Abstract
Background
Colorectal cancer (CRC) incidence rates have been decreasing in the United States (US), but there is limited information about differences in these improvements among individuals from different racial and ethnic subgroups across different regions of the US.
Methods
Data from the National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology, and End Results (SEER) databases were used to examine trends in CRC incidence from 2001 to 2020 using a population-based retrospective cohort study. We obtained annual estimates of CRC incidence and used meta-regression analyses via weighted linear models to identify main effects and interactions that explained differences in CRC incidence trends among groups defined by race/ethnicity and US region while also considering CRC stage and sex. To summarize overall trends over time in incidence rates for specific racial and ethnic groups within and across US regions, we obtained average annual percentage change (AAPC) estimates.
Results
The greatest differences in CRC incidence trends were among groups defined by race/ethnicity and US region. Non-Hispanic Black (NHB) persons had the largest declines in CRC incidence, with AAPC estimates ranging from −2.27 (95% CI: −2.49 to −2.06) in the South to −3.03 (95% CI: −3.59 to −2.47) in the West, but had higher-than-average incidence rates at study end. The AAPC estimate for American Indian/Alaska Native (AIAN) persons suggested no significant change over time (AAPC: −0.41, 95% CI: −2.51 to 1.73).
Conclusion
CRC incidence trends differ among racial/ethnic groups residing in different US regions. Notably, CRC incidence rates have not changed noticeably for AIAN persons from 2001-2020. These findings highlight the importance of reinvigorating collaborative efforts to develop geographic and population-specific screening and preventative approaches to reduce the CRC burden experienced by Native American communities and members of other minoritized groups.
Keywords: colorectal cancer, incidence, trends, minority health, disparities
Plain language summary
Colorectal cancer (CRC) incidence rates have been decreasing in the United States (US), but there is limited information about differences in these improvements among individuals from different racial and ethnic subgroups across different regions of the US. Data from the National Program of Cancer Registries from 2001-2020 were used to compare trends in CRC incidence rates among racial/ethnic groups, US regions, and other characteristics. Non-Hispanic Black (NHB) individuals displayed the largest reductions in CRC incidence, but their incidence rates remained higher than the national average at the end of the study study period. Incidence rates decreased significantly for all racial/ethnic groups, except for American Indian/Alaska Native individuals, whose incidence rates were essentially constant over time. These findings highlight the importance of reinvigorating collaborative efforts to develop geographic and population-specific screening and preventative approaches to reduce the CRC burden experienced by minoritized groups.
Introduction
Colorectal cancer (CRC) has the third highest incidence of all cancers in the United States (US), and it results in the third and fourth highest number of cancer deaths in men and women, respectively.1-4 Recently, it has been noted that CRC incidence has been trending higher for younger persons, and this has led to the recent recommendation that CRC screening begin at age 45 rather than at age 50.5,6 In spite of this concerning development, overall CRC incidence rates have been trending downwards over the past decades.2,7-11
In the US, differences in incidence rates have been noted among persons of different racial and ethnic groups, and in particular, evidence suggests that non-Hispanic Black (NHB) and American Indian/Alaska Native (AIAN) populations have historically experienced the highest CRC incidence.12-14 In addition to reports of differences in incidence rates overall, there is also evidence suggesting that trends in incidence differ among racial and ethnic groups in the US.8,10,12,15,16 Drivers of these differences can be attributed to racial and ethnic group-level differences encountered throughout the CRC clinical care continuum, beginning with risk factors and prevention, but also continuing through diagnosis, treatment, and surveillance. 17 Socioeconomic disadvantages such as low income and low education, which have disproportionately impacted NHB and AIAN populations historically, resulted in higher exposure to CRC risk factors for CRC including high-fat diets, tobacco and alcohol use, obesity and diabetes.18,19 NHB and AIAN persons are also less likely than NHW persons to have ever been screened for CRC. 20 Even among those who are screened, NHB populations are less likely than NHW populations to receive screening colonoscopy, which removes precancerous polyps. 21 Similarly, due to poor access to endoscopy services for many AIAN persons who reside in rural areas, this population relies more heavily on mailed fecal immunochemical tests (FITs) and may struggle to access follow-up diagnostic colonoscopy.22,23
In spite of these reports, much remains unknown about differences in CRC incidence trends among population subgroups. Not only is limited information published that compares and contrasts CRC incidence trends among multiple racial and ethnic groups, but much that is reported also fails to differentiate among different regions of the US, something that has been shown to be important, particularly for AIAN populations.12,15
Understanding CRC incidence trends within subgroups of the population defined by different demographic characteristics such as race/ethnicity and gender, and across regions of the US, can provide a comprehensive picture of the distribution of CRC burden. Such information can help highlight priorities for CRC prevention and control research and programmatic efforts. Therefore, our primary purpose is to estimate and compare CRC incidence trends for individuals from distinct racial/ethnic groups across regions of the US while accounting for differences arising due to sex and age. In this effort, we focus on data from the past two decades, 2001 through 2020, and estimate separate trends according to summary stage of CRC at diagnosis: localized/non-metastatic or regional metastases only vs distant metastasis. Our hypotheses were that distinct CRC incidence trends would be apparent for individuals from different racial/ethnic groups, and these trends might differ according to the region of the US in which individuals reside.
Methods
This study was based on data from United States Cancer Statistics (USCS) for the period 2001 through 2020. USCS data were accessed from the National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program SEER*Stat Database: NPCR and SEER Incidence – U.S. Cancer Statistics 2001-2020 Public Use Research Database, 2022 submission (2001-2020). 24 Data are from retrospective population-based registries that participate in CDC’s National Program of Cancer Registries and/or NCI’s Surveillance, Epidemiology, and End Results Program and meet high-quality data criteria. These registries cover approximately 98% of the U.S. population, 25 and data are available for download in SEER*Stat v8.4.2. This project, Study ID: 21-102, was reviewed by the University of New Mexico Health Sciences Institutional Review Board, and was granted exemption according to Category 4: Secondary research on data or specimens (no consent required). Furthermore, the current report complies to RECORD guidelines for SEER/USCS studies. 26 Although this study received financial support from the National Cancer Institute of the United States, the funders played no role in the work (Supplemental Material).
We extracted information about incident CRC cases reported in individuals ages 20 and older for the stated time period from the data source described above. CRC cases were defined according to their locations in SEER*Stat with the codes C18.0 through C18.9 and C19.9 and C20.9. As recommended, 27 we extracted incidence estimates using only those cancer registries that provided appropriate data in each year from 2001 through 2020 using the “R0120” indicator in SEER*Stat. The demographic groups of primary interest in this analysis were: US regions (Midwest, Northeast, South, West), race/ethnicity (SEER/USCS labels are non-Hispanic American Indian/Alaska Native [AIAN], non-Hispanic Asian/Pacific Islander [API], Hispanic of any race [Hispanic], non-Hispanic Black [NHB], and non-Hispanic White [NHW]), and sex (female and male). We excluded individuals of unknown race from analysis. Because trends in age at onset have been explored recently from this data source, we did not focus on this important demographic grouping. 28 Even so, we extracted data for three age groups (20-49, 50-64, 65+) for summary purposes. We obtained counts of incident CRC cases, classified according to their overall summary stage, within each demographic group by year of diagnosis (2001-2020). As summary stage definitions changed markedly after 2017, particularly with respect to localized vs regional CRC definitions, 29 we extracted only two categories of summary stage – distant vs localized or regional – to enable stable assessments of trends over time. In addition to obtaining total counts within groups defined separately according to the levels of each demographic variable, we used the tools incorporated into SEER*Stat to obtain age-adjusted estimates of CRC incidence (events per 100 000 individuals), within groups of individuals simultaneously defined by combinations of the demographic, CRC, and year variables. Age adjustments were accomplished according to 19 age groups and standardized to the US Census P25-1130. 30 We obtained 95% confidence intervals using the Tiwari modification. 31
In our primary analyses, we used the SEER-estimated age-adjusted incidence rates, and their standard errors, within subgroups of individuals simultaneously defined by the categories of the factors of interest: US region, race/ethnicity, sex, distant vs localized or regional summary stage, and year at diagnosis. Some of these combinations resulted in small enough groups that fewer than 16 incident CRC cases were observed. Because of this, we obtained estimates of CRC incidence rates while pooling across years, in 2-, 3-, 4-, 5-, and 7-year intervals. We then used the estimates obtained from combined years to impute incidence rates successively, along with standard errors that reflected the added uncertainty due to the prediction values. We used 7-year estimates to impute suppressed incidence rates from the 5-year data, the 5-year estimates to impute suppressed 4-year incidence rates, and so on until annual incidence rates were available for all combinations of the factors of interest.
Using the natural logarithm-transformed estimates of incidence rates from all the possible combinations of the different factors listed above, and the inverse of their variances as analysis weights, we performed meta-regression analyses via linear models to identify combinations of factors associated with differences in CRC incidence trends over time. In this analysis, we included year of CRC diagnosis using natural cubic splines with knots placed at 2001, 2005.75, 2010.5, 2015.25, and 2020 to estimate CRC incident trends without making parametric assumptions about their form.32,33 We selected a model by minimizing Akaike’s Information Criterion (AIC) in a step-by-step process. In the first step, we fit a main-effects-only model, and subsequently fit additional models that included all possible interactions for interaction terms of higher and higher order. Once the AIC no longer was lower after including the next higher set of interactions, we initiated the second step. In this step, we added single interactions of one higher order than the all-interactions model with the lowest AIC. We selected interactions according to their impact on the model AIC, and continued to add these interactions until the AIC was not improved with the addition of the next most important interaction.
The interactions included in the final meta-regression model defined combinations of the factors of interest which explained important differences in CRC incidence, and in CRC incidence trends. To interpret the differences suggested by these interactions we returned to SEER*Stat and extracted age-adjusted incidence rates and standard errors within the combinations of the factors of interest identified in the interaction. We chose to obtain these marginal data summaries to avoid the need to make specific comparisons that might have been based on imputed annual incidence rates. We summarized these CRC incidence estimates, including the indicated CRC incidence trends over time, and compared them among those combinations of factors. Using the results of these models, we obtained estimates of incidence rate ratios, and their confidence intervals, to make specific between-group comparisons. We summarized the average trends over the study time period with estimates of average annual percentage change (AAPC) within specific subgroups. We obtained these estimates via linear regression models with log-transformed incidence rates as the outcome variable and year of diagnosis as the predictor variable. Exponentiating and scaling the resulting regression coefficients and confidence limits provided the estimates corresponding to AAPC. In addition to estimating AAPC estimates, we also obtained between-group incidence rate ratios using estimates from the meta-regression regression model. Analyses were performed in SAS (v9.4) and R (v4.2.3).
Results
This study reports on CRC incident trends from more than 2.5 million incident cases of CRC from 2001 to 2020 in the US, as contained in the USCS. Over 75% of these cases occurred among individuals identified as being NHW, with more than 10% occurring among those identified as NHB. This data resource reported more than 130 000 incident cases for each of the first five years of this time interval. The lowest counts of incident cases were reported between 2010 and 2013, where there were approximately 120 000 incident cases per year. These incident counts occurred during a time period of increasing population size, and this is reflected in decreases in age- and population-adjusted incidence rates over the course of this study (Table 1). After accounting for differences in population sizes and age distributions, the rates in Table 1 suggest that CRC incidence was consistently highest among NHB persons and lowest for API persons between 2001 and 2020.
Table 1.
Colorectal Cancer Incidence Rates From 2001-2020.
| AIAN | API | Hispanic | NHB | NHW | ||
|---|---|---|---|---|---|---|
| Rate (95%CI) | Rate (95%CI) | Rate (95%CI) | Rate (95%CI) | Rate (95%CI) | ||
| Total | 37.1 (36.5-37.8) | 30.5 (30.3-30.7) | 33.1 (32.9-33.2) | 45.0 (44.9-45.2) | 39.6 (39.5-39.6) | |
| Sex | Female | 33.5 (32.7-34.3) | 26.4 (26.1-26.6) | 27.9 (27.7-28.0) | 39.6 (39.4-39.9) | 34.5 (34.4-34.6) |
| Male | 41.5 (40.5-42.5) | 35.8 (35.5-36.1) | 39.7 (39.5-40.0) | 52.8 (52.5-53.1) | 45.5 (45.4-45.6) | |
| Age | <50y | 7.7 (7.4-8.0) | 5.6 (5.5-5.7) | 5.5 (5.4-5.6) | 8.0 (7.9-8.1) | 7.2 (7.2-7.3) |
| 50-64y | 69.8 (68.0-71.7) | 57.5 (56.9-58.2) | 60.4 (60-60.9) | 90.3 (89.8-90.9) | 67.4 (67.2-67.6) | |
| 65+y | 167.0 (162.9-171.1) | 141.1 (139.7-142.4) | 158.6 (157.6-159.7) | 203.3 (202.2-204.3) | 191.6 (191.3-192) | |
| Stage | Localized or Regional | 28.5 (28.0-29.1) | 24.3 (24.1-24.5) | 25.7 (25.6-25.9) | 33.5 (33.3-33.6) | 31.4 (31.3-31.4) |
| Distant | 8.6 (8.3-8.9) | 6.3 (6.2-6.4) | 7.4 (7.3-7.4) | 11.6 (11.5-11.6) | 8.2 (8.2-8.2) | |
| Region | Northeast | 21.6 (19.8-23.4) | 28.8 (28.3-29.2) | 36.5 (36.1-36.9) | 43.1 (42.7-43.5) | 41.8 (41.6-41.9) |
| Midwest | 39.4 (37.6-41.3) | 27.8 (27.1-28.5) | 32.5 (31.8-33.1) | 47.8 (47.4-48.2) | 41.3 (41.1-41.4) | |
| South | 35.4 (34.4-36.4) | 24.4 (23.9-24.8) | 32.4 (32.2-32.7) | 45.0 (44.8-45.3) | 39.1 (39.0-39.2) | |
| West | 40.5 (39.6-41.5) | 33.8 (33.5-34.1) | 32.5 (32.2-32.7) | 43.7 (43.1-44.2) | 36.2 (36.0-36.3) | |
| Year | 2001 | 39.2 (35.7-42.8) | 39.6 (38.1-41.0) | 40.2 (39.2-41.2) | 54.5 (53.5-55.4) | 50.2 (49.9-50.5) |
| 2002 | 38.7 (35.3-42.4) | 39.4 (38.1-40.8) | 39.0 (38.1-40.0) | 54.9 (54.0-55.8) | 49.1 (48.8-49.4) | |
| 2003 | 40.7 (37.3-44.4) | 37.6 (36.3-38.9) | 39.1 (38.2-40.1) | 55.4 (54.5-56.4) | 48.2 (47.9-48.5) | |
| 2004 | 38.0 (34.7-41.4) | 37.1 (35.9-38.4) | 40.1 (39.2-41.0) | 54.9 (54.0-55.8) | 47.3 (47.0-47.6) | |
| 2005 | 37.7 (34.6-41.1) | 36.4 (35.2-37.6) | 38.7 (37.8-39.5) | 53.6 (52.7-54.5) | 46.1 (45.8-46.3) | |
| 2006 | 39.8 (36.5-43.2) | 35.7 (34.6-36.9) | 38.7 (37.9-39.6) | 52.7 (51.8-53.6) | 44.5 (44.2-44.8) | |
| 2007 | 41.6 (38.3-45.0) | 34.9 (33.8-36.0) | 37.5 (36.7-38.3) | 51.3 (50.5-52.2) | 43.7 (43.4-44.0) | |
| 2008 | 38.8 (35.7-42.1) | 34.9 (33.8-36.0) | 36.8 (36.0-37.6) | 50.6 (49.8-51.4) | 42.4 (42.1-42.7) | |
| 2009 | 38.0 (35.1-41.1) | 33.1 (32.1-34.1) | 35.6 (34.9-36.4) | 48.7 (47.9-49.5) | 40.3 (40.0-40.5) | |
| 2010 | 38.5 (35.6-41.5) | 32.6 (31.6-33.6) | 33.3 (32.6-34.1) | 46.6 (45.8-47.4) | 38.6 (38.3-38.8) | |
| 2011 | 37.9 (35.1-40.9) | 31.7 (30.8-32.7) | 33.4 (32.7-34.2) | 44.8 (44.1-45.6) | 37.8 (37.6-38.1) | |
| 2012 | 36.3 (33.6-39.1) | 29.9 (29.0-30.8) | 31.9 (31.2-32.5) | 43.6 (42.8-44.3) | 37.0 (36.8-37.3) | |
| 2013 | 37.6 (34.9-40.5) | 29.4 (28.5-30.2) | 31.8 (31.2-32.4) | 43.2 (42.5-43.9) | 36.5 (36.3-36.8) | |
| 2014 | 36.7 (34.1-39.4) | 29.1 (28.3-30.0) | 31.9 (31.3-32.5) | 42.4 (41.7-43.1) | 36.6 (36.3-36.8) | |
| 2015 | 39.6 (37.0-42.4) | 28.3 (27.5-29.1) | 31.7 (31.1-32.3) | 41.5 (40.9-42.2) | 36.5 (36.2-36.7) | |
| 2016 | 40.6 (38.0-43.4) | 28.4 (27.7-29.2) | 30.9 (30.3-31.5) | 40.0 (39.4-40.7) | 35.8 (35.6-36.1) | |
| 2017 | 38.6 (36.1-41.2) | 27.5 (26.7-28.2) | 30.7 (30.2-31.3) | 38.4 (37.8-39.1) | 35.2 (34.9-35.4) | |
| 2018 | 35.8 (33.4-38.3) | 26.8 (26.1-27.6) | 30.2 (29.6-30.7) | 38.5 (37.9-39.2) | 34.8 (34.6-35.0) | |
| 2019 | 34.4 (32.1-36.8) | 26.8 (26.1-27.5) | 29.6 (29.1-30.1) | 37.9 (37.3-38.5) | 34.8 (34.5-35.0) | |
| 2020 | 31.7 (29.5-34.0) | 22.7 (22.1-23.4) | 25.6 (25.1-26.1) | 32.6 (32.0-33.2) | 30.9 (30.7-31.1) |
Notes. Rates are per 100 000 and age-adjusted to the 2000 US Std Population (19 age groups - Census P25-1130) standard; Confidence intervals (Tiwari 2006 mod) are 95% for rates.
Abbreviations: AIAN: Non-Hispanic American Indian or Alaska Native; API: Non-Hispanic Asian or Pacific Islander; Hispanic: Hispanic of any race; NHB: Non-Hispanic Black; NHW: Non-Hispanic White.
Midwest US region includes Ohio, Indiana, Illinois, Michigan, Wisconsin, Minnesota, Iowa, Missouri, North Dakota, South Dakota, Nebraska, Kansas; Northeast US region includes Maine, New Hampshire, Vermont, Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Pennsylvania; South US region includes Delaware, Maryland, District of Columbia, Virginia, West Virginia, North Carolina, South Carolina, Georgia, Florida, Kentucky, Tennessee, Alabama, Mississippi, Arkansas, Louisiana, Oklahoma, Texas; West US region includes Montana, Idaho, Wyoming, Colorado, New Mexico, Arizona, Utah, Nevada, Washington, Oregon, California, Alaska, Hawaii.
Our meta-regression model-building efforts identified a final model that included four significant three-way interactions among the factors of interest. The most important interaction in this meta-regression model estimated separate incidence trends by race/ethnicity within regions of the US. The other three interactions, in order of importance, modeled separate incidence trends within groups defined by combinations of summary stage and sex, sex and race/ethnicity, and summary stage and race/ethnicity.
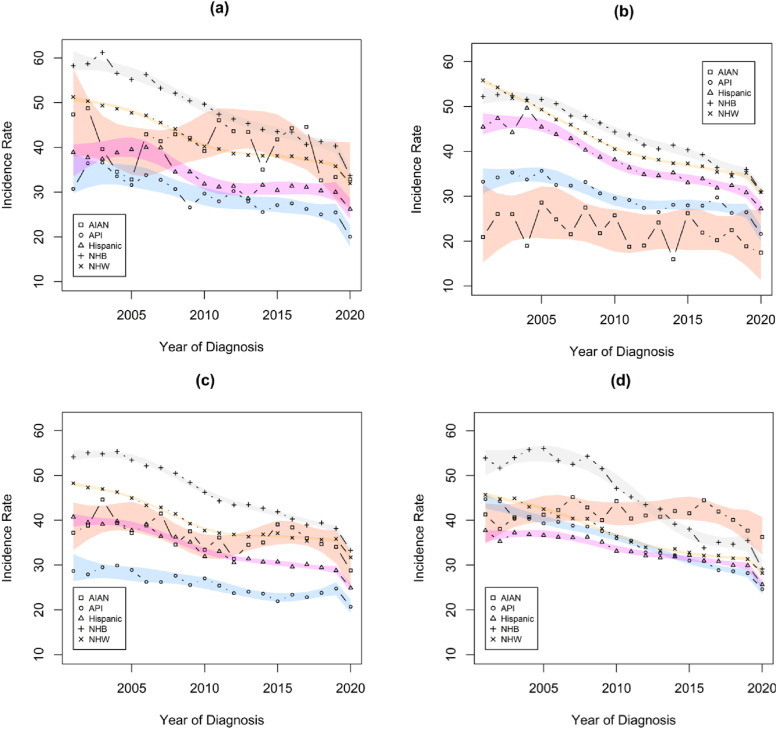
Figure 1 illustrates the most important interaction identified in the meta-regression linear model, with spline estimates of CRC incidence trends over time for each racial/ethnic group within four US regions. The estimated trends shown in the four panels of this figure highlight several key points. CRC incidence rates trended lower over the 20-year study period for almost all racial/ethnic groups in each US region, with the exception of AIAN individuals. The greatest, and least, heterogeneity in CRC incidence rates among racial/ethnic groups is apparent in the northeast, and west, US regions, respectively. All four regions have greater parity among these population subgroups toward the end of our study period when compared to the beginning. The levels of CRC incidence rates for different racial/ethnic groups appear to vary considerably across different regions of the US, particularly in the early years of the study period. This is illustrated further in Table 2, where rate ratios compare the within-region incidence rates for each racial and ethnic group to the overall incidence rates within each US region for selected study years.
Figure 1.
Trends in incidence rates for each racial/ethnic group within each region of the US. Rates are per 100 000 and age-adjusted to the 2000 US Std Population (19 age groups - Census P25-1130) standard. Estimates were obtained from the results of the meta-regression linear models analyses. (a) Midwest (b) Northeast (c) South (d) West.
Table 2.
Comparisons of the Relative CRC Incidence Rates Between Each Region and the Nationwide Incidence Rate in That Year, for Each Racial and Ethnic Group in Each of Three Study Years.
| Incidence rate ratios (95% CI), relative to nationwide estimates | |||||
|---|---|---|---|---|---|
| Race/Ethnicity | Year | Midwest | Northeast | South | West |
| AIAN | 2001 | 1.19 (0.93 - 1.52) | 0.53 (0.33 - 0.84) | 0.94 (0.78 - 1.12) | 1.04 (0.90 - 1.21) |
| 2010 | 1.01 (0.81 - 1.25) | 0.66 (0.46 - 0.94) | 0.86 (0.74 - 1.00) | 1.14 (1.00 - 1.29) | |
| 2019 | 0.96 (0.78 - 1.17) | 0.54 (0.39 - 0.76) | 0.98 (0.86 - 1.11) | 1.08 (0.97 - 1.21) | |
| API | 2001 | 0.76 (0.65 - 0.89) | 0.82 (0.75 - 0.91) | 0.71 (0.63 - 0.80) | 1.11 (1.06 - 1.15) |
| 2010 | 0.90 (0.79 - 1.01) | 0.89 (0.83 - 0.96) | 0.82 (0.75 - 0.89) | 1.09 (1.04 - 1.13) | |
| 2019 | 0.95 (0.86 - 1.04) | 0.98 (0.92 - 1.05) | 0.92 (0.86 - 0.98) | 1.05 (1.00 - 1.09) | |
| Hispanic | 2001 | 0.96 (0.86 - 1.08) | 1.13 (1.06 - 1.20) | 1.01 (0.96 - 1.06) | 0.94 (0.89 - 0.98) |
| 2010 | 0.95 (0.87 - 1.05) | 1.14 (1.08 - 1.21) | 0.96 (0.92 - 1.00) | 0.99 (0.95 - 1.03) | |
| 2019 | 1.01 (0.94 - 1.09) | 1.04 (0.99 - 1.09) | 0.97 (0.94 - 1.01) | 1.01 (0.98 - 1.05) | |
| NHB | 2001 | 1.07 (1.02 - 1.12) | 0.96 (0.92 - 1.00) | 0.99 (0.97 - 1.02) | 0.99 (0.93 - 1.05) |
| 2010 | 1.07 (1.02 - 1.11) | 0.95 (0.91 - 0.99) | 0.99 (0.97 - 1.02) | 1.01 (0.96 - 1.07) | |
| 2019 | 1.06 (1.02 - 1.11) | 0.95 (0.91 - 0.99) | 1.01 (0.98 - 1.03) | 0.94 (0.88 - 0.99) | |
| NHW | 2001 | 1.02 (1.00 - 1.03) | 1.11 (1.09 - 1.13) | 0.96 (0.95 - 0.97) | 0.91 (0.89 - 0.92) |
| 2010 | 1.04 (1.02 - 1.06) | 1.05 (1.03 - 1.07) | 0.98 (0.96 - 0.99) | 0.94 (0.92 - 0.96) | |
| 2019 | 1.03 (1.01 - 1.04) | 1.01 (0.99 - 1.03) | 1.03 (1.01 - 1.04) | 0.90 (0.88 - 0.92) | |
Notes. Estimates were obtained from the results of the meta-regression linear models analyses.
Rates are per 100 000 and age-adjusted to the 2000 US Std Population (19 age groups - Census P25-1130) standard; Confidence intervals (Tiwari 2006 mod) are 95% for rates.
Abbreviations: AIAN: Non-Hispanic American Indian or Alaska Native; API: Non-Hispanic Asian or Pacific Islander; Hispanic: Hispanic of any race; NHB: Non-Hispanic Black; NHW: Non-Hispanic White.
Midwest US region includes Ohio, Indiana, Illinois, Michigan, Wisconsin, Minnesota, Iowa, Missouri, North Dakota, South Dakota, Nebraska, Kansas; Northeast US region includes Maine, New Hampshire, Vermont, Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Pennsylvania; South US region includes Delaware, Maryland, District of Columbia, Virginia, West Virginia, North Carolina, South Carolina, Georgia, Florida, Kentucky, Tennessee, Alabama, Mississippi, Arkansas, Louisiana, Oklahoma, Texas; West US region includes Montana, Idaho, Wyoming, Colorado, New Mexico, Arizona, Utah, Nevada, Washington, Oregon, California, Alaska, Hawaii.
Although CRC incidence trends for racial/ethnic groups within US regions are typically not linear in nature, summarizing the overall trends over time with average annual percentage change (AAPC) estimates can provide additional insights into differences in overall trends over time. Table 3 contains AAPC estimates for each racial/ethnic group within each region of the US. The AAPC estimates differ significantly across the four US regions for each racial/ethnic group (P < 0.001 for each), except for AIAN persons (P = 0.864). The AAPC estimates demonstrate that NHB persons had the highest declines in CRC incidence, ranging from −2.27 (95% CI: −2.49 to −2.06) in the South to −3.03 (95% CI: −3.59 to −2.47) in the West. On the other end of the spectrum, AIAN persons had the smallest changes in CRC incidence. None of the per-region AAPC estimates for this population group demonstrated a significant decline, nor did the pooled-across-regions AAPC, which had an estimate of −0.41, with a 95% CI ranging from −2.51 to 1.73).
Table 3.
Estimates of Average Annual Percentage Change (AAPC) for Persons of Each Racial/Ethnic Group Residing in Each US Region, and Separately for Diagnoses of CRC at Different Summary Stages.
| Race/Ethnicity | US region | AAPC | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| AIAN | Midwest | −0.63 | −2.73 | 1.50 |
| Northeast | −1.06 | −4.64 | 2.65 | |
| South | −0.72 | −2.00 | 0.59 | |
| West | −0.07 | −1.18 | 1.05 | |
| API | Midwest | −1.85 | −2.98 | −0.71 |
| Northeast | −1.60 | −2.33 | −0.86 | |
| South | −1.33 | −2.16 | −0.49 | |
| West | −2.53 | −2.91 | −2.15 | |
| Hispanic | Midwest | −1.73 | −2.59 | −0.86 |
| Northeast | −2.53 | −3.02 | −2.05 | |
| South | −2.04 | −2.37 | −1.71 | |
| West | −1.36 | −1.70 | −1.02 | |
| NHB | Midwest | −2.35 | −2.74 | −1.96 |
| Northeast | −2.37 | −2.76 | −1.99 | |
| South | −2.27 | −2.49 | −2.06 | |
| West | −3.03 | −3.59 | −2.47 | |
| NHW | Midwest | −2.09 | −2.23 | −1.96 |
| Northeast | −2.76 | −2.89 | −2.63 | |
| South | −1.89 | −2.00 | −1.79 | |
| West | −2.33 | −2.47 | −2.18 |
Notes. Estimates were obtained from the results of the meta-regression linear models analyses.
An annual percentage change of −1 represents the situation where the group experienced an average 1% reduction in CRC incidence for each year of observation.
Abbreviations: AIAN: Non-Hispanic American Indian or Alaska Native; API: Non-Hispanic Asian or Pacific Islander; Hispanic: Hispanic of any race; NHB: Non-Hispanic Black; NHW: Non-Hispanic White.
Midwest US region includes Ohio, Indiana, Illinois, Michigan, Wisconsin, Minnesota, Iowa, Missouri, North Dakota, South Dakota, Nebraska, Kansas; Northeast US region includes Maine, New Hampshire, Vermont, Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Pennsylvania; South US region includes Delaware, Maryland, District of Columbia, Virginia, West Virginia, North Carolina, South Carolina, Georgia, Florida, Kentucky, Tennessee, Alabama, Mississippi, Arkansas, Louisiana, Oklahoma, Texas; West US region includes Montana, Idaho, Wyoming, Colorado, New Mexico, Arizona, Utah, Nevada, Washington, Oregon, California, Alaska, Hawaii.
The second most important interaction in the meta-regression model demonstrates significant differences in sex-specific incidence trends for CRC diagnoses for localized or regional vs distant summary stages. The key differences suggested by this finding are summarized here, rather than in tables or figures. In 2001, age-adjusted localized or regional summary stage CRC incidence rates for women and men were 34.9 (95% CI: 34.6 to 35.2) and 48.1 (95% CI: 47.7 to 48.5) events per 100 000 individuals, respectively. The distant summary stage incidence rates were 7.7 (95% CI: 7.5 to 7.8) per 100 000 for women and 10.9 (95% CI: 10.7 to 11.1) per 100 000 for men. Incidence rates decreased faster for men than for women for both summary stage groups. The female:male incidence rate ratio for CRC diagnoses of localized or regional summary stages was 0.73 (95% CI: 0.72 to 0.74) in 2001 and this rate ratio increased linearly to a value of 0.78 (95% CI: 0.77 to 0.79) by 2020. For CRC diagnoses at distant summary stages, the female:male incidence rate ratio increased linearly from 0.71 (95% CI: 0.69 to 0.73) in 2001 to a maximum of 0.76 (95% CI: 0.74 to 0.78) in 2006. After 2006, this incidence rate ratio decreased to a value of 0.73 (95% CI: 0.71 to 0.74) in 2020.
The third most impactful interaction in the meta-regression linear model indicates that there were differences in sex-specific incidence trends according to different racial/ethnic groups. The key differences suggested by this finding are summarized here, rather than in tables or figures. Incidence rates are higher for males than for females throughout the study period for all racial/ethnic groups. In 2001, the female:male incidence rate ratios varied among persons of different races/ethnicities; these female:male incidence rate ratios were 0.79 (95% CI: 0.72 to 0.86) for AIAN, 0.75 (95% CI: 0.73 to 0.78) for API, 0.67 (95% CI: 0.66 to 0.69) for Hispanic, 0.75 (95% CI: 0.74 to 0.77) for NHB, and 0.722 (95% CI: 0.717 to 0.727) for NHW persons. The trends in female:male incidence rate ratios did not change significantly over time for AIAN, API, or NHB persons. For Hispanic persons, the female:male rate ratios increased on average by 0.34% (95% CI: 0.11% to 0.56%) for each year from 2001 to 2020, and for NHW persons the female:male rate ratios increased on average by 0.44% (95% CI: 0.38% to 0.51%) for each year.
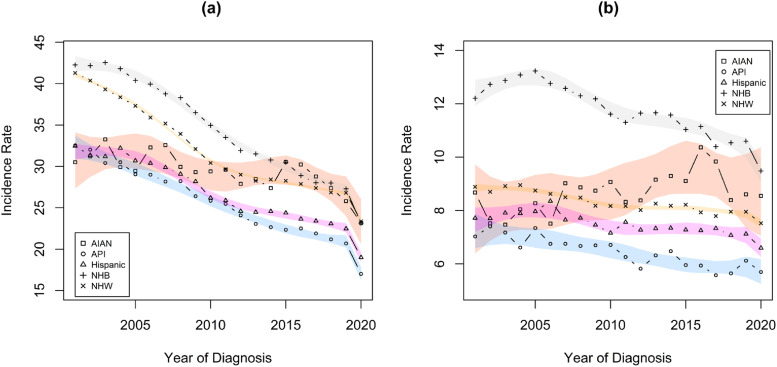
Figure 2 demonstrates that there are differences in summary stage-specific CRC incidence rates for persons of different racial and ethnic groups. Declines in incidence rates are evident for all race/ethnic groups for localized or regional CRC diagnoses, and these rates of decline are significantly faster for localized or regional than for distant summary stages for each racial/ethnic group. In 2020, there was an average 12.3% (95% CI: 11.0% to 13.6%) decrease in the localized or regional incidence rate for CRC diagnoses, compared to the prevailing trends. There was also a significant decrease in the incidence rates of CRC diagnosed at distant summary stages (3.7%, 95% CI: 1.1% to 6.2%), but this decrease was significantly lower than that for those not diagnosed at distant summary stages. Table 3 shows AAPC estimates for changes in incidence rates for localized or regional CRC, and for distant CRC, within each racial/ethnic group. AIAN persons experienced the slowest declines in CRC incidence at localized and regional stage, and these differences were significant when compared to each other racial/ethnic group. Hispanic persons experienced significantly slower declines in localized or regional CRC incidence than NHB and NHW persons. AIAN persons also appeared to experience a slight, though non-significant, increase in incidence rates for distant stage CRC, and this trend differed significantly from those of all other racial/ethnic groups. Hispanic and NHW persons experienced significantly slower rates of decline in the incidence of distant stage CRC than NHB persons. Summaries of additional findings are shown in the Supplemental materials.
Figure 2.
Trends in Localized or Regional and Distant summary stage CRC incidence rates, separately for each racial/ethnic group. Rates are per 100 000 and age-adjusted to the 2000 US Std Population (19 age groups - Census P25-1130) standard. Estimates were obtained from the results of the meta-regression linear models analyses. (a) Localized or regional (b) Distant.
Discussion
Our analyses of trends in CRC incidence identified four key effects that simultaneously captured differences in CRC incidence trends among the factors that were studied. The most important finding is that there are differences in overall CRC incidence trends that differed significantly among regions of the US according to race and ethnicity. This finding suggests that although overall CRC incidence rates are declining in the US, there are population subgroups for whom improvement has been markedly slower. This is illustrated in both Table 2 and Figure 1. Table 2 illustrates that incidence rates for individuals from distinct racial and ethnic groups varied over time according to the region of the US in which they resided. For instance, NHW individuals in the northeast had significantly higher than average CRC incidence rates in 2001, but their incidence rates in 2019 were not distinguishable from the regional average. In comparison, NHB individuals displayed CRC incidence rates that were relatively consistent over time when compared to the regional averages. Pairing this observation from Table 2 with the visual representation from Figure 1, one observes that although overall declines in CRC incidence were greatest for NHB individuals, they were not sufficient to reduce their most recent rates below those of other racial/ethnic subgroups. Of particular importance, the curves in Figure 1 illustrate that although the CRC incidence rates for AIAN individuals were among the lowest observed in each region in 2001, this was no longer the case in most regions of the US by the end of the study period.
This finding is concordant with prior reports of incidence trends differing between AIAN and NHW populations in various regions in the US.15,34 Although our findings do not focus on the Contract Health Service Delivery Area (CHSDA) counties containing or adjacent to federally recognized tribal lands, and the regions in the US available in our analyses are less refined, our findings are remarkably consistent. CRC incidence rates in 2001 tended to be lower for AIAN persons than for NHW, with considerable heterogeneity across regions. These patterns changed over the course of our study period, with CRC incidence rates changing negligibly among AIAN persons while CRC incidence rates declined over the same period for NHW persons. Unique to what has been reported previously,8,10,12,15,16 our analyses suggest that similar patterns are present when comparing CRC incidence rates between AIAN persons and individuals of all other racial/ethnic groups we considered.
The reported declines in CRC incidence rates, particularly among NHW individuals,15,35 have been at least partially ascribed to the effectiveness of screening colonoscopy, which can remove precancerous polyps before they can develop into CRC. 36 Indeed, the gap for screening rates between NHB and NHW populations has narrowed over time, from 41.9% among NHB vs 49.6% among NHW in 2000 to 70.0% among NHB vs 71.0% among NHW by 2018. 17 The narrowing of the incidence gap between NHB and NHW populations observed in our results provides further evidence of this screening-incidence relationship. Interestingly, comparing AIAN and NHW populations in the same time period, the screening gap did not narrow to the same extent (2000: 39.2% AIAN vs 49.6% NHW; 2018: 62.1% AIAN vs 71.0% NHW), which may partially explain why incidence did not fall among AIAN persons. Variations in the availability of and access to colonoscopy for CRC screening across different regions of the US, particularly for minoritized populations, may therefore be a contributor to the differences in CRC incidence trends we report.15,37 There are many possible causes for disparities in CRC incidence trends among different racial/ethnic groups. These include differences in the prevalence of risk factors such as diet, obesity, smoking, and alcohol use, the gut microbiome, and access to screening and treatment.15,37-40 Differences in these exposures and challenges with healthcare access may result in the disparities in CRC incidence trends that are apparent across the different regions in the US. Challenges to access include the geographic density of gastroenterologists, which has been shown to be the highest in the Northeast and Midwest, and lowest in the West, 41 as well as high variability in the uninsured rate, which has been shown to be the highest in the South. 42
The distinct CRC incidence trends reported here suggest that there are unique patterns of CRC incidence over time among racial/ethnic groups according to the four US regions considered in this analysis. There also appear to be differences between males and females, and among different racial/ethnic groups in CRC incidence trends according to the stage at diagnosis. For instance, it appears that AIAN women engage in CRC screening at significantly lower rates than women from other racial and ethnic groups. 43 This suggests that differences in screening participation may lead to the distinct trends among racial and ethnic groups according to stage at diagnosis. This could in turn have an impact on the overall differences in the CRC incidence trends for different racial/ethnic groups residing in different regions of the US. The differences reported here suggest a need to develop and implement interventions that are targeted towards improving screening rates for men, and for AIAN persons, where higher incidence rates or poorer incidence trends are present.
It is apparent that incidence of CRC incidence dropped in the year 2020 (see Figure 1). In fact, the pooled estimate of CRC incidence in the year 2020 was 10.3% lower (95% CI: 9.3% to 11.2% lower) than would have been expected given the trends from 2001 to 2019. This observation is consistent with other reports that the COVID-19 pandemic caused healthcare disruptions that led to delays in the diagnosis of cancer. 1
The findings of this report need to be considered within the context of several limitations. This is a retrospective analysis of data from the SEER and NPCR registries, with relatively little information on comorbidities, access to care, CRC screening, and insurance status. The provenance of the data also suggests that the reported racial and ethnic classifications may reflect misclassification, 44 which may bias our estimates. Also, several of the factors of primary interest to us were only available in broad categories in the data source to which we had access. This is particularly noteworthy for the regions of the US available for use, which were limited to four broad categories, and in the racial/ethnic groups, which only enabled the consideration of five groups: non-Hispanic American Indian/Alaska Native (AIAN), non-Hispanic Asian/Pacific Islander (API), Hispanic of any race (Hispanic), non-Hispanic Black (NHB), and non-Hispanic White (NHW).
Conclusion
This analysis highlights the significant differences in CRC incidence trends among persons of different racial/ethnic population subgroups according to the region in the US in which these individuals reside. Notable declines in CRC incidence were evident in all four US regions for all racial/ethnic groups, except for AIAN persons. Despite these improvements, heterogeneity in CRC incidence among racial and ethnic population subgroups persists, particularly for diagnoses made at distant summary stages. That CRC incidence rates appear to be unchanged for AIAN persons over the two-decade interval considered for this study is a notable concern, particularly within the context of significant declines for all other racial/ethnic groups reported here. Coupling this observation with recent reports that AIAN persons experience poorer CRC-specific survival highlights the importance of addressing CRC among this population subgroup.45,46 A number of interventions are being implemented to support Native American communities in their efforts to access recommended CRC screening tests, promote healthy behaviors, and reduce risk exposures.23,47,48 At least one study has shown that some evidence-based interventions for increasing CRC screening that may be effective in non-AIAN populations—such as using social media to increase CRC awareness—were not preferred and respondents instead preferred culturally appropriate printed materials and mailed reminders. 47 Still another tribe-specific study revealed that improving access to a regular source of care was the most preferred strategy for increasing CRC screening rates, as Indian Health Service health centers must often prioritize acute care services over preventive health.23,49 Additional efforts will be required to fully address the disparities highlighted in this paper. Engagement with Native American communities as they develop comprehensive community and systems-based approaches is likely to offer the greatest potential to finally reduce and overcome these historical and distressingly persistent health disparities.
Supplemental Material
Supplemental Material for Racial and Ethnic Disparities in Colorectal Cancer Incidence Trends Across Regions of the United States From 2001 to 2020 – A United States Cancer Statistics Analysis by Vernon Shane Pankratz, Deborah Kanda, Mikaela Kosich, Nicholas Edwardson, Kevin English, Prajakta Adsul, and Shiraz I. Mishra Cancer Control
Supplemental Material for Racial and Ethnic Disparities in Colorectal Cancer Incidence Trends Across Regions of the United States From 2001 to 2020 – A United States Cancer Statistics Analysis by Vernon Shane Pankratz, Deborah Kanda, Mikaela Kosich, Nicholas Edwardson, Kevin English, Prajakta Adsul, and Shiraz I. Mishra Cancer Control
Acknowledgments
This work would not have been possible without the extensive efforts of the research staff of the registries participating in the SEER Program of the National Cancer Institute.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Cancer Institute of the National Institutes of Health grant R01CA192967 (Mishra, PI), the University of New Mexico Comprehensive Center Support Grant 3P30CA118100 (Sanchez, PI), a supplement to the University of New Mexico Comprehensive Cancer Center P30 Cancer Center Support grant 3P30CA118100-16S4 (Sanchez, PI; Mishra, PD), and the Biostatistics Shared Resource of the University of New Mexico Comprehensive Cancer Center.
Supplemental Material: Supplemental material for this article is available online.
Ethical Statement
Ethical Approval
This project, Study ID: 21-102, was reviewed by the University of New Mexico Health Sciences Institutional Review Board, and was granted exemption according to Category 4: Secondary research on data or specimens (no consent required).
ORCID iDs
Vernon Shane Pankratz https://orcid.org/0000-0002-3742-040X
Deborah Kanda https://orcid.org/0000-0002-0182-5050
Prajakta Adsul https://orcid.org/0000-0003-2860-4378
Data Availability Statement
The data analyzed for this study are available from SEER and NPCR, and restrictions apply to its availability. Data are available data are available for download in SEER*Stat v8.4.2. The authors will provide programming code and analysis files upon reasonable request, and with the permission of SEER and NPCR.*
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022 [Internet]. Atlanta, GA: American Cancer Society; 2020. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf (accessed on May 24, 2024) [Google Scholar]
- 4.National Cancer Institute . Cancer stat facts: colorectal cancer [Internet]. 2023. Available from: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on May 24, 2024)
- 5.Akimoto N, Ugai T, Zhong R, et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol. 2021;18(4):230-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Preventive Services Task Force . Final recommendation statement colorectal cancer: screening [Internet]. 2021. Available from: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening (accessed on May 24, 2024)
- 7.Primm KM, Malabay AJ, Curry T, Chang S. Who, where, when: colorectal cancer disparities by race and ethnicity, subsite, and stage. Cancer Med. 2023;12(13):14767-14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohri A, Robinson A, Liu B, Bhuket T, Wong R. Updated assessment of colorectal cancer incidence in the U.S. by age, sex, and race/ethnicity. Dig Dis Sci. 2020;65(6):1838-1849. [DOI] [PubMed] [Google Scholar]
- 9.Chang SH, Patel N, Du M, Liang PS. Trends in early-onset vs late-onset colorectal cancer incidence by race/ethnicity in the United States cancer statistics database. Clin Gastroenterol Hepatol. 2022;20(6):e1365-e1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansa B, Coughlin S, Alema-Mensah E, Smith S. Evaluation of colorectal cancer incidence trends in the United States (2000–2014). J Clin Med. 2018;7(2):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopalani SV, Janitz AE, Martinez SA, Gutman P, Khan S, Campbell JE. Trends in cancer incidence among American Indians and Alaska natives and non-hispanic whites in the United States, 1999–2015. Epidemiology. 2020;31(2):205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haverkamp D, Melkonian SC, Jim MA. Growing disparity in the incidence of colorectal cancer among non-hispanic American Indian and Alaska native populations—United States, 2013–2017. Cancer Epidemiol Biomarkers Prev. 2021;30(10):1799-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramai D, Barakat M, Dhaliwal A, et al. Gender and racial disparities in colorectal cancer incidence and mortality: a national cancer registry study. Int J Colorectal Dis. 2021;36(8):1801-1804. [DOI] [PubMed] [Google Scholar]
- 14.Melkonian SC, Jim MA, Haverkamp D, et al. Disparities in cancer incidence and trends among American Indians and Alaska natives in the United States, 2010–2015. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1604-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perdue DG, Haverkamp D, Perkins C, Daley CM, Provost E. Geographic variation in colorectal cancer incidence and mortality, age of onset, and stage at diagnosis among American Indian and Alaska native people, 1990–2009. Am J Public Health. 2014;104 Suppl 3(S3):S404-S414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin H, Henley J, King J, Richardson L, Eheman C. Changes in colorectal cancer incidence rates in young and older adults in the United States: what does it tell us about screening. Cancer Causes Control. 2014;25(2):191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLeod MR, Galoosian A, May FP. Racial and ethnic disparities in colorectal cancer screening and outcomes. Hematol Oncol Clin North Am. 2022;36(3):415-428. [DOI] [PubMed] [Google Scholar]
- 18.Kelly JJ, Alberts SR, Sacco F, Lanier AP. Colorectal cancer in Alaska Native people, 2005-2009. Gastrointest Cancer Res. 2012;5(5):149-154. [PMC free article] [PubMed] [Google Scholar]
- 19.Carethers JM. Racial and ethnic disparities in colorectal cancer incidence and mortality. Advances in Cancer Research. Adv Cancer Res; 2021:151, 197-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimou A, Syrigos KN, Saif MW. Disparities in colorectal cancer in African-Americans vs Whites: before and after diagnosis. World J Gastroenterol. 2009;15(30):3734-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson NB, Murff HJ, Fowke JH, et al. Use of colonoscopy and flexible sigmoidoscopy among African Americans and whites in a low-income population. Prev Chronic Dis. 2008;5:A28. [PMC free article] [PubMed] [Google Scholar]
- 22.Haverkamp D, English K, Jacobs-Wingo J, Tjemsland A, Espey D. Effectiveness of interventions to increase colorectal cancer screening among American Indians and Alaska natives. Prev Chronic Dis. 2020;17:E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwardson N, Cartwright K, Sheche J, et al. Colorectal cancer screening among adults in Zuni Pueblo: factors associated with FOBT and colonoscopy utilization. J Community Health. 2023;48(4):565-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program SEER*Stat Database: NPCR and SEER Incidence – U.S. Cancer Statistics 2001–2020 Public Use Research Database , 2022 Submission (2001–2020) [Internet]. USA: United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2023. Available from: https://www.cdc.gov/cancer/uscs/public-use (accessed on May 24, 2024) [Google Scholar]
- 25.U.S. Cancer Statistics Public Use Database Technical Documentation [Internet]. USA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2021. Available from: https://www.cdc.gov/cancer/uscs/public-use/pdf/uscs-public-use-database-technical-documentation-us-2001-2019-508.pdf (accessed on May 24, 2024) [Google Scholar]
- 26.Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nov. https://www.cdc.gov/cancer/uscs/public-use/dictionary/data-variable.htm?dataVarDesc=uscs-since-2001&dataVarAlt=USCS-since-2001&dbName=us CDC’s national program of cancer registries. USCS0120 [Internet]. [cited 2023. 21]. Available from: (accessed on May 24, 2024)
- 28.Shah RR, Millien VO, Da Costa WL, Oluyomi AO, Gould Suarez M, Thrift AP. Trends in the incidence of early‐onset colorectal cancer in all 50 United States from 2001 through 2017. Cancer. 2022;128(2):299-310. [DOI] [PubMed] [Google Scholar]
- 29.Ruhl JL, Callaghan C, Schussler N. Summary Stage 2018: Codes and Coding Instructions [Internet]. Bethesda, MD: National Cancer Institute; 2023. [cited 2024 Apr 16]. Available from: https://seer.cancer.gov/tools/ssm/ (accessed on May 24, 2024) [Google Scholar]
- 30.U.S. Department of Commerce . Population Projections of the United States by Age, Sex, Race, and Hispanic Origin: 1995 to 2050. USA: Bureau of the Census; 1996. [Google Scholar]
- 31.Tiwari R, Clegg L, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547-569. [DOI] [PubMed] [Google Scholar]
- 32.Chambers J, Hastie T. Generalized Additive Models. 1st ed. Pacific Grove, CA, USA: Wadsworth & Brooks/Cole; 1992. [Google Scholar]
- 33.Perperoglou A, Sauerbrei W, Abrahamowicz M, Schmid M. A review of spline function procedures in R. BMC Med Res Methodol. 2019;19:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perdue DG, Perkins C, Jackson-Thompson J, et al. Regional differences in colorectal cancer incidence, stage, and subsite among American Indians and Alaska natives, 1999–2004. Cancer. 2008;113(5 suppl):1179-1190. [DOI] [PubMed] [Google Scholar]
- 35.Jemal A, Simard EP, Dorell C, et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)–associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Day LW, Espey DK, Madden E, Segal M, Terdiman JP. Screening prevalence and incidence of colorectal cancer among American Indian/Alaskan natives in the Indian health service. Dig Dis Sci. 2011;56:2104-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson JS, Nobmann ED, Asay E, Lanier AP. Dietary intake of Alaska native people in two regions and implications for health: the Alaska native dietary and subsistence food assessment project. Int J Circumpolar Health. 2009;68(2):109-122. [DOI] [PubMed] [Google Scholar]
- 39.Cobb N, Espey D, King J. Health behaviors and risk factors among American Indians and Alaska Natives, 2000–2010. Am J Public Health. 2014;104 Suppl 3(S3):S481-S489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn J, Sinha R, Pei Z, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105(24):1907-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee BP, Dodge JL, Terrault NA. Geographic density of gastroenterologists is associated with decreased mortality from alcohol-associated liver disease. Clin Gastroenterol Hepatol. 2023;21(6):1542-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen RA, Terlizzi EP, Cha AE, et al. Geographic Variation in Health Insurance Coverage: United States, 2019. USA: NCHS Natl Health Stat Rep [Internet]; 2021. Available from: https://stacks.cdc.gov/view/cdc/107558 (accessed on May 24, 2024) [PubMed]
- 43.Shah SK, Narcisse MR, Hallgren E, Felix HC, McElfish PA. Assessment of colorectal cancer screening disparities in U.S. men and women using a demographically representative sample. Cancer Res Commun. 2022;2(6):561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melkonian SC, Weir HK, Jim MA, Preikschat B, Haverkamp D, White MC. Incidence of and trends in the leading cancers with elevated incidence among American Indian and Alaska native populations, 2012–2016. Am J Epidemiol. 2020;190(4):528-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pankratz VS, Kosich M, Edwardson N, et al. American Indian/Alaska Native and black colon cancer patients have poorer cause-specific survival based on disease stage and anatomic site of diagnosis. Cancer Epidemiol. 2022;80:102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pankratz VS, Kanda D, Edwardson N, et al. Colorectal cancer survival trends in the United States from 1992 to 2018 differ among persons from five racial and ethnic groups according to stage at diagnosis: a SEER-based study. Cancer Control. 2022;29:10732748221136440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edwardson N, Kosich M, Shane Pankratz V, et al. Preferences for CPSTF-recommended intervention approaches for increasing cancer screening among screen-eligible adults in Zuni Pueblo, USA. Prev Med Rep. 2023;36:102453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coronado GD, Ferrari RM, Barnes A, et al. Characteristics of patient navigation programs in the cancer moonshot ACCSIS colorectal cancer screening initiative. J Natl Cancer Inst. 2023;115(6):680-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narva AS, Romancito G, Faber T, Steele ME, Kempner KM. Managing CKD by telemedicine: the Zuni telenephrology clinic. Adv Chronic Kidney Dis. 2017;24(1):6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Racial and Ethnic Disparities in Colorectal Cancer Incidence Trends Across Regions of the United States From 2001 to 2020 – A United States Cancer Statistics Analysis by Vernon Shane Pankratz, Deborah Kanda, Mikaela Kosich, Nicholas Edwardson, Kevin English, Prajakta Adsul, and Shiraz I. Mishra Cancer Control
Supplemental Material for Racial and Ethnic Disparities in Colorectal Cancer Incidence Trends Across Regions of the United States From 2001 to 2020 – A United States Cancer Statistics Analysis by Vernon Shane Pankratz, Deborah Kanda, Mikaela Kosich, Nicholas Edwardson, Kevin English, Prajakta Adsul, and Shiraz I. Mishra Cancer Control
Data Availability Statement
The data analyzed for this study are available from SEER and NPCR, and restrictions apply to its availability. Data are available data are available for download in SEER*Stat v8.4.2. The authors will provide programming code and analysis files upon reasonable request, and with the permission of SEER and NPCR.*