this study generally adhered to the RAND/UCLA Appropriateness Method user manual by: (1) conducting a literature review and sharing with invited experts a synthesis of the evidence; (2) conducting a first-round online survey and a second-round, in-person panel meeting; (3) using specific RAND/UCLA criteria for grading of consensus agreement, disagreement, or uncertainty about statements; and (4) prospectively developing a clinical decision aid for exercise preparticipation screening prior to patient enrollment in the LAMFit program.
Purposive sampling28 was used to identify and invite a diverse panel of 16 providers, researchers, and other professionals in LAM and ILD. Our panel size was informed by other Delphi studies in health care,29,30 as there is no standard size.31 Physician directors of at least five geographically distinct LAM Centers of Excellence; other clinician scientists with expertise and recent publications in the pathophysiology of LAM and ILD, hypoxia, altitude, and exercise; and leadership from The LAM Foundation were invited to participate.
Review of the Literature
A MEDLINE database search was performed for relevant articles published in the English language between 1992 and 2022. MeSH headings of “exercise” and “exercise training” were combined with other relevant search terms, including guidelines, preparticipation screening, risk of event, remote, physical activity, chronic respiratory disease, interstitial lung disease, and lymphangioleiomyomatosis. Additional online resources were compiled and reviewed from the websites of medical professional associations, including the ATS, European Respiratory Society, American College of Cardiology, American Heart Association, International Society for Heart and Lung Transplantation, American College of Sports Medicine, and the American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Full articles of available clinical practice guidelines and evidence-based exercise preparticipation screening tools for chronic diseases were reviewed and summarized by two investigators. Prior to Round 1, all invited expert panelists were asked to read a summary of existing literature regarding exercise preparticipation screening in chronic diseases.
Existing tools for exercise preparticipation screening have been developed and disseminated17,32,33 but are less specific to LAM and ILD. Population-based screening tools consider cardiovascular risks first and foremost—without intact cardiac electrophysiology and pump function, increasing the cardiovascular workload can result in an abnormal exercise response.34 Patients with ILD can develop comorbid cardiovascular conditions, especially as they age.35 Thus, in addition to risk for exertional desaturation,4 current literature suggests that patients with LAM and ILD should also be prescreened for cardiovascular risks17 prior to initiating an unsupervised exercise program.
Round 1
A Round 1 survey for experts was developed by the research team utilizing concepts from literature on population-based exercise preparticipation screening, CPR, and LAM-specific exercise physiology. Initial survey questions were reviewed by three content experts in medicine, pulmonology, and LAM, and revisions were made based on feedback. The Round 1 survey for experts consisted of short-answer, ranking, and open-ended questions about the risks and benefits of unsupervised exercise in LAM (Supplemental Material). The Round 1 survey was administered to expert panelists via REDCap (Research Electronic Data Capture), a secure, web-based platform designed to support research data capture,36 and remained open for 3 weeks. To maximize response rates, the tailored design method devised by Dillman et al37 was used with reminder emails three additional times, 4 days apart until completion.
Patient Survey
Recognizing that patients are key stakeholders in a community-engaged approach to the development of a health promotion program,16 patients with LAM were also invited to complete a separate online survey (different than the Round 1 survey for experts) about their own lived experience and their perceptions of exercise in LAM (Supplemental Material). Since there is little available literature about the patient experience of exercising with LAM, the research team felt that the patient voice was important for experts to consider during panel activities, in addition to their own professional experience in LAM. A convenience sample of patients with LAM were recruited via email, social media, and word of mouth, in collaboration with designated patient liaisons for The LAM Foundation. The survey was administered anonymously via REDCap and remained open for 3 weeks. Importantly, patient survey results were not used in the drafting of statements, and patients did not comment on the statements or the preparticipation screening tool that are the primary outcomes of this study. The patient survey results were summarized as major themes with example raw quotes and provided to experts as background material prior to the in-person panel meeting.
Round 2
Round 1 expert survey results were shared electronically with panelists 4 weeks before the Round 2 panel meeting. For this, two investigators (C. E. C. and M. B. B.) drafted initial statements based on review of published exercise preparticipation screening guidelines in the literature4,17,32,38 and final themes from the primary risks and concerns expressed by LAM experts. Two other content experts (L. A. H. and A. M. Turner) in medicine, pulmonology, and LAM reviewed and provided feedback on the initial statements for clinical and methodological appropriateness. Four experts in total participated in an iterative process of review, revision, and interpretation to arrive at the final statements that were reviewed by the expert panel. Round 2 occurred as an in-person meeting at the May 2023 ATS International Conference in Washington, DC. Panelists received complimentary breakfast and no monetary compensation. The moderator presented each statement individually for panel discussion. Final level of agreement assessment occurred via anonymous paper survey at the end of the Round 2 meeting, with options to select “Agree,” “Disagree,” or “Uncertain” for 9 of the 10 statements. One statement provided numerical options for acceptable desaturation threshold for exertional oxygen use in LAM. Consensus was defined a priori as ≥ 78% of panelists selecting the same option (eg, “Agree”) for a statement, similar to the RAND/UCLA Appropriateness mean threshold of ≥ 7 of 9 on a 9-point Likert scale.22
The moderator also presented for panel discussion a draft of the LAMFit preparticipation screening tool based on the primary risks perceived by experts in Round 1. During the Round 2 meeting, each component and decision point of the screening tool was openly discussed and evaluated by panel members. After the Round 2 meeting, a revised LAMFit preparticipation screening tool was designed and shared with experts electronically for another round of feedback and consensus approval. Statements that did not reach consensus in Round 2 were not included in the final design of the LAMFit preparticipation screening tool.
Post-Delphi Panel Assessment Survey
Panelists were also asked to complete an anonymous post-Delphi assessment survey about their experience, with questions adapted from an example survey in the RAND/UCLA Appropriateness Method user manual22 (Supplemental Material). The post-Delphi assessment survey remained open for 3 weeks.
Data Analysis
Qualitative data from the Round 1 expert survey, patient survey, and expert post-Delphi panel assessment survey were analyzed using content analysis39 and inductive thematic analysis,40,41 employing constant comparative coding methods42 in Dedoose version 9.0.107 (SocioCultural Research Consultants). Determination of the major themes occurred in a three-step process. Open-ended survey responses were closely read by two investigators (C. E. C. and M. B. B.) to create code categories, and continuously revise and refine them. In the second step, open coding terms were triangulated and grouped into salient themes via axial coding. Finally, a third reviewer and content expert (L. A. H.) in pulmonology confirmed the selection of a final list of themes and assessed whether they reflected the main constructs of expert-perceived risks of remote, unsupervised exercise in LAM. Final themes from the expert survey data structured the drafting of guideline statements.
In addition to qualitative data analysis as described above, descriptive statistics were used to characterize study participants and survey responses. Quantitative group results were analyzed using counts and frequency analysis in GraphPad Prism version 10.1.0 to identify statements meeting the definitions of consensus. Group survey ratings and other quantitative data are presented as mean ± SD.
Results
One hundred percent of invited experts in LAM (N = 16) completed the Round 1 online survey. One hundred percent of Round 1 survey questions were answered by experts, and most respondents utilized free text response boxes for optional elaboration. Of the 16 experts who completed Round 1, a total of 14 (87.5%) attended the Round 2 panel meeting and completed the Round 2 survey. Wide geographic representation was achieved in both rounds, with five regions of the United States represented and two international experts from Europe and Australia (Table 1). Sixty patients with LAM completed the anonymous patient survey.
Table 1.
Delphi Panel Characteristics
| Round 1 (Online Survey) | |
|---|---|
| PatientsWith LAM | |
| Response rate, No. | 60 |
| Female sex, No. (%) | 60 (100) |
| LAM subtype, No. (%) | |
| Sporadic LAM | 55 (91.7) |
| TSC-associated LAM | 5 (8.3) |
| Responded “I don’t know” | 1 (1.7) |
| Age, mean ± SD, y | 52.2 ± 11.4 |
| Time since diagnosis, mean ± SD, y | 8.1 ± 7.3 |
| Home supplemental oxygen available, No. (%) | 20 (33.3) |
| Uses supplemental oxygen at rest, No. (%) | 4 (6) |
| Uses supplemental oxygen during exertion, No. (%) | 20 (33.3) |
| Uses supplemental oxygen during sleep, No. (%) | 19 (31.7) |
| Receives care from a pulmonologist at a designated LAM Center of Excellence, No. (%) | 33 (55) |
| Has participated in a supervised pulmonary rehabilitation program, No. (%) | 17 (28.3) |
| Currently participates in regular exercise or physical activity program, No. (%) | 37 (61.7) |
| Round 1 (Online Survey) | Round 2 (In-person Survey) | |
|---|---|---|
| LAMExperts | ||
| Response/participation rate, No. of total (%) | 16 of 16 (100) | 14 of 16 (87.5) |
| Years working with patients with LAM and other chronic respiratory diseases, mean ± SD | 13.5 ± 7.1 | 12.2 ± 6.5 |
| Female sex, No. of total (%) | 7 of 16 (44) | 7 of 14 (50) |
| Geographic location, No. (%) | ||
| Northeast United States | 1 (6) | 1 (7) |
| Southeast United States | 2 (13) | 2 (14) |
| Mid-Atlantic United States | 2 (13) | 2 (14) |
| Central United States | 4 (25) | 4 (56) |
| Northwest United States | 5 (31) | 3 (21) |
| Europe | 1 (6) | 1 (7) |
| Australia | 1 (6) | 1 (7) |
| Occupation, No. (%) | ||
| Physician | 12 (75) | 10 (71) |
| Physical therapist | 3 (19) | 3 (21) |
| Nurse | 1 (6) | 1 (7) |
| Actively conducts research in LAM, No. of total (%) | 14 of 16 (87.5) | 12 of 14 (85.7) |
| Physician director of a designated LAM Center of Excellence, No. (%) | 6 (37.5) | 6 (42.9) |
LAM = lymphangioleiomyomatosis; TSC = tuberous sclerosis complex.
Round 1 Expert and Patient Surveys
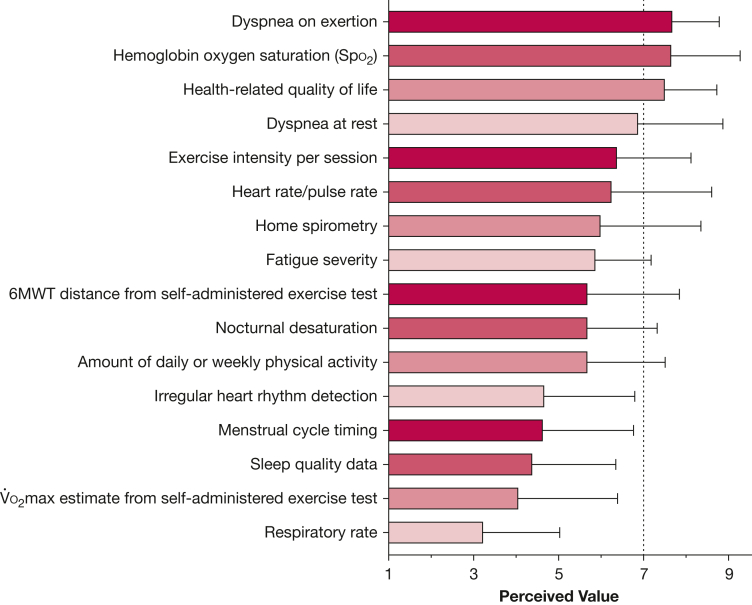
Round 1 expert and patient survey results revealed that the top five perceived fears and concerns related to unsupervised exercise in LAM differed somewhat between patients and experts (Table 2). Major themes and example raw quotes from patients can be found in e-Table 2, Supplemental Material; these data in the patients’ own voice add rich insights into the patient experience associated with exercising with LAM. Despite these perceived risks during exercise, most patients (83.3%) and experts (81.3%) were comfortable participating in or prescribing remote exercise training, without direct supervision of a physician or exercise professional. Remote exercise monitoring variables of oxygen saturation (Spo2), dyspnea on exertion, and health-related quality of life were rated as highly valuable data by experts (mean > 7 on a 9-point Likert scale), while respiratory rate and sleep quality data were rated lower (Fig 2). Crosscutting themes related to safety included the importance of: (1) an in-person medical screening prior to starting an exercise program; (2) a clinical exercise test to ensure adequate home supplemental oxygen availability during exercise; and (3) a patient-centered approach to screening, including asking the patient about exertional dyspnea and exercise tolerance to guide prescription of supplemental oxygen. An overview of the results for expert-perceived risks of unsupervised exercise in LAM and statements created for the Delphi panel is presented in e-Table 3, Supplemental Material.
Table 2.
Specific Risks, Fears, and Concerns About Unsupervised Exercise in LAM
| Patients with LAM (N = 60) | LAM Experts (N = 16) | ||
|---|---|---|---|
| Fear or Concern | Present (% of Respondents) | Primary Potential Risk | Present (% of Respondents) |
| Oxygen desaturation during exercise | Yes (39.9) | Exercise-induced desaturation | Yes (87.5) |
| Pulse oximetry not detecting desaturation | Yes (27.5) | Syncope/other cardiovascular events | Yes (50) |
| Other health complications during exercise | Yes (24.1) | Pneumothorax during exercise | Yes (50) |
| Pneumothorax during exercise | Yes (22.4) | Pulmonary hypertension | Yes (35.7) |
| Wearing oxygen during exercise | Yes (8.6) | Falls | Yes (31.3) |
LAM = lymphangioleiomyomatosis.
Figure 2.
Perceived value of remote exercise monitoring variables in lymphangioleiomyomatosis (LAM), as rated by LAM experts (N = 16) on a 9-point Likert scale. Variables of hemoglobin oxygen saturation (Spo2), dyspnea on exertion, and health-related quality of life ranked highly at > 7 of 9. Respiratory rate, estimate of maximal oxygen consumption (O2max) from a self-administered exercise test, irregular rhythm detection, menstrual cycle timing, and sleep quality data scored lower than 5 of 9. 6MWT = 6-min walk test.
Round 2 Panel Consensus
Of the 10 statements presented to the expert panel in the Round 2 survey, seven met consensus for agreement (Table 3). All experts agreed that patients with LAM and recent pneumothorax should not immediately start a remote exercise program but instead should wait until after resolution of the pneumothorax and clearance by their physician. There was strong agreement that patients with LAM with severe resting pulmonary hypertension and/or risk for falls may be better served by a center-based PR program until they are medically optimized, consistent with current clinical practice guidelines.43,44
Table 3.
Delphi Panel Statements and Levels of Agreement for Exercise Preparticipation Screening in LAM
| Agree (No.) | Disagree (No.) | Uncertain (No.) | |
|---|---|---|---|
| Statements That Achieved Consensus | |||
| Exercise-Induced Desaturation | |||
| Patients with LAM should complete an in-person, standardized exercise test (eg, 6MWT) to screen for exercise-induced desaturation prior to initiating a remote exercise program. | 11 | 2 | 1 |
| Pneumothorax | |||
| In the absence of symptoms suggestive of pneumothorax, patients with LAM do not need to be prescreened by chest radiograph prior to initiating a remote exercise program. | 12 | 2 | 0 |
| Patients with LAM who have recently experienced pneumothorax should not start a remote exercise program until at least 4 weeks after the pneumothorax has resolved and they have been cleared by their physician. | 14 | 0 | 0 |
| Syncope and Other Cardiovascular Events | |||
| Patients with LAM with known cardiovascular disease should be prescreened for risk of cardiovascular events, as defined by the 2012 AACVPR risk stratification tool, prior to initiating a remote exercise program. | 13 | 1 | 0 |
| Patients with LAM who are at high risk for cardiovascular exercise event(s), as defined by the 2012 AACVPR risk stratification tool, should exercise only under the direct supervision of a health care provider. | 13 | 1 | 0 |
| Pulmonary Hypertension | |||
| Patients with LAM and severe resting pulmonary hypertension should exercise only under the direct supervision of a health care provider. | 12 | 0 | 2 |
| Isolated exertional hypoxemia does not lead to the development of pulmonary hypertension in patients with LAM. | 0 | 0 | 14 |
| Falls | |||
| Patients with LAM with a recent history of fall(s) should be prescreened for fall risk by their health care provider prior to initiating a remote exercise program. | 11 | 0 | 3 |
| Agree (n) | Disagree (n) | Uncertain (n) | |
|---|---|---|---|
| Statements That Did Not Achieve Consensus | |||
| Exercise-Induced Desaturation | |||
| What should be the recommended threshold for supplemental oxygen use during exertion in patients with LAM? | |||
| A. < 80% | A. 1 | 2 | |
| B. < 85% | B. 10 | ||
| C. < 89% | C. 0 | ||
| D. < 90% | D. 1 | ||
| E. None of the above | E. 0 | ||
| Syncope and Other Cardiovascular Events | |||
| Patients with LAM should complete an in-person standardized exercise test (eg, 6MWT) to screen for inappropriate cardiovascular response to exercise prior to initiating a remote exercise program. | 9 | 4 | 1 |
6MWT = 6-min walk test; AACVPR = American Association of Cardiovascular and Pulmonary Rehabilitation; LAM = lymphangioleiomyomatosis.
Round 2 Panel Non-Consensus and Uncertainty
Some disagreement arose regarding whether patients with LAM needed to complete an in-person clinical exercise test, such as 6-minute walk test (6MWT), to screen for exercise-induced desaturation prior to starting a remote exercise program. Two panelists felt that the 6MWT protocol is not vigorous enough to detect exertional desaturation for some patients with LAM who are more fit or have less severe disease. After discussion, the language in the first statement was changed from recommending completion of an “in-person 6MWT” to “in-person, standardized exercise test [eg, 6-minute walk test (6MWT)]” to screen for exercise-induced desaturation prior to initiating a remote exercise program. Consensus was reached for this revised statement, with the caveat that some individuals with LAM and mild to moderate disease severity may require maximal cardiopulmonary exercise test (CPET) to screen adequately for exertional desaturation.
Three statements did not reach the consensus threshold for expert agreement (Table 3). All experts were uncertain whether isolated exertional hypoxemia during exercise training leads to the development of pulmonary hypertension in patients with LAM. Consensus was not met concerning a desaturation threshold for recommending exertional oxygen use in patients with LAM. Experts felt that a cardiac workup is priority only for patients with LAM exhibiting cardiac symptoms and/or with known history of cardiovascular disease.
Exercise Preparticipation Screening Tool
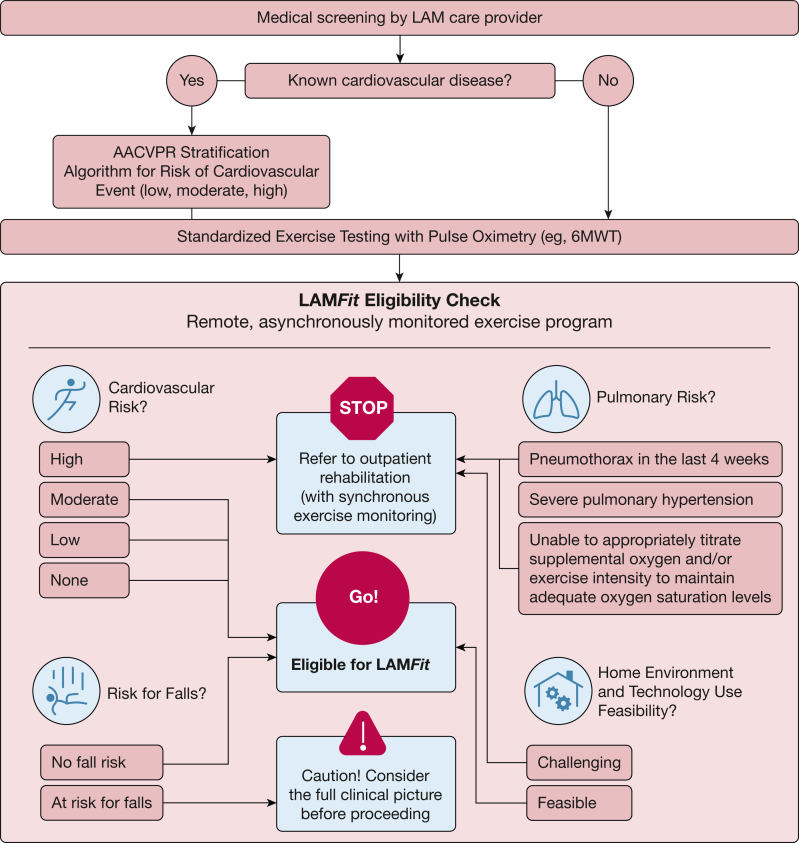
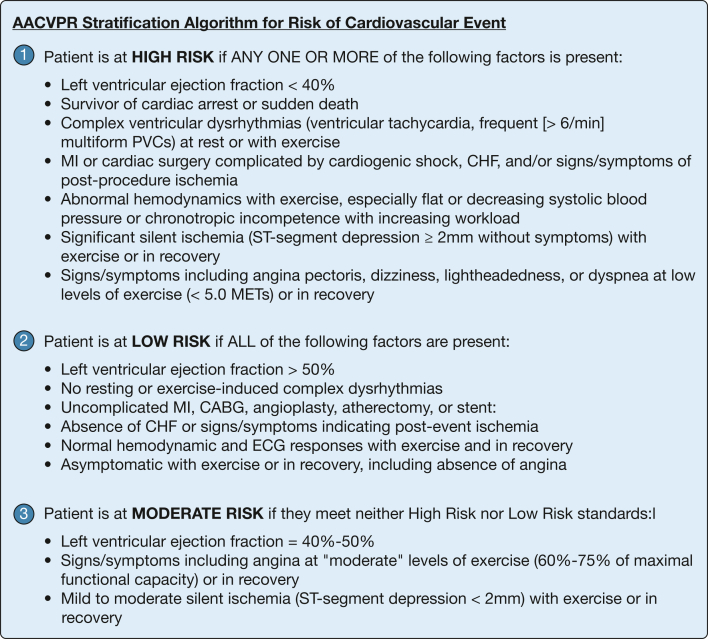
During the Round 2 meeting, each component and decision node of an initial draft of the LAMFit exercise preparticipation screening tool were openly discussed and evaluated by the panel. Panelists agreed with the visual layout, major categories, and clinical decisions prompted by the screening tool. The initial draft suggested use of the AACVPR Stratification Algorithm for Risk of Cardiovascular Event45 for all patients with LAM, but the panel recommended addition of a decision node at the beginning of the tool for “known cardiovascular disease—yes/no.” Requiring a cardiac workup for all patients with LAM prior to starting an exercise program was felt by the panel to be excessively cautious, not cost-effective, and inconsistent with other clinical practice guidelines in chronic respiratory diseases. Clarification was added to the tool that only those exhibiting cardiac symptoms and/or with known history of cardiovascular disease need a cardiac workup as part of LAMFit prescreening. After the Round 2 meeting, the final LAMFit Exercise Preparticipation Screening Tool (Fig 3) was designed to integrate consensus statements from the Delphi panel, panel feedback about the tool, and planned LAMFit study eligibility criteria. In accordance with panel discussion, an adapted version of the 2012 AACVPR Stratification Algorithm for Risk of Cardiovascular Event45 (Fig 4) was incorporated into the decision support tool (Fig 3) for use with patients with LAM and known history of cardiovascular disease.
Figure 3.
The LAMFit Exercise Preparticipation Screening Tool incorporates the consensus statements from the Delphi panel and the American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) Stratification Algorithm for Risk of Cardiovascular Event45 (Fig 4) into a decision support algorithm. The LAMFit screening tool is intended to help providers decide whether a patient with lymphangioleiomyomatosis (LAM) is medically appropriate to participate in LAMFit, a home exercise program delivered on a digital health platform with remote monitoring. 6MWT = 6-min walk test.
Figure 4.
The 2012 American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) Stratification Algorithm for Risk of Cardiovascular Event45 was perceived by panel members to be useful when screening patients with lymphangioleiomyomatosis with a known history of cardiovascular disease. During preparticipation screening for LAMFit, providers can use this stratification algorithm to classify a patient as high, low, or moderate risk for cardiovascular event during exercise, and choose whether to refer them to participate in LAMFit (no, low, or moderate risk) or to a center-based rehabilitation program instead (high risk). Providers can refer to the AACVPR Registry Resources website46 for more information about the algorithm. CABG = coronary artery bypass graft; CHF = congestive heart failure; MET = metabolic equivalent; MI = myocardial infarction; PVC = premature ventricular contraction.
Evaluation of the Delphi Process
According to the post-Delphi assessment survey results, panelists (n = 15) generally felt that the instructions were clear, the moderators functioned well as group leaders, the panel’s ratings reflected expert consensus in LAM well, and participation in the expert panel was highly satisfying. Other assessment survey results are presented in e-Figure 1. One hundred percent of experts agreed that the final version of the LAMFit Exercise Preparticipation Screening Tool (Fig 3) is appropriate for screening use by LAM care providers prior to patient enrollment in LAMFit.
Discussion
In this study, we demonstrate the utility and outcomes of a modified Delphi study to develop consensus recommendations for ILDs such as LAM that have limited research available to guide provider referral to new models of exercise programming delivered outside of center-based rehabilitation settings. The primary product of this Delphi study is a patient-centered, disease-specific exercise preparticipation screening tool for patients with LAM who want to become more active and participate in LAMFit. Experts in LAM achieved consensus that individuals with LAM that have no, low, or moderate cardiovascular risk of event during exercise, no recent pneumothorax within the last 4 weeks, and no risk for falls can safely participate in remote exercise during the LAMFit program. In the post-Delphi assessment survey, Delphi panelists agreed that the panel’s ratings reflected expert consensus in LAM well and gave unanimous approval for the final version of the tool, showing support of future plans to implement the consensus exercise preparticipation screening tool into LAM provider clinical practices during the upcoming LAMFit program.
Other population-based exercise preparticipation screening tools consider cardiovascular risks first and foremost,17,32,33 recommend center-based risk stratification processes only,47 and do not account for ILD-specific exercise risks such as exertional desaturation or pulmonary hypertension. Extant literature shows that even in patients with known cardiovascular disease, there are only few patients for whom unsupervised exercise at home should be avoided or requires significant caution.7,48, 49, 50 Higher risk patients with heart failure receiving continuous inotropic support, mechanical circulatory support, or who are symptomatic at very low workloads (≤ 2 metabolic equivalents) may be at an increased risk of complication.51,52 Otherwise, when considering cardiovascular risks during exercise, most patients with chronic, stable cardiopulmonary disease(s) should be able to exercise on their own at a lower risk of complication.
The LAMFit exercise preparticipation screening tool incorporates cardiovascular screening for patients with LAM who also have a known history of cardiovascular disease using the 2012 AACVPR Stratification Algorithm for Risk of Exercise Event,45 a validated resource that is widely used in cardiopulmonary rehabilitation centers. The tool also recommends completion of an in-person clinical exercise test prior to LAMFit enrollment to screen for exercise-induced desaturation. Completion of a submaximal or maximal exercise test is standard practice for assessing whether patients with ILD are at risk for exercise-induced hypoxemia and would benefit from short-term supplemental oxygen therapy.53,54 This screening step is anticipated to increase the safety and outcomes of the LAMFit program, without adding additional costs and burden to the current standard of care for ILD in the United States. Additional steps should be taken by the screening provider to ensure that the participant has adequate home supplemental oxygen available for use during exercise, including determining whether oxygen supply is currently present in the home, whether a necessary type of oxygen source can be available at the specific location of exercise, and whether the oxygen delivery system and accessories constitute any significant safety hazards during exercise.
The 6MWT is a low-resource test55 that is routinely used as an outcome measure in rehabilitation settings, especially among patients without evidence of ischemic heart disease, and when providers do not need the additional data provided by a maximal CPET.56 However, 6MWT performance may represent a lower percentage of maximal oxygen consumption in some individuals with LAM and may not identify exercise-induced desaturation in LAM as well as in other ILDs.57 Other standardized exercise testing protocols, including maximal CPET, may be preferred on a case-by-case basis when screening for exercise-induced desaturation.
All panelists expressed uncertainty whether isolated exertional hypoxemia leads to the development of pulmonary hypertension in patients with LAM. In a 2019 Delphi study by Lim et al58 on oxygen use in fibrotic ILD, 74% of experts agreed that resting hypoxemia leads to the development of pulmonary hypertension in patients with fibrotic ILD, but consensus was not achieved for whether isolated exertional hypoxemia leads to the development of pulmonary hypertension. Similar to our study, Lim et al reported no consensus for a recommended Spo2 threshold for supplemental oxygen use during exertion in patients with fibrotic ILD. Forty-five percent of experts in that study recommended oxygen use below an Spo2 threshold of < 89%, 21% at a threshold < 90%, and 21% at a threshold < 85%.58 Experts on our panel tended to be more accepting of lower exertional Spo2 thresholds, with 10 (71%) of 14 recommending oxygen use at a threshold < 85%, and emphasized the importance of considering patient symptoms and exercise tolerance when recommending exertional oxygen use. The LAMFit screening tool asks providers to judge whether patients are able to titrate supplemental oxygen and/or exercise intensity to maintain adequate Spo2 levels, but providers are challenged by a lack of expert consensus on Spo2 threshold for supplemental oxygen use during exertion. More research is needed to elucidate safety concerns and risk mitigation strategies related to exertional desaturation in patients with ILD.
Other published recommendations for virtual or remote exercise programming in pulmonary and cardiac rehabilitation include screening for risk for falling and ability to exercise independently.4,49,51,59 Consistent with panel recommendations, the LAMFit tool recommends screening for risk for falls and assessing home environment and technology use feasibility to further ensure safety. Digital literacy and technology readiness may be barriers to uptake of new models of telerehabilitation,14,59,60 but to our knowledge, there are no universally accepted methods of measuring these patient factors in telerehabilitation. Thus, LAMFit prescreening providers will rely on their own knowledge of the patient in judging home exercise environment and technology use feasibility.
Evidence and momentum are growing for new models of exercise programming and telerehabilitation for people living with chronic, stable diseases such as LAM. The important benefits of structured exercise training in chronic respiratory diseases are recognized perhaps now more than ever, as reflected in the higher level of recommendation for PR in ILD in recent clinical practice guidelines.4 New strategies are being sought to help bridge gaps in delivery of PR around the world, since only a very small percentage of eligible patients are currently participating.61 Exercise preparticipation screening by the patient’s own provider and, as appropriate, referral to participate in a remote, self-monitored exercise program such as LAMFit, is another strategy to increase uptake of structured exercise training as an essential component of disease management.
Next Steps
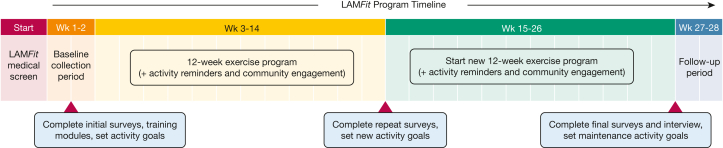
Subsequent to the development of the exercise preparticipation screening tool, a launch of LAMFit, a home-based, self-monitored exercise program, is planned that incorporates target heart rate-guided aerobic exercise, resistance training, daily activity goal setting, reminder messaging, and LAM-specific social connection (Fig 5). In collaboration with The LAM Foundation, a 6-month prospective pilot test of LAMFit will be conducted (www.lamfit.org). Pilot study results will inform any necessary changes to the program before invitations to enroll in LAMFit are extended to all individuals with LAM who are medically safe to participate.
Figure 5.
LAMFit 6-mo pilot study protocol. After preparticipation screening, eligible patients with lymphangioleiomyomatosis will be invited to enroll in a 6-mo pilot study of LAMFit, a home-based, self-monitored exercise program that incorporates two subsequent 12-wk exercise programs, daily activity goal setting, reminder messaging, and lymphangioleiomyomatosis-specific social connection. Wk = week.
Key to the LAMFit program is upfront involvement of the patient’s own provider to determine medical appropriateness for unsupervised exercise, using the LAMFit Exercise Preparticipation Screening Tool developed here (Fig 3). The screening provider’s knowledge of the patient’s risk for exertional desaturation and other systems risks will help the study team to maximize safety and outcomes of remote exercise programming. The screening tool developed in this study is intended for clinical use by LAM care providers; any future modifications for patient-facing use should be mindful of patients with LAM who are not yet established with a specialized LAM pulmonologist, as one expert emphasized in the post-Delphi assessment survey (Supplemental Material), and optimize readability for diverse patient literacy levels. Offering LAMFit as a digital program designed for adults with LAM with sufficient technology literacy may have some implications for equity of accessibility,13 but efforts will be made to minimize inequities wherever possible, including providing smartphones with active cellular plans and additional training in technology use as necessary. Since LAM is a rare lung disease, and patients may be located far from specialists, it is possible that the use of digital technology will actually increase access to exercise programming for this community, similar to other innovative, evidence-based rural health approaches that integrate technology to increase access.62,63
Limitations
Our study has some limitations. Expert consensus obtained during a Delphi study is considered a lower level of evidence than other study designs64 but was useful in our circumstances given the urgent need to develop practical guidance for LAMFit exercise program prescreening, and given the smaller literature base available for the rare ILD LAM. Our methods differed from published guidelines for the RAND/UCLA Appropriateness Method in a few ways. Many Delphi studies do not include the patient voice in any rounds as a key stakeholder.65 We chose not to include patients as named panelists in Rounds 1 or 2 to facilitate candid discussion among experts; other studies have shown that panelists tend to be less open about their medical opinions when patient stakeholders are present.22 However, we did choose to conduct an anonymous patient survey alongside the expert surveys to add more patient perspective. Our choice for convenience patient sampling limited representation of patient voice to those with Internet access and existing connections to The LAM Foundation patient advocacy group, and not the broader population of patients living with LAM. While we did collect several demographics from respondents, we did not collect data about patient race/ethnic identity, sex, or socioeconomic status, and thus cannot describe these demographic trends in patient experience. In addition to the patient themes obtained during this study (e-Table 2, Supplemental Material), continued solicitation and incorporation of patient feedback and experience during the LAMFit pilot study (Fig 5) will help optimize the patient-centeredness66,67 of the program.
Instead of engaging the full expert panel to develop statements for consensus rating, a smaller team of only four experts drafted initial statements from the literature and expert surveys. Furthermore, given time limitations, additional Delphi rounds were not held to refine and increase the appropriateness of statements that did not reach consensus. These modifications to the traditional RAND/UCLA Appropriateness Method helped expedite the panel process to create a useful consensus screening tool but may have added some bias to statements and group ratings. The expert panel was relatively small in size (N = 16); although the size was in line with other published Delphi studies,29,30 a larger panel size may have yielded more diverse representation of expert opinion. The panel primarily included LAM providers and researchers from the United States, with limited international representation. Future development of guidelines should seek to recruit experts from around the world. Given these limitations, the screening tool is not intended to change the current standard of care or policy for all patients with LAM, and will be tested and revised as necessary during the LAMFit pilot study (Fig 5). If shown to be useful during the LAMFit study, screening tool appropriateness should subsequently be studied for expanded clinical use in other chronic respiratory diseases.
Interpretation
Experts in LAM and ILD achieved consensus on 7 of 10 items to guide exercise preparticipation screening by a LAM provider and determine medical appropriateness prior to enrollment in an unsupervised exercise program. The resulting preparticipation screening tool will be used to inform provider screening and referral processes for the upcoming LAMFit digital fitness exercise program.
Funding/Support
This work was funded by an Established Investigator Award (LAM0130PB07-18) to M. B. B. from The LAM Foundation. N. G. was supported by The LAM Foundation Professorship for LAM Research. A. J. and J. M. were supported by the Division of Intramural Research, National Institutes of Health, National Heart, Lung, and Blood Institute.
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Author contributions: C. E. C. and M. B. B. conceived and designed the study. C. E. C. and M. B. B. helped with the acquisition, analysis, and interpretation of study data. C. E. C. drafted the manuscript, and all other authors reviewed the manuscript for important intellectual content. M. B. B. provided final approval of the version to be published and agrees to be accountable for all aspects of the work.
Role of sponsors: The sponsors provided no input or contributions in the development of the research and manuscript.
Other contributions: The authors acknowledge the invaluable guidance and expertise of Anne M. Turner, MD, MLIS, MPH, FACMI, Professor and Associate Director of the Health Promotion Research Center, Department of Health Systems and Population Health, School of Public Health, University of Washington, during the design and completion of the study.
Additional information: The e-Figure, e-Tables, and Supplemental Material are available online under “Supplementary Data.”
Supplementary Data
References
- 1.Bondarenko J., Babic C., Burge A.T., Holland A.E. Home-based pulmonary rehabilitation: an implementation study using the RE-AIM framework. ERJ Open Res. 2021;7(2):00469–02020. doi: 10.1183/23120541.00469-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seron P., Oliveros M.J., Gutierrez-Arias R., et al. Effectiveness of telerehabilitation in physical therapy: a rapid overview. Physical Therapy. 2021;101(6) doi: 10.1093/ptj/pzab053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox N.S., Dal Corso S., Hansen H., et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2021;1(1) doi: 10.1002/14651858.CD013040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rochester C.L., Alison J.A., Carlin B., et al. Pulmonary rehabilitation for adults with chronic respiratory disease: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2023;208(4):e7–e26. doi: 10.1164/rccm.202306-1066ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Child C.E., Kelly M.L., Sizelove H., et al. A remote monitoring-enabled home exercise prescription for patients with interstitial lung disease at risk for exercise-induced desaturation. Respir Med. 2023;218 doi: 10.1016/j.rmed.2023.107397. [DOI] [PubMed] [Google Scholar]
- 6.Queiroz D.S., da Silva C., Amaral A.F., et al. Desaturation-distance ratio during submaximal and maximal exercise tests and its association with lung function parameters in patients with lymphangioleiomyomatosis. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.659416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowman L., Hill C.J., May A., Holland A.E. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev. 2021;(2) doi: 10.1002/14651858.CD006322.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy C., Gupta N., Johnson S.R., Yu J.J., McCormack F.X. Lymphangioleiomyomatosis: pathogenesis, clinical features, diagnosis, and management. Lancet Respir Med. 2021;9(11):1313–1327. doi: 10.1016/S2213-2600(21)00228-9. [DOI] [PubMed] [Google Scholar]
- 9.Gupta N., Lee H.S., Ryu J.H., et al. The NHLBI LAM Registry: prognostic physiologic and radiologic biomarkers emerge from a 15-year prospective longitudinal analysis. Chest. 2019;155(2):288–296. doi: 10.1016/j.chest.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu J.H., Moss J., Beck G.J., et al. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173(1):105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belkin A., Albright K., Fier K., Desserich J., Swigris J.J. “Getting stuck with LAM”: patients perspectives on living with lymphangioleiomyomatosis. Health Qual Life Outcomes. 2014;12:79. doi: 10.1186/1477-7525-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman M., Mellerick C., Symons K., Glaspole I., Holland A.E. Pulmonary rehabilitation for interstitial lung disease: referral and patient experiences. Chron Respir Dis. 2021;18 doi: 10.1177/14799731211046022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holko M., Litwin T.R., Munoz F., et al. Wearable fitness tracker use in federally qualified health center patients: strategies to improve the health of all of us using digital health devices. NPJ Digit Med. 2022;5(1):1–6. doi: 10.1038/s41746-022-00593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slevin P., Kessie T., Cullen J., Butler M.W., Donnelly S.C., Caulfield B. Exploring the barriers and facilitators for the use of digital health technologies for the management of COPD: a qualitative study of clinician perceptions. QJM. 2020;113(3):163–172. doi: 10.1093/qjmed/hcz241. [DOI] [PubMed] [Google Scholar]
- 15.Polgar O., Patel S., Walsh J.A., et al. Digital habits of pulmonary rehabilitation service-users following the COVID-19 pandemic. Chron Respir Dis. 2022;19 doi: 10.1177/14799731221075647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eldredge L.K.B., Markham C.M., Ruiter R.A.C., Fernández M.E., Kok G., Parcel G.S. John Wiley & Sons; 2016. Planning Health Promotion Programs: An Intervention Mapping Approach. [Google Scholar]
- 17.Riebe D., Franklin B.A., Thompson P.D., et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47(11):2473–2479. doi: 10.1249/MSS.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 18.Piercy K.L., Troiano R.P. Physical activity guidelines for Americans from the US Department of Health and Human Services. Circ Cardiovasc Qual Outcomes. 2018;11(11) doi: 10.1161/CIRCOUTCOMES.118.005263. [DOI] [PubMed] [Google Scholar]
- 19.Riebe D., Ehrman J.K., Liguori G., Magal M., Medicine AC. of S. Wolters Kluwer; 2018. ACSM’s Guidelines for Exercise Testing and Prescription. [Google Scholar]
- 20.Fink A., Kosecoff J., Chassin M., Brook R.H. Consensus methods: characteristics and guidelines for use. Am J Public Health. 1984;74(9):979–983. doi: 10.2105/ajph.74.9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niederberger M., Spranger J. Delphi technique in health sciences: a map. Front Public Health. 2020;8:457. doi: 10.3389/fpubh.2020.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitch K. The RAND/UCLA Appropriateness Method user’s manual. Library of Congress catalog website. http://catdir.loc.gov/catdir/enhancements/fy1003/00045830-d.html
- 23.De Schreye R., Houttekier D., Deliens L., Cohen J. Developing indicators of appropriate and inappropriate end-of-life care in people with Alzheimer’s disease, cancer or chronic obstructive pulmonary disease for population-level administrative databases: a RAND/UCLA appropriateness study. Palliat Med. 2017;31(10):932–945. doi: 10.1177/0269216317705099. [DOI] [PubMed] [Google Scholar]
- 24.Milton-Jones H., Soussi S., Davies R., et al. An international RAND/UCLA expert panel to determine the optimal diagnosis and management of burn inhalation injury. Critical Care. 2023;27(1):459. doi: 10.1186/s13054-023-04718-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verburg A.C., van Dulmen S.A., Kiers H., Ypinga J.H., Nijhuis-van der Sanden M.W., van der Wees P.J. Development of a standard set of outcome domains and proposed measures for chronic obstructive pulmonary disease in primary care physical therapy practice in the Netherlands: a modified RAND/UCLA appropriateness method. Int J Chronic Obstruct Pulmonary Dis. 2019;14:2649–2661. doi: 10.2147/COPD.S219851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin V.V., Channick R., De Marco T., et al. Results of an expert consensus survey on the treatment of pulmonary arterial hypertension with oral prostacyclin pathway agents. Chest. 2020;157(4):955–965. doi: 10.1016/j.chest.2019.10.043. [DOI] [PubMed] [Google Scholar]
- 27.Khodyakov D., Grant S., Kroger J., Gadwah-Meaden C., Motala A., Larkin J. Disciplinary trends in the use of the Delphi method: a bibliometric analysis. PLoS One. 2023;18(8) doi: 10.1371/journal.pone.0289009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patton M.Q. 2nd ed. Sage Publications; 1990. Qualitative evaluation and research methods. [Google Scholar]
- 29.Isla D., Felip E., Garrido P., et al. A Delphi consensus panel about clinical management of early-stage EGFR-mutated non-small cell lung cancer (NSCLC) in Spain: a Delphi consensus panel study. Clin Transl Oncol. 2023;25(1):283–291. doi: 10.1007/s12094-022-02941-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAllister-Williams R.H., Arango C., Blier P., et al. The identification, assessment and management of difficult-to-treat depression: an international consensus statement. J Affect Dis. 2020;267:264–282. doi: 10.1016/j.jad.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Nasa P., Jain R., Juneja D. Delphi methodology in healthcare research: how to decide its appropriateness. World J Methodol. 2021;11(4):116–129. doi: 10.5662/wjm.v11.i4.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribeiro F., Takahashi C., Vanzella L.M., et al. An investigation into whether cardiac risk stratification protocols actually predict complications in cardiac rehabilitation programs? Clin Rehabil. 2021;35(5):775–784. doi: 10.1177/0269215520978499. [DOI] [PubMed] [Google Scholar]
- 33.Ilarraza-Lomeli H., Rojano-Castillo J., Flores-Carrillo P.S., et al. Risk stratification model for telemedicine-based cardiac rehabilitation. Eur Heart J. 2021;42(suppl 1) 2687. [Google Scholar]
- 34.Franklin B.A., Whaley M.H., Howley E.T., Balady G.J. ACSM’s guidelines for exercise testing and prescription. 6th edition. Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 35.Clarson L.E., Bajpai R., Whittle R., et al. Interstitial lung disease is a risk factor for ischaemic heart disease and myocardial infarction. Heart. 2020;106(12):916–922. doi: 10.1136/heartjnl-2019-315511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dillman D.A. 2nd Ed. John Wiley & Sons Inc; 2007. Mail and Internet Surveys: The Tailored Design Method; p. 523. xviii. [Google Scholar]
- 38.Garvey C., Bayles M.P., Hamm L.F., et al. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: review of selected guidelines: an official statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev. 2016;36(2):75–83. doi: 10.1097/HCR.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 39.Leavy P. In: The Oxford Handbook of Qualitative Research. Second Edition. Leavy P., editor. Oxford University Press; 2020. Introduction to The Oxford Handbook of Qualitative Research. 540-568. [Google Scholar]
- 40.Braun V., Clarke V. Using thematic analysis in psychology. Qualit Res Psychol. 2006;3(2):77–101. [Google Scholar]
- 41.Braun V., Clarke V. SAGE Publications; 2021. Thematic Analysis: A Practical Guide. [Google Scholar]
- 42.Glaser B.G. The constant comparative method of qualitative analysis. Social Problems. 1965;12(4):436–445. [Google Scholar]
- 43.Grünig E., Eichstaedt C., Barberà J.A., et al. ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur Respir J. 2019;53(2) doi: 10.1183/13993003.00332-2018. [DOI] [PubMed] [Google Scholar]
- 44.Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG) Eur Heart J. 2022;43(38):3618–3731. [Google Scholar]
- 45.AACVPR 2012 AACVPR Stratification Algorithm for Risk of Event. https://registry.dev.aacvpr.org/Documents/AACVPR%20Risk%20Stratification%20Algorithm_June2012.pdf
- 46.AACVPR Registry Resources & Information. https://www.aacvpr.org/registry-resources
- 47.da Silva A.K.F., da Costa de Rezende Barbosa M.P., Bernardo A.F.B., Vanderlei F.M., Pacagnelli F.L., Vanderlei L.C.M. Cardiac risk stratification in cardiac rehabilitation programs: a review of protocols. Rev Bras Cir Cardiovasc. 2014;29(2):255–265. doi: 10.5935/1678-9741.20140067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hwang R., Bruning J., Morris N.R., Mandrusiak A., Russell T. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: a randomised trial. J Physiother. 2017;63(2):101–107. doi: 10.1016/j.jphys.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Thomas R.J., Beatty A.L., Beckie T.M., et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140(1):e69–e89. doi: 10.1161/CIR.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 50.Thomas R.J., Petersen C.E., Olson T.P., Beatty A.L., Ding R., Supervia M. Asynchronous and synchronous delivery models for home-based cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2021;41(6):407–412. doi: 10.1097/HCR.0000000000000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keteyian S.J., Ades P.A., Beatty A.L., et al. A review of the design and implementation of a hybrid cardiac rehabilitation program: an expanding opportunity for optimizing cardiovascular care. J Cardiopulm Rehabil Prev. 2022;42(1):1–9. doi: 10.1097/HCR.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 52.Adamopoulos S, Corrà U, Laoutaris ID, et al. Exercise training in patients with ventricular assist devices: a review of the evidence and practical advice. A position paper from the Committee on Exercise Physiology and Training and the Committee of Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 19;21(1):3-13. [DOI] [PubMed]
- 53.Holland A.E., Spruit M.A., Troosters T., et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 54.Singh S.J., Puhan M.A., Andrianopoulos V., et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–1478. doi: 10.1183/09031936.00150414. [DOI] [PubMed] [Google Scholar]
- 55.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 56.AACVPR. Guidelines for Pulmonary Rehabilitation Programs. Human Kinetics; 2011. [Google Scholar]
- 57.Holland A.E., Dowman L., Fiore J., Brazzale D., Hill C.J., McDonald C.F. Cardiorespiratory responses to 6-minute walk test in interstitial lung disease: not always a submaximal test. BMC Pulmonary Medicine. 2014;14(1):136. doi: 10.1186/1471-2466-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim R.K., Humphreys C., Morisset J., Holland A.E., Johannson K.A. Oxygen in patients with fibrotic interstitial lung disease: an international Delphi survey. Eur Respir J. 2019;54(2) doi: 10.1183/13993003.00421-2019. [DOI] [PubMed] [Google Scholar]
- 59.Beatty A.L., Beckie T.M., Dodson J., et al. A new era in cardiac rehabilitation delivery: research gaps, questions, strategies, and priorities. Circulation. 2023;147(3):254–266. doi: 10.1161/CIRCULATIONAHA.122.061046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nouri S.S., Avila-Garcia P., Cemballi A.G., Sarkar U., Aguilera A., Lyles C.R. Assessing mobile phone digital literacy and engagement in user-centered design in a diverse, safety-net population: mixed methods study. JMIR Mhealth Uhealth. 2019;7(8) doi: 10.2196/14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holland A.E., Cox N.S., Houchen-Wolloff L., et al. Defining modern pulmonary rehabilitation. An Official American Thoracic Society Workshop Report. Annals ATS. 2021;18(5):e12–e29. doi: 10.1513/AnnalsATS.202102-146ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The University of New Mexixo (UNM) About the ECHO Model. UNM Health Sciences website. https://projectecho.unm.edu/model/
- 63.Zhou C., Crawford A., Serhal E., Kurdyak P., Sockalingam S. The impact of Project ECHO on participant and patient outcomes: a systematic review. Academic Medicine. 2016;91(10):1439–1461. doi: 10.1097/ACM.0000000000001328. [DOI] [PubMed] [Google Scholar]
- 64.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrington H., Young B., Williamson P.R. Patient participation in Delphi surveys to develop core outcome sets: systematic review. BMJ Open. 2021;11(9) doi: 10.1136/bmjopen-2021-051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aronson K.I., Danoff S.K., Russell A.M., et al. Patient-centered outcomes research in interstitial lung disease: an official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2021;204(2):e3–e23. doi: 10.1164/rccm.202105-1193ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.NEJM Catalyst. What is patient-centered care? Catalyst Carryover. 2017;3(1) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.