ABSTRACT
Herpes simplex virus type 1 (HSV-1) is a globally widespread virus that causes and associates with a wide range of diseases, including herpes simplex encephalitis, herpes simplex keratitis, and herpes labialis. The interaction between HSV-1 and the host involves complex immune response mechanisms, including recognition of viral invasion, maintenance of latent infection, and triggering of reactivation. Antiviral therapy is the core treatment for HSV-1 infections. Meanwhile, vaccine development employs different strategies and methods, and several promising vaccine types have emerged, such as live attenuated, protein subunit, and nucleic acid vaccines, offering new possibilities for the prevention of HSV-1 infection. Moreover, HSV-1 can be modified into a therapeutic vector for gene therapy and tumour immunotherapy. This review provides an in-depth summary of HSV-1 infection-associated innate and adaptive immune responses, disease pathogenesis, current therapeutic approaches, recent advances in vaccine development, and vector therapy applications for cancer treatment. Through a systematic review of multiple aspects of HSV-1, this study aims to provide a comprehensive and detailed reference for the public on the prevention, control, and treatment of HSV-1.
KEYWORDS: Herpes simplex virus 1, immune response, HSV-1 vaccine, herpes simplex meningitis, herpes labialis, herpes simplex keratitis
Introduction
Herpes simplex virus (HSV) is an alphaherpesvirinae with a fairly high prevalence of infection and is highly susceptible to causing various diseases in humans [1,2]. HSV is a ubiquitous double-stranded DNA virus that includes herpes simplex virus type 1 (HSV-1) and HSV-2 [1]. Globally, approximately two-thirds of the population is infected by HSV-1 [3,4]. Moreover, according to the World Health Organization, as of 2016, a whopping 3.7 billion people under the age of 50 (67% of that age group) were infected with HSV-1 worldwide. Notably, seropositivity for HSV-1 tended to increase with age [5]. Occult infections account for the vast majority of initial HSV infections, with a proportion as high as 80 to 90 %, while overt infections are in the minority. This high prevalence of occult infections has become a key factor in HSV infections, as it invariably exacerbates the spread of the virus.
HSV-1 is transmitted primarily through direct skin-to-mucosal contact (such as touching, kissing, and airborne droplets) and infects the skin and mucous membranes, including the central nervous system, eye, mouth, lips, metabolic system, respiratory system, and genitals [6–9]. It can cause diseases such as herpes simplex encephalitis (HSE), herpes simplex keratitis (HSK), and herpes labialis, resulting in lifelong recurrent clinical or subclinical episodes [10–13]. In addition, HSV-1 infection significantly increases the risk of acquired immunodeficiency syndrome (AIDS) in humans [14]. Due to the intimate and hidden nature of HSV-1 infection and the large number of recently infected individuals, it poses a great challenge for society to interrupt the transmission of HSV-1. Currently, the main therapeutic tools for HSV-1 are antiviral drugs; however, these drugs cannot completely remove the virus with drug resistance for long-term use. Therefore, the development of an effective vaccine against HSV-1 is of great significance in controlling the infection.
In this review, we focus on the structural features, replication process, innate and acquired immune responses, and common diseases (HSE, HSK, and herpes labialis) triggered by HSV-1, as well as the latest progress in the development of live attenuated vaccines, subunit vaccines, nucleic acid vaccines, and replication-defective vaccines. Moreover, the advanced applications of HSV-1 vectors for therapy will be summarized. Through this review, we aim to provide new ideas and clues for the prevention and control of HSV-1 to address this global health challenge more effectively.
Structure and infection of HSV-1
HSV-1 structure and replication
HSV consists of four main components: the viral genome, capsid, tegument, and envelope (Figure 1) [15]. The viral genome, which is 125–240 kb in length, consists of two unique regions, including a unique long (UL) region and a unique short (US) region, both of which are flanked by inverted repetitive sequences (TRL/IRL and TRS/IRS). There were 56 encoding genes in the UL, 12 encoding genes in the US, and one encoding gene in the TRL, IRL, TRS, and IRS regions. This structure is typical of the alphaherpesvirus family of genomes and encodes more than 80 proteins [16–18] (Figure 1).
Figure 1.
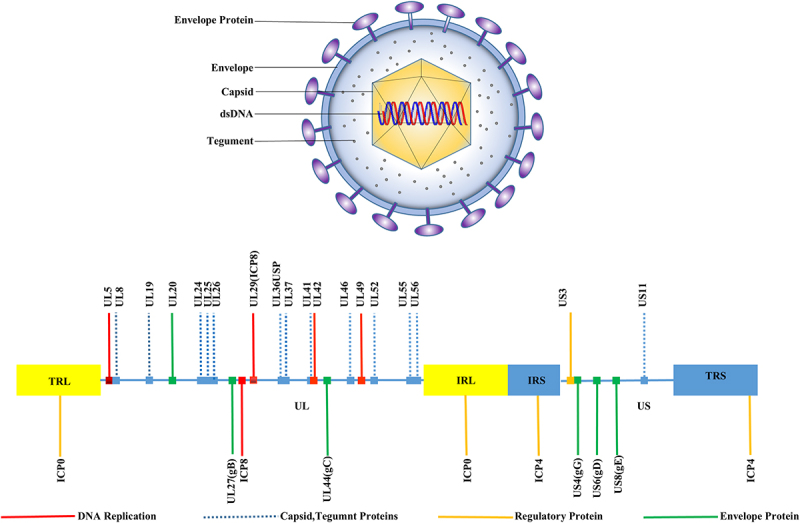
HSV virus structure (above). HSV consists of four main components, including the viral genome, an icosahedral capsid consisting of 162 capsid particles, a tegument composed of 20-23 different viral periplasmic proteins with structural and regulatory roles, and a lipid bilayer envelope containing multiple glycoproteins. Schematic diagram of the HSV genome (below). The herpesvirus genome consists of two unique regions, UL (unique long sequence) indicated as light yellow shading, and US (unique short sequence – dark blue), both of which are flanked by reverse repetitive sequences (TRL/IRL and TRS/IRS).
The capsid structure, approximately 125 nm in diameter, is an icosahedron composed of 162 capsids with six different viral proteins. The tegument is composed of 20–23 different viral periplasmic proteins with structural and regulatory roles [19]. Some of these play important roles in counteracting the host antiviral immune response during the early stages of the infection. The envelope is covered by 12 different glycoproteins, namely, gB, gC, gD, gE, gG, gH, gI, gJ, gK, gL, gM, and gN (Table 1). The surfaces of these glycoproteins present diverse shapes and sizes, with some existing as heterodimers, such as gH/gL and gE/gI, while most of the rest exist as monomers [20].
Table 1.
Glycoproteins of HSV-1.
| Glycoprotein | Coding gene | function |
|---|---|---|
| gB | HSV-1 UL27 | Composition of vesicle membranes; membrane fusion proteins |
| gC | HSV UL44 | Adhesion protein; C3b receptor; protective effect against HSV-1 |
| gD | HSV US6 | Composition of the vesicle membrane; viral entry; membrane fusion |
| gE | HSV-1 US8 | Virus transmission, Fc receptor; immune escape; subunit vaccine |
| gI | HSV-1 US7 | Virus transmission, Fc receptor; immune escape; subunit vaccine |
| gG | HSV US4 | Antigen-specific, subunit vaccines |
| gH | HSV-1 UL22 | Viral entry, membrane fusion; gH and gL bind to be active |
| gL | UL1 | Viral entry, membrane fusion; gH and gL bind to be active |
| gJ | US5 | virus replication |
| gK | UL53 | Vesicle membrane composition; viral replication; viral transmission and escape |
| gM | UL10 | Virus assembly, membrane fusion |
| gN | UL49.5 | Lack of leads to slow penetration of the membrane |
During the replication process, viral particles of HSV-1 first bind to specific receptors (nectin-1, etc.) on the surface of host cells via glycoproteins on their envelope, specifically gB and gC. Once attached, HSV-1 can enter the cell in one of two ways, direct membrane fusion or endocytosis [21,22]. The viral particles are encapsulated in vesicles (endosomes) formed by the host cell, and then through a series of complex membrane transport processes, the viral DNA is finally released into the cytoplasm. Upon entering the cytoplasm, the viral DNA rapidly interacts with virus-encoded proteases and other cofactors to be released from the capsid of the viral particle. The decapsulated viral DNA enters the nucleus through the nuclear pore complex [23].
Within the nucleus, the viral DNA begins to express a series of early genes. The proteins encoded by these genes consist mainly of regulatory proteins (ICP0, ICP4, and ICP27), which create a favourable environment for the replication of the viral DNA by regulating the transcription and translation machinery of the host cell. In the presence of early gene expression products, viral DNA begins to replicate. Viral DNA replication proceeds in a semiconserved manner, producing new strands of viral DNA. As DNA replication proceeds, late genes begin to be expressed. The proteins encoded by these genes include mainly structural proteins such as viral coat proteins and envelope glycoproteins. These proteins are synthesized within the nucleus or in the cytoplasm and are prepared for the assembly of the viral particle. Inside the nucleus, newly synthesized viral DNA binds to coat proteins to form new capsids. Subsequently, these capsids enter the cytoplasm through the nuclear pore complex and acquire an envelope in the cytoplasm. Eventually, the newly formed virus particles complete assembly in the cytoplasm. The assembled viral particles are released into the extracellular environment by either cell lysis or cytotoxicity [24,25] (Figure 2).
Figure 2.
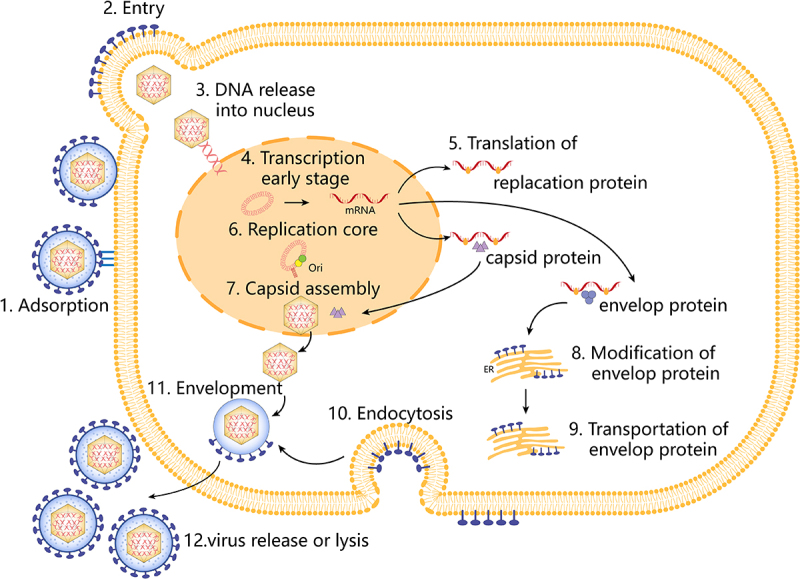
Replication process of HSV-1. Steps as follows, (1) viral attachment, (2) entry of HSV-1, (3) entry of HSV-1 capsid into the nucleus, (4) transcription, (5) translation, (6) replication, (7) capsid assembly, (8) modification of envelope proteins, (9) translocation of envelope proteins, (10) endocytosis, (11) envelope, and (12) release of HSV-1.
Latent infection and reactivation of HSV-1
HSV-1 infection is complex and difficult to eradicate due in large part to its unique characteristics of latent infection. The establishment of latent infection is a consequence of the inability to successfully express the HSV-1 gene in a linear fashion and is dictated by the unique differentiated morphology. During the latent infection process, the viral genome exists in the form of circular DNA, and its expression is severely restricted, mainly expressing a few genes such as latency-associated transcripts (LATs), thus avoiding a strong immune response [26].
The establishment of latent infection involves complex interactions between the virus and the host cell, including viral regulation of the host cell cycle and epigenetic modifications. Reactivation of latent infection is a major cause of HSV-1 disease recurrence. This process is regulated by a variety of internal and external factors, including host immune status, stress response, hormonal fluctuations, and signalling. During reactivation, the viral genome changes from a quiescent state to an active state, initiating a replication cycle that produces new viral particles and spreads them to the peripheral tissues, triggering new infections or disease relapses. Molecularly, this involves multiple steps such as reactivation of viral gene expression, replication of viral DNA, and assembly and release of viral particles [26–28].
HSV-1-related innate and acquired immune responses
Both innate and adaptive immunity play important roles in the process of latent HSV-1 infection and reactivation. The innate immune system recognizes viral components through pattern recognition receptors and initiates an antiviral response, although its role may be relatively limited during latent infection. In contrast, the adaptive immune system, particularly T and B cells, responds rapidly upon viral reactivation, controlling viral replication and transmission through cytotoxic effects, antibody production, and immune memory [29].
Innate immune signals
HSV-1 evasion induces early host antiviral responses through innate immune signals [25]. Over the past decade, a variety of pattern recognition receptors (PRRs) have been associated with innate recognition of HSV infection, including Toll-like receptors (TLRs), nucleotide-binding oligomerization structural domain nucleotide-binding and leucine-rich repeat (NLRs), Retinoic acid-inducible gene l-like receptors (RLRs) and several DNA sensors (Figure 3) [30–32].
Figure 3.
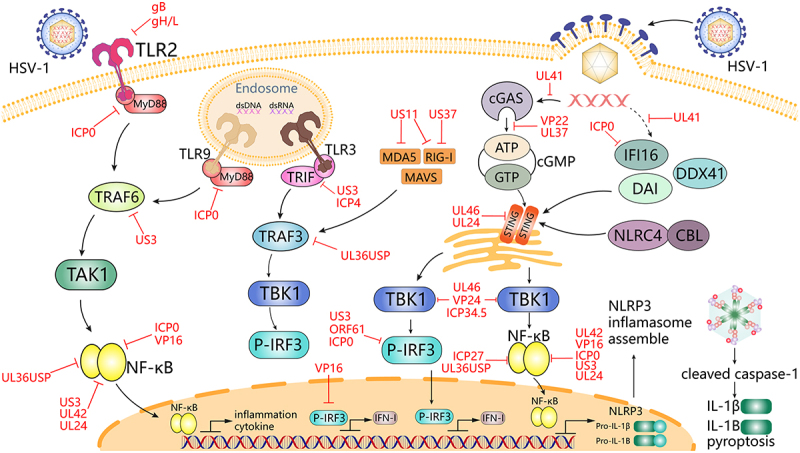
HSV-1 mediates innate immune signalling and escape strategies. IFN-I and inflammatory cytokines induce antiviral immunity, whereas HSV-1 proteins can hijack multiple steps downstream of the TLRs, RLRs, and DNA sensor signalling pathways. TLRs are located at the plasma membrane and in the nuclear endosomes, where they sense different ligands such as viral dsRNA, dsDNA, and glycoproteins and switch signals through TRIF and MyD88, which then leads to activation of IRF and NF-κB. Various viral proteins, including ICP0, US3, UL36USP, ICP4, and VP16 are sufficient to abrogate the TLR signalling pathway. rig-I and MDA5 in the RLR receptor detect distinct RNA structures and signals through the junction protein MAVS protein, triggering IRF3 and nf-κB activation. US11, UL36USP, and UL37 block MDA5 and RIG-I-mediated antiviral signalling pathways. Cytoplasmic DNA sensors, such as cGAS, IFI16, DDX41, and DAI, recognize double-stranded DNA in the cytoplasm and trigger the production of IFN-I through STING signalling. VP22, UL37, and UL41 can directly interact with cGAS and inhibit its enzymatic activity, subsequently inhibiting downstream signalling. ICP0, ICP27, ICP34.5, US3, US11, VP16, VP24, and UL46 interrupt signal transduction at the level of the TBK1-IRF3 axis. US3, ICP0, UL24, VP16, ORF61, UL42, and VP24 interrupt signal transduction at the level of the TBK1-nf-κB axis. HSV-1 also affects NLRP3 inflammatory factor assembly, which in turn inhibits IL-1β and IL-1B-mediated apoptosis. Black lines indicate cellular processes. Red lines indicate processes regulated by HSV.
Among the TLRs that play a major role in HSV infection are TLR2, TLR3, and TLR9 [33,34]. TLR2 activates the NF-κB signalling pathway through the recognition of gB and promotes the secretion of inflammatory factors such as interleukin 8 (IL-8) [35–37]. In addition, TLR2 detects viral gH and gL present on the viral envelope when HSV interacts with the plasma membrane and signals through myeloid differentiation primary response 88 (MyD88), which can lead to the activation of type I interferons (IFNs) and pro-inflammatory cytokine expression [38–40]. In addition, CpG oligonucleotides in HSV-1 viral DNA can also be recognized by intracellular TLR9, which activates the IRF7 and NF-κB signalling pathways and promotes the secretion of type I interferon (IFN-I) and other inflammatory factors [41]. Both signalling pathways, TLR2 and TLR9, are dependent on MyD88, and their dual synergistic action is a key factor in preventing HSV mucosal infections [42,43] (Figure 3).
The cGAS-STING pathway plays a key role in host antiviral immune responses, and interactions with viral immune escape mechanisms are critical for limiting HSV-1 lysis and latent infection [44,45]. During the replication of the HSV-1 virus, the cGAS enzyme senses aberrant DNA and catalyzes the cyclic GMP-AMP (cGAMP) to activate STING receptors inside the cell [46]. This recognition process, in turn, activates the IRF3 and NF-κB signalling pathways, thereby promoting the secretion of interferon IFN-I and other inflammatory factors. This mechanism can efficiently recognize and defend against viral infections, thereby protecting the safety and health of host cells [47]. Following HSV-1 infection and cytoplasmic DNA stimulation, STING recruits NLRP3 in the endoplasmic reticulum and attenuates NLRP3 polyubiquitination associated with K48 and K63, thereby promoting inflammasome activation, which is essential for the host defence against HSV-1 infection [48]. Beta-conjugated proteins can also promote type I IFN production in the cGAS-cGAMP-STING pathway, which can better exert anti-HSV-1 effects. In addition, NLRC4 was found to play a positive regulatory role in the cGAS-STING signalling pathway and antiviral innate immunity [30]. While STING can also be packaged to act in HSV-1-infected extracellular vesicles via the CD63 cytosolic pathway [49]. To combat STING, HSV-1 has evolved a number of strategies, including interfering with its oligomerization, deamidation, and post-translational modification of downstream signalling mechanisms [50,51] (Figure 3).
As a DNA sensor, interferon (IFN)-γ inducible protein 16 (IFI16) plays a major role in the response to viral infections by activating the canonical STING/TBK-1/IRF3 signalling pathway [52]. During HSV infection, IFI16 recognizes and binds DNA nucleic acids mainly in the nucleus, restricting its gene expression and leading to the production of type I IFN, which is the most important receptor in innate antiviral defence, inducing the production of interferon and many antiviral proteins, such as mucosal viral resistance protein A GTPase, 2”−5” oligo adenosine synthetase and ribonuclease L, thereby inhibiting viral infection [53–55]. Moreover IFN expression is significantly reduced in the absence of IFI16. [56] USP12 promotes antiviral responses by deubiquitinating and stabilizing IFI16 [31]. Similar to the TLR2/TLR9 duality, cGAS and IFI16 co-recognize HSV and stimulate the IRF3 pathway [57] (Figure 3).
As a large DNA virus, HSV-1 replicates transiently to produce RNAs, which may trigger several RNA recognition receptor pathways simultaneously or sequentially [58]. Double-stranded RNA (dsRNA) produced during HSV-1 viral replication can be recognized by intracellular TLR3 or RLRs, which activate the IRF3 and NF-κB signalling pathways and promote the secretion of IFN-I and other inflammatory factors [33,59,60].
RIG-1 is a cytoplasmic RLR that mediates intrinsic cellular immunity against viral pathogens and plays an important role in DNA virus recognition [61,62]. HSV-1 triggers RIG-I activation via RNA polymerase III, which produces 5’-pppRNA that induces an antiviral immune response in cells [58,63]. HSV-1 infection causes the host 5S ribosomal pseudogene transcript RNA5SP141 to travel from the nucleus to the cytoplasm, where it is exposed by the absence of its binding protein, leading to the activation of RIG-I, as a unique pathway to initiate antiviral immunity [63,64]. On the other hand, the protein of HSV-1 can interfere with RIG signals [65]. Herpesvirus accessory protein γ134.5 (ICP34.5) facilitates viral replication by disabling the mitochondrial translocation of RIG-I [66]. HSV-1 UL37 deamidates and inhibits RIG-I sensing [65,67]. The US11 of HSV-1 impairs RIG-I by inactivating PACT [68,69].
Recent studies have shown that nuclear 40 S ribosomal protein SA (RPSA) also recognizes HSV nucleic acids and promotes an inflammatory response in antiviral innate immunity [70]. An mtDNA replicase DNA polymerase gamma POLG1 has a role in antiviral defence mechanisms against HSV-1 infection [71].
Innate immune cells
The oropharynx, genital skin, and mucosal tissues were the main susceptible sites for HSV-1 infection. Various resident innate immune cells are present in these tissues. A large number of dendritic cells (DCs) are densely packed in the epidermis. In the dermis, there is an even richer distribution of multiple types of resident immune cells, including DCs, macrophages, natural killer (NK) cells, and γδ-T cells. These immune cells play an important role in maintaining tissue homoeostasis and defence against HSV-1 infection [72].
DCs
DCs are pivotal for eliciting a defensive HSV-specific T-cell response. Their primary role in infection is to patrol pathogens; once detected, they phagocytose and present antigens to the initial T cells in the lymph node [72]. Langerhans cells (LCs), one of the earliest DCs to contact HSV, migrate to the dermis after infection. In the dermis, they converge with dermal DCs, which are responsible for presenting HSV-1 antigens to T cells, thereby triggering a corresponding immune response [73,74].
Recognition of HSV-1 by DCs also stimulates the secretion of multiple antiviral cytokines, as well as the release of IFN-I, thereby exhibiting potent antiviral activity. This process can block the expression of viral genes in the early stages of HSV infection and effectively prevent the excretion of viral particles from the infected cells. DCs play an important role in limiting the spread of HSV infection from the peripheral tissues to the nervous system, providing a key defence mechanism for the host [75]. However, HSV-1 infection also exerts an inhibitory effect on the maturation of DCs, leading to impaired migratory ability, thus affecting the effectiveness of the immune response [76,77].
Although virus-infected DCs are unable to mature, they are still capable of releasing cytokines including TNF, CXCL10, and IL-8. These cytokines play an important role in promoting the maturation of uninfected DCs [78]. Virus-infected DCs undergo apoptosis through the down-regulation of cellular caspase-8 inhibitory protein (c-FLIP) or phagocytosis by uninfected and mature DCs. After phagocytosis, these infected DCs are processed and present viral antigens to adaptive immune cells, which trigger a specific immune response [74,79].
Plasma cell-like dendritic cells (pDCs)
Plasmacytoid dendritic cells (pDCs) differ from myeloid dendritic cells in their immune function. They are the main producers of IFN-I, with more IFN-α than IFN-β. pDCs are mainly found in the bloodstream and lymph nodes, where they play an important role in immunomodulation. HSV-1 infection induces the infiltration of pDCs into the infected area, which is particularly evident in the upper dermis and lamina propria [80]. pDCs can produce IFN-I through TLR7 in a direct response to HSV-1. pDCs have also been shown to produce other cytokines and chemokines such as TNF, IL-6, IL-18, CXCL10, and CCL3, leading to the recruitment and activation of NK and T cells [81].
Macrophages
Macrophages are sentinel cells in the innate immune response that can produce chemokines to control HSV-1 infection and reduce the viral load [82–84]. HSV-1 induced higher levels of pro-inflammatory cytokines from M1 than M2 macrophages [85]. Macrophages are recruited to primary and recurrent lesions in mice at an early stage via chemokines produced [84]. During infection, the main functions of macrophages are phagocytosis of cellular debris and secretion of cytokines, such as TNF, IFN-I, IL-6, and IL-12 [86]. Recent studies have shown that the primary role of macrophages during HSV infection is to participate in the processing of HSV antigens and their presentation to T cells, which is consistent with the role of DCs [72].
HSV-1 can inhibit the immune response in macrophages and suppress downstream molecules associated with the cGAS-STING-TBK1 axis, particularly through the direct interaction of ICP27 with STING and TBK1, leading to a reduction in IFN-I secretion [87].
NK cells
NK cells play an important role in the recognition and rapid clearance of virus-infected cells. NK cells contribute to HSV clearance through two main mechanisms: direct cytotoxic effects on infected cells with down-regulated MHC-I expression via the CD56dimCD16+ subpopulation, and through the CD56bright subpopulation to release IFN-γ [88]. Chemokine receptor expression in NK cells varies according to the subtype [89]. CXCR1 and CXCR2 are expressed on CD56dim NK cells, whereas CXCR3, CCR5, and CCR7 are expressed on CD56bright NK cells [90]. NK cells induce the apoptosis of HSV-1-infected peripheral epithelial cells and control viral levels through cell death [75]. In addition, TLR2 is present on the surface of NK cells that can bind to the HSV-1 gD protein, thereby activating NK cells [91]. In short, NK cells play a critical role in clearing HSV infection.
HSV-1 via ICP47 blocks the expression of MHC-I on the surface of infected cells [92]. Reduced MHC-I antigen presentation is indicative of NK cell-targeted virus-infected cells [93]. HSV-1 infection has also been shown to reduce the expression of MHC-I chain-related A (MICA) gene and UL16 binding proteins (ULBP1, ULBP2, and ULBP3) on the surface of infected cells, and these proteins are NK-cell activators that mediate signalling events through the involvement of NKG2D [94].
Adaptive immune responses
Innate immunity is the key to establishing a rapid immune response against HSV-1, and adaptive immunity is critical for maintaining protective immunity through neutralizing antibodies and memory T cells (Figure 4) [95]. The adaptive immune response under HSV-1 infection is a complex process that involves the interaction of multiple immune cells and cytokines. It is divided into cellular- and humoral- mediated immunity, carried out by T or B lymphocytes, respectively.
Figure 4.
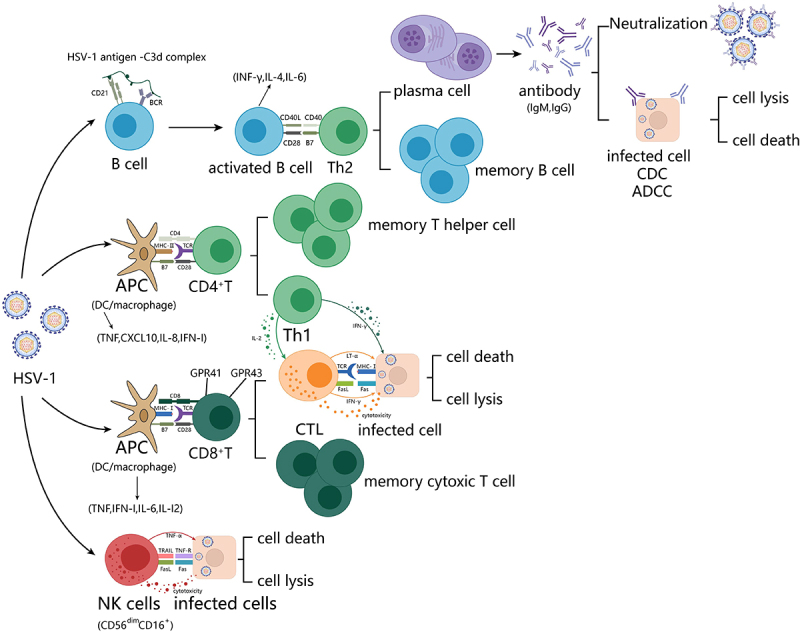
HSV-1 elicits immune cell responses. (1) B lymphocytes generate a first signal of activation by recognizing HSV-1 antigen-C3d complexes via BCR and its co-receptor CD21. A second signal of activation is induced by surface CD40 binding to surface CD40L on Th2 cells. Differentiation into plasma cells and memory B cells in response to IL-4 and IL-13. Antibodies cause apoptosis of infected cells through the neutralization of antigens, ADCC effect, and the CDC effect. (2) initial T lymphocytes activated by classical DCs produce and receive IL-2 themselves and differentiate into Th0 cells. Th0 cells receive CD40, IL-12, and ifn-γ stimulation produced by classical DCs and differentiate into Th1 cells for CTL activation. (3) apc-activated CTL cells receive IL-2 stimulation from themselves and Th1, and differentiate into effector CTLs and memory CTLs. Effector CTLs release various toxic mediators and secrete ifn-γ to induce the role of macrophages and NK cells through the binding of TCR-CD3 complexes with antigenic peptide-mhc class I molecules complexes on the surface of target cells. (4) after NK cells recognize infected cells, they trigger the caspase-3 cascade reaction through FasL or TNF, leading to apoptosis.
HSV-1 infection induces the activation and differentiation of CD4+T cells and CD8+T cells, which are a key part of the adaptive immune response against HSV-1. CD4+T cells are involved in the immune response to recurrent human HSV-1 infections as early as 12–48 hours post-infection [96]. CD4+T cells help DCs activate CD8+T cells to form an immune response and produce IFN-γ, which limits HSV-1 replication and transmission and activates B cells [97–99].
Different subpopulations of CD4+T cells coordinate with each other during the clearance and control of viral infection. Th1, Th2, and Th17 cells play distinct roles in the immune response to HSV-1 infection. Th1 cells are the key cells that produce IFN-γ and help clear the infection involving antiviral cell-mediated immunity. IFN-γ production by Th1 cells stimulates the secretion of CXCL9 and CXCL10 by keratinocytes, which then recruit CD8+T cells to the site of infection [100]. IFN-γ also activates and enhances the antiviral capacities of other immune cells. Th2 cells are primarily involved in humoral-mediated immunity. They mainly secrete cytokines, such as IL-4, IL-5, and IL-13, which promote antibody production by B cells. Th17 cells can help regulate the inflammatory response by promoting the migration of inflammatory cells through IL-17 and IL-22 secretion. HSV-1 infection also induces the production of regulatory T (Treg) cells, which inhibit excessive immune response and attenuate tissue damage and inflammatory responses. Tregs use a variety of mechanisms to modulate the immune response, such as the release of the immunomodulatory cytokines IL-10, TGF-β, and IL-35 [101,102].
CD8+T cells play a key role in suppressing HSV-1 infection [103]. CD8+ T cells infiltrate HSV-1 lesions later than CD4+T cells, and their presence correlates with HSV-1 clearance [104]. CD8+ T cells eliminate virus-infected cells by inducing apoptosis via Fas-FasL signalling, perforin, and granzyme B release [105]. Importantly, CD8+T cells are also capable of secreting antiviral IFN-γ in response to HSV-1 infection. This cytokine also helps to further promote the DC presentation of antigens to CD8+T cells and enhances MHC-I expression in HSV-infected target cells [106]. With the clearance of HSV, CD8+Ttrm cells remain as sentinels at the dermal-epidermal junction in preparation for HSV-1 reactivation. These cells prepare for encounters by expressing IFN-γ, TNF-α, perforin, and granzyme in response to a viral infection [107,108]. A recent study found that GPR41 and GPR43 regulate CD8+T cell priming during HSV-1 infection, emphasizing the importance of metabolite sensing in fine-tuning antiviral CD8+T cell priming [105].
HSV-1 infection also induces the activation and differentiation of B cells, which produce specific antibodies that neutralize viral particles and prevent viral binding to the host cells. In addition to the antibody response, B cells also secrete IFN-γ, IL-4, IL-6, and several other cytokines [109,110]. B cell-deficient mice have an increased susceptibility to HSV-1 encephalomyelitis and mortality [111]. HSV-1 also induces the formation of memory B-cells, which provide long-term immunoprotection against viral relapse or reinfection.
HSV-1 infection disease
In recent decades, HSV-1 infection has caused a series of serious diseases, including HSE, HSK, and herpes labialis. These diseases not only seriously affect the physical health and quality of life of patients, but also pose great challenges to the public health system of society. To date, some therapeutic drugs have been successfully developed and provided an effective treatment way for treatment (Table 2).
Table 2.
Treatment medicine formula.
| Disease | Medicine | Chemical formula |
|---|---|---|
| HSE | nucleoside analog vidarabine | 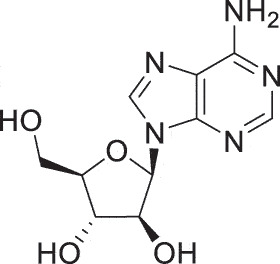 |
| HSK | Iododeoxycytidine | 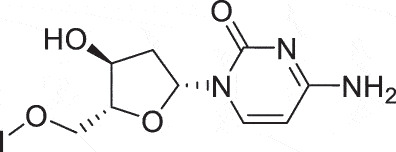 |
| HSK | Idoxuridine | 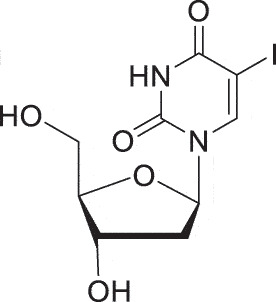 |
| HSK | Famciclovir | 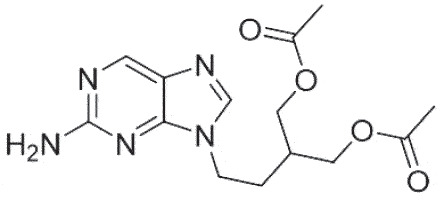 |
| HSK | Ganciclovir | 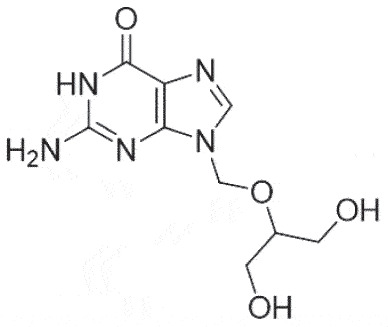 |
| HSK | Trifluridine | 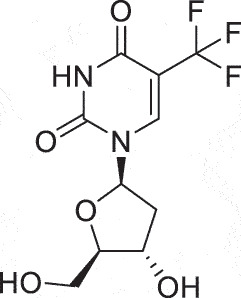 |
| HSK | 6-TG | 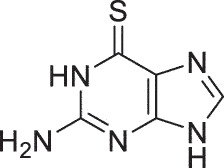 |
| HSK | Harringtonine | 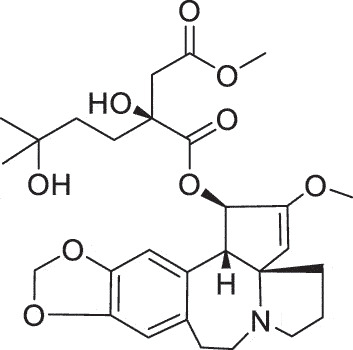 |
| HSK | BX795 | 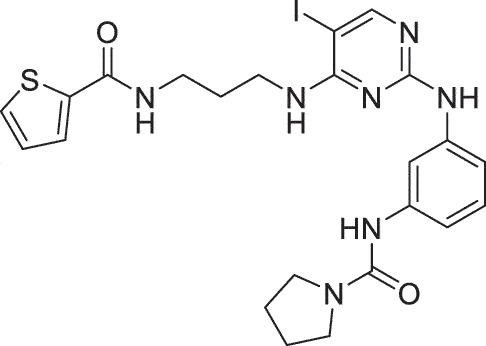 |
| HSK | SC93305 | 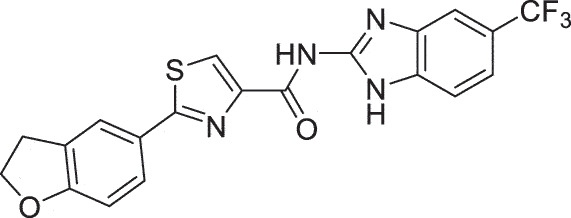 |
| HSK Herpes Labialis | valacyclovir | 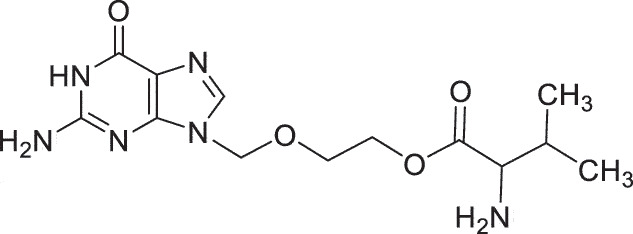 |
| Herpes Labialis | Docosanol | 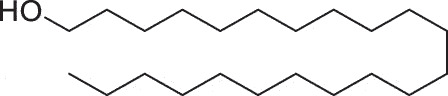 |
| Herpes Labialis | SADBE | 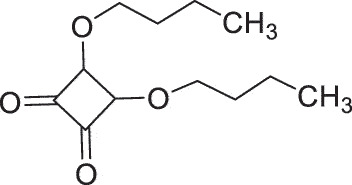 |
HSV-1 and HSE
Invasion of the central nervous system by pathogens leads to inflammation and swelling of the brain, causing encephalitis [12]. Nearly all HSEs are caused by HSV-1, except during the neonatal period, which is the most common cause of fatal encephalitis and occurs globally in a disseminated and non-seasonal pattern [112]. The mortality rate of patients with HSE who do not receive antiviral treatment is 70%, and most survivors suffer from permanent neurological sequelae [113,114].
The nucleoside analog vidarabine was the first antiviral drug to be used for the treatment of HSE [115]. Its mechanism of action is to bind to the viral DNA polymerase and reduce its activity, thereby inhibiting viral DNA synthesis. However, vidarabine is easily converted into deamination compounds by deaminase in the blood to lose its activity [116]. However, it has been shown in clinical trials to be less therapeutically effective than acyclovir. Therefore, the nucleoside analog acyclovir is currently the first-choice treatment for HSE. Foscarnet is used as an alternative antiviral drug when acyclovir resistance is observed [117]. Foscarnet is a pyrophosphate analog that reversibly inhibits DNA polymerase in many herpesviruses by binding to and blocking the viral polymerase’s pyrophosphate binding site, which interferes with pyrophosphate cleavage from incoming deoxynucleoside triphosphates and impedes viral replication [118]. Acyclovir is an acyclic guanosine analog that lacks both the 2’- and 3’-OH moieties and the 2’- and 3’-carbons [119]. Three phosphorylation steps are necessary to activate acyclovir into its triphosphate form. The initial step is catalysed by the virus-encoded thymidine kinase (TK), followed by two successive phosphorylation steps carried out by cellular guanosine monophosphate (GMP) kinase and nucleoside diphosphate (NDP) kinase, respectively. The active triphosphate form of acyclovir competes with deoxyguanosine triphosphate (dGTP) as a substrate for viral DNA polymerase (DNA pol), ultimately being incorporated into the elongating DNA chain, its incorporation results in chain termination [119].
Other anti-HSV drugs are currently under investigation. PHA767491 is a novel anti-herpes simplex virus drug. It showed a strong inhibitory effect on the expression of the essential HSV-I genes such as ICP4 and ICP27 [120]. It effectively blocked HSV-1 proliferation and cell death. It has been shown that this drug reduces viral titres in mice infected with HSV-1, but further evaluation is required for its use in the treatment of encephalitis in severe HSV-1 infections [117]. In addition, a recent study found that ferroptosis is the key mechanism in the pathogenesis of HSV-1 infection by upregulation of PTGS2 and PGE2, which promotes the replication of HSV-1, increases inflammation and tissue damage, and contributes to encephalitis. Inhibition of ferroptosis significantly attenuates neuropathological damage and inflammation in the brains of HSV-1-infected mice, which could be a promising immunotherapeutic strategy for the treatment of HSV-1 encephalitis [121].
Despite effective treatment, HSE is associated with high mortality and long-term neurological impairment [122]. Therefore, improving awareness of the clinical features, diagnosis, and treatment of HSE over time are the keys to improving patient prognosis. Further research on the biological characteristics of HSV-1 and the pathological mechanisms of HSE will help to develop more effective prevention and treatment strategies.
HSV-1 and HSK
HSK is a corneal disease caused by an HSV-1 infection. It is estimated that approximately 1.5 million cases of HSV-associated eye infections occur globally each year, and 95% of ocular HSV infections are caused by HSV-1 [123]. Of these 40,000 populations ultimately result in long-term visual impairment [124]. HSV-1 can be transmitted by ocular contact with a source of infection or ocular secretions from virus carriers.
HSV infection of corneal cells not only causes direct lysis of corneal cells but also induces a cascade of immune responses. Moreover, during HSV-1 corneal infection, polymorphonuclear leukocytes (PMN), macrophages, NK cells, and LCs flood the underlying corneal stroma [125]. It is thought that recruited inflammatory cells release pro-inflammatory cytokines, chemokines, and growth factors that initially contribute to viral clearance, leading to tissue destruction and scarring [126,127]. The connective tissue in the corneal scar continues to rebuild, but unlike uninjured corneal tissue, it increases light scattering and reduces transparency [128]. The pathogenesis of corneal fibrosis is not yet fully understood. HSK includes epithelial keratitis, stromal keratitis, and endothelial keratitis. The specific typical features and clinical manifestations are shown in Table 3.
Table 3.
Classification of HSK.
| Classification | Type | Typical Features | Clinical Symptoms |
|---|---|---|---|
| Epithelial Keratitis | (1) Punctate corneal keratitis (2) Dendritic keratitis (Most common type) (3) Geographic ulcer |
Corneal dendrites are linear and branching with terminal bulbs | Eye pain, Eye redness, Eye tearing, Foreign body sensation in the eyes |
| Stromal Keratitis | (1) Necrotizing (2) Non-necrotizing/disciform (Most common type) |
Recurrent or chronic stromal inflammation | Stromal infiltrates, Stromal oedema, An immune ring, Cornea thinning, Sectoral or diffuse stromal neovascularization |
| Endotheliitis | (1) Disciform Keratitis (Most common type) (2) Disciform endothelins (3) Linear endothelins |
Keratic precipitates, Iritis, Stromal oedema, Without infiltration or neovascularization |
Decreased vision, Photophobia, Eye pain |
HSK treatment
Antiviral drugs are the first choice against HSV-related ocular symptoms and mainly consist of nucleoside analogs, which interrupt viral DNA synthesis by the irreversible binding of viral DNA polymerase in infected cells (Figure 5). Nucleoside analog drugs mainly include idoxuridine, iododeoxycytidine, trifluridine, vidarabine, acyclovir, valacyclovir, famciclovir, and ganciclovir. Trifluridine operates through a mechanism of action similar to that of acyclovir. Its triphosphate derivative has the ability to bind to DNA and competitively inhibit DNA polymerase alongside thymidine triphosphate. Trifluridine was approved by the FDA in 1980 as a 1% solution for treatment and has become the most widely used treatment for HSK with significant efficacy. Acyclovir, valacyclovir, and famciclovir are FDA-approved oral medications used for the treatment of HSV. Ganciclovir is a new drug that has been approved for the treatment of acute herpes simplex epithelial dendritic ulcerative keratitis in the form of an aqueous 0.15% gel [129]. In order to effectively suppress the significant inflammation caused by HSV, particularly in stromal keratitis, endotheliitis, trabeculitis, and uveitis, topical corticosteroids have been widely adopted as the treatment option.
Figure 5.
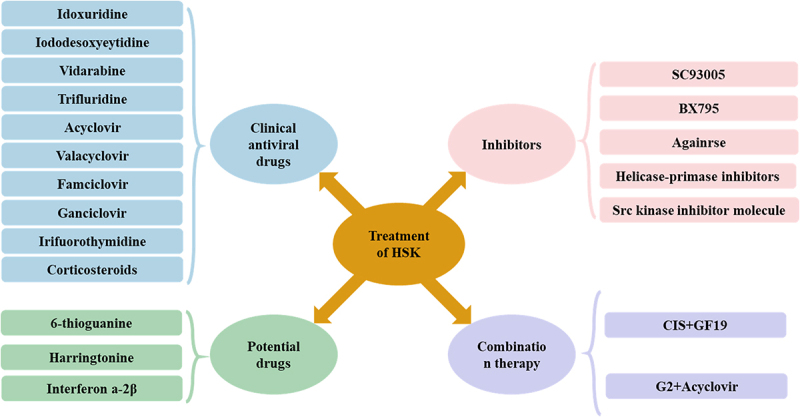
Treatment of HSK. Drugs for the treatment of HSK can be divided into four categories, including clinical antiviral drugs, potential drugs, inhibitors, and combination therapy.
Although existing treatment options have shown some effectiveness and feasibility in treating HSK, their efficacy is still subject to many limitations, especially the emerging problem of antiviral drug resistance. In addition, topical interferon α-2B has shown promising applications in the treatment of HSV epithelial keratitis; however, this drug has not yet been formally approved by the FDA [130]. 6-Thioguanine (6-TG) is a thiopurine analog that has received considerable attention in the treatment of HSV eye infections. 6-TG suppresses Rac1 function, and Rac1 promotes HSV replication and HSK development. One study found that 6-TG demonstrated greater efficacy than acyclovir and ganciclovir in the treatment of HSV eye infections [131]. Harringtonine also plays a significant role in the treatment of drug-resistant HSV-1. It can prevent HSV-1 from entering host cells by inhibiting the expression level of herpesvirus entry mediator (HVEM) in the early stage of viral infection. This mechanism contributes to its effectiveness against drug-resistant strains of HSV-1 [132].
In recent years, an increasing number of inhibitors have been developed for the potential treatment (Figure 5). A new class of antiviral drugs, known as helicase-primase inhibitors, works by blocking viral DNA synthesis, and antiviral drugs such as proteolipid and amenamevir work by inhibiting the activity of the helicase-primase enzyme, effectively decreasing viral shedding and replication levels [133]. In addition, SC93305 and B×795 demonstrated potential antiviral replication capabilities. Specifically, the dual-specificity tyrosine phosphorylation-regulated kinase (DYRK) inhibitor SC93305 inhibits protein expression during the replication phase of HSV-1, thereby blocking the viral replication process [134]. TANK-binding kinase 1 inhibitor B×795 exerts its antiviral effect by reducing phosphorylation of Akt at Ser473 and prevents hyperphosphorylation of 4-binding protein 1 in infected cells, thus blocking viral protein synthesis [134,135]. The drug Aganirsen, an insulin receptor substrate 1 (IRS-1) inhibitor, showed similar effects in clinical trials. It significantly reduced corneal neovascularization but did not show significant results in improving vision [136]. In addition, Src kinase inhibitors have been shown to reduce the severity of HSV keratitis and subsequent corneal angiogenesis in a mouse model. Src kinase inhibitors interrupt the chain of Src in the pathology of HSV keratitis by specifically blocking the phosphorylation activity of Src kinase and inhibiting the activation of its downstream signalling pathways. These include reduced release of inflammatory factors, inhibition of cell proliferation and migration, and decreased expression of angiogenesis-related factors. Therefore, Src kinase inhibitors are able to reduce the severity of HSV keratitis and the subsequent corneal angiogenesis, helping to improve the pathological state of the cornea and protect vision [137].
Combination therapy was performed for HSK treatment. The effective peptide-acyclovir combination regimens have also been reported for the treatment of HSV ocular infections. G2 is a cationic membrane-penetrating peptide that binds to 3-O-sulphated heparin sulphate, effectively preventing HSV-1 from entering the cell [138]. When G2 was used in combination with acyclovir, its antiviral activity was significantly enhanced, and it was able to block HSV cellular invasion more effectively. A new study also developed an injectable Cornea-in-a-Syringe (CIS) cell-free biomaterial to treat damaged corneas. Composite injectable biomaterials can be regenerated by modulating inflammation and blocking viral activity in the infected tissues [139,140]. CIS consists of a modified antiviral host-defence peptide, GF19. When GF19 is released from silica nanoparticles, it can block the activity of HSV-1 [141]. In a surgically perforated and HSV-1-infected rabbit cornea model, the CIS closed a full-layer perforation in the rabbit cornea and suppressed inflammation, allowing corneal regeneration [142].
Despite advances in the pathogenesis of and therapeutic approaches to HSV infection, it remains one of the leading causes of ocular diseases [143]. Antiviral medications are considered as an effective treatment for HSK. However, the virus is able to retrogradely transit through the ocular meridian to the trigeminal ganglion after initial infection and replicative reproduction, where establishes a long-lasting latent reservoir that persists in the body. Current treatments are unable to effectively deal with the virus in its latent state or completely eradicate the infection. These limitations render the treatment of HSK challenging [143]. Early and accurate diagnosis is essential to prevent visually devastating complications resulting from HSV-1 infection. Moreover, HSV-1-associated vaccines are also being developed to prevent infections.
HSV-1 and herpes labialis
Oral HSV-1 infection is very common, and its appearance usually affects the facial skin (lips and nostrils), with characteristic febrile vesicles known as herpes simplex labialis [144]. According to a 2016 report, approximately 3,583 million people were infected with oral HSV, with an overall prevalence of 63.6% [5]. The average incidence of recurrent herpes labialis (RHL) is approximately 1.6 cases per 1000 patients per year, whereas the prevalence is 2.5 cases per 1000 patients, with a higher incidence in women [145].
Most primary infections of herpes labialis are caused by direct contact with body fluids, such as saliva or exudates from progressive lesions, as well as close contact with lesions from infected individuals [146]. At the site of contact, HSV-1 invades tissues, and the viral envelope fuses with the epidermal and dermal skin membranes to initiate primary infection.
Herpes labialis treatment
Many management strategies have been used for herpes labialis treatment, including conventional therapies, antivirals, laser immunotherapies, and probiotics (Figure 6).
Figure 6.
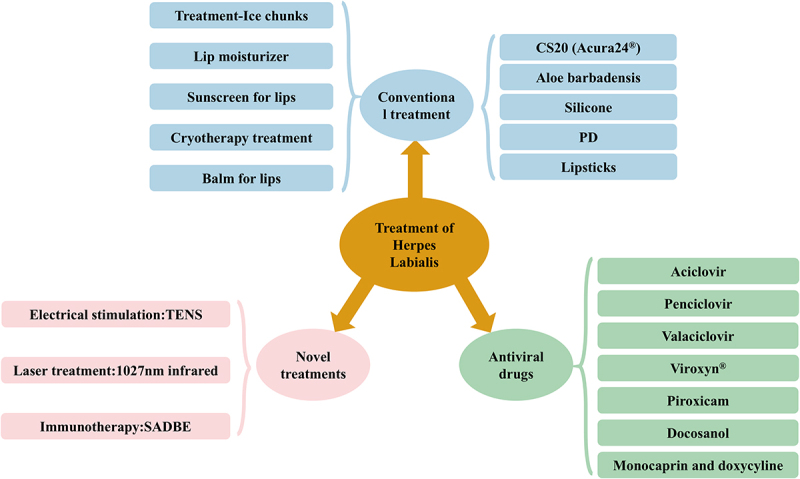
Treatment of herpes labialis. Herpes labialis treatment medications can be divided into three categories, including conventional treatments, antiviral drugs, and novel treatments.
One of the first long-term effective methods with RHL was cryotherapy, in which self-treatment with ice provided immediate relief from stinging, itching, and pain. In addition, lip moisturizers, balms, and sunscreens may also provide relief. CS20 (Acura24®) protective barrier gel demonstrated superior efficacy for the treatment of functional symptoms associated with HSV-1 lip recurrence [147]. Studies have shown that Aloe vera gel extract significantly inhibits the growth of HSV-1 at the site of infection without any toxic side effects [148]. In addition, the use of silicone as an alternative therapy is also effective in treating RHL, with fewer side effects and faster onset of action [149]. A randomized double-blind clinical trial evaluating the efficacy of 1,5-pentanediol (1.5-PD) in the treatment of herpes labialis found that the PD gel did not demonstrate a significant effect in preventing herpes recurrences, but that it demonstrated a role in the relief of swelling and pain [150].
The use of antiviral drugs, such as acyclovir, penciclovir, and valacyclovir, is mainly for the treatment of herpes labialis. Viroxyn® is marketed for the treatment of herpes labialis. It contains benzalkonium chloride, which, according to one study, exhibits virucidal properties against HSV [151]. In addition, piroxicam, a potent non-steroidal anti-inflammatory drug, is suitable as a topical treatment for HSV infections because of its lipophilic nature, antipyretic properties, and direct antiviral response to HSV-1 infection [152]. Docosanol 10% ointment is a medication for the treatment of skin lesions caused by viruses and is packaged as a topical cream for the treatment of cold sores. A study showed that treatment of herpes labialis with 10% docosanol cream reduced the recovery time by 18 h [153]. Its mechanism of action is to inhibit HSV-1 by preventing the fusion of the virus with the cell membrane [154]. Combination therapy with monocaprin and low-dose doxycycline has shown remarkable results in the treatment of herpes labialis [155]. This treatment can be considered highly effective and reliable as it not only shortens the healing time of herpes but also reduces pain.
Novel treatments, such as electrical stimulation, laser therapy, and immunotherapy, have shown great potential and promise in addressing RHL [145]. The use of transcutaneous electrical nerve stimulation (TENS) for the treatment of herpes labialis has been proposed either independently as an alternative therapy or in combination with antiviral drugs to meet the needs of different patients [156,157]. Laser treatment of herpes labialis using 1072 nm infrared low-intensity light resulted in a significant reduction in healing time [158]. Squaric acid dibutyl ester (SADBE) immunotherapy has been adopted as an innovative approach in the treatment of recurrent herpes labialis. As a topical immune sensitizer with a unique mechanism for inducing a delayed hypersensitivity response, SADBE in turn activates the T-cell response and effectively removes virus-infected cells by inducing cytotoxic lymphocytes [159].
HSV-1 vaccines development
HSV-1 infection has spread widely across the globe owing to its high prevalence, leading to millions of infections and posing a serious threat to human health [160]. Traditional treatments rely on antiviral drugs to control HSV-1 infection. However, these drugs do not completely eradicate the virus and are susceptible to drug resistance during long-term use. Therefore, the development of a vaccine against HSV-1-induced diseases is particularly important and will be an important preventive and therapeutic approach, which is expected to provide a more effective and long-lasting solution for controlling HSV-1 infections.
Traditional vaccines are divided into three main types: inactivated, live attenuated, and subunit vaccines. These vaccines stimulate the host immune system to produce antibodies and induce cellular immune responses. However, the development of conventional vaccines against HSV-1 still faces many challenges, such as ensuring that the replication capacity of the virus is effectively controlled, improving the durability of the immune response, and ensuring vaccine safety.
Novel vaccine strategies, including recombinant vaccines, viral vector vaccines, and nucleic acid (DNA/RNA) vaccines, are being increasingly developed for HSV-1 vaccine. These vaccines use genetic engineering techniques, viral vectors, or nucleic acid molecules to express HSV-1-associated antigens precisely, thereby effectively activating the immune system and inducing immune protection. Currently, these vaccines have demonstrated immunoprotective effects in animal models and in early clinical trials. In particular, viral vector and DNA/RNA vaccines have been shown to be safe and immunogenic. The main advantages of these novel vaccine strategies are their high degree of customizability and excellent safety profile; however, key issues such as immune escape and persistence of the immune response still need to be addressed.
Overall, studies on novel HSV-1 vaccines are progressing rapidly, providing strong support for future vaccine development and applications. The main vaccines that have been developed include live attenuated, subunit, nucleic acid, and replication-deficient vaccines (Table 4).
Table 4.
Classification of HSV-1-related vaccines.
| Class | Name | Building Strategies | Result | Stage | Refs |
|---|---|---|---|---|---|
| Live attenuated vaccines | HSV-1 0NLS | Removal of codons 19 ~ 104 and 489 ~ 694 from the ICP0 gene, lack of the NLS region, and a portion of the C-terminal oligomerization domain of ICP0 | Protection against ocular HSV-1 attacks | Mouse model | [161] |
| Elicits a strong B-lymphocyte response; maintains vision in the absence of HSV-1 glycoprotein M or thymidine kinase recognition | Mouse model | [162] | |||
| HSV-l 0RlNG | Removal of codons 19 ~ 162 of the ICP0 gene removes several cysteine residues required for ICP0 E3 ligase activity | Significant short-term efficacy in ocular HSV-1 infection, but poor long-term prophylaxis | Mouse model | [163] | |
| RVx-201 | HSV-2 0ΔNLS Mutation | Fatal HSV-2 genital excitation is extremely protective against HSV-2 reactivation | Guinea pig model | https://rationalvaccines.com/ | |
| R2 | Encodes a pUL37 periplasmic protein with a mutation in region 2 | Reduces the severity of acute and recurrent HSV-2 disease. Does not cause neurological complications and provides better prophylaxis |
Guinea pig model | [164] | |
| HSV-1-GS6264 | Five missense mutations in the gene encoding UL37 | HSV cannot invade nerves and prevent HSV infection | Guinea pig model | [165,166] | |
| VC2 | gK aa31-68 and UL20 aa4-22 deletions in recombinant VC2 | Protective effect of live attenuated HSV-1 VC2 vaccine against HSV-2 genital infection in a guinea pig model of genital herpes. | Guinea pig model | [167] | |
| Protective immune responses can be generated at the site of attack to protect against HSV-1-induced ocular pathogenesis | Mouse model | [168] | |||
| Subunit vaccine | gB1 vaccine | Delivery of HSV-1 glycoprotein B (gB1) using feline immunodeficiency virus (FIV) vector LAW34 | Cross-neutralizing antibodies and cell-mediated responses were elicited, protecting against HSV-1 infection in mice. | Mouse model | [169–172] |
| gD-2 | HSV-2 glycoprotein D (gD2-AS04) and the adjuvants aluminium hydroxide and 3-o-deacylated monophosphoryl lipid a (MPL) | Effective in preventing HSV-1 genital disease and infection, but not effective in preventing HSV-2 disease or infection | Clinical Phase III | [173] | |
| GEN-003 | gD2/ICP4 + matrix M2 adjuvant | Demonstrates good safety and efficacy in clinical trials and stimulates humoral and cellular immune responses | Clinical Phase III | [174] | |
| Trivalent subunit vaccine | HSV-2 subunits containing (gC2, gD2 and gE2) + CpG | Neutralizing antibodies and significant CD4+ T-cell responses were produced in rhesus monkeys. Protection against vaginal infections was up to 95 percent in guinea pigs after vaccination. |
Rhesus monkey model; Guinea pig model; Clinical Phase I |
[175, 16] |
|
| Nucleic acid vaccine | Polyvalent DNA vaccine SL-V20 |
IL-21 and MIP-1α |
Enhanced antiviral T-cell response; Significant increase in 15-day survival and a significant reduction in vaginal viral load. |
Mouse model; Clinical Phase I |
[176] |
| mRNA vaccine BNT163 |
Encodes three HSV-2 glycoproteins | Prevention of HSV cell entry and transmission, as well as counteracting the immunosuppressive properties of HSV. | Clinical Phase I | http://www.biontech.de/ | |
| Nucleotide-modified mRNA lipid nanoparticle vaccines | mRNA lipid nanoparticles expressing glycoproteins gC, gD, gE (mRNA-LNP) | Prevents HSV-1 and HSV-2 genital herpes; ability to prevent nHSV in offspring is similar to subunit vaccines; BioNTech to begin Phase I clinicals in 2022. |
Mouse model; Guinea pig model; Clinical Phase I |
[177, 178] |
|
| Replication-deficient vaccine | gH-deficient vaccine | HSV-1 gH coding deletion (SC16∆gH) | Significant reduction in oedema and erythema; Clinical trials were inconclusive |
Guinea Pig Model; Clinical trials |
[179, 166] |
| ΔgD-2 | gD2-deficient HSV | Activation of a long-lasting ADCC effect providing active and passive protection against HSV-1 and HSV-2 | Mouse Model; Preclinical |
[180, 181] |
Live attenuated vaccines
Live attenuated vaccines provide greater immunity to specific pathogens than other vaccines do [182]. The application of live attenuated HSV vaccines involves genetic alterations that prevent the virus from causing disease in an infected host while triggering a broad immune response. However, the ability of HSV to evade host immune surveillance makes it difficult to construct live attenuated HSV vaccines.
An HSV-1 0ΔNLS mutation is adopted on the live attenuated vaccine [161]. Many HSV-1-encoded proteins with protective epitopes have been recognized in the HSV-1 0∆NLS vaccine [183,184]. Prophylactic vaccination with HSV-1 0ΔNLS can effectively block the pathogenesis of HSV-1 without causing ocular pathology or visual disorders, as well as preventing corneal pathology and protecting the visual axis [185]. Another study found that the HSV-1 0∆NLS vaccine elicited a strong B-lymphocyte response in the absence of recognition by the HSV-1 glycoprotein M or thymidine kinase [162]. Another mutant attenuated virus HSV-1 ICP0, named 0ΔRING, similar to 0ΔNLS, showed significant efficacy as a prophylactic vaccine against ocular HSV-1 infections in short-term takedown studies in mice. However, for long-term prophylaxis, these vaccines did not perform as well. When mice were vaccinated and then attacked by the virus one year later, 0ΔRING and 0ΔNLS were significantly less effective in controlling viral replication and protecting the optic axis [163].
In addition, RVx-201, a live attenuated vaccine HSV-2 0ΔNLS mutation against HSV-1 and HSV-2, is currently in the IND/IMPD Enabling phase, and RVx-201 provides significant protection against HSV-2 reactivation in latently infected animals. The results of the study, as well as the limited available human studies, suggest that RVx-201 could be an effective prophylactic and therapeutic vaccine approach against HSV-1 and HSV-2 infections (https://www.prnewswire.com).
The R2 vaccine is an attenuated live HSV-1 strain characterized by the encoding of the UL37 periplasmic protein with a region 2 mutation. This protein plays a crucial role in neurological invasion and the establishment of latency. In a model of HSV-2 attacking the genitalia of guinea pigs, the study showed that the R2 vaccination positively induced the production of neutralizing antibodies and reduced the severity of both acute and recurrent HSV-2 disease in guinea pigs. Compared to the subunit vaccine gD2+monophosphoryl lipid A (MPL)-aluminium hydroxide (alum), the R2 vaccine showed a more obvious reduction in viral shedding during recurrence, reaching 33–64%. The next generation of the R2 recombinant virus, HSV-1-GS6264, encodes five missense mutations in UL37 and is derived from a variant of the HSV-1 strain [95,165]. The live attenuated HSV-1-GS6264 vaccine in preventing HSV infections is remarkably effective and renders the virus incapable of invading nerves [164,166].These data suggest that the R2 vaccine has potential advantages for the prevention and treatment of HSV infections.
The live attenuated VC2 vaccine consists of two separate protein deletions, one in the gK and the other in UL20, preventing the virus from entering neurons and establishing latency [186]. VC2 is an effective live attenuated vaccine with protective effects against HSV-1 and HSV-2 infection of genital herpes in mice and guinea pigs [187,188].
The efficacy of VC2 against herpetic keratitis and ocular infections caused by a lethal human clinical strain of HSV-1 has been evaluated in mice. Mice injected intramuscularly reported no ocular attacks or complete recovery from conjunctivitis originally caused by HSV-1. Immunized mice also showed higher virus-neutralizing titres and an increase in CD3+T, CD4+T, and CD8+T cells. VC2 is a safe vaccine that is highly effective in protecting mice against ocular attacks caused by the HSV-1 McKrae strain [95,168]. In addition, the VC2 vaccine was protective against HSV-2 genital infection in a guinea pig model of genital herpes [167,188]. A variety of live attenuated HSV vaccines have been tested in both the preclinical and clinical stages. Although live-attenuated vaccines are particularly effective in eliciting humoral and cell-mediated immune responses, vaccine safety remains a major challenge [16].
Subunit vaccines
Subunit vaccines have emerged as powerful tools and are one of the most common types of immunological vaccines against HSV-1; further progress has been made in recent years. Recombinant protein HSV subunit vaccines usually select gB and gD as the primary immunogens. Compared to other HSV glycoproteins, gB and gD can induce neutralizing antibodies more precisely after infection, triggering a cross-reaction covering both humoral and cellular immunity. In addition, because of the high sequence similarity between HSV-1 and HSV-2, these vaccines are potentially protective against both types of HSV infection [95,189,190].
HSV-1 gB1 was delivered using the feline immunodeficiency virus (FIV) vector LAW34 and tested against HSV-1 and HSV-2 infection in C57BL/6 mice. The results showed that the gB1 vaccine was able to trigger cross-neutralizing antibodies and cell-mediated immune responses, effectively protecting mice against serious HSV-1- and HSV-2-related diseases. Specifically, the vaccine protected 100% of the animals against HSV-1-associated disease, while protection 75% of the animals against HSV-2-associated disease. This finding provides new insights for the treatment and prevention of HSV infections [169–172].
Another gD-2 vaccine is a subunit vaccine consisting of HSV-2 gD (gD2-AS04) as well as the adjuvants aluminium hydroxide and 3-O-deacylated monophosphoryl lipid A (MPL). In a herpevac clinical trial, significant protection against genital HSV-1 infection was observed. However, the vaccine did not show significant protection against HSV-2 infection [173]. The gD-2 vaccine protected against ocular HSV-1 infection in CD-1 mice. However, the gD-2 subunit vaccine was ineffective in C57BL/6 mice [185]. This suggests that the protective effects of this vaccine may vary among different strains of mice.
A specific region of the gD protein covers most of the binding sites for the HVEM, an HSV receptor, which plays a crucial role in HSV infection of epithelial cells and lymphocytes. Inoculation with a human monoclonal antibody targeting the HVEM-binding domain of HSV-1 gD not only neutralizes infection of HSV-1 cells expressing HVEM but also effectively mediates HSV-1-specific antibody-dependent cellular cytotoxicity (ADCC). Additionally, the antibody significantly attenuated HSV-1-induced corneal disease and reduced viral shedding in a mouse model [191]. This finding provides a new strategy for the treatment and prevention of HSV-1 infection.
In addition to gB and gD, other target antigens of HSV-1 could be used as vaccine development sites because the herpesvirus genome is large and diverse (encoding more than 80 polypeptides), [192] suggesting that any of these proteins could elicit the desired immune response. After exposure of a set of polymeric peptides to CD8+ T cells via autologous DCs, strong immune responses to HSV proteins UL25, UL27, UL39, UL46, UL47, and ICP0 were observed in HSV seropositive individuals [193,194]. These studies have demonstrated the potential of these six proteins as vaccine candidates against HSV, however, their efficacy in both symptomatic and asymptomatic individuals remains to be tested.
GEN-003 is a subunit vaccine that incorporates two HSV-2 proteins, gD2 and ICP4, and matrix-M2 adjuvant. This vaccine is currently in clinical trials (NCT02515175). This study has shown that the lesion rate was reduced by approximately 40%, the duration of recurrence was reduced by approximately 20 days, and an adequate humoral and cellular immune response was successfully elicited. A phase III clinical trial of GEN-003 supported the clinical and virological efficacy of this therapeutic genital herpes vaccine and suggested that a dose of 60 mg of viral antigen and 50 mg of M2 adjuvant would be the preferred dose for future phase III trials. These positive results provide strong support for the further development and application of GEN-003 [174].
The trivalent subunit vaccine is a combination of glycoproteins C, D, and E, with CpG/aluminium adjuvant [195]. Studies in rhesus monkeys have shown that this trivalent vaccine can produce specific antibodies against the immune evasion activities of gC2 and gE2. In addition, the vaccine stimulated immune responses in CD4+T cells. Further testing of the efficacy of the vaccine in a guinea pig model showed that it was effective in reducing the duration and incidence of genital lesions [175].
Nucleic acid vaccines
Nucleic acid vaccines consist mainly of DNA and mRNA vaccines, which are designed to transfer genetic material into cells for the expression and production of proteins.
DNA vaccines
DNA vaccines, referred to as gene vaccines, are made from plasmid DNA (pDNA) that can be engineered to express the appropriate viral gene when it enters the host cell [176]. Naked DNA vaccines can present HSV antigenic genes directly to DCs and stimulate immune responses in CD4+T and CD8+T cells via a cross-pollination mechanism. They can also augment DNA through the use of adjuvants or cytokines to enhance the antibody response of the host to the HSV surface glycoproteins gB, gD, or gH/L complexes [95].
Polyvalent DNA vaccines contain more than one antigen, which is designed to induce a stronger immune response. The multivalent DNA vaccine SL-V20 has been reported to be developed and is being tested for its efficacy [176]. The novel vaccine was constructed using the pGX27 plasmid expressing the following antigenic genes, Tissue-type Plasminogen Activator (t-PA), Flt3L, HSV-2 gB, and UL39 antigens. Other pGX27 plasmids encoding HSV gD2, ICP4, and ICP0, as well as the immune response-stimulating factors IL-12, IL-21, and MIP-1α were also incorporated. In a mouse model, SL-V20 administered intramuscularly successfully attenuated HSV-2-induced pathology and effectively facilitated viral clearance. The SL-V20 vaccine protected mice against antigen presentation via a combination of DC subpopulations to augment the antiviral T-cell response. The 15 days survival rate increased by 85%, and the vaginal viral load was reduced by 67% [176]. The vaccine is currently in the preclinical stage and recruits trialists for further study. Polyvalent DNA vaccines are a relatively new method of viral vaccination with great potential and promise [95,176].
mRNA vaccines
BNT163 from BioNTech is an mRNA prophylactic vaccine against HSV-2, with potential benefits for HSV-1. The mRNA vaccine encodes three HSV-2 glycoproteins designed to prevent HSV cell entry and transmission and counteract the immunosuppressive properties of HSV. Patient administration was completed in the first phase of phase I clinical study in 2022. This trial (NCT05432583) is evaluating the safety, tolerability, and immunogenicity of the vaccine for the prevention of genital lesions caused by HSV. This is expected to be concluded by 2025.
Trivalent nucleoside-modified mRNA vaccines in lipid nanoparticles are currently being investigated for their effectiveness in providing protection against HSV-1 and HSV-2 infections. Evidence suggests that they have the potential to be superior to conventional subunit vaccines. The mRNA lipid nanoparticle (mRNA-LNP) vaccines express the same immunogens as nucleoside-modified mRNAs in lipid nanoparticles (LNPs) [196]. Nucleoside-modified mRNA-LNP stimulates T follicular helper and germinal B-cell responses, leading to high titres and long-lasting antibody responses [197]. The nucleoside-modified HSV mRNA vaccine was more effective than the trivalent subunit vaccine in limiting viral replication in the genital tract, providing complete protection from genital disease. This vaccine also blocked HSV invasion of the dorsal root ganglia at the site of viral latency in most of the mice infected with HSV-1 or HSV-2. It is effective for the prevention of HSV-1 and HSV-2 in genital herpes and is a promising candidate for clinical trials [177]. In addition, the vaccine was evaluated for its ability to prevent HSV in the offspring (a mouse model) and showed the same preventive efficacy as the subunit vaccine [178].
Replication-deficient vaccines
Replication-defective vaccines have been developed by creating a mutant virus that fails to function properly in viral replication by deleting an essential gene [198]. The gH protein is an essential glycoprotein required for HSV-1 entry into host cells and membrane fusion [199,200]. Using animal models, gH-deficient HSV viruses have been selected as potential vaccine candidates [201,202]. By deletion of the gene encoding the gH glycoprotein, this HSV-1 mutant becomes disabled during replication, which can only grow in a cell line and replicate for one full cycle to produce progeny viral particles lacking gH. The gH-deficient HSVs have been reported to significantly protect against HSV disease and have significantly less viral shedding in primary lesions [201]. A vaccine for a deletion of the gH (SC16AgH) was inoculated through the mucous membranes and found to have significantly reduced oedema and erythema symptoms, with a 78% reduction in the extent of lesions in a guinea-pig model [179]. However, in a clinical trial, subjects with significantly lower lesion healing times, number of recurrences, and severity of first recurrence were not significantly improved [166].
HSV, which encodes gD2, is essential for viral entry and spread. Therefore, the progeny of gD2-deficient viruses are unable to infect new cells and do not develop diseases in mice [203]. Accordingly a ΔgD2 vaccine was developed with high immunogenicity and produced high titres of IgG in mice. ΔgD2 showed better protection in an ocular model than the adjuvant gD2 subunit vaccine. In addition, ∆gD2 inoculation elicited a long-lasting ADCC-mediated response and provided complete protection at all-time points in mice, providing both active and passive protection against HSV-1 and HSV-2, and predicting that ∆gD2 will elicit durable responses in humans [180,181]. In addition, X-Vax is expected to initiate clinical trials by 2024. One of the most common challenges for replication-deficient vaccines is the activation of latent viruses. Although it may not be infectious, it is still a cause for concern [204].
Several HSV-1 vaccines, including live attenuated, subunit, and DNA vaccines, have entered clinical trials. Although these vaccines have demonstrated safety and immunogenicity in clinical trials, with the potential to prevent HSV-1 infections and alleviate disease symptoms, no HSV-1 vaccine has been approved [95].
The development of a vaccine against HSV-1 still faces several challenges. First, HSV-1 has a complex immune escape mechanism, which makes vaccine development difficult. Second, ensuring the safety of the vaccine and the durability of the immune response are key issues that must be addressed during the development process. Third, factors such as vaccination strategies, vaccine costs, and accessibility on a global scale must be considered. Nevertheless, the future of HSV-1 vaccine development remains promising, as the understanding of the vaccine grows with further research and technological innovations.
HSV-1 as a therapeutic vector
HSV-1 as a therapeutic vector possesses unique advantages, including efficient infectivity, broad host cell adaptability, and relatively safe genome editing ability. These features make HSV-1 a hot topic in the field of therapeutic research. Using HSV-1 as a vector, a variety of therapeutic strategies, including gene therapy, immunotherapy, and targeted therapy, can be achieved, providing new options and opportunities for clinical treatment (Table 5). With the development of genetic engineering techniques, HSV viruses have been transformed into mutant strains with new potential [213].
Table 5.
Application of HSV-1 as a therapeutic vector.
| Name | Diseases | Developmental Phase | characteristics | therapeutic mechanism | Refs |
|---|---|---|---|---|---|
| T-VEC (OncoVEXGM-CSF) |
human melanoma | Approved drugs | Genetically modified herpesviruses target cancer cells for replication, enhance immune response, promote antigen presentation, and induce systemic antitumor immune response. | Infected cancer cells replicate and cause lysis, release antigen-promoting immunity, GM-CSF helps recruitment, and T cells activate to kill tumours. | [205,206] |
| VC2 | mouse melanoma | Mouse model | VC2 Is an attenuated HSV-1 virus that removes gK (31-68 aa) and UL 20 (4-22 aa), retaining infectious capacity while reducing toxicity and improving safety, may be targeted and immunogenic, and suitable for specific studies or treatment | VC2 Infected cells have unique strategies and may enter through non-canonical receptors, using the remaining viral proteins and host metabolic replication, avoiding strong immune responses. Finally releasing the virus by lysis or apoptosis, infecting neighbouring cells, or triggering an immune response, the specific mechanism needs further investigation | [207] |
| OV-mOX40L | Pancreatic ductal adenocarcinoma (PDAC) | Mouse model | Combining oncolysis with immune enhancement, activating anti-tumour immunity, and regulating the immune microenvironment. | Virus lysis of tumour release antigen, T cell activation increases immunity, and microenvironment transformation to promote cancer | [208] |
| KB105 | Autosomal recessive congenital ichthyosis (ARCI) | Preclinical | KB105 is an HSV-1 based gene therapy that targets the TGM 1-deficient ARCI. The TGM 1 gene was delivered to the skin cells using the STAR-D technology to restore the protein function. As a non-integrated viral vector, with high loading and low immunogenicity, suitable for repeat delivery. | 1.Gene delivery: effective TGM 1 gene delivery to skin cells. 2.Gene expression: intracellular expression of TGM 1 protein. 3. Functional recovery: improve the skin barrier and reduce the symptoms of ichthyosis. 4.Immune regulation: low immunogenicity, reduces immune response, and promotes gene transmission and expression. |
[209] |
| G47Δ-mIL12 | triple-negative breast cancer (TNBC) | Mouse model | G47 Δ -mIL 12 is a genetically modified oncolytic virus based on triple mutant HSV-1, which carries the murine-derived IL-12 gene designed to enhance the specific killing of the tumour and activate the immune system. | 1. Oncolysis: infection and lysis of tumour cells. 2. Immune activation: express IL-12, activate T cells, NK cells, etc. 3. Microenvironment regulation: reduce immune suppression and increase anti-tumour immunity. 4. Synergism: used in combination with immune checkpoint inhibitors to improve the efficacy. |
[210] |
| B-VEC | Dystrophic epidermolysis bullosa (DEB) | Approved drugs | B-VEC is a non-invasive gene therapy for DEB. Gel dosage form, directly applied to the DEB wound, carrying the COL7A1 gene fragment by the HSV-1 vector | The COL7A1 gene fragment was introduced into skin cells to express COL 7 protein, restore the basement membrane band structure and function, and enhance skin junctions. Promote wound healing, reduce blisters and tears, and improve skin condition. | [211] |
| HSV-1dko-B7H3nb/CD3 | Glioblastoma (GBM) or colorectal adenocarcinoma (COAD) | mouse model | HSV-1dko-B7H3nb/CD3 is a genetically modified herpes simplex virus (HSV-1) vector with the properties of a dual gene knockout (dko) and the expression of a chimeric antigen receptor (CAR). It aims to specifically target and kill tumour cells by redirecting T cells. | CAR recognizes tumour B7H3, activates T cells, and releases toxins to kill the tumour and initiate antitumor immunity | [212] |
In oncolytic virus (OVs) therapy, these modified viruses can selectively kill cancer cells through their cytopathic effects or induce safer and more effective antitumor immune responses, thereby reducing damage to normal tissues. This therapy offers new ideas and approaches for cancer treatment and has broad application prospects [214]. The oHSV is a thoroughly studied OV that selectively infects cancer cells and enhances antigen presentation. Unlike other lysosomal viruses, oHSV has a large genome (152 kb) that allows multiple transgenes to be modified and inserted into the intergenic and nonessential gene regions [215].
Several HSV-1 genes have been deleted or mutated to improve the safety and efficacy of oHSV. An oHSV carrying a bispecific antibody (BsAb) to B7H3/CD3 (HSV-1dko-B7H3nb/CD3) was established, and the ICP6, ICP47, and ICP34.5 genes of the HSV-1 genome were deleted and that the exogenous gene was inserted into the ICP34.5 gene region. This oHSV provides local delivery of B7H3/CD3 BsAbs for the treatment of glioblastoma (GBM) or colorectal adenocarcinoma (COAD) through a combination of direct tumour lysis, tumour immune checkpoint blockade, and innate immune activation. In the GL261 and MC38 models, HSV-1dko-B7H3nb/CD3 showed strong antitumor activity in vitro when bound to T cells, superior to that of HSV-1dko bound to T cells, suggesting that expression of the B7H3nb/CD3 BsAb enhances the antitumor effect of HSV-1 lysogenic virus [212].
The HSV-1 virus has a strong ability to insert multiple transgenes and exhibits excellent tumour-lysing activity. HSV-1 expresses a variety of cytokines, and immune checkpoints (ICs) are used in tumour therapy [216]. For example, a lysogenic HSV encoding IL-12 (G47Δ-mIL12) significantly reduced primary tumour volume and metastasis in triple-negative breast cancer (TNBC) [210].
OX40 plays an important role in brain tumour therapy, and its ligand OX40L has demonstrated a robust antitumor immune response in preclinical models of cancers and has successfully prolonged survival [217]. A mouse lysogenic virus, OV-mOX40L, was developed by HSV-1 modification, which expresses OX40L and was used to treat pancreatic ductal adenocarcinoma (PDAC). This therapeutic approach combines the direct tumour lysis effect of OV with OX40L-mediated immune-stimulating activity to achieve better therapeutic outcomes. Compared with the original oncolytic virus, OV-mOX40L showed enhanced efficacy in a mouse model of PDAC, effectively slowing tumour growth and prolonging the survival time of the mice [208]. Other studies have also shown that multiple administrations of HSV-1 with CD40L improved survival and provided long-term protection against tumour recurrence in PDAC mice [218].
Modified HSV-1 has been used in melanoma treatment. HSV-1-derived Talimogene laherparepvec (T-VEC) (OncovexGM-CSF) has become the first HSV-1 type granulocyte-macrophage colony-stimulating factor (GM-CSF), which received FDA approval for the treatment of human melanoma. This innovative therapy provides a new treatment option for patients with melanoma and is expected to improve patient prognosis in the future [219–222]. Treatment with OncovexGM-CSF can induce the production of local and systemic melanoma antigens recognized by T-cell (MART)-1-specific CD8+T effector cells in melanoma patients [205,206]. Another HSV-1 vaccine VC2 also induced effective antitumor immune responses in a mouse model of melanoma [188,207]. VC2 can be easily adapted to promote specific immune responses to tumour-associated antigens, thereby prolonging survival time and reducing tumour growth rate in mice [223].
Modified HSV-1 has been used in dystrophic epidermolysis bullosa (DEB) treatment. B-VEC is a modified viral vector characterized by the absence of ICP4. Owing to the absence of ICP4, the viral life cycle is blocked at the immediate early (IE) stage of infection, resulting in the virus being incapable of reproduction. DEB is a rare hereditary skin disorder primarily triggered by mutations in the COL7A1 gene encoding the alpha chain of collagen type VII. A non-replicative HSV-1 vector, B-VEC, has demonstrated the potential to treat this condition. The ability of B-VEC to deliver the COL7A1 gene locally to the skin is an important step towards the treatment of DEB [224]. In an exploratory phase I and phase II trial involving patients with DEB, repeated topical B-VEC application was associated with durable wound closure, full-length cutaneous C7 expression, and AF assembly with minimal adverse events [225].
Local delivery, reproducible dosing, and high loading capacity of non-replicating HSV-1 provide significant advantages in dermatological therapies. These properties enable this viral vector to effectively deliver therapeutic genes directly to diseased tissues, thereby improving therapeutic efficacy and reducing systemic side effects. Currently, similar vectors are being studied for other diseases, such as autosomal recessive congenital ichthyosis (ARCI, in which the vector expresses transglutaminase 1 (TGM1)), Nethersole’s syndrome (in which the vector expresses SPINK5), and cystic fibrosis (CF, in which the vector expresses the full-length cystic fibrosis transmembrane conductance regulator (CFTR) gene) [211]. These studies are expected to provide new options for the treatment of more diseases and to further advance the field of gene therapy.
ARCI is a heterogeneous and rare form of ichthyosis that affects approximately 1:200,000 people and is associated with severe clinical complications and reduced quality of life. (Orphadata-Orphanet datasets) TGM1 is an intracellular enzyme [226], and germline mutations in the TGM1 gene are a major cause of ARCI, these TGM1 mutations trigger the aberrant epidermal differentiation and impaired skin barrier function observed in patients with ARCI. Freedman et al. investigated the effect of germline mutations in the TGM1 gene encoding a full-length human TGM1 modification using KB105, a replication-deficient HSV-1 gene therapy vector encoding TGM1. Rescuing ARCI patients with TGM1 deficiency by topical application of KB105 is a safe and non-invasive therapeutic strategy [209].
HSV-1, as a therapeutic vector, has demonstrated great potential for applications in a variety of fields such as gene therapy, immunotherapy, and oncology treatment [227]. However, there are still some challenges that must be overcome to make it widely available for clinical practice, such as ensuring its safety, improving its therapeutic efficacy, and realizing large-scale production.
Conclusion
HSV-1 is a widespread viral infection that can cause a wide range of diseases and poses a major threat to human health. Although the host immune system plays a key role in resisting HSV-1 infection, the highly evasive immune surveillance properties of HSV-1, as well as its complex mechanisms of infection and latent infection, make treatment extremely challenging. With an increase in medical research, treatments for HSV-1 infection are constantly being innovated and developed. In addition to traditional antiviral drugs, emerging therapeutic strategies such as gene therapy and immune therapy have shown encouraging therapeutic effects. However, a great deal of research and clinical trials are still needed to further increase the efficacy, reduce side effects, and improve the management and prognosis of HSV-1 infection.
With the rapid development of biotechnology, remarkable achievements have been made in the treatment of HSV-1 infection, vaccine development, and vector therapy, but a series of complex challenges still need to be faced. The latent infection and frequent recurrence of HSV-1 make it difficult to completely eliminate the virus, especially in immunocompromised patients, which may cause serious diseases. At the same time, as a gene therapy vector, the safety and efficiency of HSV-1 need to be solved urgently, especially the replication ability and potential cytotoxicity of the vector need to be strictly evaluated. In addition, the research and development of therapeutic vaccines also face many challenges, and how to induce a specific immune response to eliminate latent viruses becomes the key.
In order to solve the faced problems, we need to deepen our understanding of the latent infection mechanism of HSV-1 and explore new strategies to break the latent state; optimize the design of the carrier platform to reduce cytotoxicity and improve safety; strengthen the research and development of therapeutic vaccines, develop new adjuvants, optimize vaccine formulations and explore new immunization strategies; promote interdisciplinary cooperation and technological innovation and accelerate the development and application of new therapies and technologies. Through these comprehensive measures, we are expected to better cope with the challenges brought by HSV-1 infection and make greater contributions to human health.
Funding Statement
This work was supported by grants from the National Natural Science Foundation [81500675, 82070895, and 81671226] of China, the Natural Science Foundation of Henan Province [232300421047], Science and Technology Innovation Talents in Universities of Henan Province [24HASTIT067], Henan Province Young and Middle-aged Health Science and Technology Innovation Talent Project [JQRC2023001], Key Research Projects for Higher Education in Henan Province [22A320043], and Henan Province Graduate Education Reform and Quality Improvement Project [YJS2023AL058].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Dan Su: data collection, writing original draft and editing; Liping Han: data collection; Chengyu Shi: data collection;Yaoxin Li: editing; Shaoju Qian: editing and review; Zhiwei Feng: editing and review; LiliYu : Conceptualization, Supervision, Writing and editing. All authors have read and agreed to the submitted version of the manuscript. All listed authors meet the criteria for authorship as per the ICMJE guidelines.
Data availability statement
Data sharing is not applicable to this article, as no datasets were generated or analysed during the current study.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- ARCI
autosomal recessive congenital ichthyosis
- TGM1
transglutaminase 1
- CF
cystic fibrosis
- CFTR
Cystic Fibrosis Transmembrane Conductance Regulator
- c-FLIP
cellular caspase-8 inhibitory protein
- CIS
cornea-in-a-syringe
- COAD
colorectal adenocarcinoma
- DCs
dendritic cells
- DEB
dystrophic epidermolysis bullosa
- dsRNA
double-stranded RNA
- DYRK
dual-specificity tyrosine phosphorylation-regulated kinase
- FIV
feline immunodeficiency virus
- GBM
glioblastoma
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HSE
herpes simplex encephalitis
- HSK
herpes simplex keratitis
- ICs
immune checkpoints
- IE
immediate early
- IFI16
inducible protein 16
- IFNs
interferons
- IFN-I
type I interferon
- IRS-1
insulin receptor substrate 1
- LCs
Langerhans cells
- MyD88
myeloid differentiation primary response 88
- NK
natural killer
- NLRs
nucleotide-binding and leucine-rich repeat receptors
- Ovs
oncolytic viruses
- PDAC
pancreatic ductal adenocarcinoma
- pDCs
Plasma cell-like dendritic cells
- pDNA
plasmid DNA
- PMN
polymorphonuclear leukocytes
- PRRs
pattern recognition receptors
- RHL
recurrent herpes labialis
- RLRs
Retinoic acid-inducible gene l-like receptors
- SADBE
squaric acid dibutyl ester
- TENS
transcutaneous electrical nerve stimulation
- TLRs
toll-like receptors
- TNF
tumour necrosis factor
- T-VEC
talimogene laherparepvec
- 1,5-PD
1,5-pentanediol
- 6-TG
6-Thioguanine.
References
- [1].Whitley RJ, Roizman B.. Herpes simplex virus infections. Lancet. 2001;357(9267):1513–30. doi: 10.1016/S0140-6736(00)04638-9 [DOI] [PubMed] [Google Scholar]
- [2].Heldwein EE. Up close with herpesviruses. Science. 2018;360(6384):34–35. doi: 10.1126/science.aat3990 [DOI] [PubMed] [Google Scholar]
- [3].Looker KJ, Magaret AS, May MT, et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLOS ONE. 2015;10(10):e0140765. doi: 10.1371/journal.pone.0140765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brown ZA, Vontver LA, Benedetti J, et al. Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med. 1987;317(20):1246–1251. doi: 10.1056/NEJM198711123172002 [DOI] [PubMed] [Google Scholar]
- [5].James C, Harfouche M, Welton NJ, et al. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98(5):315–329. doi: 10.2471/BLT.19.237149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fredrikson JP, Domanico LF, Pratt SL, et al. Single-cell herpes simplex virus type 1 infection of neurons using drop-based microfluidics reveals heterogeneous replication kinetics. Sci Adv. 2024;10(9):eadk9185. doi: 10.1126/sciadv.adk9185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pegg CE, Zaichick SV, Bomba-Warczak E, et al. Herpesviruses assimilate kinesin to produce motorized viral particles. Nature. 2021;599(7886):662–666. doi: 10.1038/s41586-021-04106-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Suryawanshi RK, Patil CD, Agelidis A, et al. Pathophysiology of reinfection by exogenous HSV-1 is driven by heparanase dysfunction. Sci Adv. 2023;9(17):eadf3977. doi: 10.1126/sciadv.adf3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sun B, Yang X, Hou F, et al. Regulation of host and virus genes by neuronal miR-138 favours herpes simplex virus 1 latency. Nat Microbiol. 2021;6(5):682–696. doi: 10.1038/s41564-020-00860-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang Z, Li S, Zhong L, et al. Effect of resveratrol on herpesvirus encephalitis: evidences for its mechanisms of action. Phytomedicine. 2024;127:155476. doi: 10.1016/j.phymed.2024.155476 [DOI] [PubMed] [Google Scholar]
- [11].Zhao M, Ma G, Yan X, et al. Microbial infection promotes amyloid pathology in a mouse model of Alzheimer’s disease via modulating gamma-secretase. Mol Psychiatry. 2024:1–10. doi: 10.1038/s41380-024-02428-5 [DOI] [PubMed] [Google Scholar]
- [12].Bloch KC, Glaser C, Gaston D, et al. State of the art: acute encephalitis. Clin Infect Dis. 2023;77(5):e14–e33. doi: 10.1093/cid/ciad306 [DOI] [PubMed] [Google Scholar]
- [13].Hussain MS, Gupta G, Samuel VP, et al. Immunopathology of herpes simplex virus-associated neuroinflammation: unveiling the mysteries. Rev Med Virol. 2024;34(1):e2491. doi: 10.1002/rmv.2491 [DOI] [PubMed] [Google Scholar]
- [14].Mosca JD, Bednarik DP, Raj NB, et al. Is there a role for herpesvirus in AIDS? Nature. 1988;331(6152):122. doi: 10.1038/331122a0 [DOI] [PubMed] [Google Scholar]
- [15].Copeland AM, Newcomb WW, Brown JC. Herpes simplex virus replication: roles of viral proteins and nucleoporins in capsid-nucleus attachment. J Virol. 2009;83(4):1660–1668. doi: 10.1128/JVI.01139-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Krishnan R, Stuart PM. Developments in vaccination for herpes simplex virus. Front Microbiol. 2021;12:798927. doi: 10.3389/fmicb.2021.798927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jiao X, Sui H, Lyons C, et al. Complete genome sequence of herpes simplex virus 1 strain McKrae. Microbiol Resour Announc. 2019;8(39). doi: 10.1128/MRA.00993-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Denes CE, Everett RD, Diefenbach RJ. Tour de herpes: cycling through the life and biology of HSV-1. Methods Mol Biol. 2020;2060:1–30. doi: 10.1007/978-1-4939-9814-2_1 [DOI] [PubMed] [Google Scholar]
- [19].Maruzuru Y, Ichinohe T, Sato R, et al. Herpes simplex virus 1 VP22 inhibits AIM2-dependent inflammasome activation to enable efficient viral replication. Cell Host Microbe. 2018;23(2):254–265 e257. doi: 10.1016/j.chom.2017.12.014 [DOI] [PubMed] [Google Scholar]
- [20].Madavaraju K, Koganti R, Volety I, et al. Herpes simplex virus cell entry mechanisms: an update. Front Cell Infect Microbiol. 2020;10:617578. doi: 10.3389/fcimb.2020.617578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sharthiya H, Seng C, Van Kuppevelt TH, et al. HSV-1 interaction to 3-O-sulfated heparan sulfate in mouse-derived DRG explant and profiles of inflammatory markers during virus infection. J Neurovirol. 2017;23(3):483–491. doi: 10.1007/s13365-017-0521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Akhtar J, Shukla D. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009;276(24):7228–7236. doi: 10.1111/j.1742-4658.2009.07402.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].He D, Mao A, Li Y, et al. TRPC1 participates in the HSV-1 infection process by facilitating viral entry. Sci Adv. 2020;6(12):eaaz3367. doi: 10.1126/sciadv.aaz3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhu S, Viejo-Borbolla A. Pathogenesis and virulence of herpes simplex virus. Virulence. 2021;12(1):2670–2702. doi: 10.1080/21505594.2021.1982373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tognarelli EI, Palomino TF, Corrales N, et al. Herpes simplex virus evasion of early host antiviral responses. Front Cell Infect Microbiol. 2019;9:127. doi: 10.3389/fcimb.2019.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cen P, Wray CJ, Zhang S, et al. Durable response for ampullary and duodenal adenocarcinoma with a nab-paclitaxel plus gemcitabine ± cisplatin combination. Cancer Med. 2019;8(7):3464–3470. doi: 10.1002/cam4.2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rock DL, Fraser NW. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983;302(5908):523–525. doi: 10.1038/302523a0 [DOI] [PubMed] [Google Scholar]
- [28].Wang S, Song X, Rajewski A, et al. Stacking the odds: multiple sites for HSV-1 latency. Sci Adv. 2023;9(4):eadf4904. doi: 10.1126/sciadv.adf4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xiong TC, Wei MC, Li FX, et al. The E3 ubiquitin ligase ARIH1 promotes antiviral immunity and autoimmunity by inducing mono-ISGylation and oligomerization of cGAS. Nat Commun. 2022;13(1):5973. doi: 10.1038/s41467-022-33671-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang R, Yang W, Zhu H, et al. NLRC4 promotes the cGAS-sting signaling pathway by facilitating cbl-mediated K63-linked polyubiquitination of TBK1. J Med Virol. 2023;95(8):e29013. doi: 10.1002/jmv.29013 [DOI] [PubMed] [Google Scholar]
- [31].Fu Y, Zhan X, You X, et al. USP12 promotes antiviral responses by deubiquitinating and stabilizing IFI16. PloS Pathog. 2023;19(7):e1011480. doi: 10.1371/journal.ppat.1011480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang JP, Bowen GN, Zhou S, et al. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J Virol. 2012;86(4):2273–2281. doi: 10.1128/JVI.06010-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ma Y, He B. Recognition of herpes simplex viruses: toll-like receptors and beyond. J Mol Biol. 2014;426(6):1133–1147. doi: 10.1016/j.jmb.2013.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Malmgaard L, Melchjorsen J, Bowie AG, et al. Viral activation of macrophages through tlr-dependent and -independent pathways. J Immunol. 2004;173(11):6890–6898. doi: 10.4049/jimmunol.173.11.6890 [DOI] [PubMed] [Google Scholar]
- [35].Kurt-Jones EA, Chan M, Zhou S, et al. Herpes simplex virus 1 interaction with toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101(5):1315–1320. doi: 10.1073/pnas.0308057100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Aravalli RN, Hu S, Rowen TN, et al. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 2005;175(7):4189–4193. doi: 10.4049/jimmunol.175.7.4189 [DOI] [PubMed] [Google Scholar]
- [37].Cai M, Li M, Wang K, et al. The herpes simplex virus 1-encoded envelope glycoprotein B activates nf-κB through the toll-like receptor 2 and MyD88/TRAF6-dependent signaling pathway. PLOS ONE. 2013;8(1):e54586. doi: 10.1371/journal.pone.0054586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Leoni V, Gianni T, Salvioli S, et al. Herpes simplex virus glycoproteins gH/gL and gB bind toll-like receptor 2, and soluble gH/gL is sufficient to activate nf-κB. J Virol. 2012;86(12):6555–6562. doi: 10.1128/JVI.00295-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gianni T, Leoni V, Campadelli-Fiume G. Type I interferon and nf-κB activation elicited by herpes simplex virus gH/gL via αvβ3 integrin in epithelial and neuronal cell lines. J Virol. 2013;87(24):13911–13916. doi: 10.1128/JVI.01894-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gianni T, Campadelli-Fiume G, Sandri-Goldin RM. The epithelial αvβ3-integrin boosts the MYD88-dependent TLR2 signaling in response to viral and bacterial components. PloS Pathog. 2014;10(11):e1004477. doi: 10.1371/journal.ppat.1004477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hochrein H, Schlatter B, O’Keeffe M, et al. Herpes simplex virus type-1 induces ifn-α production via toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci USA. 2004;101(31):11416–11421. doi: 10.1073/pnas.0403555101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sorensen LN, Reinert LS, Malmgaard L, et al. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J Immunol. 2008;181(12):8604–8612. doi: 10.4049/jimmunol.181.12.8604 [DOI] [PubMed] [Google Scholar]
- [43].Uyangaa E, Choi JY, Patil AM, et al. Dual TLR2/9 recognition of herpes simplex virus infection is required for recruitment and activation of monocytes and NK cells and restriction of viral dissemination to the central nervous system. Front Immunol. 2018;9:905. doi: 10.3389/fimmu.2018.00905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Motwani M, Pesiridis S, Fitzgerald KA. DNA sensing by the cGAS–sting pathway in health and disease. Nat Rev Genet. 2019;20(11):657–674. doi: 10.1038/s41576-019-0151-1 [DOI] [PubMed] [Google Scholar]
- [45].Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–678. doi: 10.1038/nature07317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xu P, Liu Y, Liu C, et al. The CRL5–SPSB3 ubiquitin ligase targets nuclear cGAS for degradation. Nature. 2024;627(8005):873–879. doi: 10.1038/s41586-024-07112-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang Y, Li F, Wang Z, et al. Luteolin inhibits herpes simplex virus 1 infection by activating cyclic guanosine monophosphate-adenosine monophosphate synthase-mediated antiviral innate immunity. Phytomedicine. 2023;120:155020. doi: 10.1016/j.phymed.2023.155020 [DOI] [PubMed] [Google Scholar]
- [48].Wang W, Hu D, Wu C, et al. STING promotes NLRP3 localization in ER and facilitates NLRP3 deubiquitination to activate the inflammasome upon HSV-1 infection. PLOS Pathog. 2020;16(3):e1008335. doi: 10.1371/journal.ppat.1008335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dogrammatzis C, Saud R, Waisner H, et al. Tracing the STING exocytosis pathway during herpes viruses infection. MBio. 2024;15(4):e0037324. doi: 10.1128/mbio.00373-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jia M, Chai L, Wang J, et al. S-nitrosothiol homeostasis maintained by ADH5 facilitates sting-dependent host defense against pathogens. Nat Commun. 2024;15(1):1750. doi: 10.1038/s41467-024-46212-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang J, Zhao J, Xu S, et al. Species-specific deamidation of cGAS by herpes simplex virus UL37 protein facilitates viral replication. Cell Host Microbe. 2018;24(2):234–248 e235. doi: 10.1016/j.chom.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Liu D, Lum KK, Treen N, et al. IFI16 phase separation via multi-phosphorylation drives innate immune signaling. Nucleic Acids Res. 2023;51(13):6819–6840. doi: 10.1093/nar/gkad449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Soby S, Laursen RR, Ostergaard L, et al. HSV-1-induced chemokine expression via IFI16-dependent and IFI16-independent pathways in human monocyte-derived macrophages. Herpesviridae. 2012;3(1):6. doi: 10.1186/2042-4280-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Merkl PE, Orzalli MH, Knipe DM, et al. Mechanisms of host IFI16, PML, and daxx protein restriction of herpes simplex virus 1 replication. J Virol. 2018;92(10):10 .1128/jvi. 00057–00018. doi: 10.1128/JVI.00057-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Johnson KE, Bottero V, Flaherty S, et al. IFI16 restricts HSV-1 replication by accumulating on the hsv-1 genome, repressing HSV-1 gene expression, and directly or indirectly modulating histone modifications. PloS Pathog. 2014;10(11):e1004503. doi: 10.1371/journal.ppat.1004503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Danastas K, Miranda-Saksena M, Cunningham AL. Herpes simplex virus type 1 interactions with the interferon system. Int J Mol Sci. 2020;21(14):5150. doi: 10.3390/ijms21145150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Orzalli MH, Broekema NM, Diner BA, et al. cGAS-mediated stabilization of IFI16 promotes innate signaling during herpes simplex virus infection. Proc Natl Acad Sci USA. 2015;112(14):E1773–1781. doi: 10.1073/pnas.1424637112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chiang JJ, Sparrer KMJ, van Gent M, et al. Viral unmasking of cellular 5S rRNA pseudogene transcripts induces RIG-I-mediated immunity. Nat Immunol. 2018;19(1):53–62. doi: 10.1038/s41590-017-0005-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gao D, Ciancanelli MJ, Zhang P, et al. TLR3 controls constitutive ifn-β antiviral immunity in human fibroblasts and cortical neurons. J Clin Invest. 2021;131(1). doi: 10.1172/JCI134529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lafaille FG, Pessach IM, Zhang SY, et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature. 2012;491(7426):769–773. doi: 10.1038/nature11583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rehwinkel J, Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. 2020;20(9):537–551. doi: 10.1038/s41577-020-0288-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhao Y, Karijolich J, Tarakanova VL. Know thyself: RIG-I-Like receptor sensing of DNA virus infection. J Virol. 2019;93(23). doi: 10.1128/JVI.01085-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138(3):576–591. doi: 10.1016/j.cell.2009.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ablasser A, Bauernfeind F, Hartmann G, et al. RIG-I-dependent sensing of poly(dA: dT) through the induction of an RNA polymerase iii–transcribed RNA intermediate. Nat Immunol. 2009;10(10):1065–1072. doi: 10.1038/ni.1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gupta S, Yla-Anttila P, Callegari S, et al. Herpesvirus deconjugases inhibit the IFN response by promoting TRIM25 autoubiquitination and functional inactivation of the RIG-I signalosome. PloS Pathog. 2018;14(1):e1006852. doi: 10.1371/journal.ppat.1006852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Liu X, Ma Y, Voss K, et al. The herpesvirus accessory protein γ134.5 facilitates viral replication by disabling mitochondrial translocation of RIG-I. PloS Pathog. 2021;17(3):e1009446. doi: 10.1371/journal.ppat.1009446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhao J, Zeng Y, Xu S, et al. A viral deamidase targets the helicase domain of RIG-I to block RNA-Induced activation. Cell Host Microbe. 2016;20(6):770–784. doi: 10.1016/j.chom.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kew C, Lui PY, Chan CP, et al. Suppression of pact-induced type I interferon production by herpes simplex virus 1 Us11 protein. J Virol. 2013;87(24):13141–13149. doi: 10.1128/JVI.02564-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Xing J, Wang S, Lin R, et al. Herpes simplex virus 1 tegument protein US11 downmodulates the RLR signaling pathway via direct interaction with rig-I and MDA-5. J Virol. 2012;86(7):3528–3540. doi: 10.1128/JVI.06713-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jiang Y, Sun S, Quan Y, et al. Nuclear RPSA senses viral nucleic acids to promote the innate inflammatory response. Nat Commun. 2023;14(1):8455. doi: 10.1038/s41467-023-43784-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kang Y, Hepojoki J, Maldonado RS, et al. Ancestral allele of DNA polymerase gamma modifies antiviral tolerance. Nature. 2024;628(8009):844–853. doi: 10.1038/s41586-024-07260-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Smith JB, Herbert JJ, Truong NR, et al. Cytokines and chemokines: the vital role they play in herpes simplex virus mucosal immunology. Front Immunol. 2022;13:936235. doi: 10.3389/fimmu.2022.936235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kim M, Truong NR, James V, et al. Relay of herpes simplex virus between Langerhans cells and dermal dendritic cells in human skin. PloS Pathog. 2015;11(4):e1004812. doi: 10.1371/journal.ppat.1004812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bosnjak L, Miranda-Saksena M, Koelle DM, et al. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174(4):2220–2227. doi: 10.4049/jimmunol.174.4.2220 [DOI] [PubMed] [Google Scholar]
- [75].Amin I, Younas S, Afzal S, et al. Herpes simplex virus type 1 and host antiviral immune responses: an update. Viral Immunol. 2019;32(10):424–429. doi: 10.1089/vim.2019.0097 [DOI] [PubMed] [Google Scholar]
- [76].Prechtel AT, Turza NM, Kobelt DJ, et al. Infection of mature dendritic cells with herpes simplex virus type 1 dramatically reduces lymphoid chemokine-mediated migration. J Gen Virol. 2005;86(6):1645–1657. doi: 10.1099/vir.0.80852-0 [DOI] [PubMed] [Google Scholar]
- [77].Theodoridis AA, Eich C, Figdor CG, et al. Infection of dendritic cells with herpes simplex virus type 1 induces rapid degradation of CYTIP, thereby modulating adhesion and migration. Blood. 2011;118(1):107–115. doi: 10.1182/blood-2010-07-294363 [DOI] [PubMed] [Google Scholar]
- [78].Cheng H, Tumpey TM, Staats HF, et al. Role of macrophages in restricting herpes simplex virus type 1 growth after ocular infection. Journal. 2000;41(Issue):1402–1409. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10798656 [PubMed] [Google Scholar]
- [79].Kather A, Raftery MJ, Devi-Rao G, et al. Herpes simplex virus type 1 (HSV-1)-induced apoptosis in human dendritic cells as a result of downregulation of cellular flice-inhibitory protein and reduced expression of HSV-1 antiapoptotic latency-associated transcript sequences. J Virol. 2010;84(2):1034–1046. doi: 10.1128/JVI.01409-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Donaghy H, Bosnjak L, Harman AN, et al. Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J Virol. 2009;83(4):1952–1961. doi: 10.1128/JVI.01578-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Barr DP, Belz GT, Reading PC, et al. A role for plasmacytoid dendritic cells in the rapid IL-18-dependent activation of NK cells following HSV-1 infection. Eur J Immunol. 2007;37(5):1334–1342. doi: 10.1002/eji.200636362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Taylor PR, Martinez-Pomares L, Stacey M, et al. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23(1):901–944. doi: 10.1146/annurev.immunol.23.021704.115816 [DOI] [PubMed] [Google Scholar]
- [83].Melchjorsen J, Siren J, Julkunen I, et al. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting nf-κB and IRF-3. J Gen Virol. 2006;87(5):1099–1108. doi: 10.1099/vir.0.81541-0 [DOI] [PubMed] [Google Scholar]
- [84].Brun P, Scarpa M, Marchiori C, et al. Herpes simplex virus type 1 engages toll like receptor 2 to recruit macrophages during infection of enteric neurons. Front Microbiol. 2018;9:2148. doi: 10.3389/fmicb.2018.02148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lee DH, Ghiasi H, Longnecker RM. Roles of M1 and M2 macrophages in herpes simplex virus 1 infectivity. J Virol. 2017;91(15):3616–3616. doi: 10.1128/JVI.00578-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ellermann-Eriksen S. Macrophages and cytokines in the early defence against herpes simplex virus. Virol J. 2005;2(1):59. doi: 10.1186/1743-422X-2-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Christensen MH, Jensen SB, Miettinen JJ, et al. HSV-1 ICP27 targets the TBK1-activated STING signalsome to inhibit virus-induced type I IFN expression. Embo J. 2016;35(13):1385–1399. doi: 10.15252/embj.201593458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Fehniger TA, Cooper MA, Nuovo GJ, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101(8):3052–3057. doi: 10.1182/blood-2002-09-2876 [DOI] [PubMed] [Google Scholar]
- [89].Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/S1471-4906(01)02060-9 [DOI] [PubMed] [Google Scholar]
- [90].Lima M, Leander M, Santos M, et al. Chemokine receptor expression on normal blood CD56+ NK-Cells elucidates cell partners that comigrate during the innate and adaptive immune responses and identifies a transitional NK-Cell population. J Immunol Res. 2015;2015:1–18. doi: 10.1155/2015/839684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kim M, Osborne NR, Zeng W, et al. Herpes simplex virus antigens directly activate NK cells via TLR2, thus facilitating their presentation to CD4 T lymphocytes. J Immunol. 2012;188(9):4158–4170. doi: 10.4049/jimmunol.1103450 [DOI] [PubMed] [Google Scholar]
- [92].Orr MT, Edelmann KH, Vieira J, et al. Inhibition of MHC class I is a virulence factor in herpes simplex virus infection of mice. PloS Pathog. 2005;1(1):e7. doi: 10.1371/journal.ppat.0010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290(5489):84–89. doi: 10.1126/science.290.5489.84 [DOI] [PubMed] [Google Scholar]
- [94].Schepis D, D’Amato M, Studahl M, et al. Herpes simplex virus infection downmodulates NKG2D ligand expression. Scand J Immunol. 2009;69(5):429–436. doi: 10.1111/j.1365-3083.2009.02241.x [DOI] [PubMed] [Google Scholar]
- [95].Wijesinghe VN, Farouk IA, Zabidi NZ, et al. Current vaccine approaches and emerging strategies against herpes simplex virus (HSV). Expert Rev Vaccines. 2021;20(9):1077–1096. doi: 10.1080/14760584.2021.1960162 [DOI] [PubMed] [Google Scholar]
- [96].Cunningham AL, Turner RR, Miller AC, et al. Evolution of recurrent herpes simplex lesions. An immunohistologic study. J Clin Invest. 1985;75(1):226–233. doi: 10.1172/JCI111678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cella M, Scheidegger D, Palmer-Lehmann K, et al. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184(2):747–752. doi: 10.1084/jem.184.2.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11(8):674–680. doi: 10.1038/ni.1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Iijima N, Linehan MM, Zamora M, et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med. 2008;205(13):3041–3052. doi: 10.1084/jem.20082039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Steiner I, Benninger F. Update on herpes virus infections of the nervous system. Curr Neurol Neurosci Rep. 2013;13(12):414. doi: 10.1007/s11910-013-0414-8 [DOI] [PubMed] [Google Scholar]
- [101].Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–645. doi: 10.1016/j.immuni.2009.04.010 [DOI] [PubMed] [Google Scholar]
- [103].Knickelbein JE, Khanna KM, Yee MB, et al. Noncytotoxic lytic granule–mediated CD8 + T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322(5899):268–271. doi: 10.1126/science.1164164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Blondeau C, Pelchen-Matthews A, Mlcochova P, et al. Tetherin restricts herpes simplex virus 1 and is antagonized by glycoprotein M. J Virol. 2013;87(24):13124–13133. doi: 10.1128/JVI.02250-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Lee AR, Wilson KR, Clarke M, et al. GPR41 and GPR43 regulate CD8+ T cell priming during herpes simplex virus type 1 infection. Front Immunol. 2024;15:1332588. doi: 10.3389/fimmu.2024.1332588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mikloska Z, Kesson AM, Penfold ME, et al. Herpes simplex virus protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-γ. J Infect Dis. 1996;173(1):7–17. doi: 10.1093/infdis/173.1.7 [DOI] [PubMed] [Google Scholar]
- [107].Peng T, Zhu J, Phasouk K, et al. An effector phenotype of CD8+ T cells at the junction epithelium during clinical quiescence of herpes simplex virus 2 infection. J Virol. 2012;86(19):10587–10596. doi: 10.1128/JVI.01237-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zhu J, Peng T, Johnston C, et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature. 2013;497(7450):494–497. doi: 10.1038/nature12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15(7):441–451. doi: 10.1038/nri3857 [DOI] [PubMed] [Google Scholar]
- [110].Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol. 2004;172(6):3422–3427. doi: 10.4049/jimmunol.172.6.3422 [DOI] [PubMed] [Google Scholar]
- [111].Beland JL, Sobel RA, Adler H, et al. B cell-deficient mice have increased susceptibility to HSV-1 encephalomyelitis and mortality. J Neuroimmunol. 1999;94(1–2):122–126. doi: 10.1016/S0165-5728(98)00238-0 [DOI] [PubMed] [Google Scholar]
- [112].Matthews E, Beckham JD, Piquet AL, et al. Herpesvirus-associated encephalitis: an update. Curr Trop Med Rep. 2022;9(3):92–100. doi: 10.1007/s40475-022-00255-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Piret J, Boivin G. Immunomodulatory strategies in herpes simplex virus encephalitis. Clin Microbiol Rev. 2020;33(2):10 .1128/cmr. 00105–00119. doi: 10.1128/CMR.00105-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Li F, Wang Y, Zheng K. Microglial mitophagy integrates the microbiota-gut-brain axis to restrain neuroinflammation during neurotropic herpesvirus infection. Autophagy. 2023;19(2):734–736. doi: 10.1080/15548627.2022.2102309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Whitley RJ, Soong SJ, Dolin R, et al. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. National institute of allergy and infectious diseases collaborative antiviral study. N Engl J Med. 1977;297(6):289–294. doi: 10.1056/NEJM197708112970601 [DOI] [PubMed] [Google Scholar]
- [116].Wang Z, Zang R, Niu Z, et al. Synthesis and antiviral effect of phosphamide modified vidarabine for treating HSV 1 infections. Bioorg Med Chem Lett. 2021;52:128405. doi: 10.1016/j.bmcl.2021.128405 [DOI] [PubMed] [Google Scholar]
- [117].Stahl JP, Mailles A. Herpes simplex virus encephalitis update. Curr Opin Infect Dis. 2019;32(3):239–243. doi: 10.1097/QCO.0000000000000554 [DOI] [PubMed] [Google Scholar]
- [118].James SH, Prichard MN. Current and future therapies for herpes simplex virus infections: mechanism of action and drug resistance. Curr Opin Virol. 2014;8:54–61. doi: 10.1016/j.coviro.2014.06.003 [DOI] [PubMed] [Google Scholar]
- [119].Schalkwijk HH, Snoeck R, Andrei G. Acyclovir resistance in herpes simplex viruses: prevalence and therapeutic alternatives. Biochem Pharmacol. 2022;206:115322. doi: 10.1016/j.bcp.2022.115322 [DOI] [PubMed] [Google Scholar]
- [120].Hou J, Zhang Z, Huang Q, et al. Antiviral activity of PHA767491 against human herpes simplex virus in vitro and in vivo. BMC Infect Dis. 2017;17(1):217. doi: 10.1186/s12879-017-2305-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Xu XQ, Xu T, Ji W, et al. Herpes simplex virus 1-induced ferroptosis contributes to viral encephalitis. MBio. 2023;14(1):e0237022. doi: 10.1128/mbio.02370-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhang L, Ashraf U, Chen H, et al. Potential therapeutic strategies to halt viral neuroinvasion. Rev Med Virol. 2024;34(3):e2539. doi: 10.1002/rmv.2539 [DOI] [PubMed] [Google Scholar]
- [123].Hill GM, Ku ES, Dwarakanathan S. Herpes simplex keratitis. Dis Mon. 2014;60(6):239–246. doi: 10.1016/j.disamonth.2014.03.003 [DOI] [PubMed] [Google Scholar]
- [124].Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57(5):448–462. doi: 10.1016/j.survophthal.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Stuart PM, Keadle TL. Recurrent herpetic stromal keratitis in mice: a model for studying human HSK. Clin Dev Immunol. 2012;2012:1–10. doi: 10.1155/2012/728480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Darougar S, Wishart MS, Viswalingam ND. Epidemiological and clinical features of primary herpes simplex virus ocular infection. Br J Ophthalmol. 1985;69(1):2–6. doi: 10.1136/bjo.69.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006;25(4):355–380. doi: 10.1016/j.preteyeres.2006.05.001 [DOI] [PubMed] [Google Scholar]
- [128].Tsatsos M, MacGregor C, Athanasiadis I, et al. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin Exp Ophthalmol. 2016;44(9):824–837. doi: 10.1111/ceo.12785 [DOI] [PubMed] [Google Scholar]
- [129].Chou TY, Hong BY. Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: background, effectiveness, tolerability, safety, and future applications. Ther Clin Risk Manag. 2014;10:665–681. doi: 10.2147/TCRM.S58242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Lobo AM, Agelidis AM, Shukla D. Pathogenesis of herpes simplex keratitis: the host cell response and ocular surface sequelae to infection and inflammation. Ocul Surf. 2019;17(1):40–49. doi: 10.1016/j.jtos.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Chen D, Liu Y, Zhang F, et al. 6-thioguanine inhibits herpes simplex virus 1 infection of eyes. Microbiol Spectr. 2021;9(3):e0064621. doi: 10.1128/Spectrum.00646-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Liu Y, You Q, Zhang F, et al. Harringtonine inhibits herpes simplex virus type 1 infection by reducing herpes virus entry mediator expression. Front Microbiol. 2021;12:722748. doi: 10.3389/fmicb.2021.722748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sadowski LA, Upadhyay R, Greeley ZW, et al. Current drugs to treat infections with herpes simplex viruses-1 and -2. Viruses. 2021;13(7):1228. doi: 10.3390/v13071228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Zinser E, Krawczyk A, Muhl-Zurbes P, et al. A new promising candidate to overcome drug resistant herpes simplex virus infections. Antiviral Res. 2018;149:202–210. doi: 10.1016/j.antiviral.2017.11.012 [DOI] [PubMed] [Google Scholar]
- [135].Jaishankar D, Yakoub AM, Yadavalli T, et al. An off-target effect of BX795 blocks herpes simplex virus type 1 infection of the eye. Sci Transl Med. 2018;10(428):eaan5861. doi: 10.1126/scitranslmed.aan5861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Cursiefen C, Viaud E, Bock F, et al. Aganirsen antisense oligonucleotide eye drops inhibit keratitis-induced corneal neovascularization and reduce need for transplantation: the I-CAN study. Ophthalmology. 2014;121(9):1683–1692. doi: 10.1016/j.ophtha.2014.03.038 [DOI] [PubMed] [Google Scholar]
- [137].Sharma S, Mulik S, Kumar N, et al. An anti-inflammatory role of VEGFR2/Src kinase inhibitor in herpes simplex virus 1-induced immunopathology. J Virol. 2011;85(12):5995–6007. doi: 10.1128/JVI.00034-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Bosze S, Zsila F, Biri-Kovacs B, et al. Tailoring uptake efficacy of HSV-1 gD derived carrier peptides. Biomolecules. 2020;10(5):721. doi: 10.3390/biom10050721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Islam MM, Buznyk O, Reddy JC, et al. Biomaterials-enabled cornea regeneration in patients at high risk for rejection of donor tissue transplantation. NPJ Regen Med. 2018;3(1):2. doi: 10.1038/s41536-017-0038-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Simpson FC, McTiernan CD, Islam MM, et al. Collagen analogs with phosphorylcholine are inflammation-suppressing scaffolds for corneal regeneration from alkali burns in mini-pigs. Commun Biol. 2021;4(1):608. doi: 10.1038/s42003-021-02108-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].McTiernan CD, Simpson FC, Haagdorens M, et al. LiQD Cornea: pro-regeneration collagen mimetics as patches and alternatives to corneal transplantation. Sci Adv. 2020;6(25):eaba2187. doi: 10.1126/sciadv.aba2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Simoliunas E, Ruedas-Torres I, Jimenez-Gomez Y, et al. Inflammation-suppressing cornea-in-a-syringe with anti-viral GF19 peptide promotes regeneration in HSV-1 infected rabbit corneas. NPJ Regen Med. 2024;9(1):11. doi: 10.1038/s41536-024-00355-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Glick JE, Bar J. Herpes simplex virus-associated keratitis. J Emerg Med. 2021;61(1):e11–e12. doi: 10.1016/j.jemermed.2021.02.032 [DOI] [PubMed] [Google Scholar]
- [144].Crimi S, Fiorillo L, Bianchi A, et al. Herpes virus, oral clinical signs and QoL: systematic review of recent data. Viruses. 2019;11(5):463. doi: 10.3390/v11050463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gopinath D, Koe KH, Maharajan MK, et al. A comprehensive overview of epidemiology, pathogenesis and the management of herpes labialis. Viruses. 2023;15(1):225. doi: 10.3390/v15010225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Roizman B, Whitley RJ. An inquiry into the molecular basis of HSV latency and reactivation. Annu Rev Microbiol. 2013;67(1):355–374. doi: 10.1146/annurev-micro-092412-155654 [DOI] [PubMed] [Google Scholar]
- [147].Khemis A, Duteil L, Coudert AC, et al. Evaluation of the efficacy and safety of a CS20 ® protective barrier gel containing OGT compared with topical aciclovir and placebo on functional and objective symptoms of labial herpes recurrences: a randomized clinical trial. J Eur Acad Dermatol Venereol. 2012;26(10):1240–1246. doi: 10.1111/j.1468-3083.2011.04269.x [DOI] [PubMed] [Google Scholar]
- [148].Rezazadeh F, Moshaverinia M, Motamedifar M, et al. Assessment of anti HSV-1 activity of aloe vera gel extract: an in vitro study. Journal. 2016;17(Issue):49–54. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26966709 [PMC free article] [PubMed] [Google Scholar]
- [149].Zschocke I, Reich C, Zielke A, et al. Silica gel is as effective as acyclovir cream in patients with recurrent herpes labialis: results of a randomized, open-label trial. J Dermatolog Treat. 2008;19(3):176–181. doi: 10.1080/09546630701593457 [DOI] [PubMed] [Google Scholar]
- [150].Busch R, Graubaum HJ, Gruenwald J, et al. Therapeutic effect of 1,5-pentanediol for herpes simplex labialis: a randomized, double-blind, placebo-controlled clinical trial. Adv Ther. 2009;26(7):719–727. doi: 10.1007/s12325-009-0049-y [DOI] [PubMed] [Google Scholar]
- [151].Belec L, Tevi-Benissan C, Bianchi A, et al. In vitro inactivation of Chlamydia trachomatis and of a panel of DNA (HSV-2, CMV, adenovirus, BK virus) and RNA (RSV, enterovirus) viruses by the spermicide benzalkonium chloride. J Antimicrob Chemother. 2000;46(5):685–693. doi: 10.1093/jac/46.5.685 [DOI] [PubMed] [Google Scholar]
- [152].Astani A, Albrecht U, Schnitzler P. Piroxicam inhibits herpes simplex virus type 1 infection in vitro. Pharmazie. 2015;70(5):331–336. doi: 10.1691/ph.2015.4791 [DOI] [PubMed] [Google Scholar]
- [153].Sacks SL, Thisted RA, Jones TM, et al. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45(2):222–230. doi: 10.1067/mjd.2001.116215 [DOI] [PubMed] [Google Scholar]
- [154].Pope LE, Marcelletti JF, Katz LR, et al. The anti-herpes simplex virus activity of n-docosanol includes inhibition of the viral entry process. Antiviral Res. 1998;40(1–2):85–94. doi: 10.1016/S0166-3542(98)00048-5 [DOI] [PubMed] [Google Scholar]
- [155].Skulason S, Holbrook WP, Thormar H, et al. A study of the clinical activity of a gel combining monocaprin and doxycycline: a novel treatment for herpes labialis. J Oral Pathol Med. 2012;41(1):61–67. doi: 10.1111/j.1600-0714.2011.01037.x [DOI] [PubMed] [Google Scholar]
- [156].Billet B, Wynendaele R, Vanquathem NE. A novel minimally invasive wireless technology for neuromodulation via percutaneous intercostal nerve stimulation for post-herpetic neuralgia: a case report with short-term follow-up. Pain Pract. 2018;18(3):374–379. doi: 10.1111/papr.12607 [DOI] [PubMed] [Google Scholar]
- [157].Cervino G, Cicciu M, Biondi A, et al. Antibiotic prophylaxis on third molar extraction: systematic review of recent data. Antibiotics (Basel). 2019;8(2):53. doi: 10.3390/antibiotics8020053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Dougal G, Lee SY. Evaluation of the efficacy of low-level light therapy using 1072 nm infrared light for the treatment of herpes simplex labialis. Clin Exp Dermatol. 2013;38(7):713–718. doi: 10.1111/ced.12069 [DOI] [PubMed] [Google Scholar]
- [159].Palli MA, McTavish H, Kimball A, et al. Immunotherapy of recurrent herpes labialis with squaric acid. JAMA Dermatol. 2017;153(8):828–829. doi: 10.1001/jamadermatol.2017.0725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex Infections: an evidence-based review. Arch Intern Med. 2008;168(11):1137–1144. doi: 10.1001/archinte.168.11.1137 [DOI] [PubMed] [Google Scholar]
- [161].Gmyrek GB, Filiberti A, Montgomery M, et al. Herpes simplex virus 1 (HSV-1) 0ΔNLS live-attenuated vaccine protects against ocular HSV-1 infection in the absence of neutralizing antibody in HSV-1 gB T cell receptor-specific transgenic mice. J Virol. 2020;94(24):10 .1128/jvi. 01000–01020. doi: 10.1128/JVI.01000-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Gmyrek GB, Berube AN, Sjoelund VH, et al. HSV-1 0∆NLS vaccine elicits a robust B lymphocyte response and preserves vision without HSV-1 glycoprotein M or thymidine kinase recognition. Sci Rep. 2022;12(1):15920. doi: 10.1038/s41598-022-20180-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Carr DJJ, Berube A, Gershburg E. The durability of vaccine efficacy against ocular HSV-1 infection using ICP0 mutants 0∆NLS and 0∆RING is lost over time. Pathogens. 2021;10(11):1470. doi: 10.3390/pathogens10111470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Bernstein DI, Cardin RD, Smith GA, et al. The R2 non-neuroinvasive HSV-1 vaccine affords protection from genital HSV-2 infections in a guinea pig model. NPJ Vaccines. 2020;5(1):104. doi: 10.1038/s41541-020-00254-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Tanaka M, Kagawa H, Yamanashi Y, et al. Construction of an excisable bacterial artificial chromosome containing a full-length infectious clone of herpes simplex virus type 1: viruses reconstituted from the clone exhibit wild-type properties in vitro and in vivo. J Virol. 2003;77(2):1382–1391. doi: 10.1128/JVI.77.2.1382-1391.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].de Bruyn G, Vargas-Cortez M, Warren T, et al. A randomized controlled trial of a replication defective (gH deletion) herpes simplex virus vaccine for the treatment of recurrent genital herpes among immunocompetent subjects. Vaccine. 2006;24(7):914–920. doi: 10.1016/j.vaccine.2005.08.088 [DOI] [PubMed] [Google Scholar]
- [167].Bernstein DI, Pullum DA, Cardin RD, et al. The HSV-1 live attenuated VC2 vaccine provides protection against HSV-2 genital infection in the guinea pig model of genital herpes. Vaccine. 2019;37(1):61–68. doi: 10.1016/j.vaccine.2018.11.042 [DOI] [PubMed] [Google Scholar]
- [168].Naidu SK, Nabi R, Cheemarla NR, et al. Intramuscular vaccination of mice with the human herpes simplex virus type-1(HSV-1) VC2 vaccine, but not its parental strain HSV-1(F) confers full protection against lethal ocular HSV-1 (McKrae) pathogenesis. PLOS ONE. 2020;15(2):e0228252. doi: 10.1371/journal.pone.0228252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Chiuppesi F, Vannucci L, De Luca A, et al. A lentiviral vector-based, herpes simplex virus 1 (HSV-1) glycoprotein B vaccine affords cross-protection against HSV-1 and HSV-2 genital infections. J Virol. 2012;86(12):6563–6574. doi: 10.1128/JVI.00302-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Suenaga T, Satoh T, Somboonthum P, et al. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc Natl Acad Sci USA. 2010;107(2):866–871. doi: 10.1073/pnas.0913351107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Campadelli-Fiume G, Amasio M, Avitabile E, et al. The multipartite system that mediates entry of herpes simplex virus into the cell. Rev Med Virol. 2007;17(5):313–326. doi: 10.1002/rmv.546 [DOI] [PubMed] [Google Scholar]
- [172].Taylor JM, Lin E, Susmarski N, et al. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe. 2007;2(1):19–28. doi: 10.1016/j.chom.2007.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Belshe RB, Leone PA, Bernstein DI, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366(1):34–43. doi: 10.1056/NEJMoa1103151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Bernstein DI, Flechtner JB, McNeil LK, et al. Therapeutic HSV-2 vaccine decreases recurrent virus shedding and recurrent genital herpes disease. Vaccine. 2019;37(26):3443–3450. doi: 10.1016/j.vaccine.2019.05.009 [DOI] [PubMed] [Google Scholar]
- [175].Awasthi S, Hook LM, Shaw CE, et al. An HSV-2 trivalent vaccine is immunogenic in rhesus macaques and highly efficacious in Guinea pigs. PloS Pathog. 2017;13(1):e1006141. doi: 10.1371/journal.ppat.1006141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Kim HC, Oh DS, Park JH, et al. Multivalent DNA vaccine protects against genital herpes by T-cell immune induction in vaginal mucosa. Antiviral Res. 2020;177:104755. doi: 10.1016/j.antiviral.2020.104755 [DOI] [PubMed] [Google Scholar]
- [177].Egan KP, Hook LM, Naughton A, et al. An HSV-2 nucleoside-modified mRNA genital herpes vaccine containing glycoproteins gC, gD, and gE protects mice against HSV-1 genital lesions and latent infection. PLOS Pathog. 2020;16(7):e1008795. doi: 10.1371/journal.ppat.1008795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].LaTourette PC 2nd, Awasthi S, Desmond A, et al. Protection against herpes simplex virus type 2 infection in a neonatal murine model using a trivalent nucleoside-modified mRNA in lipid nanoparticle vaccine. Vaccine. 2020;38(47):7409–7413. doi: 10.1016/j.vaccine.2020.09.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].McLean CS, Ni Challanain D, Duncan I, et al. Induction of a protective immune response by mucosal vaccination with a DISC HSV-1 vaccine. Vaccine. 1996;14(10):987–992. doi: 10.1016/0264-410X(95)00259-4 [DOI] [PubMed] [Google Scholar]
- [180].Kuraoka M, Aschner CB, Windsor IW, et al. A non-neutralizing glycoprotein B monoclonal antibody protects against herpes simplex virus disease in mice. J Clin Invest. 2023;133(3). doi: 10.1172/JCI161968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Mahant AM, Gromisch MS, Kravets L, et al. Greater durability and protection against herpes simplex viral disease following immunization of mice with single-cycle ΔgD-2 compared to an adjuvanted glycoprotein D protein vaccine. Vaccines (Basel). 2023;11(8):1362. doi: 10.3390/vaccines11081362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Vartak A, Sucheck SJ. Recent advances in subunit vaccine carriers. Vaccines (Basel). 2016;4(2):12. doi: 10.3390/vaccines4020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Srivastava R, Khan AA, Huang J, et al. A herpes simplex virus type 1 human asymptomatic CD8+ T-Cell epitopes-based vaccine protects against ocular herpes in a “humanized” HLA transgenic rabbit model. Invest Ophthalmol Vis Sci. 2015;56(6):4013–4028. doi: 10.1167/iovs.15-17074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Khan AA, Srivastava R, Chentoufi AA, et al. Bolstering the number and function of HSV-1–specific CD8+ effector memory T cells and tissue-resident memory T cells in latently infected trigeminal ganglia reduces recurrent ocular herpes infection and disease. J Immunol. 2017;199(1):186–203. doi: 10.4049/jimmunol.1700145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Royer DJ, Hendrix JF, Larabee CM, et al. Vaccine-induced antibodies target sequestered viral antigens to prevent ocular HSV-1 pathogenesis, preserve vision, and preempt productive neuronal infection. Mucosal Immunol. 2019;12(3):827–839. doi: 10.1038/s41385-019-0131-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Bernstein DI, Cardin RD, Pullum DA, et al. Duration of protection from live attenuated vs. sub unit HSV-2 vaccines in the guinea pig model of genital herpes: reassessing efficacy using endpoints from clinical trials. PLOS ONE. 2019;14(3):e0213401. doi: 10.1371/journal.pone.0213401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Clark CM, Jambunathan N, Collantes TMA, et al. Inactivation of the UL37 deamidase enhances virus replication and spread of the HSV-1(VC2) oncolytic vaccine strain and secretion of GM-CSF. Viruses. 2023;15(2):367. doi: 10.3390/v15020367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Stanfield BA, Stahl J, Chouljenko VN, et al. A single intramuscular vaccination of mice with the HSV-1 VC2 virus with mutations in the glycoprotein K and the membrane protein UL20 confers full protection against lethal intravaginal challenge with virulent HSV-1 and HSV-2 strains. PLOS ONE. 2014;9(10):e109890. doi: 10.1371/journal.pone.0109890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Chentoufi AA, Kritzer E, Yu DM, et al. Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown. Clin Dev Immunol. 2012;2012:1–16. doi: 10.1155/2012/187585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Koelle DM, Ghiasi H. Prospects for developing an effective vaccine against ocular herpes simplex virus infection. Curr Eye Res. 2005;30(11):929–942. doi: 10.1080/02713680500313153 [DOI] [PubMed] [Google Scholar]
- [191].Wang K, Tomaras GD, Jegaskanda S, et al. Monoclonal antibodies, derived from humans vaccinated with the RV144 hIV vaccine containing the HVEM binding domain of herpes simplex virus (HSV) glycoprotein D, neutralize HSV infection, mediate antibody-dependent cellular cytotoxicity, and protect mice from ocular challenge with HSV-1. J Virol. 2017;91(19):JVI.00411–00417. doi: 10.1128/JVI.00411-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Lachmann R. Herpes simplex virus-based vectors. Int J Exp Pathol. 2004;85(4):177–190. doi: 10.1111/j.0959-9673.2004.00383.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Srivastava R, Coulon PA, Prakash S, et al. Human epitopes identified from herpes simplex virus tegument protein VP11/12 (UL46) recall multifunctional effector memory CD4+ T(EM) cells in asymptomatic individuals and protect from ocular herpes infection and disease in “humanized” HLA-DR transgenic mice. J Virol. 2020;94(7):10 .1128/jvi. 01991–01919. doi: 10.1128/JVI.01991-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Hosken N, McGowan P, Meier A, et al. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol. 2006;80(11):5509–5515. doi: 10.1128/JVI.02659-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Hook LM, Awasthi S, Dubin J, et al. A trivalent gC2/gd2/ge2 vaccine for herpes simplex virus generates antibody responses that block immune evasion domains on gC2 better than natural infection. Vaccine. 2019;37(4):664–669. doi: 10.1016/j.vaccine.2018.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Awasthi S, Hook LM, Pardi N, et al. Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Sci Immunol. 2019;4(39):eaaw7083. doi: 10.1126/sciimmunol.aaw7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Pardi N, Hogan MJ, Naradikian MS, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215(6):1571–1588. doi: 10.1084/jem.20171450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Dudek T, Knipe DM. Replication-defective viruses as vaccines and vaccine vectors. Virology. 2006;344(1):230–239. doi: 10.1016/j.virol.2005.09.020 [DOI] [PubMed] [Google Scholar]
- [199].Beilstein F, Cohen GH, Eisenberg RJ, et al. Dynamic organization of herpesvirus glycoproteins on the viral envelope revealed by super-resolution microscopy. PloS Pathog. 2019;15(12):e1008209. doi: 10.1371/journal.ppat.1008209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Johnson DC, Wisner TW, Wright CC. Herpes simplex virus glycoproteins gB and gD function in a redundant fashion to promote secondary envelopment. J Virol. 2011;85(10):4910–4926. doi: 10.1128/JVI.00011-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Boursnell ME, Entwisle C, Blakeley D, et al. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J Infect Dis. 1997;175(1):16–25. doi: 10.1093/infdis/175.1.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Farrell HE, McLean CS, Harley C, et al. Vaccine potential of a herpes simplex virus type 1 mutant with an essential glycoprotein deleted. J Virol. 1994;68(2):927–932. doi: 10.1128/jvi.68.2.927-932.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Ramsey NLM, Visciano M, Hunte R, et al. A single-cycle glycoprotein D deletion viral vaccine candidate, ΔgD-2, elicits polyfunctional antibodies that protect against ocular herpes simplex virus. J Virol. 2020;94(13):10 .1128/jvi. 00335–00320. doi: 10.1128/JVI.00335-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Johnston C, Koelle DM, Wald A. Current status and prospects for development of an HSV vaccine. Vaccine. 2014;32(14):1553–1560. doi: 10.1016/j.vaccine.2013.08.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Kaufman HL, Kim DW, DeRaffele G, et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17(3):718–730. doi: 10.1245/s10434-009-0809-6 [DOI] [PubMed] [Google Scholar]
- [206].Uche IK, Stanfield BA, Rudd JS, et al. Utility of a recombinant HSV-1 vaccine vector for personalized cancer vaccines. Front Mol Biosci. 2022;9:832393. doi: 10.3389/fmolb.2022.832393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Uche IK, Kousoulas KG, Rider PJF. The effect of herpes simplex virus-type-1 (HSV-1) oncolytic immunotherapy on the tumor microenvironment. Viruses. 2021;13(7):1200. doi: 10.3390/v13071200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Liu S, Li F, Ma Q, et al. OX40L-Armed oncolytic virus boosts T-cell response and remodels tumor microenvironment for pancreatic cancer treatment. Theranostics. 2023;13(12):4016–4029. doi: 10.7150/thno.83495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Freedman JC, Parry TJ, Zhang P, et al. Preclinical evaluation of a modified herpes simplex virus type 1 vector encoding human TGM1 for the treatment of autosomal recessive congenital ichthyosis. J Invest Dermatol. 2021;141(4):874–882 e876. doi: 10.1016/j.jid.2020.07.035 [DOI] [PubMed] [Google Scholar]
- [210].Ghouse SM, Nguyen HM, Bommareddy PK, et al. Oncolytic herpes simplex virus encoding IL12 controls triple-negative breast cancer growth and metastasis. Front Oncol. 2020;10:384. doi: 10.3389/fonc.2020.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Epstein AL, Haag-Molkenteller C. Herpes simplex virus gene therapy for dystrophic epidermolysis bullosa (DEB). Cell. 2023;186(17):3523–3523 e3521. doi: 10.1016/j.cell.2023.07.031 [DOI] [PubMed] [Google Scholar]
- [212].Zhang Z, Yang N, Lu H, et al. Improved antitumor effects elicited by an oncolytic HSV-1 expressing a novel B7H3nb/CD3 BsAb. Cancer Lett. 2024;588:216760. doi: 10.1016/j.canlet.2024.216760 [DOI] [PubMed] [Google Scholar]
- [213].Epstein AL. HSV-1’s contribution as a vector for gene therapy. Nat Biotechnol. 2022;40(9):1316. doi: 10.1038/s41587-022-01449-1 [DOI] [PubMed] [Google Scholar]
- [214].Russell L, Peng KW. The emerging role of oncolytic virus therapy against cancer. Chin Clin Oncol. 2018;7(2):16. doi: 10.21037/cco.2018.04.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Jahan N, Ghouse SM, Martuza RL, et al. In situ cancer vaccination and immunovirotherapy using oncolytic HSV. Viruses. 2021;13(9):1740. doi: 10.3390/v13091740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Raja J, Ludwig JM, Gettinger SN, et al. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer. 2018;6(1):140. doi: 10.1186/s40425-018-0458-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Springgay LK, Strauwald AM, Ewald B, et al. Abstract 6713: oncolytic adenoviruses expressing immune modulators enhance tumor cell killing in human cancer 3D microtumor models. Cancer Res. 2020;80(16_Supplement):6713–6713. doi: 10.1158/1538-7445.AM2020-6713 [DOI] [Google Scholar]
- [218].Wang R, Chen J, Wang W, et al. CD40L-armed oncolytic herpes simplex virus suppresses pancreatic ductal adenocarcinoma by facilitating the tumor microenvironment favorable to cytotoxic T cell response in the syngeneic mouse model. J Immunother Cancer. 2022;10(1):e003809. doi: 10.1136/jitc-2021-003809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor–encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27(34):5763–5771. doi: 10.1200/JCO.2009.24.3675 [DOI] [PubMed] [Google Scholar]
- [220].Ferrucci PF, Pala L, Conforti F, et al. Talimogene Laherparepvec (T-VEC): an intralesional cancer immunotherapy for advanced melanoma. Cancers (Basel). 2021;13(6):1383. doi: 10.3390/cancers13061383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Bommareddy PK, Patel A, Hossain S, et al. Talimogene Laherparepvec (T-VEC) and other oncolytic viruses for the treatment of melanoma. Am J Clin Dermatol. 2017;18(1):1–15. doi: 10.1007/s40257-016-0238-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Ramelyte E, Tastanova A, Balazs Z, et al. Oncolytic virotherapy-mediated anti-tumor response: a single-cell perspective. Cancer Cell. 2021;39(3):394–406.e394. doi: 10.1016/j.ccell.2020.12.022 [DOI] [PubMed] [Google Scholar]
- [223].Uche IK, Fowlkes N, Vu L, et al. Novel oncolytic herpes simplex virus 1 VC2 promotes long-lasting, systemic anti-melanoma tumor immune responses and increased survival in an immunocompetent B16F10-derived mouse melanoma model. J Virol. 2021;95(3):10 .1128/jvi. 01359–01320. doi: 10.1128/JVI.01359-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Glorioso JC. Herpes simplex viral vectors: late bloomers with big potential. Hum Gene Ther. 2014;25(2):83–91. doi: 10.1089/hum.2014.2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Gurevich I, Agarwal P, Zhang P, et al. In vivo topical gene therapy for recessive dystrophic epidermolysis bullosa: a phase 1 and 2 trial. Nat Med. 2022;28(4):780–788. doi: 10.1038/s41591-022-01737-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6(4):328–340. doi: 10.1038/nrm1619 [DOI] [PubMed] [Google Scholar]
- [227].Chowaniec H, Slubowska A, Mroczek M, et al. New hopes for the breast cancer treatment: perspectives on the oncolytic virus therapy. Front Immunol. 2024;15:1375433. doi: 10.3389/fimmu.2024.1375433 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analysed during the current study.


