Abstract
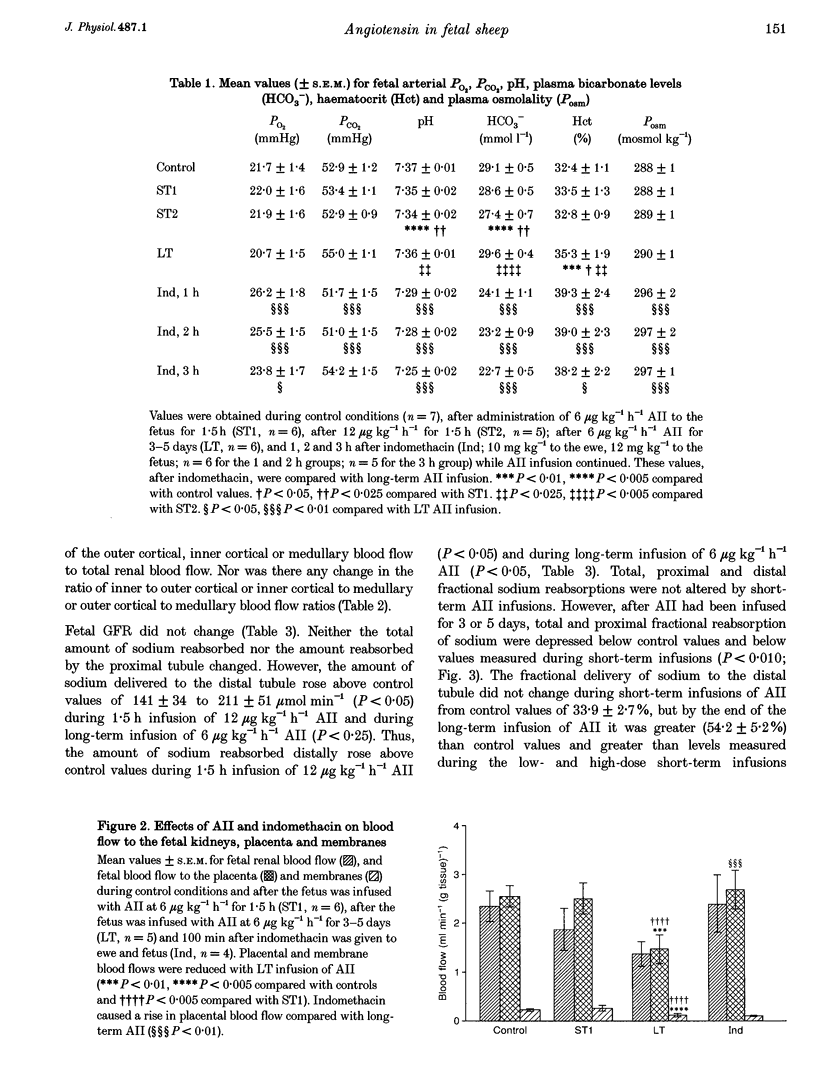
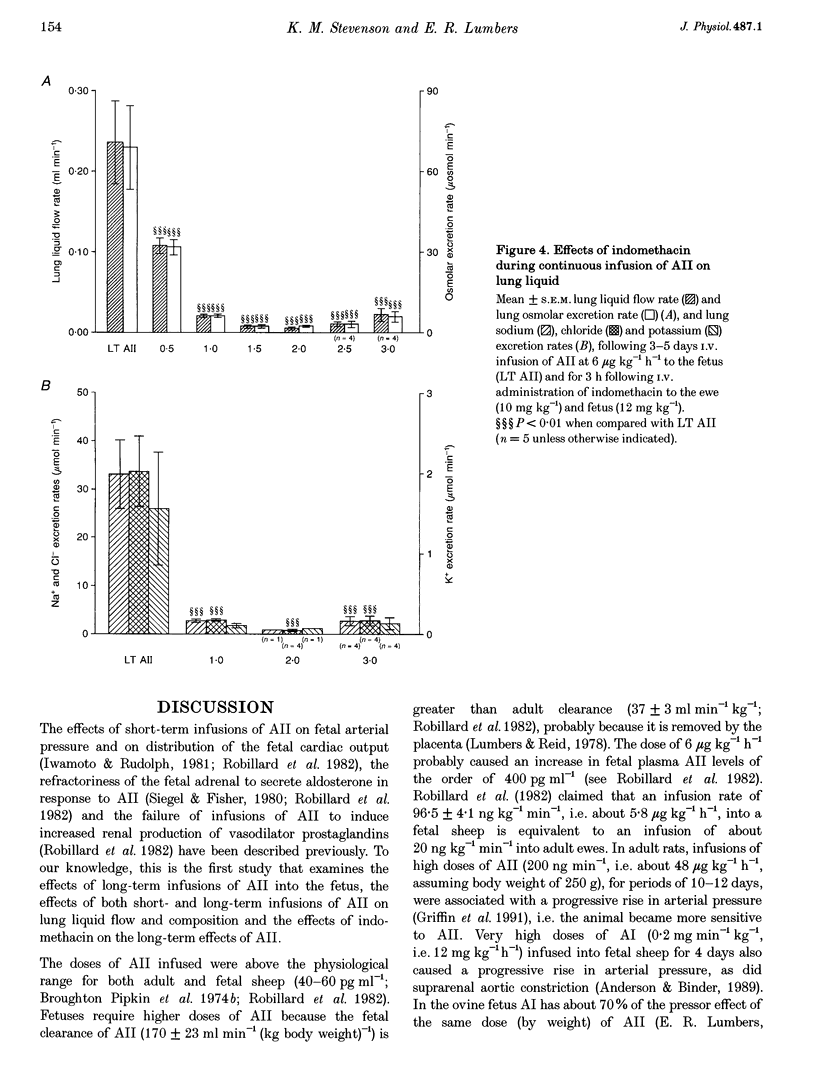
1. Angiotensin II (AII) was infused I.V. into seven chronically catheterized fetal sheep (gestational age, 120-136 days). The effects of short-term infusions of 6 and 12 micrograms kg-1 h-1 for 1.5 h were compared with the effects of infusing 6 micrograms kg-1 h-1 for 3 or 5 days (long-term infusion). AII produced an immediate rise in fetal arterial blood pressure (P < 0.025). When infused for 3 or 5 days, 6 micrograms kg-1 h-1 AII caused a greater increase in arterial blood pressure (P < 0.05). 2. Infusions of 6 micrograms kg-1 h-1 AII for 1.5 h had no effect on fetal placental blood flow or on flow to the fetal membranes, but after AII infusion for 3 or 5 days both flows were reduced (P < 0.01 and P < 0.005, respectively). Fetal blood gas status and pH were maintained. The only change in fetal renal function observed with short-term infusions of AII was a rise in sodium excretion when 12 micrograms kg-1 h-1 AII was given (P < 0.05). Infusion of 6 micrograms kg-1 h-1 for 3 or 5 days also caused a rise in sodium excretion (P < 0.025) because total and proximal fractional sodium reabsorptions were depressed (P < 0.01). Infusions of AII had no effects on the volume of lung liquid produced or on its composition. 3. Administration of indomethacin to the ewe (10 mg kg-1) and to the fetus (12 mg kg-1), during the infusion of AII, caused a rise in maternal arterial pressure (P < 0.01) but no change in fetal arterial pressure. 4. After indomethacin, umbilicoplacental blood flow rose (P < 0.05), as did fetal arterial PO2 (P < 0.05). Fetal arterial PCO2, pH and bicarbonate levels fell (P < 0.01). Glomerular filtration rate (GFR) rose (P < 0.01); there was a natriuresis (P < 0.01), chloriuresis (P < 0.01) and a kaliuresis (P < 0.05) but urine flow rate did not change. Lung liquid flow fell (P < 0.01). 5. It is concluded that in the fetus, long-term infusions of AII at a constant dose rate cause a progressive rise in arterial pressure. In addition, effects of AII on placental blood flow and on renal function develop. Thus, short-term infusions of AII cannot be used to predict the renal and cardiovascular effects of sustained high levels of this peptide in the fetus.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J. W., Vane J. R. Intrarenal prostaglandin release attenuates the renal vasoconstrictor activity of angiotensin. J Pharmacol Exp Ther. 1973 Mar;184(3):678–687. [PubMed] [Google Scholar]
- Alexander D. P., Forsling M. L., Martin M. J., Nixon D. A., Ratcliffe J. G., Redstone D., Tunbridge D. The effect of maternal hypoxia on fetal pituitary hormone release in the sheep. Biol Neonate. 1972;21(3):219–228. doi: 10.1159/000240510. [DOI] [PubMed] [Google Scholar]
- Anderson D. F., Binder N. D. Suprarenal aortic flow reduction versus angiotensin I infusion in fetal sheep. J Dev Physiol. 1989 May;11(5):317–321. [PubMed] [Google Scholar]
- Anderson D. F., Faber J. J. Animal model for polyhydramnios. Am J Obstet Gynecol. 1989 Feb;160(2):389–390. doi: 10.1016/0002-9378(89)90454-7. [DOI] [PubMed] [Google Scholar]
- Armentrout T., Katz S., Thornburg K. L., Faber J. J. Osmotic flow through the placental barrier of chronically prepared sheep. Am J Physiol. 1977 Oct;233(4):H466–H474. doi: 10.1152/ajpheart.1977.233.4.H466. [DOI] [PubMed] [Google Scholar]
- Bonjour J. P., Malvin R. L. Stimulation of ADH release by the renin-angiotensin system. Am J Physiol. 1970 Jun;218(6):1555–1559. doi: 10.1152/ajplegacy.1970.218.6.1555. [DOI] [PubMed] [Google Scholar]
- Brace R. A., Andres R. L. Left thoracic duct lymph flow responses to angiotensin II or atrial natriuretic factor infusion in the ovine fetus. Am J Obstet Gynecol. 1991 Dec;165(6 Pt 1):1607–1613. doi: 10.1016/0002-9378(91)90003-a. [DOI] [PubMed] [Google Scholar]
- Brace R. A. Blood volume and its measurement in the chronically catheterized sheep fetus. Am J Physiol. 1983 Apr;244(4):H487–H494. doi: 10.1152/ajpheart.1983.244.4.H487. [DOI] [PubMed] [Google Scholar]
- Brace R. A., Gold P. S. Fetal whole-body interstitial compliance, vascular compliance, and capillary filtration coefficient. Am J Physiol. 1984 Nov;247(5 Pt 2):R800–R805. doi: 10.1152/ajpregu.1984.247.5.R800. [DOI] [PubMed] [Google Scholar]
- Broughton Pipkin F., Kirkpatrick S. M., Lumbers E. R., Mott J. C. Renin and angiotensin-like levels in foetal, new-born and adult sheep. J Physiol. 1974 Sep;241(3):575–588. doi: 10.1113/jphysiol.1974.sp010672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton Pipkin F., Lumbers E. R., Mott J. C. Factors influencing plasma renin and angiotensin II in the conscious pregnant ewe and its foetus. J Physiol. 1974 Dec;243(3):619–636. doi: 10.1113/jphysiol.1974.sp010769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. J., Olver R. E., Ramsden C. A., Strang L. B., Walters D. V. Effects of adrenaline and of spontaneous labour on the secretion and absorption of lung liquid in the fetal lamb. J Physiol. 1983 Nov;344:137–152. doi: 10.1113/jphysiol.1983.sp014929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H. A., Horton E. W. Output of prostaglandins from the rabbit kidney, its increase on renal nerve stimulation and its inhibition by indomethacin. Br J Pharmacol. 1972 Dec;46(4):658–675. doi: 10.1111/j.1476-5381.1972.tb06891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne I., Lumbers E. R., Hill K. J. The secretion of organic acids and bases by the ovine fetal kidney. Exp Physiol. 1990 Mar;75(2):211–221. doi: 10.1113/expphysiol.1990.sp003395. [DOI] [PubMed] [Google Scholar]
- FELDBERG W., LEWIS G. P. THE ACTION OF PEPTIDES ON THE ADRENAL MEDULLA. RELEASE OF ADRENALINE BY BRADYKININ AND ANGIOTENSIN. J Physiol. 1964 May;171:98–108. doi: 10.1113/jphysiol.1964.sp007364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman W. F., Molony D. A., Kirkpatrick S. E. Prostaglandins: physiological and clinical correlations. Adv Pediatr. 1978;25:151–204. [PubMed] [Google Scholar]
- Gibson K. J., Lumbers E. R. The roles of arginine vasopressin in fetal sodium balance and as a mediator of the effects of fetal "stress". J Dev Physiol. 1993 Mar;19(3):125–136. [PubMed] [Google Scholar]
- Glance D. G., Elder M. G., Myatt L. Prostaglandin production and stimulation by angiotensin II in the isolated perfused human placental cotyledon. Am J Obstet Gynecol. 1985 Feb 1;151(3):387–391. doi: 10.1016/0002-9378(85)90309-6. [DOI] [PubMed] [Google Scholar]
- Gold P. S., Brace R. A. Fetal whole-body permeability--surface area product and reflection coefficient for plasma proteins. Microvasc Res. 1988 Nov;36(3):262–274. doi: 10.1016/0026-2862(88)90027-1. [DOI] [PubMed] [Google Scholar]
- Griffin S. A., Brown W. C., MacPherson F., McGrath J. C., Wilson V. G., Korsgaard N., Mulvany M. J., Lever A. F. Angiotensin II causes vascular hypertrophy in part by a non-pressor mechanism. Hypertension. 1991 May;17(5):626–635. doi: 10.1161/01.hyp.17.5.626. [DOI] [PubMed] [Google Scholar]
- Harris P. J., Young J. A. Dose-dependent stimulation and inhibition of proximal tubular sodium reabsorption by angiotensin II in the rat kidney. Pflugers Arch. 1977 Jan 17;367(3):295–297. doi: 10.1007/BF00581370. [DOI] [PubMed] [Google Scholar]
- Iwamoto H. S., Rudolph A. M. Effects of angiotensin II on the blood flow and its distribution in fetal lambs. Circ Res. 1981 Feb;48(2):183–189. doi: 10.1161/01.res.48.2.183. [DOI] [PubMed] [Google Scholar]
- Jones O. W., 3rd, Cheung C. Y., Brace R. A. Dose-dependent effects of angiotensin II on the ovine fetal cardiovascular system. Am J Obstet Gynecol. 1991 Nov;165(5 Pt 1):1524–1533. doi: 10.1016/0002-9378(91)90400-l. [DOI] [PubMed] [Google Scholar]
- Kesby G. J., Lumbers E. R. Factors affecting renal handling of sodium, hydrogen ions, and bicarbonate by the fetus. Am J Physiol. 1986 Aug;251(2 Pt 2):F226–F231. doi: 10.1152/ajprenal.1986.251.2.F226. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum M. A., White N., Stein J. H., Ferris T. F. Redistribution of renal cortical blood flow during inhibition of prostaglandin synthesis. Am J Physiol. 1974 Oct;227(4):801–805. doi: 10.1152/ajplegacy.1974.227.4.801. [DOI] [PubMed] [Google Scholar]
- Levens N. R., Peach M. J., Carey R. M. Role of the intrarenal renin-angiotensin system in the control of renal function. Circ Res. 1981 Feb;48(2):157–167. doi: 10.1161/01.res.48.2.157. [DOI] [PubMed] [Google Scholar]
- Liu F. Y., Cogan M. G. Angiotensin II: a potent regulator of acidification in the rat early proximal convoluted tubule. J Clin Invest. 1987 Jul;80(1):272–275. doi: 10.1172/JCI113059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbers E. R., Burrell J. H., Menzies R. I., Stevens A. D. The effects of a converting enzyme inhibitor (captopril) and angiotensin II on fetal renal function. Br J Pharmacol. 1993 Oct;110(2):821–827. doi: 10.1111/j.1476-5381.1993.tb13886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbers E. R., Hill K. J., Bennett V. J. Proximal and distal tubular activity in chronically catheterized fetal sheep compared with the adult. Can J Physiol Pharmacol. 1988 Jun;66(6):697–702. doi: 10.1139/y88-111. [DOI] [PubMed] [Google Scholar]
- Lumbers E. R., Kingsford N. M., Menzies R. I., Stevens A. D. Acute effects of captopril, an angiotensin-converting enzyme inhibitor, on the pregnant ewe and fetus. Am J Physiol. 1992 May;262(5 Pt 2):R754–R760. doi: 10.1152/ajpregu.1992.262.5.R754. [DOI] [PubMed] [Google Scholar]
- Lumbers E. R., Lewes J. L. The actions of vasoactive drugs on fetal and maternal plasma renin activity. Biol Neonate. 1979;35(1-2):23–32. doi: 10.1159/000241150. [DOI] [PubMed] [Google Scholar]
- Lumbers E. R., Reid G. C. The actions of vasoactive compounds in the foetus and the effect of perfusion through the placenta on their biological activity. Aust J Exp Biol Med Sci. 1978 Feb;56(1):11–24. doi: 10.1038/icb.1978.2. [DOI] [PubMed] [Google Scholar]
- Lumbers E. R., Smith F. G., Stevens A. D. Measurement of net transplacental transfer of fluid to the fetal sheep. J Physiol. 1985 Jul;364:289–299. doi: 10.1113/jphysiol.1985.sp015745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magness R. R., Rosenfeld C. R., Faucher D. J., Mitchell M. D. Uterine prostaglandin production in ovine pregnancy: effects of angiotensin II and indomethacin. Am J Physiol. 1992 Jul;263(1 Pt 2):H188–H197. doi: 10.1152/ajpheart.1992.263.1.H188. [DOI] [PubMed] [Google Scholar]
- Mason R. T., Coghlan J. P., Denton D. A., Fei D. W., Scoggins B. A., Whitworth J. A. Haemodynamic and renin responses to prostacyclin infusion in Na depleted and Na restricted sheep. Prostaglandins. 1984 Apr;27(4):527–534. doi: 10.1016/0090-6980(84)90088-1. [DOI] [PubMed] [Google Scholar]
- McGiff J. C., Crowshaw K., Terragno N. A., Lonigro A. J. Release of a prostaglandin-like substance into renal venous blood in response to angiotensin II. Circ Res. 1970 Jul;27(1 Suppl 1):121–130. [PubMed] [Google Scholar]
- McGiff J. C., Vane J. R. Prostaglandins and the regulation of blood pressure. Kidney Int Suppl. 1975 Sep;:S262–S270. [PubMed] [Google Scholar]
- McLaughlin M. K., Brennan S. C., Chez R. A. Effects of indomethacin on sheep uteroplacental circulations and sensitivity to angiotensin II. Am J Obstet Gynecol. 1978 Oct 15;132(4):430–435. doi: 10.1016/0002-9378(78)90780-9. [DOI] [PubMed] [Google Scholar]
- Parisi V. M., Walsh S. W. Arachidonic acid metabolites and the regulation of placental and other vascular tone during pregnancy. Semin Perinatol. 1986 Oct;10(4):288–298. [PubMed] [Google Scholar]
- Rahman A. R., Motwani J. G., Lang C. C., Struthers A. D. Circulating angiotensin II and renal sodium handling in man: a dose-response study. Clin Sci (Lond) 1993 Aug;85(2):147–156. doi: 10.1042/cs0850147. [DOI] [PubMed] [Google Scholar]
- Robillard J. E., Gomez R. A., VanOrden D., Smith F. G., Jr Comparison of the adrenal and renal responses to angiotensin II fetal lambs and adult sheep. Circ Res. 1982 Jan;50(1):140–147. doi: 10.1161/01.res.50.1.140. [DOI] [PubMed] [Google Scholar]
- Ross M. G., Ervin G., Leake R. D., Fu P., Fisher D. A. Fetal lung liquid regulation by neuropeptides. Am J Obstet Gynecol. 1984 Oct 15;150(4):421–425. doi: 10.1016/s0002-9378(84)80151-9. [DOI] [PubMed] [Google Scholar]
- Rurak D. W. Plasma vasopressin levels during hypoxaemia and the cardiovascular effects of exogenous vasopressin in foetal and adult sheep. J Physiol. 1978 Apr;277:341–357. doi: 10.1113/jphysiol.1978.sp012275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. R., Fisher D. A. Ontogeny of the renin-angiotensin-aldosterone system in the fetal and newborn lamb. Pediatr Res. 1980 Feb;14(2):99–102. doi: 10.1203/00006450-198002000-00006. [DOI] [PubMed] [Google Scholar]
- Stevenson K. M., Lumbers E. R. Effects of indomethacin on fetal renal function, renal and umbilicoplacental blood flow and lung liquid production. J Dev Physiol. 1992 Jun;17(6):257–264. [PubMed] [Google Scholar]
- Thomsen K., Holstein-Rathlou N. H., Leyssac P. P. Comparison of three measures of proximal tubular reabsorption: lithium clearance, occlusion time, and micropuncture. Am J Physiol. 1981 Oct;241(4):F348–F355. doi: 10.1152/ajprenal.1981.241.4.F348. [DOI] [PubMed] [Google Scholar]
- Walker M. P., Moore T. R., Cheung C. Y., Brace R. A. Indomethacin-induced urinary flow rate reduction in the ovine fetus is associated with reduced free water clearance and elevated plasma arginine vasopressin levels. Am J Obstet Gynecol. 1992 Dec;167(6):1723–1731. doi: 10.1016/0002-9378(92)91767-5. [DOI] [PubMed] [Google Scholar]
- Wlodek M. E., Harding R., Thorburn G. D. Effects of inhibition of prostaglandin synthesis on flow and composition of fetal urine, lung liquid, and swallowed fluid in sheep. Am J Obstet Gynecol. 1994 Jan;170(1 Pt 1):186–195. doi: 10.1016/s0002-9378(94)70406-6. [DOI] [PubMed] [Google Scholar]


