Abstract
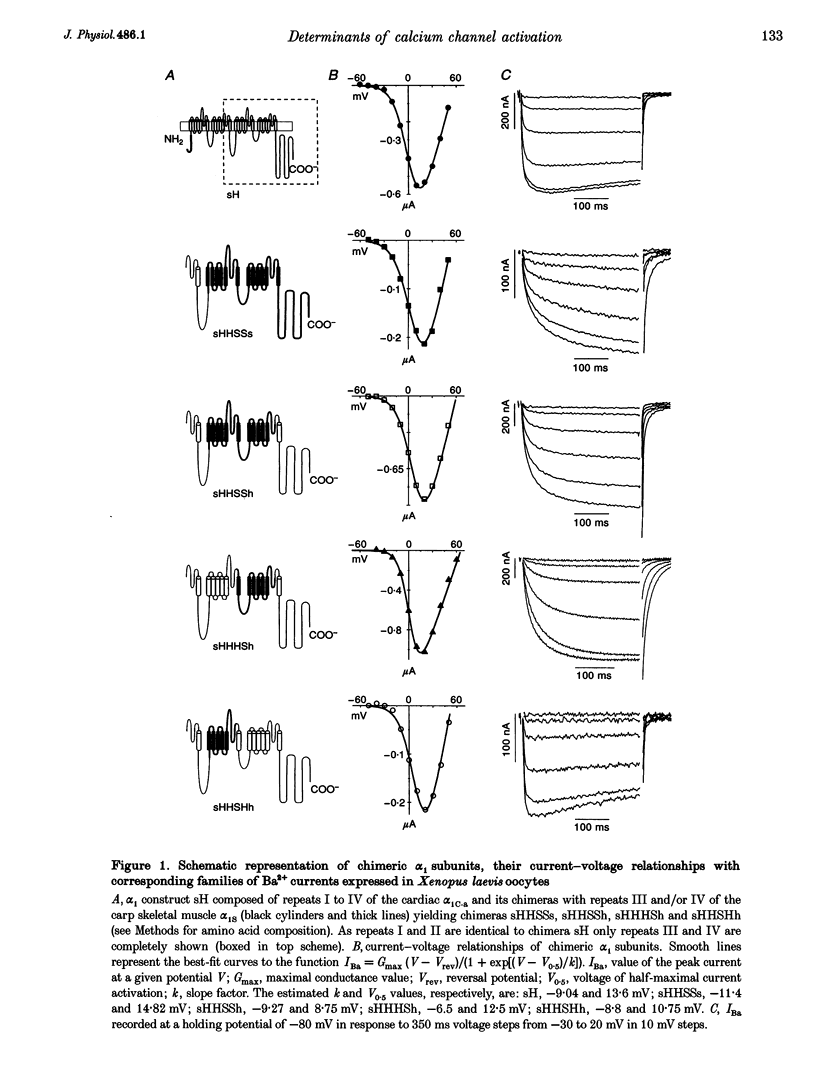
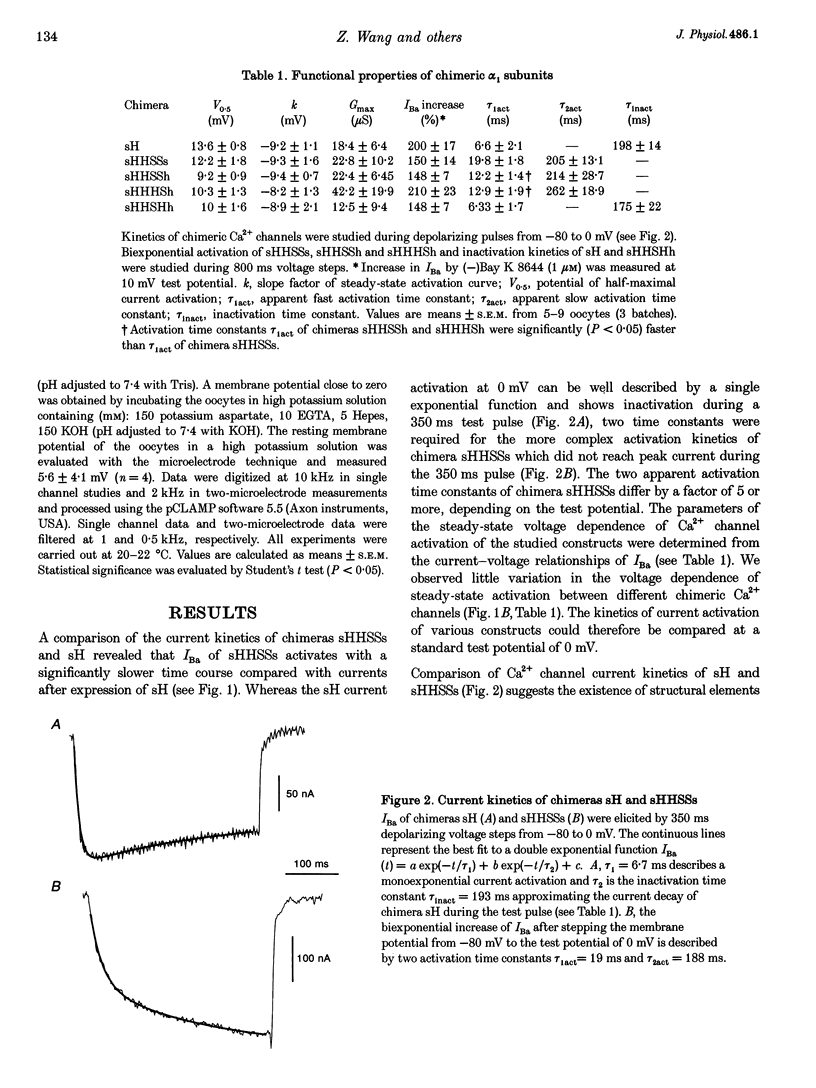
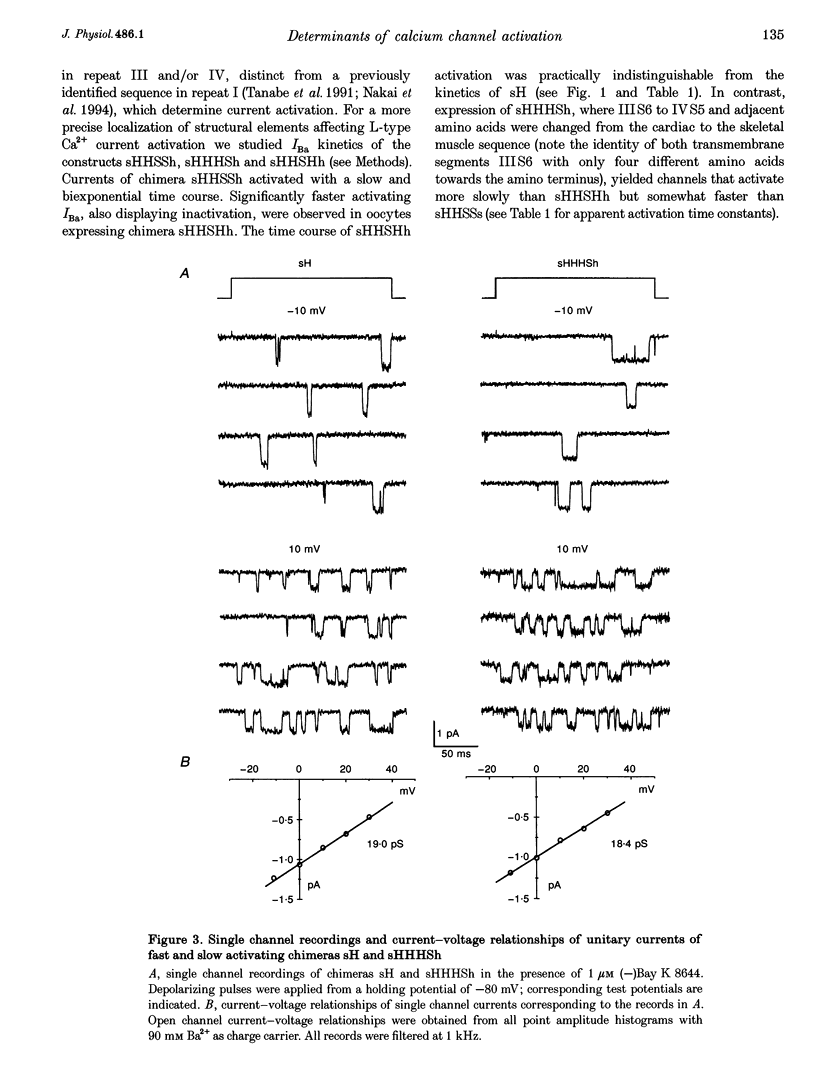
1. Chimeric alpha 1 subunits consisting of repeat I and II from the rabbit cardiac (alpha 1C-a) and repeat III and IV from the carp skeletal muscle Ca2+ channel (alpha 1S) were constructed and expressed in Xenopus laevis oocytes without co-expressing other channel subunits. Ba2+-current kinetics of five chimeric channel constructs were studied in Xenopus oocytes using the two-microelectrode technique. 2. Exchange of repeats III and IV of alpha 1C-a with sequences of alpha 1S results in a significantly slower and biexponential activation (apparent activation time constants tau 1act = 19.8 +/- 1.8 ms and tau 2act = 214 +/- 28.7 ms, n = 7) of expressed Ca2+ channel currents; no current inactivation was observable during an 800 ms test pulse to 0 mV. 3. Activation of a chimera consisting of repeats I, II and IV from the alpha 1C-a subunit and repeat III from alpha 1S was fast and monoexponential (tau 1act = 6.33 +/- 1.7 ms, n = 5) and the current inactivated during a 350 ms test pulse to 0 mV (tau inact = 175 +/- 22 ms, n = 5). The current kinetics of this construct did not significantly differ from kinetics of a construct consisting of repeats I to IV from alpha 1C-a (tau 1act = 6.6 +/- 2.1 ms; tau inact = 198 +/- 14 ms; n = 9).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beam K. G., Adams B. A., Niidome T., Numa S., Tanabe T. Function of a truncated dihydropyridine receptor as both voltage sensor and calcium channel. Nature. 1992 Nov 12;360(6400):169–171. doi: 10.1038/360169a0. [DOI] [PubMed] [Google Scholar]
- Biel M., Hullin R., Freundner S., Singer D., Dascal N., Flockerzi V., Hofmann F. Tissue-specific expression of high-voltage-activated dihydropyridine-sensitive L-type calcium channels. Eur J Biochem. 1991 Aug 15;200(1):81–88. doi: 10.1111/j.1432-1033.1991.tb21051.x. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Campbell K. P., Catterall W. A., Harpold M. M., Hofmann F., Horne W. A., Mori Y., Schwartz A., Snutch T. P., Tanabe T. The naming of voltage-gated calcium channels. Neuron. 1994 Sep;13(3):505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Caffrey J. M. Kinetic properties of skeletal-muscle-like high-threshold calcium currents in a non-fusing muscle cell line. Pflugers Arch. 1994 Jun;427(3-4):277–288. doi: 10.1007/BF00374535. [DOI] [PubMed] [Google Scholar]
- Grabner M., Friedrich K., Knaus H. G., Striessnig J., Scheffauer F., Staudinger R., Koch W. J., Schwartz A., Glossmann H. Calcium channels from Cyprinus carpio skeletal muscle. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):727–731. doi: 10.1073/pnas.88.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner M., Wang Z., Mitterdorfer J., Rosenthal F., Charnet P., Savchenko A., Hering S., Ren D., Hall L. M., Glossmann H. Cloning and functional expression of a neuronal calcium channel beta subunit from house fly (Musca domestica). J Biol Chem. 1994 Sep 23;269(38):23668–23674. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Biel M., Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- Knudson C. M., Chaudhari N., Sharp A. H., Powell J. A., Beam K. G., Campbell K. P. Specific absence of the alpha 1 subunit of the dihydropyridine receptor in mice with muscular dysgenesis. J Biol Chem. 1989 Jan 25;264(3):1345–1348. [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Nakai J., Adams B. A., Imoto K., Beam K. G. Critical roles of the S3 segment and S3-S4 linker of repeat I in activation of L-type calcium channels. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1014–1018. doi: 10.1073/pnas.91.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W. A., Tanabe T., Zhang J. F., Mori Y., Adams M. E., Tsien R. W. Distinctive biophysical and pharmacological properties of class A (BI) calcium channel alpha 1 subunits. Neuron. 1993 Aug;11(2):291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- Sánchez J. A., Stefani E. Kinetic properties of calcium channels of twitch muscle fibres of the frog. J Physiol. 1983 Apr;337:1–17. doi: 10.1113/jphysiol.1983.sp014607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Adams B. A., Numa S., Beam K. G. Repeat I of the dihydropyridine receptor is critical in determining calcium channel activation kinetics. Nature. 1991 Aug 29;352(6338):800–803. doi: 10.1038/352800a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Ellinor P. T., Horne W. A. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol Sci. 1991 Sep;12(9):349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Ellinor P. T., Aldrich R. W., Tsien R. W. Molecular determinants of voltage-dependent inactivation in calcium channels. Nature. 1994 Nov 3;372(6501):97–100. doi: 10.1038/372097a0. [DOI] [PubMed] [Google Scholar]


