Abstract
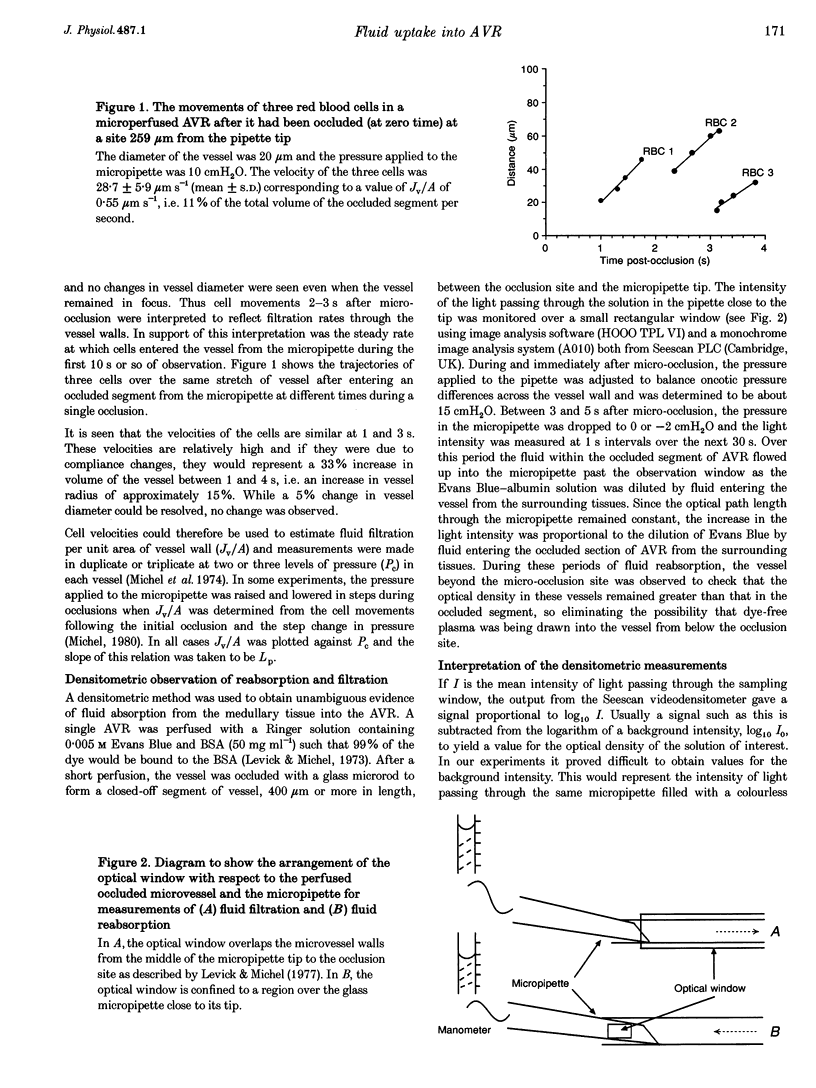
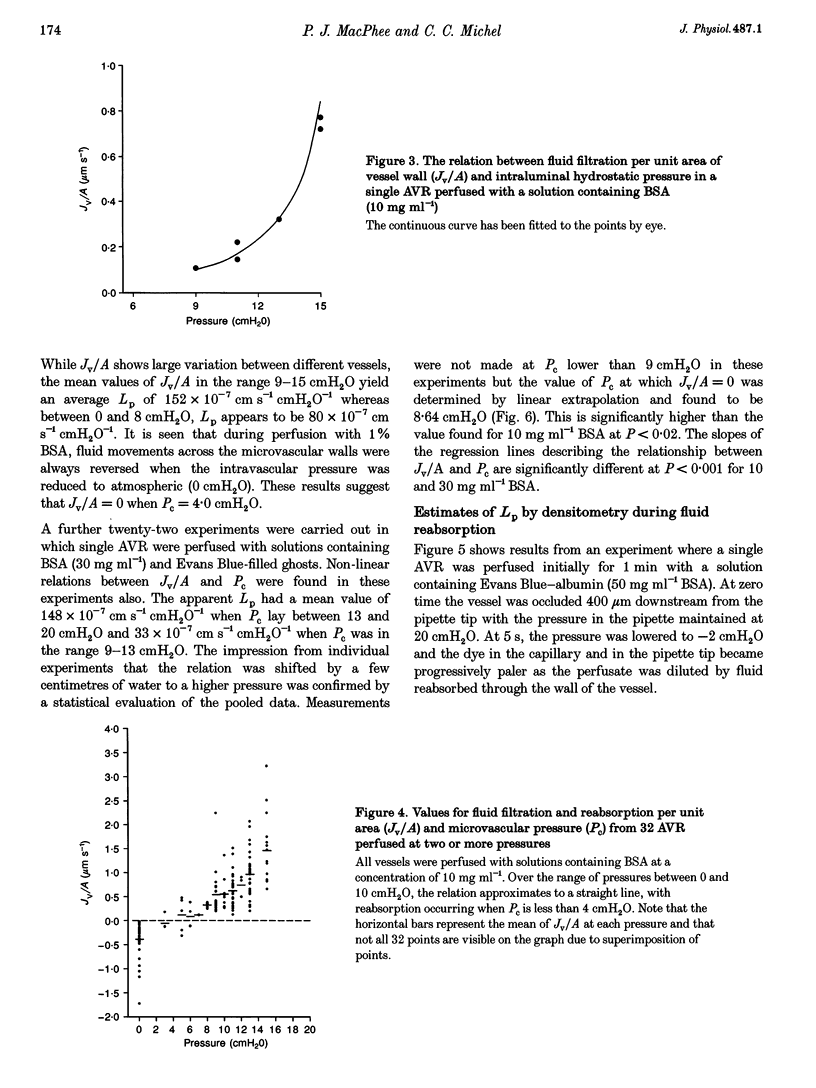
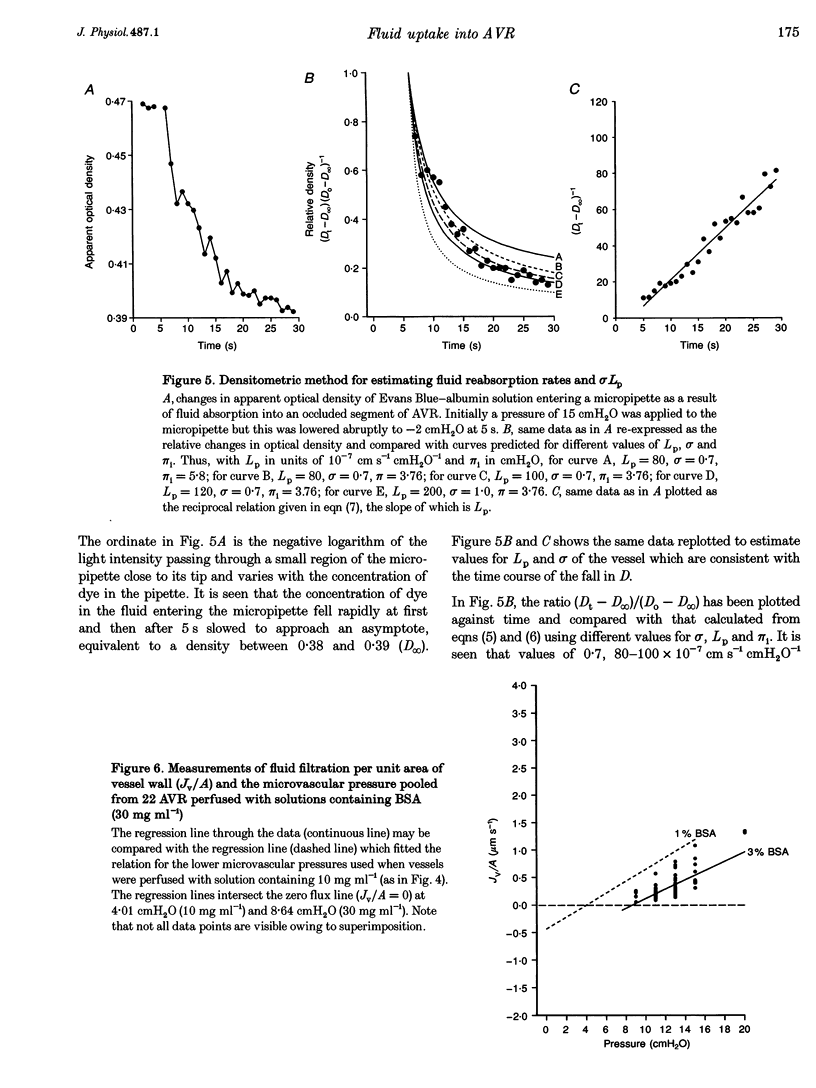
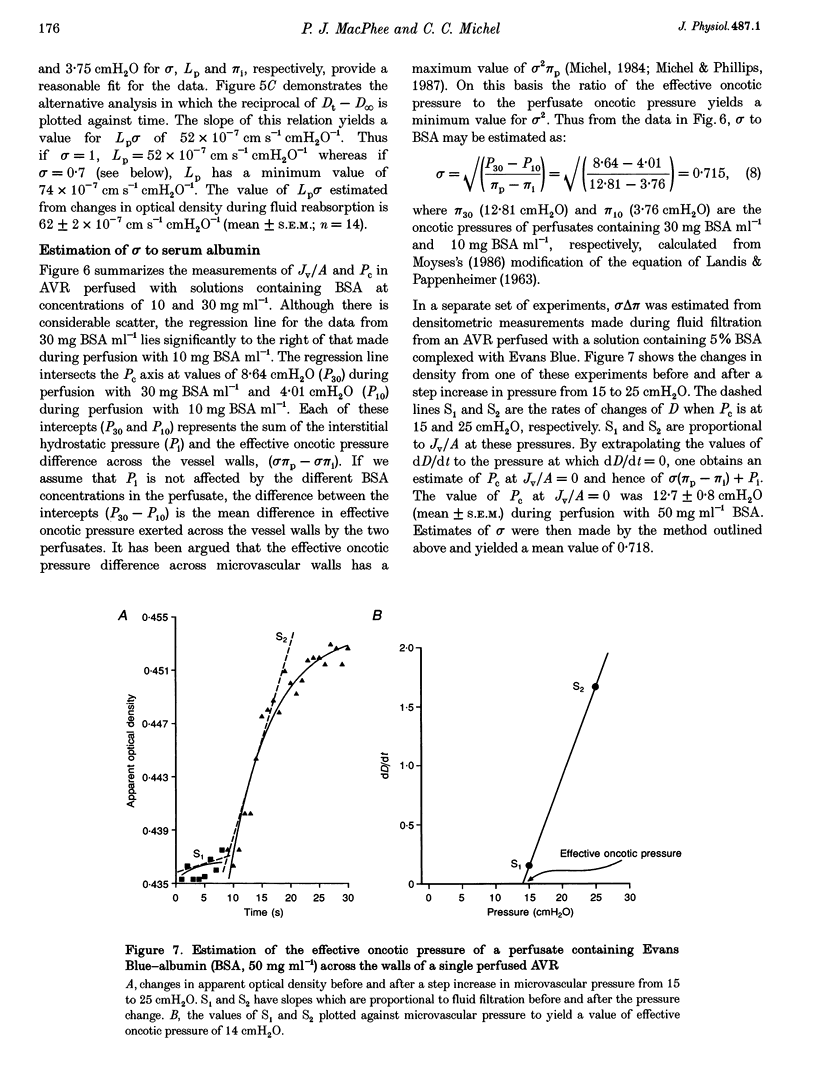
1. We have investigated fluid movements between superficial ascending vasa recta (AVR) and the interstitium of exposed papillae of the renal medullae in 15-day-old Sprague-Dawley rats anaesthetized with Hypnorm and Hypnovel. 2. Using a development of the red cell micro-occlusion technique, fluid filtration and reabsorption rates per unit area of vessel wall (Jv/A) were determined in 54 single perfused AVR at known microvascular pressures (Pc). The relation between Jv/A and Pc was non-linear suggesting hydraulic permeabilities (Lp) of 50-100 x 10(-7) cm s-1 cmH2O-1 when Pc was between 0-10 cmH2O and 150-200 x 10(-7) cm s-1 cmH2O-1 when Pc was 10-15 cmH2O. 3. Rates of fluid reabsorption into the AVR estimated by a densitometric technique in a further fourteen vessels were consistent with Lp values of 50-100 x 10(-7) cm s-1 cmH2O-1 when Pc was -2 to 0 cmH2O. 4. The effective oncotic pressures of perfusates containing bovine serum albumin (BSA) were consistent with minimum values for the reflection coefficients of the walls of the AVR to BSA of between 0.59 and 0.72. 5. The concentration of native serum albumin in the papillary interstitial fluid was 9.1 +/- 0.6 mg ml-1 (mean +/- S.E.M., n = 16, from 9 rats), which is approximately 25% of the plasma level. 6. After their microinjection into the medullary interstitium, Patent Blue V and Evans Blue-albumin cleared within 1 min. There was no evidence of preferential movement of either dye towards the base of the exposed renal medulla. 7. Because Lp of the AVR is high, mean pressures of only approximately 3 cmH2O are necessary to account for the total clearance of fluid from the medullary interstitium into the AVR. From published data and from our own observations, it appears that differences in hydrostatic and oncotic pressure across the walls of the AVR are more than sufficient to provide this driving force. The possibility of the clearance of protein from the interstitium into the AVR is discussed.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aukland K., Bogusky R. T., Renkin E. M. Renal cortical interstitium and fluid absorption by peritubular capillaries. Am J Physiol. 1994 Feb;266(2 Pt 2):F175–F184. doi: 10.1152/ajprenal.1994.266.2.F175. [DOI] [PubMed] [Google Scholar]
- Boberg U., Müller-Suur C., Persson A. E. Renal interstitial pressure measured in the subcapsular space using an in vivo oncometer. Acta Physiol Scand. 1985 Nov;125(3):377–381. doi: 10.1111/j.1748-1716.1985.tb07732.x. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Ueki I. F., Daugharty T. M. On estimating colloid osmotic pressure in pre- and postglomerular plasma in the rat. Kidney Int. 1972 Jul;2(1):51–53. doi: 10.1038/ki.1972.68. [DOI] [PubMed] [Google Scholar]
- Casley-Smith J. R. A theoretical support for the transport of macromolecules by osmotic flow across a leaky membrane against a concentration gradient. Microvasc Res. 1975 Jan;9(1):43–48. doi: 10.1016/0026-2862(75)90050-3. [DOI] [PubMed] [Google Scholar]
- Casley-Smith J. R. Endothelial fenestrae in intestinal villi: differences between the arterial and venous ends of the capillaries. Microvasc Res. 1971 Jan;3(1):49–68. doi: 10.1016/0026-2862(71)90006-9. [DOI] [PubMed] [Google Scholar]
- Cuttino J. T., Jr, Clark R. L., Jennette J. C. Microradiographic demonstration of human intrarenal microlymphatic pathways. Urol Radiol. 1989;11(2):83–87. doi: 10.1007/BF02926482. [DOI] [PubMed] [Google Scholar]
- Cuttino J. T., Jr, Jennette J. C., Clark R. L., Kwock L. Renal medullary lymphatics: microradiographic, light, and electron microscopic studies in pigs. Lymphology. 1985 Mar;18(1):24–30. [PubMed] [Google Scholar]
- Holliger C., Lemley K. V., Schmitt S. L., Thomas F. C., Robertson C. R., Jamison R. L. Direct determination of vasa recta blood flow in the rat renal papilla. Circ Res. 1983 Sep;53(3):401–413. doi: 10.1161/01.res.53.3.401. [DOI] [PubMed] [Google Scholar]
- LASSEN N. A., LONGLEY J. B., LILIENFIELD L. S. Concentration of albumin in renal papilla. Science. 1958 Sep 26;128(3326):720–721. doi: 10.1126/science.128.3326.720. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., OGSTON A. G. THE INTERACTION BETWEEN POLYSACCHARIDES AND OTHER MACROMOLECULES. 4. THE OSMOTIC PRESSURE OF MIXTURES OF SERUM ALBUMIN AND HYALURONIC ACID. Biochem J. 1963 Nov;89:249–253. doi: 10.1042/bj0890249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R. Capillary filtration-absorption balance reconsidered in light of dynamic extravascular factors. Exp Physiol. 1991 Nov;76(6):825–857. doi: 10.1113/expphysiol.1991.sp003549. [DOI] [PubMed] [Google Scholar]
- Levick J. R., Michel C. C. A densitometric method for determining the filtration coefficients of single capillaries in the frog mesentery. Microvasc Res. 1977 Mar;13(2):141–151. doi: 10.1016/0026-2862(77)90081-4. [DOI] [PubMed] [Google Scholar]
- Levick J. R., Michel C. C. The permeability of individually perfused frog mesenteric capillaries to T1824 and T1824-albumin as evidence for a large pore system. Q J Exp Physiol Cogn Med Sci. 1973 Jan;58(1):67–85. doi: 10.1113/expphysiol.1973.sp002192. [DOI] [PubMed] [Google Scholar]
- Michel C. C. Filtration coefficients and osmotic reflexion coefficients of the walls of single frog mesenteric capillaries. J Physiol. 1980 Dec;309:341–355. doi: 10.1113/jphysiol.1980.sp013512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C., Mason J. C., Curry F. E., Tooke J. E., Hunter P. J. A development of the Landis technique for measuring the filtration coefficient of individual capillaries in the frog mesentery. Q J Exp Physiol Cogn Med Sci. 1974 Oct;59(4):283–309. doi: 10.1113/expphysiol.1974.sp002275. [DOI] [PubMed] [Google Scholar]
- Michel C. C., Phillips M. E. Steady-state fluid filtration at different capillary pressures in perfused frog mesenteric capillaries. J Physiol. 1987 Jul;388:421–435. doi: 10.1113/jphysiol.1987.sp016622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C. The Malpighi lecture. Vascular permeability--the consequence of Malpighi's hypothesis. Int J Microcirc Clin Exp. 1985;4(3):265–284. [PubMed] [Google Scholar]
- Nash G. B., Meiselman H. J. Effects of preparative procedures on the volume and content of resealed red cell ghosts. Biochim Biophys Acta. 1985 May 28;815(3):477–485. doi: 10.1016/0005-2736(85)90376-1. [DOI] [PubMed] [Google Scholar]
- Ogston A. G., Michel C. C. General descriptions of passive transport of neutral solute and solvent through membranes. Prog Biophys Mol Biol. 1978;34(3):197–217. doi: 10.1016/0079-6107(79)90018-x. [DOI] [PubMed] [Google Scholar]
- Pallone T. L. Effect of sodium chloride gradients on water flux in rat descending vasa recta. J Clin Invest. 1991 Jan;87(1):12–19. doi: 10.1172/JCI114960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallone T. L. Molecular sieving of albumin by the ascending vasa recta wall. J Clin Invest. 1992 Jul;90(1):30–34. doi: 10.1172/JCI115852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallone T. L. Resistance of ascending vasa recta to transport of water. Am J Physiol. 1991 Mar;260(3 Pt 2):F303–F310. doi: 10.1152/ajprenal.1991.260.3.F303. [DOI] [PubMed] [Google Scholar]
- Pallone T. L., Robertson C. R., Jamison R. L. Renal medullary microcirculation. Physiol Rev. 1990 Jul;70(3):885–920. doi: 10.1152/physrev.1990.70.3.885. [DOI] [PubMed] [Google Scholar]
- Pallone T. L., Work J., Jamison R. L. Resistance of descending vasa recta to the transport of water. Am J Physiol. 1990 Oct;259(4 Pt 2):F688–F697. doi: 10.1152/ajprenal.1990.259.4.F688. [DOI] [PubMed] [Google Scholar]
- Pitcock J. A., Lyons H., Brown P. S., Rightsel W. A., Muirhead E. E. Glycosaminoglycans of the rat renomedullary interstitium: ultrastructural and biochemical observations. Exp Mol Pathol. 1988 Dec;49(3):373–387. doi: 10.1016/0014-4800(88)90009-3. [DOI] [PubMed] [Google Scholar]
- Sanjana V. M., Johnston P. A., Deen W. M., Robertson C. R., Brenner B. M., Jamison R. L. Hydraulic and oncotic pressure measurements in inner medulla of mammalian kidney. Am J Physiol. 1975 Jun;228(6):1921–1926. doi: 10.1152/ajplegacy.1975.228.6.1921. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen B. The renal pelvis. Kidney Int. 1987 Feb;31(2):621–628. doi: 10.1038/ki.1987.43. [DOI] [PubMed] [Google Scholar]
- Shirley D. G., Walter S. J., Sampson B. A micropuncture study of renal lithium reabsorption: effects of amiloride and furosemide. Am J Physiol. 1992 Dec;263(6 Pt 2):F1128–F1133. doi: 10.1152/ajprenal.1992.263.6.F1128. [DOI] [PubMed] [Google Scholar]
- Starling E. H. On the Absorption of Fluids from the Connective Tissue Spaces. J Physiol. 1896 May 5;19(4):312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi-Iwanaga H. The three-dimensional cytoarchitecture of the interstitial tissue in the rat kidney. Cell Tissue Res. 1991 May;264(2):269–281. doi: 10.1007/BF00313964. [DOI] [PubMed] [Google Scholar]


