Abstract
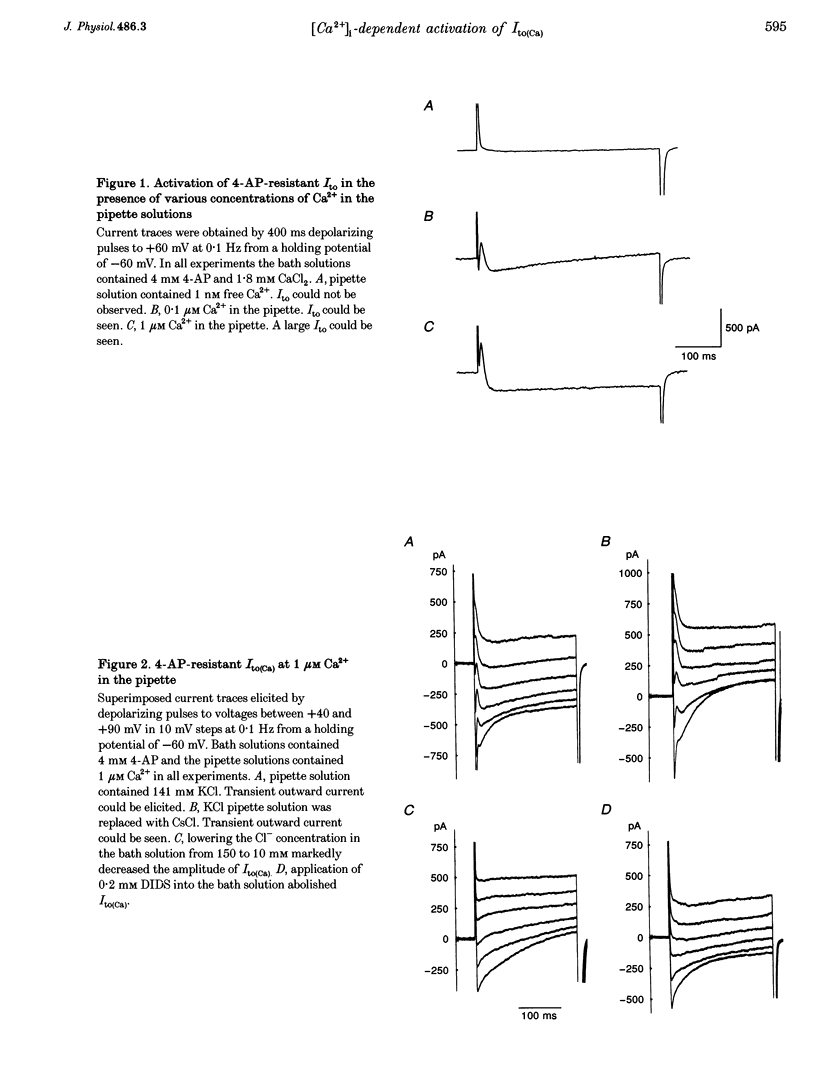
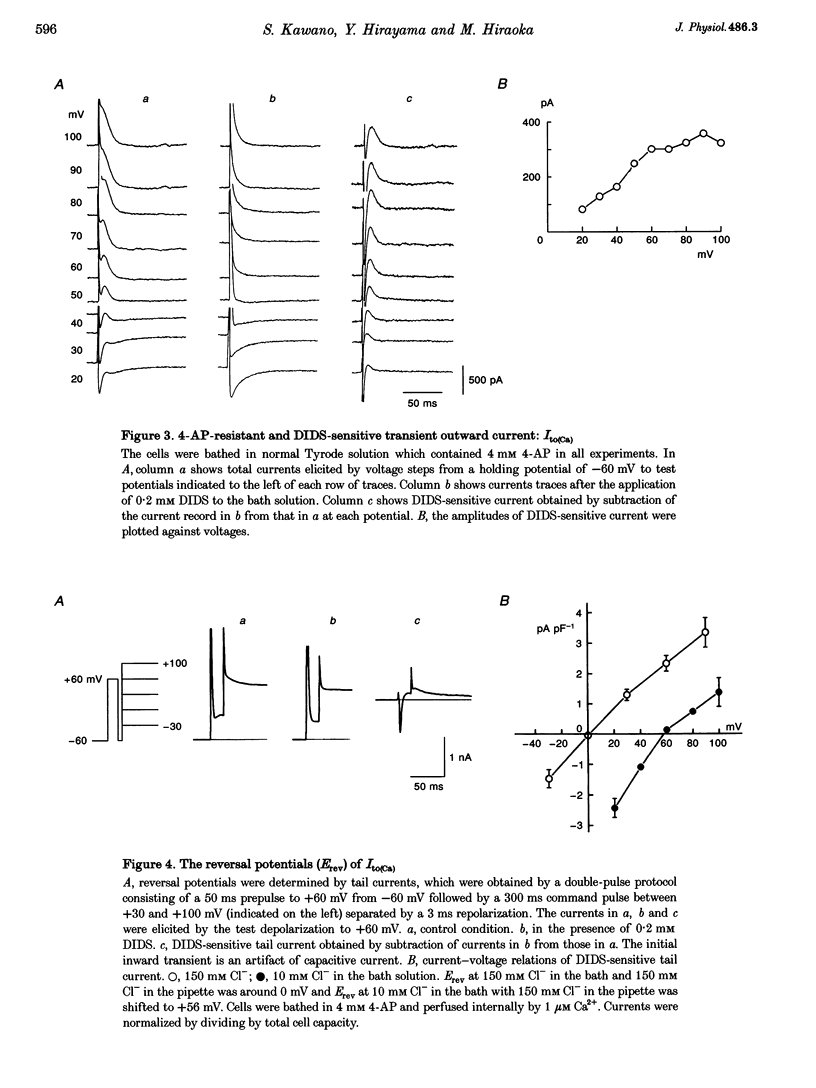
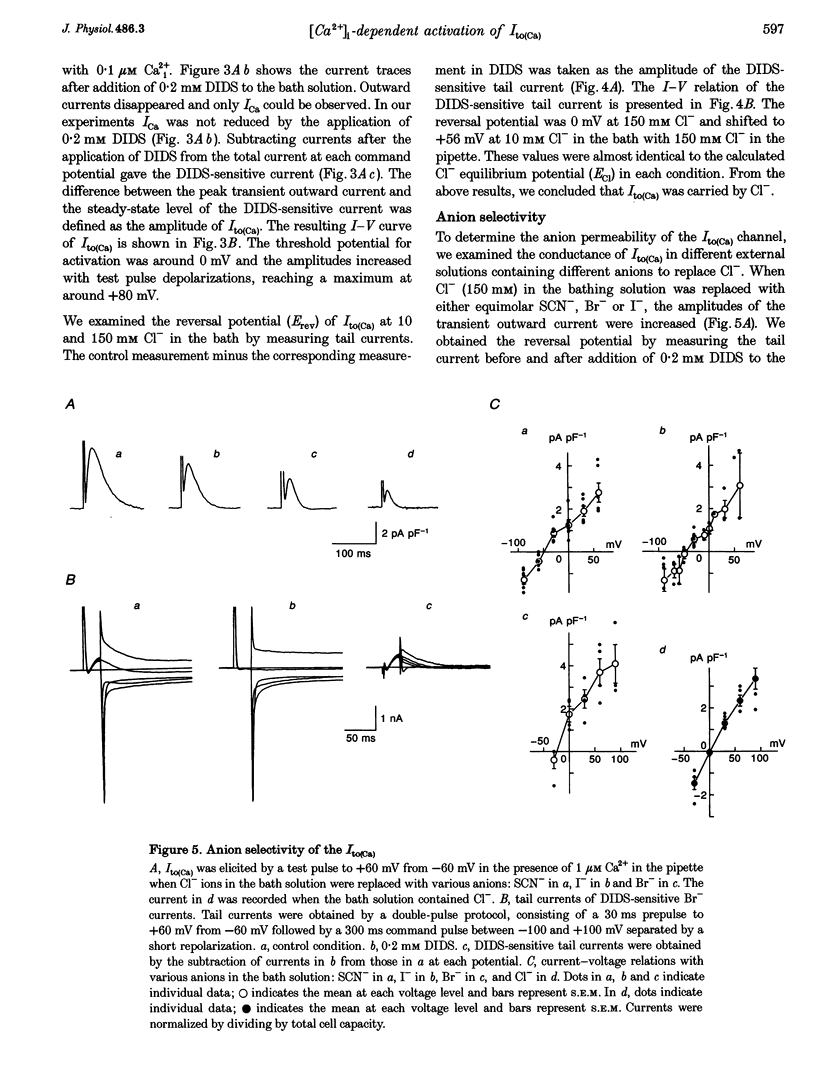
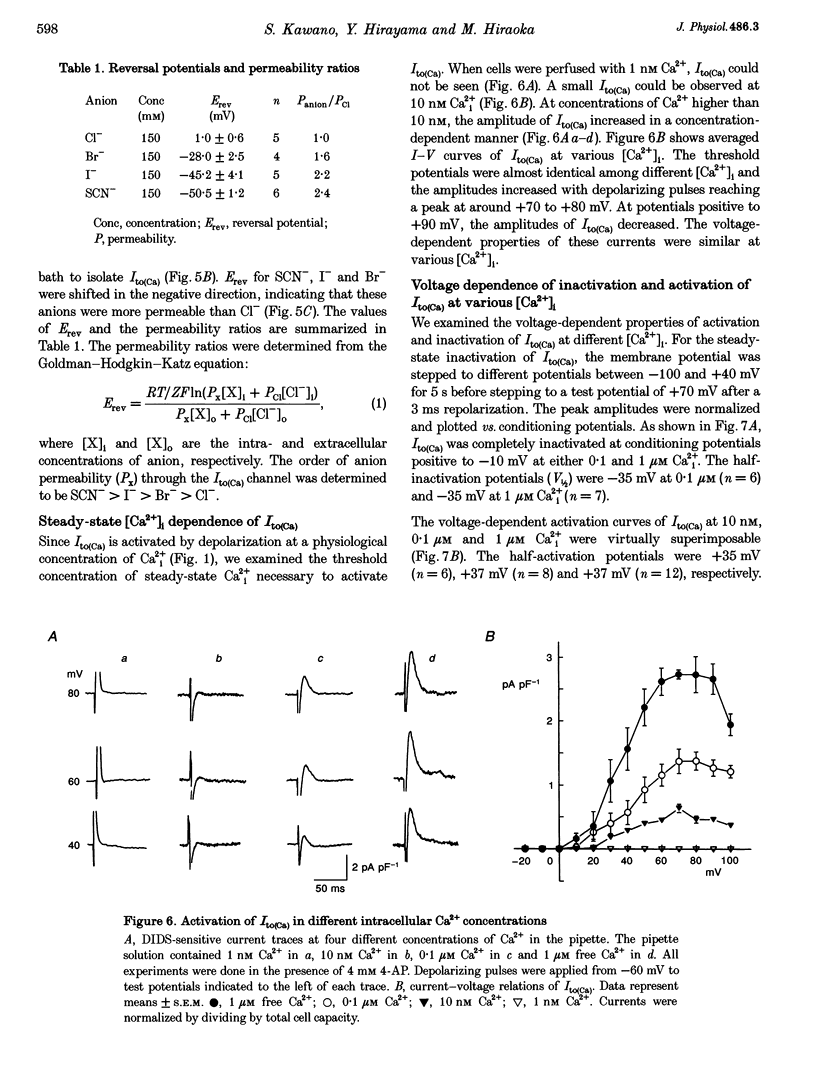
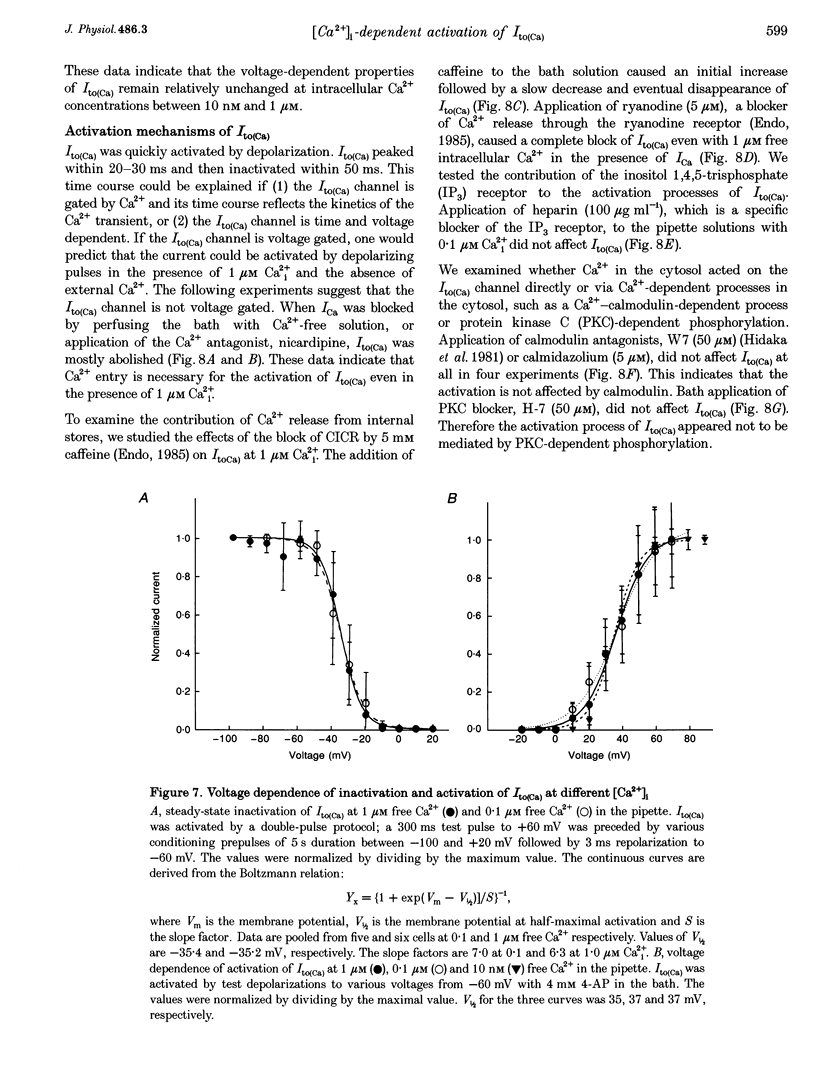
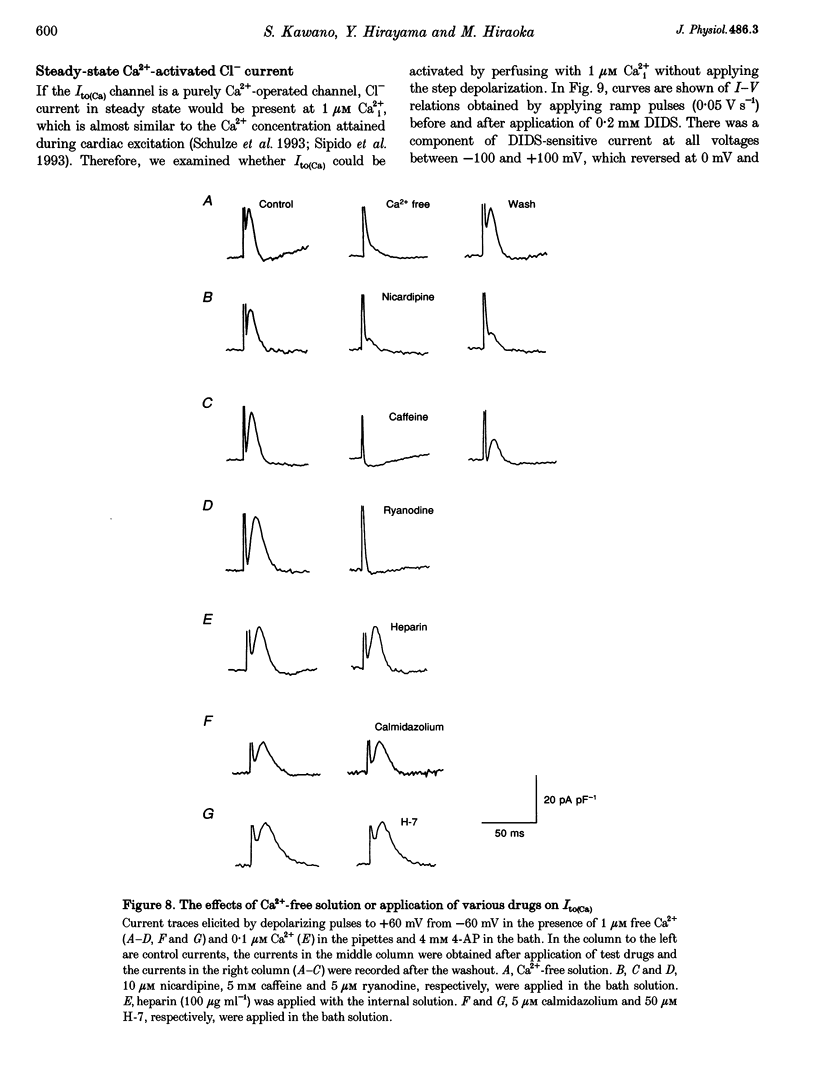
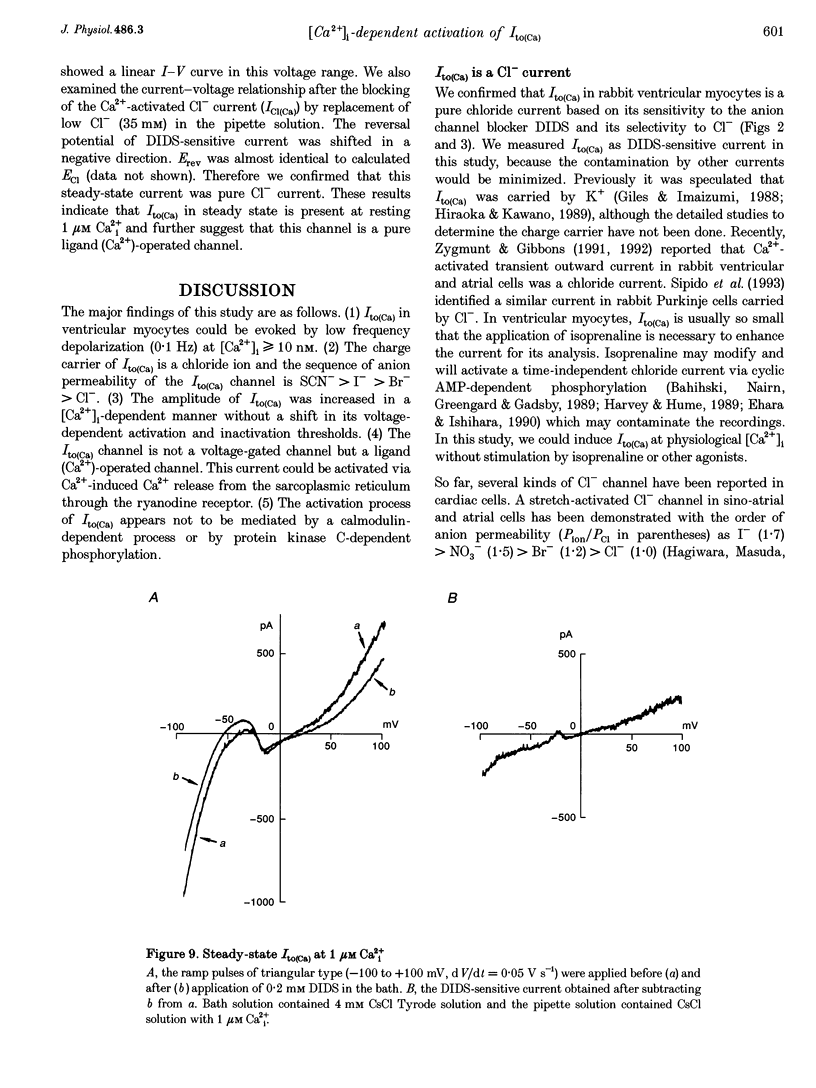
1. The mechanism of activation of the Ca(2+)-sensitive and 4-aminopyridine (4-AP)-insensitive transient outward current, I(to)(Ca), was examined in single rabbit ventricular myocytes using the whole-cell patch-clamp technique. 2. When the steady-state intracellular Ca2+ (Ca2+i) concentration ([Ca2+]i) was < 1 nM, I(to)(Ca) could not be activated by applying pulses at 0.1 Hz. When [Ca2+]i was increased to > or = 10 nM, I(to)(Ca) was activated by 0.1 Hz depolarizing pulses in all control experiments. 3. I(to)(Ca) was completely blocked by an anion transport blocker, DIDS, or by replacement of NaCl with sodium aspartate. Upon changing extracellular [Cl-], the reversal potential was shifted as predicted for a chloride-selective conductance. When intracellular K+ was replaced with Cs+, I(to)(Ca) was also observed. From these results it was concluded that I(to)(Ca) was carried by Cl-. 4. Anion selectivity of I(to)(Ca) was investigated by the replacement of C.- with various anions. The sequence of permeability was SCN- > I- > Br- > Cl-. 5. The amplitude of I(to)(Ca) was enhanced in a [Ca2+]i-dependent manner between 10 nM and 1 microM Ca2+i, while steady-state inactivation curves and the voltage-dependent activation curves were unchanged. The half-inactivation and half-activation potentials were -35 mV and +37 mV, respectively, at all [Ca2+]i. 6. I(to)(Ca) was inhibited by blocking Ca2+ influx or Ca2+ release from sarcoplasmic reticulum, suggesting that a 'Ca(2+)-induced Ca(2+)-release' mechanism is essential for the activation of I(to)(Ca). 7. A steady-state Ca(2+)-activated Cl- current with a linear I-V relationship was observed at 1 microM Ca2+, while the current activated by depolarization was strictly dependent on Ca2+ entry or Ca2+ release from the sarcoplasmic reticulum. These results suggest that the I(to)(Ca) channel is purely ligand (Ca2+) gated and its time course reflects the concentration of Ca2+i.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Wier W. G. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ Res. 1987 Jul;61(1):148–154. doi: 10.1161/01.res.61.1.148. [DOI] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Action of noradrenaline on single smooth muscle cells freshly dispersed from the rat anococcygeus muscle. J Physiol. 1987 Aug;389:513–525. doi: 10.1113/jphysiol.1987.sp016669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Coronado R., Latorre R. Detection of K+ and Cl-channels from calf cardiac sarcolemma in planar lipid bilayer membranes. Nature. 1982 Aug 26;298(5877):849–852. doi: 10.1038/298849a0. [DOI] [PubMed] [Google Scholar]
- EISENMAN G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962 Mar;2(2 Pt 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehara T., Ishihara K. Anion channels activated by adrenaline in cardiac myocytes. Nature. 1990 Sep 20;347(6290):284–286. doi: 10.1038/347284a0. [DOI] [PubMed] [Google Scholar]
- Escande D., Coulombe A., Faivre J. F., Deroubaix E., Coraboeuf E. Two types of transient outward currents in adult human atrial cells. Am J Physiol. 1987 Jan;252(1 Pt 2):H142–H148. doi: 10.1152/ajpheart.1987.252.1.H142. [DOI] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Masuda H., Shoda M., Irisawa H. Stretch-activated anion currents of rabbit cardiac myocytes. J Physiol. 1992 Oct;456:285–302. doi: 10.1113/jphysiol.1992.sp019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Sasaki Y., Tanaka T., Endo T., Ohno S., Fujii Y., Nagata T. N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4354–4357. doi: 10.1073/pnas.78.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Calcium-sensitive and insensitive transient outward current in rabbit ventricular myocytes. J Physiol. 1989 Mar;410:187–212. doi: 10.1113/jphysiol.1989.sp017528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Augustine G. J. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990 Dec 20;348(6303):735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- Kawano S., Hiraoka M. Transient outward currents and action potential alterations in rabbit ventricular myocytes. J Mol Cell Cardiol. 1991 Jun;23(6):681–693. doi: 10.1016/0022-2828(91)90978-u. [DOI] [PubMed] [Google Scholar]
- Kenyon J. L., Sutko J. L. Calcium- and voltage-activated plateau currents of cardiac Purkinje fibers. J Gen Physiol. 1987 Jun;89(6):921–958. doi: 10.1085/jgp.89.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marunaka Y., Eaton D. C. Effects of insulin and phosphatase on a Ca2(+)-dependent Cl- channel in a distal nephron cell line (A6). J Gen Physiol. 1990 May;95(5):773–789. doi: 10.1085/jgp.95.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Morad M. A transient outward current related to calcium release and development of tension in elephant seal atrial fibres. J Physiol. 1984 Dec;357:267–292. doi: 10.1113/jphysiol.1984.sp015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. A Ca-dependent Cl- conductance in cultured mouse spinal neurones. Nature. 1984 Oct 11;311(5986):567–570. doi: 10.1038/311567a0. [DOI] [PubMed] [Google Scholar]
- Schulze D., Kofuji P., Hadley R., Kirby M. S., Kieval R. S., Doering A., Niggli E., Lederer W. J. Sodium/calcium exchanger in heart muscle: molecular biology, cellular function, and its special role in excitation-contraction coupling. Cardiovasc Res. 1993 Oct;27(10):1726–1734. doi: 10.1093/cvr/27.10.1726. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido K. R., Callewaert G., Carmeliet E. [Ca2+]i transients and [Ca2+]i-dependent chloride current in single Purkinje cells from rabbit heart. J Physiol. 1993 Aug;468:641–667. doi: 10.1113/jphysiol.1993.sp019793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Neher E., Sakmann B. Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5063–5067. doi: 10.1073/pnas.84.14.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Calcium-activated chloride current in rabbit ventricular myocytes. Circ Res. 1991 Feb;68(2):424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Properties of the calcium-activated chloride current in heart. J Gen Physiol. 1992 Mar;99(3):391–414. doi: 10.1085/jgp.99.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]


