Abstract
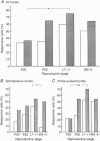
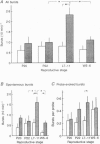
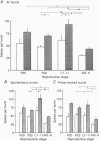
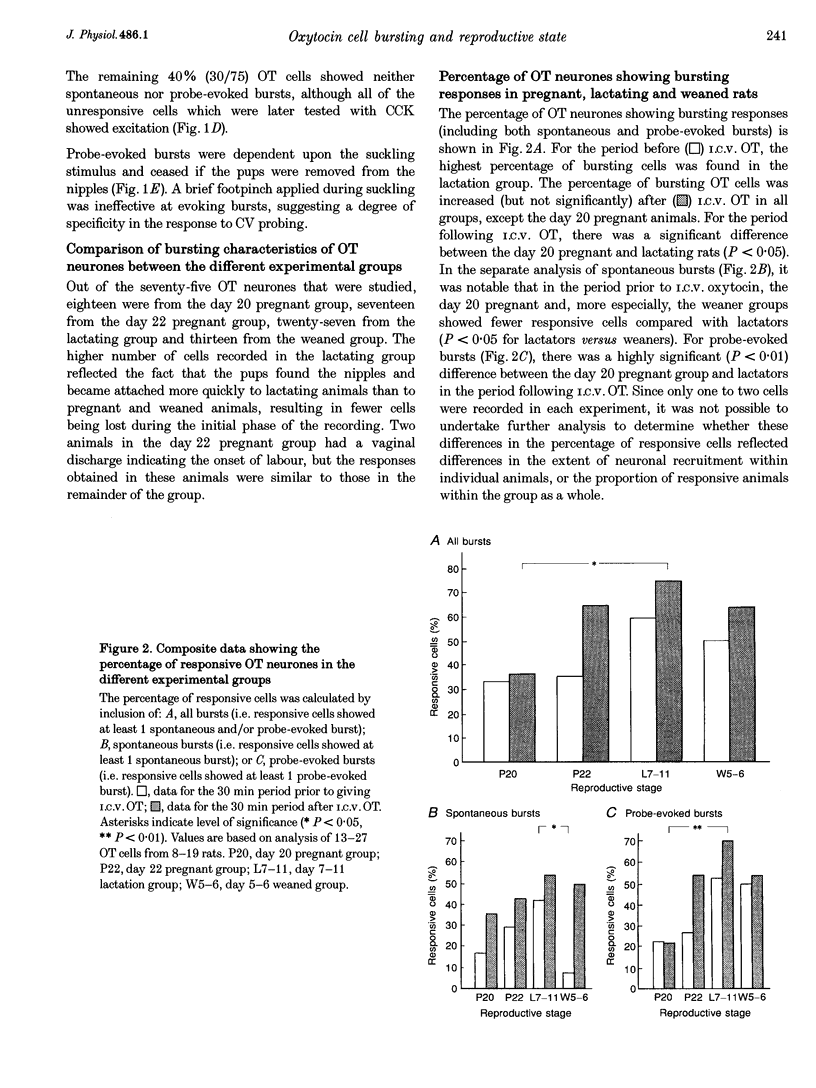
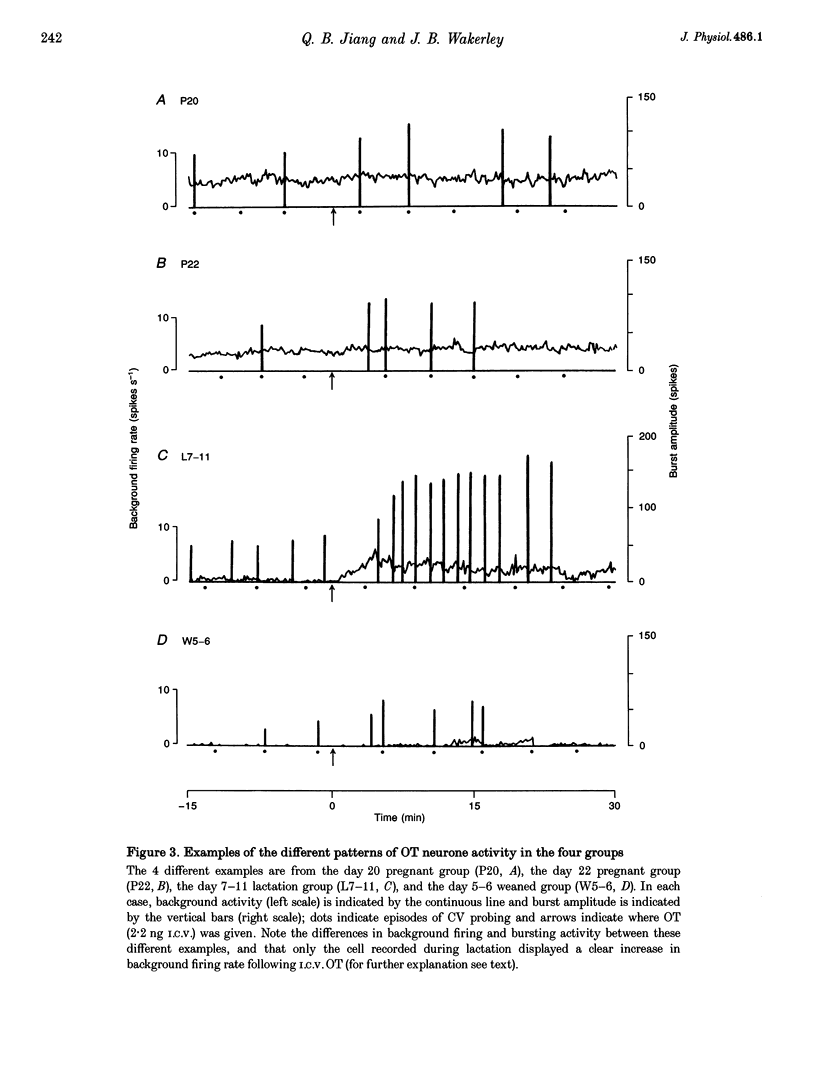
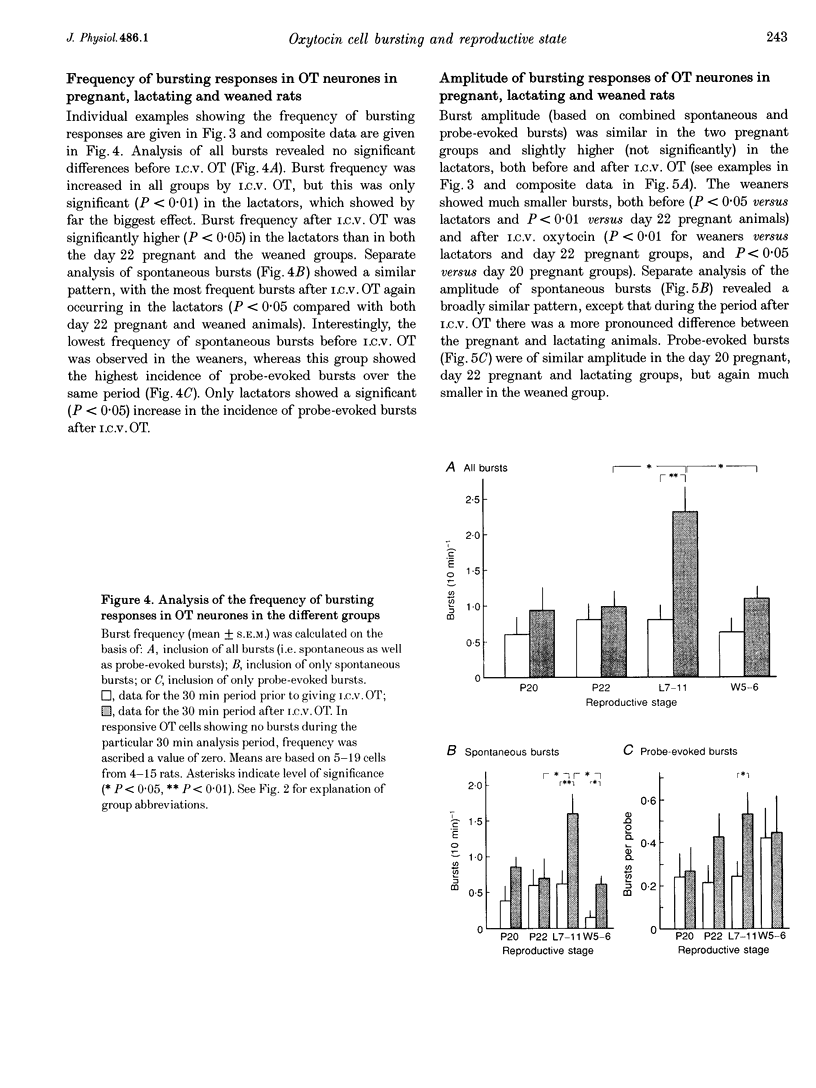
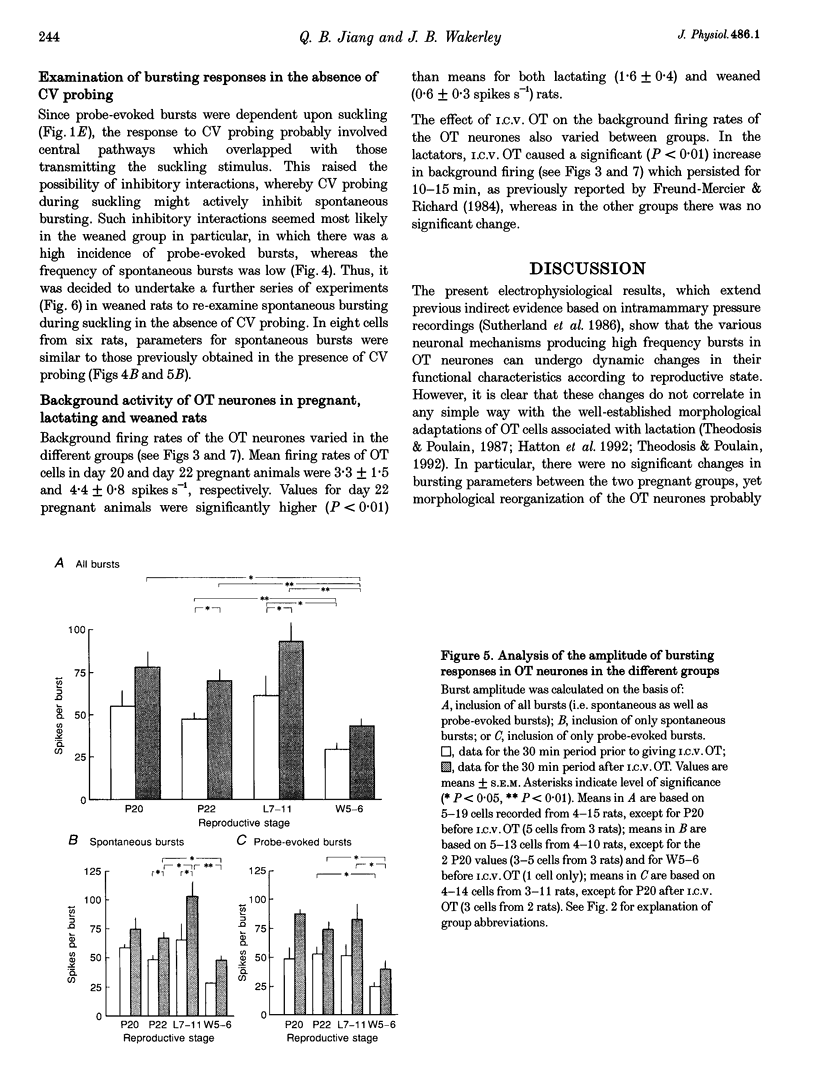
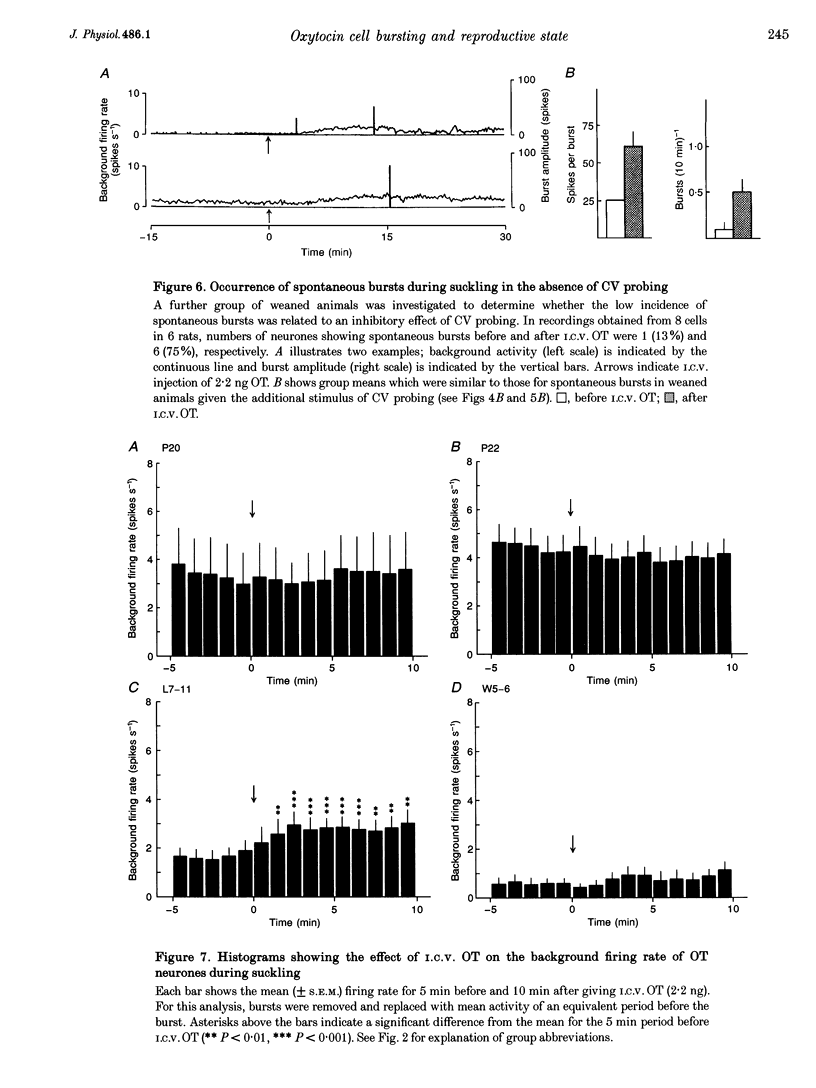
1. Electrophysiological recordings were undertaken to compare bursting characteristics of oxytocin (OT) neurones at four reproductive stages: day 20 pregnancy, day 22 of pregnancy (expected day of parturition), day 7-11 of lactation, and day 5-6 after weaning. 2. Each OT neurone was recorded for 1 h of suckling, combined with cervico-vaginal probing at 5 min intervals as an additional stimulus for bursting. Intracerebroventricular (I.C.V.) oxytocin (2.2 ng) was given after 30 min to facilitate bursting responses. Bursts observed during suckling were classified as 'spontaneous' or 'probe-evoked'. 3. The percentage of cells displaying spontaneous and/or probe-evoked bursts during the recording was low in day 20 pregnant animals, high in lactators and intermediate in day 20 pregnant and weaner groups. These differences may relate to variation in the proportion of animals with a responsive milk-ejection reflex, as well as the relative size of the population of bursting OT neurones. 4. In the period before I.C.V. OT, overall burst frequency (including both spontaneous and probe-evoked bursts) was similar across groups. After I.C.V. OT, overall burst frequency was much higher in lactators compared with other groups. Similar results were obtained when spontaneous bursts were analysed separately. 5. Burst amplitude (action potentials per burst, including both spontaneous and probe-evoked bursts) prior to I.C.V. OT was similar between the day 20 pregnant, day 22 pregnant and lactating groups, but was lower in weaners. All groups showed an increase in burst amplitude after I.C.V. OT, but values in weaners remained lower than in other groups. In a separate analysis of spontaneous bursts, burst amplitude after I.C.V. OT was higher in lactators, and lower in weaners, than in pregnant animals. 6. Background firing rates of OT cells were higher in the day 20 and day 22 pregnant groups compared with lactators, and lower in weaners. Only OT cells in lactators showed a significant increase in background firing rates following I.C.V. OT. 7. It is concluded that the bursting characteristics of OT cells change markedly between late pregnancy, mid-lactation and weaning. The factors underlying these changes, which are only loosely correlated with the sequence of morphological adaptations in OT cells surrounding lactation, remain to be established.
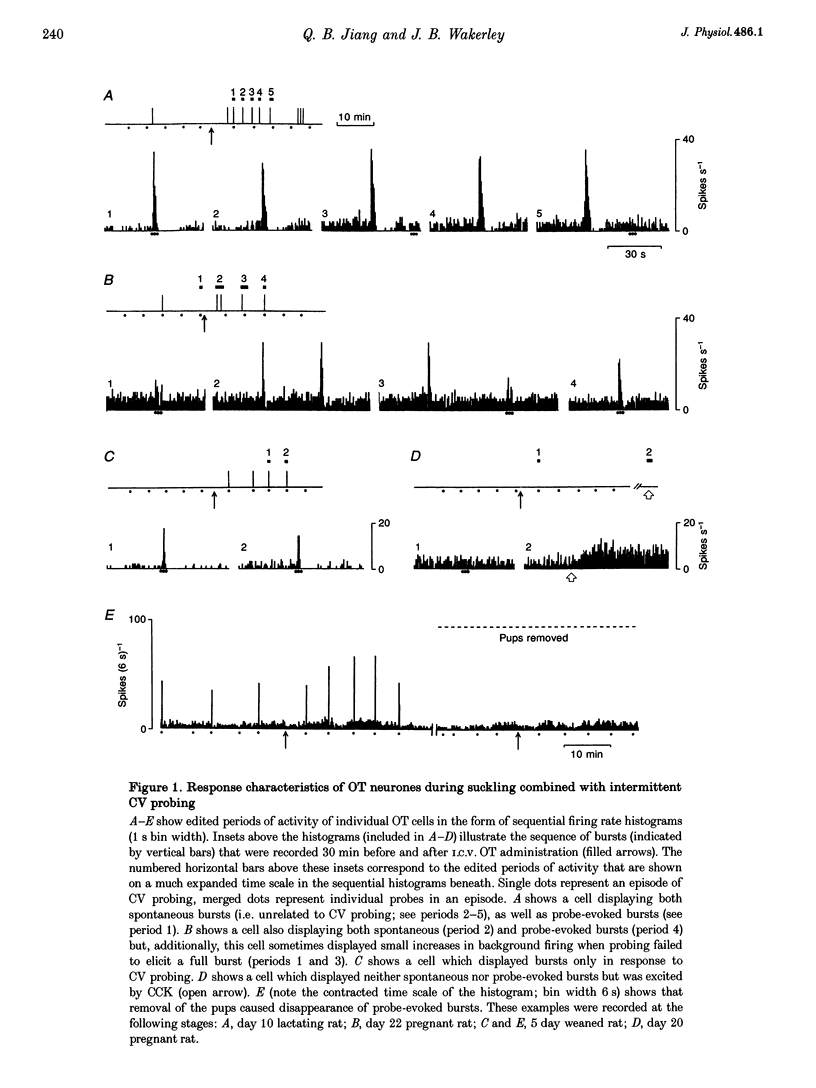
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaishi T., Sakuma Y. Estrogen excites oxytocinergic, but not vasopressinergic cells in the paraventricular nucleus of female rat hypothalamus. Brain Res. 1985 Jun 3;335(2):302–305. doi: 10.1016/0006-8993(85)90481-0. [DOI] [PubMed] [Google Scholar]
- Belin V., Moos F. Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: recruitment and synchronization. J Physiol. 1986 Aug;377:369–390. doi: 10.1113/jphysiol.1986.sp016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimble M. J., Dyball R. E., Forsling M. L. Oxytocin release following osmotic activation of oxytocin neurones in the paraventricular and supraoptic nuclei. J Physiol. 1978 May;278:69–78. doi: 10.1113/jphysiol.1978.sp012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J. A., Poulain D. A. Extracellular K+ in the supraoptic nucleus of the rat during reflex bursting activity by oxytocin neurones. J Physiol. 1991 Aug;439:383–409. doi: 10.1113/jphysiol.1991.sp018672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A. J., Dye S., Leng G., Russell J. A., Bicknell R. J. Endogenous opioid regulation of oxytocin secretion through pregnancy in the rat. J Neuroendocrinol. 1993 Jun;5(3):307–314. doi: 10.1111/j.1365-2826.1993.tb00487.x. [DOI] [PubMed] [Google Scholar]
- Dyball R. E., Leng G. Regulation of the milk ejection reflex in the rat. J Physiol. 1986 Nov;380:239–256. doi: 10.1113/jphysiol.1986.sp016283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984 Jul;352:447–466. doi: 10.1113/jphysiol.1984.sp015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamura M., Leng G., Emson P. C., Kiyama H. Electrical activation and c-fos mRNA expression in rat neurosecretory neurones after systemic administration of cholecystokinin. J Physiol. 1991 Dec;444:51–63. doi: 10.1113/jphysiol.1991.sp018865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton G. I., Modney B. K., Salm A. K. Increases in dendritic bundling and dye coupling of supraoptic neurons after the induction of maternal behavior. Ann N Y Acad Sci. 1992 Jun 12;652:142–155. doi: 10.1111/j.1749-6632.1992.tb34351.x. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Tweedle C. D. Magnocellular neuropeptidergic neurons in hypothalamus: increases in membrane apposition and number of specialized synapses from pregnancy to lactation. Brain Res Bull. 1982 Feb;8(2):197–204. doi: 10.1016/0361-9230(82)90046-6. [DOI] [PubMed] [Google Scholar]
- Higuchi T., Honda K., Fukuoka T., Negoro H., Wakabayashi K. Release of oxytocin during suckling and parturition in the rat. J Endocrinol. 1985 Jun;105(3):339–346. doi: 10.1677/joe.0.1050339. [DOI] [PubMed] [Google Scholar]
- Ingram C. D., Wakerley J. B. Post-partum increase in oxytocin-induced excitation of neurones in the bed nuclei of the stria terminalis in vitro. Brain Res. 1993 Feb 5;602(2):325–330. doi: 10.1016/0006-8993(93)90697-l. [DOI] [PubMed] [Google Scholar]
- Jirikowski G. F., Caldwell J. D., Pilgrim C., Stumpf W. E., Pedersen C. A. Changes in immunostaining for oxytocin in the forebrain of the female rat during late pregnancy, parturition and early lactation. Cell Tissue Res. 1989;256(2):411–417. doi: 10.1007/BF00218899. [DOI] [PubMed] [Google Scholar]
- Leng G., Way S., Dyball R. E. Identification of oxytoxin cells in the rat supraoptic nucleus by their response to cholecystokinin injection. Neurosci Lett. 1991 Jan 28;122(2):159–162. doi: 10.1016/0304-3940(91)90847-m. [DOI] [PubMed] [Google Scholar]
- Luckman S. M., Antonijevic I., Leng G., Dye S., Douglas A. J., Russell J. A., Bicknell R. J. The maintenance of normal parturition in the rat requires neurohypophysial oxytocin. J Neuroendocrinol. 1993 Feb;5(1):7–12. doi: 10.1111/j.1365-2826.1993.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Negoro H., Uchide K., Tadokoro Y., Honda K., Higuchi T. Vaginal distension induces milk ejection-related burst of oxytocin neurones interacting with suckling stimuli in lactating rats. Brain Res. 1987 Feb 24;404(1-2):371–374. doi: 10.1016/0006-8993(87)91397-7. [DOI] [PubMed] [Google Scholar]
- Perlmutter L. S., Tweedle C. D., Hatton G. I. Neuronal/glial plasticity in the supraoptic dendritic zone: dendritic bundling and double synapse formation at parturition. Neuroscience. 1984 Nov;13(3):769–779. doi: 10.1016/0306-4522(84)90095-2. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B., Dyball R. E. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B Biol Sci. 1977 Apr;196(1125):367–384. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Riggs C. M., Sutherland R. C., Wakerley J. B. Reappraisal of the influence of mammary distension on the frequency of milk ejection in the rat. J Endocrinol. 1985 Apr;105(1):127–132. doi: 10.1677/joe.0.1050127. [DOI] [PubMed] [Google Scholar]
- Summerlee A. J. Extracellular recordings from oxytocin neurones during the expulsive phase of birth in unanaesthetized rats. J Physiol. 1981 Dec;321:1–9. doi: 10.1113/jphysiol.1981.sp013967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. C., Aizlewood E. S., Wakerley J. B. Changing characteristics of the milk-ejection reflex during pregnancy, lactation and after weaning in the rat. J Reprod Fertil. 1986 Jan;76(1):123–130. doi: 10.1530/jrf.0.0760123. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Poulain D. A., Vincent J. D. Possible morphological bases for synchronisation of neuronal firing in the rat supraoptic nucleus during lactation. Neuroscience. 1981;6(5):919–929. doi: 10.1016/0306-4522(81)90173-1. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Clarke G., Dreifuss J. J., Lincoln D. W. The role of central adrenergic receptors in the reflex release of oxytocin. Brain Res. 1978 Feb 17;142(1):69–84. doi: 10.1016/0006-8993(78)90177-4. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Lincoln D. W. The milk-ejection reflex of the rat: a 20- to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. J Endocrinol. 1973 Jun;57(3):477–493. doi: 10.1677/joe.0.0570477. [DOI] [PubMed] [Google Scholar]
- Way S. A., Leng G. Relaxin increases the firing rate of supraoptic neurones and increases oxytocin secretion in the rat. J Endocrinol. 1992 Jan;132(1):149–158. doi: 10.1677/joe.0.1320149. [DOI] [PubMed] [Google Scholar]