Abstract
Background
Colorectal cancer (CRC) is a leading cause of cancer-related mortality, highlighting the necessity for multifaceted treatment strategies, including preoperative treatment (PT), which can enhance surgical outcomes and provide prognostic insights. This study aims to clarify the impact of PT-induced changes in mismatch repair (MMR) and human epidermal growth factor receptor 2 (HER2) expression, potentially informing tailored treatment strategies and improving clinical outcomes for CRC patients.
Methods
This retrospective study analyzed 120 paired samples from CRC patients who underwent preoperative treatment, comparing pre- and post-treatment specimens. A control group of 60 untreated surgical specimens was also included. Immunohistochemistry assessed MMR proteins (MSH6, MSH2, MLH1, PMS2) and HER2 expression. MSI status was determined in samples with low MMR expression.
Results
Compared to pre-treatment samples, post-treatment samples exhibited lower levels of MSH6, MSH2, and total MMR expression, along with higher levels of HER2 expression. However, when compared to the untreated control group, there were no significant differences in the expression of MSH6, total MMR, and HER2. All samples that exhibited weak MMR expression and those that shifted to deficient mismatch repair (dMMR) status following treatment had stable microsatellite status. No clear clinicopathological characteristics or prognostic factors were found to be associated with changes in MMR and HER2 expression, except for the use of fluorouracil or capecitabine, which was related to changes in total MMR scores. ypTNM stage and TRG scores were identified as independent factors affecting disease progression in our study.
Conclusions
PT is associated with a reduction in MMR expression, notably for the MSH2 protein, while it does not appear to influence HER2 expression.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-13120-w.
Keywords: Colorectal neoplasms, DNA mismatch repair, HER2, Neoadjuvant therapy, Microsatellite instability
Introduction
Colorectal cancer (CRC) stands as a significant contributor to cancer-related mortality globally, underscoring the need for a comprehensive, multifaceted approach to its management. While surgery is pivotal for treating localized CRC, the complexity of advanced or metastatic presentations at diagnosis can impede surgical efforts and jeopardize patient outcomes. In this regard, preoperative treatment (PT)- encompassing both neoadjuvant therapy and first line chemotherapy for unresectable tumor - has become integral to CRC management. These treatment modalities not only hold the potential to reduce tumor bulk and facilitate surgical resection but also to provide critical insights into treatment responses, thereby bolstering survival prospects [1].
Within the CRC molecular profile, the expression of mismatch repair (MMR) proteins and human epidermal growth factor receptor 2 (HER2) emerges as crucial, influencing both prognostic assessments and therapeutic strategies. MMR deficiency, frequently linked to microsatellite instability, is a positive prognostic indicator, particularly responsive to immunotherapy [2, 3], whereas HER2 overexpression possibly benefiting from targeted therapy [4, 5]. The clinical utility of accurately gauging these biomarkers underscores their importance.
It is within this context—where postoperative treatment still requires the guidance of these biomarkers—that the influence of preoperative treatment on the expression of MMR andHER2 becomes a topic of significant interest. Prior studies hint at treatment-induced alterations of these biomarkers, yet these are constrained by limitations in sample size or the lack of rigorous pre- and post-treatment comparatives [6–9]. The need for further exploration is thus imperative to clarify the clinical ramifications of such changes.
This study seeks to address this gap by analyzing changes in MMR and HER2 expression in CRC patients pre- and post-treatment and exploring the factors that may influence these alterations. By doing so, we aim to provide valuable insights that could inform personalized treatment approaches and improve clinical outcomes for CRC patients.
Materials and methods
Material source
The present study employed a comparative study design, enrolling two distinct groups for analysis. The exposed group comprises 120 pairs of CRC samples from patients who underwent treatment prior to surgery, diagnosed between October 2018 and September 2023. The control group consists of 60 surgically resected CRC samples from patients who had not received any treatment prior to surgery, all diagnosed within July 2023 (detailed in Figure S1, Supplemental Digital Content 1). The inclusion and exclusion criteria are as follows:
Inclusion criteria
Exposed Group: (1) Patients with a pathological diagnosis of primary colorectal cancer; (2) Received treatment prior to surgical resection; (3) Availability of both pre-treatment biopsy samples and surgical resection samples. (4) Complete clinical data on preoperative treatment regimens.
Control Group: (1) Patients with a pathological diagnosis of primary colorectal cancer; (2) No treatment (therapeutic treatment such as radiotherapy or systemic therapy for tumor growth or metastasis, excluding medical procedures such as biopsies for diagnostic purposes) received prior to surgical resection; (3) Availability of surgical resection samples.
Exclusion criteria
Exposed Group: (1) No residual tumor cells in the surgical resection sample or insufficient residual tumor for analysis; (2) Incomplete clinical data prior to surgery.
Control Group: (1) Diagnosis of dysplasia or carcinoma in situ; (2) Insufficient tumor cells in the sample for analysis.
Clinicopathological characteristics, tumor regression grade (TRG), treatment details, and KRAS mutation statuses were extracted from clinical records. The TNM staging adhered to the eighth edition of the AJCC staging system. Data for Progression-Free Survival (PFS) and Overall Survival (OS) were collected through clinical follow-ups and imaging records, with the follow-up period concluding in July 2024. All samples were sourced from the First Affiliated Hospital of Zhejiang University School of Medicine, with the study receiving approval from the hospital’s ethics committee.
Immunohistochemistry (IHC) analysis
All samples were fixed in 10% neutral buffered formalin and embedded in paraffin. The Leica Bond-III automated immunohistochemistry platform, using the Bond Polymer Refine detection kits and mouse monoclonal antibodies (MSH6, MSH2, MLH1, PMS2), was employed for MMR detection. HER2 expression was detected using the Roche Ventana (Tucson, AZ) fully automated immunohistochemistry system. The antibody information is detailed in Table S1(Supplemental Digital Content 2). Two experienced pathologists (W.Z., X.T.) independently scored the slides, evaluating both staining intensity and extent. Discordant results were resolved through consensus discussions.
MMR expression was scored following the criteria established by by A. Vilkin et al. [8] with percentage positivity ranging from 0 to 4 and staining intensity from 0 to 3. The overall score for each slide was calculated by multiplying these two scores. The total MMR score was the sum of the individual scores for MSH6, MSH2, MLH1, and PMS2. Proficient MMR (pMMR) was indicated by any nuclear staining in tumor cells, while deficient MMR (dMMR) was defined by a score of 0 for one or more MMR proteins. HER2 expression scoring was based on the criteria by S. Fujii et al. [10].
Expression change was categorized as follows: unchanged (pre- and post-treatment scores were identical), increased (post-treatment score exceeded pre-treatment score), and reduced (post-treatment score was lower than pre-treatment score).
Microsatellite instability (MSI) testing
MSI analysis was conducted on samples with an MMR score below 6, using the PCR-capillary electrophoresis method. Tumor tissues were selected from areas rich in tumor cells, as determined by reference HE slides, with normal control tissue obtained from the surgical resection margin. DNA extraction utilized an automated nucleic acid extraction system (HF48, Cencert, China) and its FFPE nucleic acid extraction kit. Six microsatellite loci (NR-21, NR-24, NR-27, BAT-25, BAT-26, MONO-27) were amplified for MSI testing (Sinomd, China), with fragment analysis performed on ABI 3500Dx Genetic Analyzer (Thermo Fisher Scientific, USA).
Statistical analysis
The Wilcoxon test was applied to compare paired sample scores pre- and post-treatment. The Mann-Whitney test was used to compare scores between the exposed and control groups. Categorical data were analyzed using chi-square or Fisher’s exact tests as appropriate. Log-rank test was used to compare survival between subgroups. The Cox proportional hazards model was employed to identify factors associated with survival, estimating hazard ratios and their 95% confidence intervals. PFS is defined as the time span from the date of surgical resection to the first postoperative imaging assessment that confirms disease progression. OS is defined as the time span from the date of surgical resection to the date of death from any cause. All analyses were performed using SPSS 22.0 software (IBM, USA), with a P-value of less than 0.05 considered statistically significant.
Results
Patient information
The exposed group included 120 CRC patients (84 males, 36 females), aged 28 to 80 years, with a mean age of 59.3 years. Tumor locations included 25 in the left colon, 17 in the right colon, and 78 in the rectum. A total of 87 patients were administered chemotherapy, while 30 patients received concurrent chemoradiotherapy. Additionally, 3 patients exclusively received immunotherapy. Detailed demographics and treatment regimens are presented in Table S2 (Supplemental Digital Content 2).
Changes in MMR expression after PT
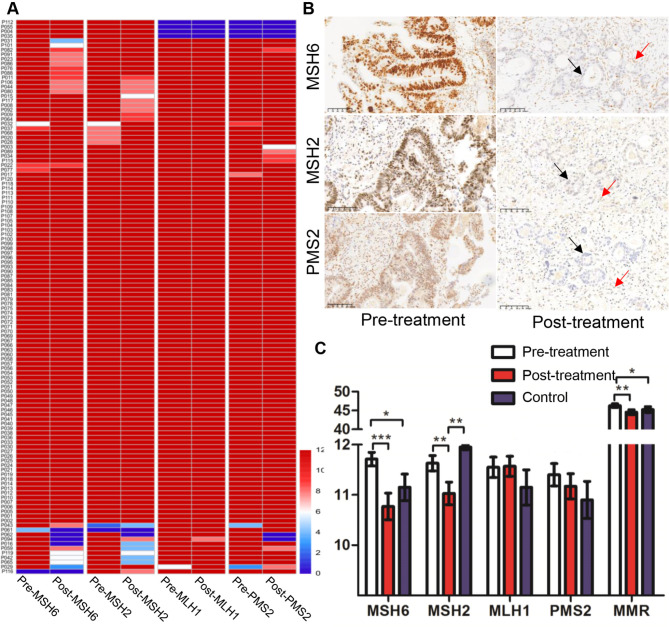
MMR expression loss was observed in 6 pre-treatment biopsy samples (1 MSH6, 1 MSH2, 4 MLH1 and PMS2). Post-treatment surgical samples revealed loss in 9 cases (4 MSH6, 2 PMS2, 2 MSH6 and MSH2, 4 MLH1 and PMS2) (Fig. 1A). Three cases showed inconsistent MMR expression post-treatment, all transitioning from pMMR to dMMR (Fig. 1B; Table 1).
Fig. 1.
MMR expression in colorectal cancer before and after preoperative treatment. (A) Heatmap of MMR proteins expression scores pre- and post-treatment; (B) Representative cases of MMR expression conversion after preoperative treatment (↑ positive staining of normal cells, ↑ negative staining of tumor cells); (C) Comparison of MMR scores between pre- and post- treatment and the control group. *P < 0.05, ** P < 0.01, ***P < 0.001
Table 1.
Clinicopathological characteristics of patients with colorectal cancer stratified by MMR expression conversion
| ID | Gender | Age | Tumor location | MSH6 | MSH2 | MLH1 | PMS2 | TRG | Treatment regimen |
|---|---|---|---|---|---|---|---|---|---|
| pre /post | pre /post | pre /post | pre /post | ||||||
| P016 | Male | 68y | Sigmoid colon | + / + | + / - | + / + | + / + | 3 | XELOXIRI plus bevacizumab (8 cycles) |
| P062 | Female | 78y | Rectum | + / - | + / - | + / + | + / - | 1 | XELOX (2 cycles) followed by FOLFOX6 plus cetuximab (3 cycles) |
| P094 | Female | 69y | Rectum | + / - | + / + | + / + | + / - | 1 | Radiotherapy plus capecitabine |
TRG: tumor regression grade; + positive; - negative
Pre-treatment samples demonstrated significantly higher scores for MSH6 (P < 0.001), MSH2 (P < 0.01), and total MMR score (P < 0.01) compared to post-treatment samples. However, no significant differences were noted for MLH1 (P = 0.655) and PMS2 (P = 0.324) (Fig. 1C).
When compared to the control group’s surgical samples, post-treatment surgical samples showed significantly lower MSH2 scores and higher MLH1 scores, with no significant differences in MSH6, PMS2, and total MMR scores (Fig. 1C). Heterogeneous staining for MSH6 was common in surgical samples, regardless of treatment, primarily manifesting as varied staining within the same gland rather than between different regions (Figure S2, Supplemental Digital Content).
Analysis of MSI status in cases with deficient or weak MMR expression
22 samples from 14 patients who with a score less than 6 underwent MSI status analysis. Among the 5 pre-treatment dMMR cases, both pre- and post-treatment samples were classified as MSI-H (Table 2). For the remaining dMMR and weakly MMR expressing cases, both pre- and post-treatment samples were classified as MSS.
Table 2.
Analysis of MSI status in cases with deficient or weakly expressed MMR proteins
| ID | Samples | Scores of MSH6 | Scores of MSH2 | Scores of MLH1 | Scores of PMS2 | MMR | MSI status |
|---|---|---|---|---|---|---|---|
| P004 | pre-treatment | 12 | 12 | 0 | 0 | dMMR | MSI-H |
| post-treatment | 12 | 12 | 0 | 0 | dMMR | MSI-H | |
| P035 | pre-treatment | 12 | 12 | 0 | 0 | dMMR | N/A |
| post-treatment | 12 | 12 | 0 | 0 | dMMR | N/A | |
| P055 | pre-treatment | 12 | 12 | 0 | 0 | dMMR | MSI-H |
| post-treatment | 12 | 12 | 0 | 0 | dMMR | MSI-H | |
| P061 | pre-treatment | 4 | 0 | 12 | 12 | dMMR | MSI-H |
| post-treatment | 0 | 0 | 12 | 12 | dMMR | MSI-H | |
| P112 | pre-treatment | 12 | 12 | 0 | 0 | dMMR | MSI-H |
| post-treatment | 12 | 12 | 0 | 0 | dMMR | MSI-H | |
| P116 | pre-treatment | 0 | 12 | 12 | 12 | dMMR | MSI-H |
| post-treatment | 0 | 8 | 12 | 12 | dMMR | MSI-H | |
| P029 | pre-treatment | 12 | 12 | 6 | 3 | pMMR | MSS |
| post-treatment | 3 | 12 | 12 | 8 | pMMR | N/A | |
| P043 | pre-treatment | 12 | 2 | 12 | 4 | pMMR | MSS |
| post-treatment | 8 | 4 | 12 | 12 | pMMR | MSS | |
| P016 | pre-treatment | 12 | 12 | 12 | 12 | pMMR | MSS |
| post-treatment | 0 | 4 | 12 | 12 | dMMR | MSS | |
| P031 | pre-treatment | 12 | 12 | 12 | 12 | pMMR | MSS |
| post-treatment | 4 | 12 | 12 | 12 | pMMR | N/A | |
| P042 | pre-treatment | 12 | 12 | 12 | 12 | pMMR | MSS |
| post-treatment | 6 | 4 | 12 | 12 | pMMR | N/A | |
| P059 | pre-treatment | 12 | 12 | 12 | 12 | pMMR | MSS |
| post-treatment | 8 | 4 | 12 | 8 | pMMR | N/A | |
| P062 | pre-treatment | 12 | 12 | 12 | 12 | pMMR | MSS |
| post-treatment | 0 | 0 | 12 | 0 | dMMR | MSS | |
| P065 | pre-treatment | 12 | 12 | 12 | 12 | pMMR | MSS |
| post-treatment | 6 | 4 | 12 | 12 | pMMR | N/A | |
| P094 | pre-treatment | 12 | 12 | 12 | 12 | pMMR | MSS |
| post-treatment | 0 | 8 | 8 | 0 | dMMR | N/A |
MMR: mismatch repair; dMMR: deficient MMR; pMMR: proficient MMR; MSI-H: microsatellite instability-high; MSS: microsatellite stable; N/A: not available
Correlation between changes in MMR expression and clinicopathologic features
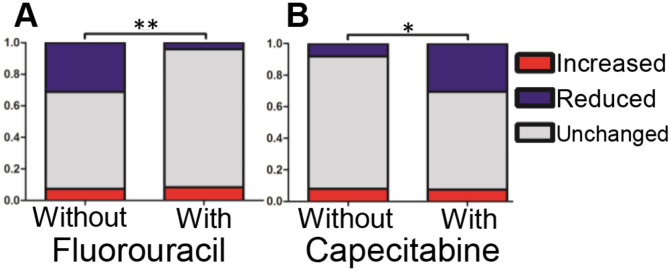
We attempted to compare the changes in protein expression with various clinicopathological features and found that, PMS2 was found to be associated with gender. However, changes in MSH6, MSH2, and MLH1 did not correlate with any clinicopathological features (Table S3, Supplemental Digital Content 2). The change in total MMR score was significantly associated with fluorouracil (5-FU) and capecitabine treatment. Specifically, cases with reduced MMR expression treated with 5-FU were significantly fewer compared to untreated cases (Fig. 2A). In contrast, cases with reduced MMR expression treated with capecitabine were significantly more frequent compared to untreated cases (Fig. 2B).
Fig. 2.
Relationship between fluorouracil (A) and capecitabine (B) and changes in total MMR scores after preoperative treatment. *P < 0.05, ** P < 0.01
Changes in HER2 expression after PT
A total of 99 cases underwent HER2 testing before and after PT (Fig. 3A). Post-treatment samples showed significantly higher HER2 scores compared to pre-treatment samples (P < 0.001; Fig. 3B), while control group scores were not significantly different. No clinicopathological features were identified as associated with HER2 expression changes except cTNM stage (Table S3, Supplemental Digital Content 2).
Fig. 3.
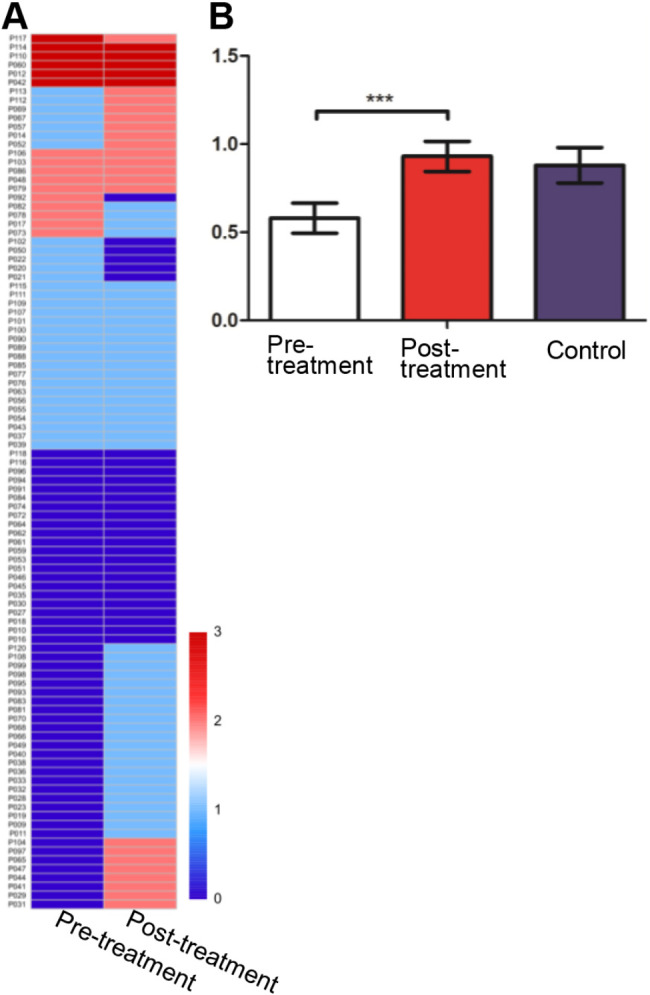
HER2 expression in colorectal cancer before and after preoperative treatment. (A) Heatmap of HER2 expression scores pre- and post-treatment; (B) Comparison of HER2 scores between pre- and post- treatment and the control group. ***P < 0.001
Prognostic analysis results
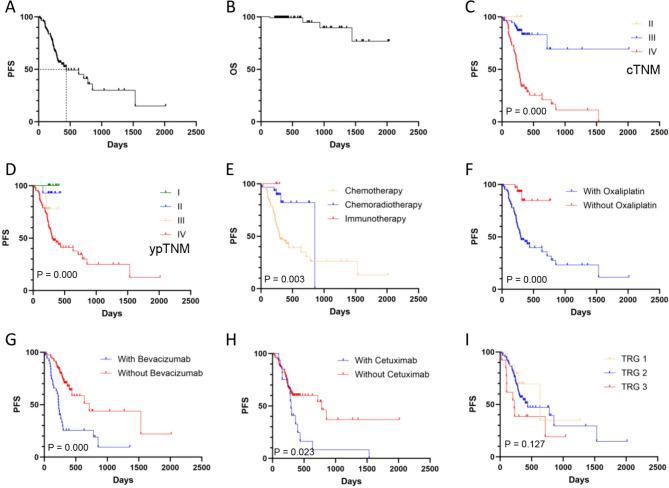
Prognostic analysis results indicated that within the exposed group, seven patients had incomplete data regarding disease progression or overall survival. Specifically, three lacked progression information, three were missing survival data, and one patient was lost to follow-up. As of the final follow-up date, four patients had deceased due to cancer-related causes. The average PFS and OS were 791.3 days and 1823.3 days, respectively (Fig. 4A-B, Table S4, Supplemental Digital Content 2).
Fig. 4.
Prognostic analysis in patients with CRC following preoperative treatment. Panel A represents the PFS curve, while Panel B represents the OS curve. Panels C-I represent the comparisons of PFS across various subgroups
Supporting information
Univariate analysis revealed significant associations between PFS and cTNM stage, ypTNM stage, treatment modalities, KRAS mutation and the use of oxaliplatin, bevacizumab, or cetuximab (Fig. 4C-H, Table S4, Supplemental Digital Content 2). However, neither the baseline expression of MMR proteins and HER2, nor the changes in their expression following treatment, showed significantly associated with PFS (Table S4, Supplemental Digital Content 2). Although univariate analysis did not identify an association between TRG scores and PFS, multivariate analysis demonstrated TRG as an independent predictor of PFS (Fig. 4I, Table S4, Supplemental Digital Content 2). Additionally, ypTNM stage was also identified as an independent factor influencing PFS in the multivariate assessment (Table S4, Supplemental Digital Content 2).
We further analyzed the relationship between MSH6, MSH2, total MMR, HER2, and their expression changes post-treatment with PFS across different clinical stages. The results indicated that in metastatic colorectal cancer, the changes in HER2 expression were significantly associated with PFS. In contrast, MMR and its expression changes did not exhibit an association with PFS, regardless of the disease being in a localized or metastatic stage (Table S5, Supplemental Digital Content 2).
It is noteworthy that no statistically significant associations were identified between any of the evaluated factors and OS (data not shown), potentially due to the limited sample size and the short follow-up duration.
Discussion
Precision medicine has increasingly shaped CRC treatment, with biomarkers such as KRAS, BRAF, MMR, and HER2 playing pivotal roles. However, the choice of specimen for biomarker testing can be influenced by tumor heterogeneity, potentially affecting treatment decisions. In untreated CRC samples, MMR expression is generally consistent between biopsy and surgical samples [9, 11], despite stronger MLH1 staining in biopsy samples [12].
Our study, in line with previous research [6, 8, 13, 14], finds that preoperative treatment can reduce MMR protein expression, especially MSH6 and MSH2. This reduction is likely due to treatment effects rather than sample variability, as untreated controls show stable MSH2 levels. Consistent with previous studies [6, 13, 14] the pMMR cases with reduced or loss MMR expression after treatment still maintain stable microsatellite status, reaffirming the importance of pre-treatment biopsies for accurate MMR evaluation.
The mechanisms underlying the reduction of MMR expression caused by treatment are currently unclear, with several hypotheses proposed. Treatment may induce secondary genetic mutations affecting MMR expression. Chemotherapy or radiation therapy-induced DNA damage can disrupt cellular signaling pathways and physically alter genomic DNA [15, 16]. Alternatively, treatment may induce a state of cell quiescence or low proliferation, leading to decreased overall transcription levels in tumor cells, as reflected by a reduced Ki67 index post-treatment [13]. Additionally, the hypoxic microenvironment induced by treatment could contribute to MMR expression dysregulation, a widely accepted rationale [17]. However, suboptimal tissue fixation can also lead to inadequate immunohistochemical staining, identifiable by comparing internal control staining.
We also observed a potential influence of fluorouracil and capecitabine on MMR expression, which has not been extensively explored in the literature. This finding is significant and suggests that the choice of chemotherapeutic agents may have implications for biomarker assessment and treatment planning in CRC.
In contrast to breast cancer [18, 19], the impact of treatment on HER2 expression in colorectal cancer (CRC) is less established. Our study observed a trend towards increased HER2 expression following treatment, though not significantly different from controls. This suggests that changes may be due to tumor heterogeneity rather than treatment effects.
The TRG score is well-documented for its prognostic utility in CRC patients undergoing neoadjuvant therapy [20]. The independent prognostic significance of TRG score in our study suggests its potential as a favorable prognostic indicator for patients undergoing initial treatment for advanced CRC, even when the treatment is palliative in intent. While the literature supports the role of dMMR/MSI-H as a predictor of good prognosis in localized CRC [21, 22], and HER2 positivity as an indicator of poor prognosis [23], these associations were not evident in our study. This discrepancy may be attributed to the small sample size of patients with dMMR/MSI-H and HER2-positive status (n = 6, both).
The lack of statistically significant between changes in MMR expression and PFS implies that the response of tumor cells to treatment may not be significantly associated with MMR expression levels. Additionally, in metastatic CRC, patients with reduced HER2 expression (n = 4) exhibited earlier disease recurrence. This implies that HER2-expressing tumor cells are less likely to survive treatment, or that treatment activates other signaling pathways thereby attenuating the tumor’s dependence on HER2, which needs to be studied on a larger scale due to the small number of cases.
While our study provides valuable insights into the impact of preoperative treatment on MMR and HER2 expressions in CRC, it is important to acknowledge its limitations. Firstly, the retrospective design of our study introduces inherent complexities, such as variability in treatment regimens and durations, which may affect the consistency of our findings. Secondly, despite representing one of the largest paired-sample analyses to date, our study includes a relatively small number of cases in certain critical subgroups, such as those with dMMR or high HER2 expression. This limitation could introduce bias and variability into our results, potentially impacting the generalizability of our findings. Therefore, future prospective studies with larger and more diverse cohorts are necessary to validate our results and to further explore the clinical implications of these biomarkers in CRC treatment strategies.
In conclusion, our findings indicate that preoperative treatment in CRC patients is associated with a reduction in MMR protein expression, particularly MSH2, and does not significantly influence HER2 expression. The lack of correlation between changes in MMR expression and PFS suggests that other factors may be more critical in determining treatment response. These observations underscore the importance of further research to clarify the mechanisms behind these changes and to refine the use of these biomarkers in personalized CRC treatment approaches.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our gratitude to all our colleagues for their valuable contributions and support.
Abbreviations
- 5-FU
Fluorouracil
- CRC
Colorectal cancer
- dMMR
Deficient mismatch repair
- MMR
Mismatch repair
- MSI
Microsatellite instability
- OS
Overall survival
- pMMR
Proficient mismatch repair
- PFS
Progression-free survival
- PT
Preoperative treatment
- TRG
Tumor regression grade
Author contributions
X.T. contributed to the conception and design of the study. Z.C. wrote the original draft. Z.C., H.C., J.Z., and W.Z. performed the data acquisition and formal analysis. H.C. and J.Z. contributed to the investigation and resources. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Beijing Jingjian Foundation for Advancement Pathology (JJTS2020-003).
Data availability
The datasets used and during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This research was conducted with the approval of the Institutional Review Board (IRB) of the First Affiliated Hospital, Zhejiang University School of Medicine. Informed consent was exempted as per the IRB guidelines.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal Cancer: a review. JAMA. 2021;325(7):669–85. [DOI] [PubMed] [Google Scholar]
- 2.Taieb J, Svrcek M, Cohen R, Basile D, Tougeron D, Phelip JM. Deficient mismatch repair/microsatellite unstable colorectal cancer: diagnosis, prognosis and treatment. Eur J Cancer. 2022;175:136–57. [DOI] [PubMed] [Google Scholar]
- 3.Lote H, Starling N, Pihlak R, Gerlinger M. Advances in immunotherapy for MMR proficient colorectal cancer. Cancer Treat Rev. 2022;111:102480. [DOI] [PubMed] [Google Scholar]
- 4.Zheng-Lin B, Bekaii-Saab TS. Treatment options for HER2-expressing colorectal cancer: updates and recent approvals. Ther Adv Med Oncol. 2024;16:17588359231225037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng X, Wang R, Huang Z, Zhang J, Feng R, Xu X, Zhu K, Dou X, Chen D, Yu J. Human epidermal growth factor receptor-2 expression in locally advanced rectal cancer: association with response to neoadjuvant therapy and prognosis. Cancer Sci. 2014;105(7):818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein JB, Wu W, Borras E, Masand G, Cuddy A, Mork ME, Bannon SA, Lynch PM, Rodriguez-Bigas M, Taggart MW, et al. Can microsatellite status of Colorectal Cancer be reliably assessed after Neoadjuvant Therapy? Clin Cancer Res. 2017;23(17):5246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ondrejka SL, Schaeffer DF, Jakubowski MA, Owen DA, Bronner MP. Does neoadjuvant therapy alter KRAS and/or MSI results in rectal adenocarcinoma testing? Am J Surg Pathol. 2011;35(9):1327–30. [DOI] [PubMed] [Google Scholar]
- 8.Vilkin A, Halpern M, Morgenstern S, Brazovski E, Gingold-Belfer R, Boltin D, Purim O, Kundel Y, Welinsky S, Brenner B, et al. How reliable is immunohistochemical staining for DNA mismatch repair proteins performed after neoadjuvant chemoradiation? Hum Pathol. 2014;45(10):2029–36. [DOI] [PubMed] [Google Scholar]
- 9.Shia J, Stadler Z, Weiser MR, Rentz M, Gonen M, Tang LH, Vakiani E, Katabi N, Xiong X, Markowitz AJ, et al. Immunohistochemical staining for DNA mismatch repair proteins in intestinal tract carcinoma: how reliable are biopsy samples? Am J Surg Pathol. 2011;35(3):447–54. [DOI] [PubMed] [Google Scholar]
- 10.Fujii S, Magliocco AM, Kim J, Okamoto W, Kim JE, Sawada K, Nakamura Y, Kopetz S, Park WY, Tsuchihara K, et al. International Harmonization of Provisional Diagnostic Criteria for ERBB2-Amplified metastatic colorectal Cancer allowing for screening by next-generation sequencing panel. JCO Precis Oncol. 2020;4:6–19. [DOI] [PubMed] [Google Scholar]
- 11.Vilkin A, Leibovici-Weissman Y, Halpern M, Morgenstern S, Brazovski E, Gingold-Belfer R, Wasserberg N, Brenner B, Niv Y, Sneh-Arbib O, et al. Immunohistochemistry staining for mismatch repair proteins: the endoscopic biopsy material provides useful and coherent results. Hum Pathol. 2015;46(11):1705–11. [DOI] [PubMed] [Google Scholar]
- 12.Grillo F, Paudice M, Gambella A, Bozzano S, Sciallero S, Puccini A, Lastraioli S, Dono M, Parente P, Vanoli A, et al. Evaluating mismatch repair deficiency in colorectal cancer biopsy specimens. Histochem Cell Biol. 2023;160(2):113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuan SF, Ren B, Brand R, Dudley B, Pai RK. Neoadjuvant therapy in microsatellite-stable colorectal carcinoma induces concomitant loss of MSH6 and Ki-67 expression. Hum Pathol. 2017;63:33–9. [DOI] [PubMed] [Google Scholar]
- 14.Bao F, Panarelli NC, Rennert H, Sherr DL, Yantiss RK. Neoadjuvant therapy induces loss of MSH6 expression in colorectal carcinoma. Am J Surg Pathol. 2010;34(12):1798–804. [DOI] [PubMed] [Google Scholar]
- 15.Buonanno M, de Toledo SM, Pain D, Azzam EI. Long-term consequences of radiation-induced bystander effects depend on radiation quality and dose and correlate with oxidative stress. Radiat Res. 2011;175(4):405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002;2(3):188–200. [DOI] [PubMed] [Google Scholar]
- 17.Shia J, Zhang L, Shike M, Guo M, Stadler Z, Xiong X, Tang LH, Vakiani E, Katabi N, Wang H, et al. Secondary mutation in a coding mononucleotide tract in MSH6 causes loss of immunoexpression of MSH6 in colorectal carcinomas with MLH1/PMS2 deficiency. Mod Pathol. 2013;26(1):131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida A, Hayashi N, Suzuki K, Takimoto M, Nakamura S, Yamauchi H. Change in HER2 status after neoadjuvant chemotherapy and the prognostic impact in patients with primary breast cancer. J Surg Oncol. 2017;116(8):1021–8. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Fan H, Xiang Q, Xu L, Zhang Z, Liu Q, Zhang T, Ling J, Zhou Y, Zhao X, et al. Prognostic value of receptor status conversion following neoadjuvant chemotherapy in breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;178(3):497–504. [DOI] [PubMed] [Google Scholar]
- 20.Trakarnsanga A, Gönen M, Shia J, Nash GM, Temple LK, Guillem JG, Paty PB, Goodman KA, Wu A, Gollub M et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst 2014, 106(10). [DOI] [PMC free article] [PubMed]
- 21.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, Richman S, Chambers P, Seymour M, Kerr D, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29(10):1261–70. [DOI] [PubMed] [Google Scholar]
- 23.Ingold Heppner B, Behrens HM, Balschun K, Haag J, Krüger S, Becker T, Röcken C. HER2/neu testing in primary colorectal carcinoma. Br J Cancer. 2014;111(10):1977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and during the current study are available from the corresponding author on reasonable request.