Abstract
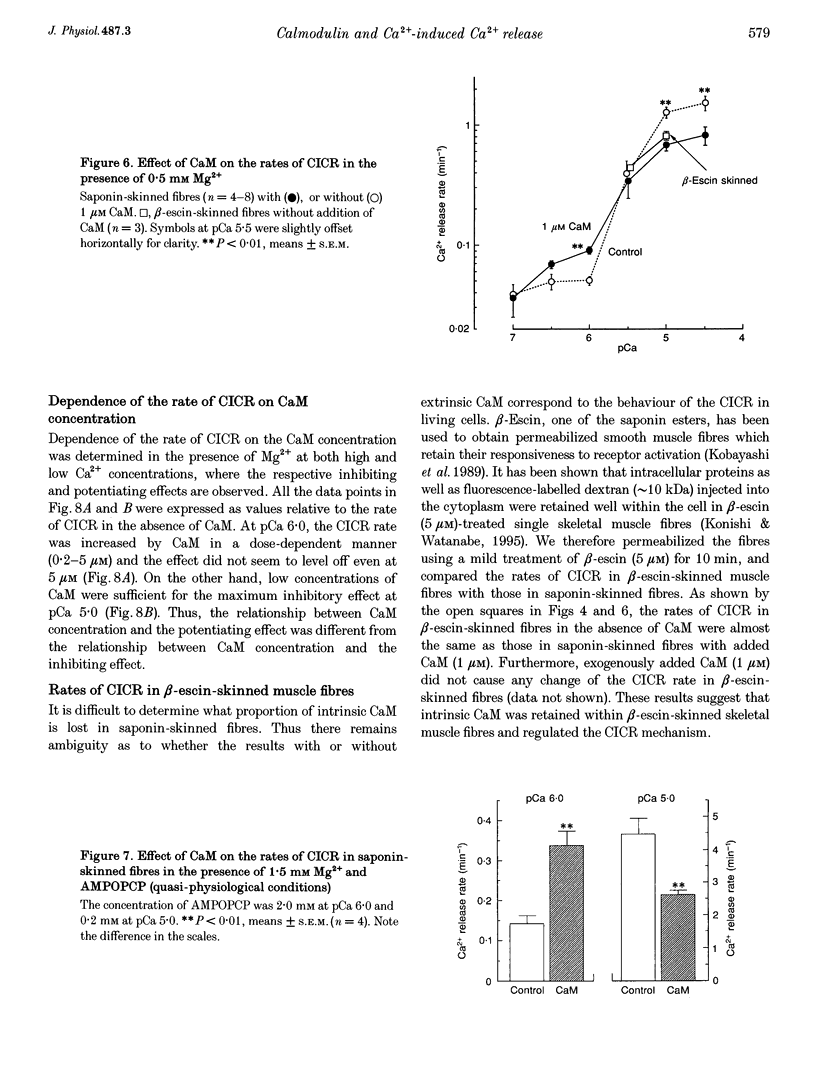
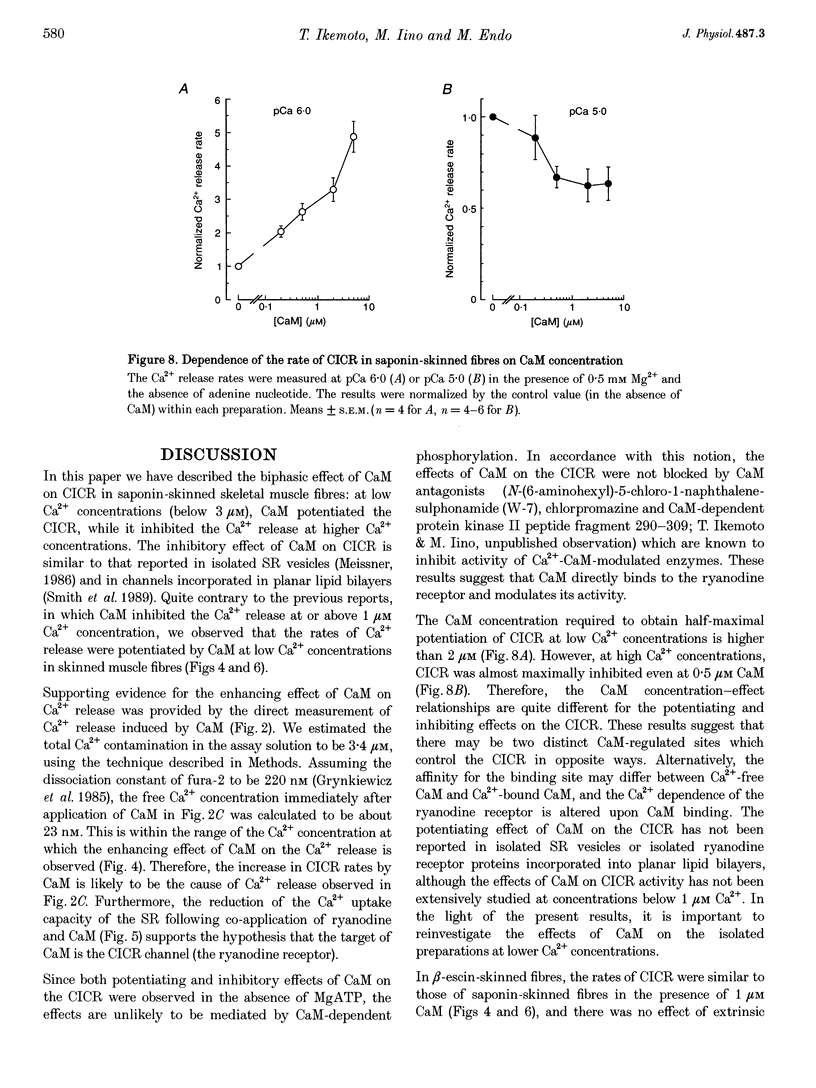
1. We analysed the effect of calmodulin on Ca(2+)-induced Ca2+ release (CICR) in the sarcoplasmic reticulum (SR) using chemically skinned fibres of rabbit psoas muscle. Ca2+ release was measured using fura-2 microfluorometry. 2. In saponin-skinned fibres, calmodulin potentiated Ca2+ release at low Ca2+ concentrations (< 3 microM), while it showed an inhibitory effect at high Ca2+ concentrations (3-30 microM). 3. Co-application of ryanodine and calmodulin at 0.3 microM Ca2+, but not ryanodine alone, induced a decline in the Ca2+ uptake capacity of the SR, an effect expected from the open-lock of active CICR channels by ryanodine. Thus, potentiation of Ca2+ release by calmodulin at low Ca2+ concentrations can be regarded as a result of the activation of the ryanodine receptor. 4. Greater concentrations of calmodulin were required for potentiation of CICR at low Ca2+ concentrations (1 microM) than for inhibition at high Ca2+ concentrations (10 microM). 5. In beta-escin-permeabilized fibres in which intrinsic calmodulin was retained, the rates of CICR were similar to those measured in the presence of 1 microM calmodulin in saponin-permeabilized fibres. 6. These results suggest that calmodulin plays an important role in the regulation of CICR channels in intact skeletal muscle fibres.
Full text
PDF

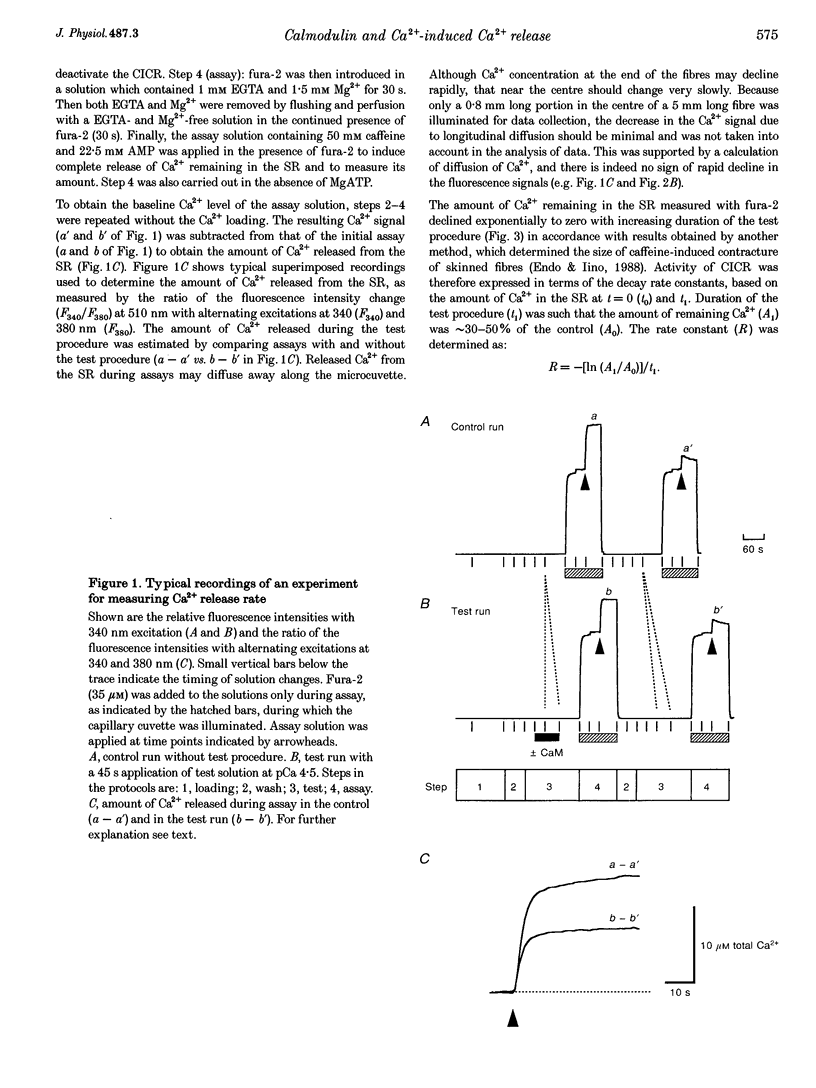
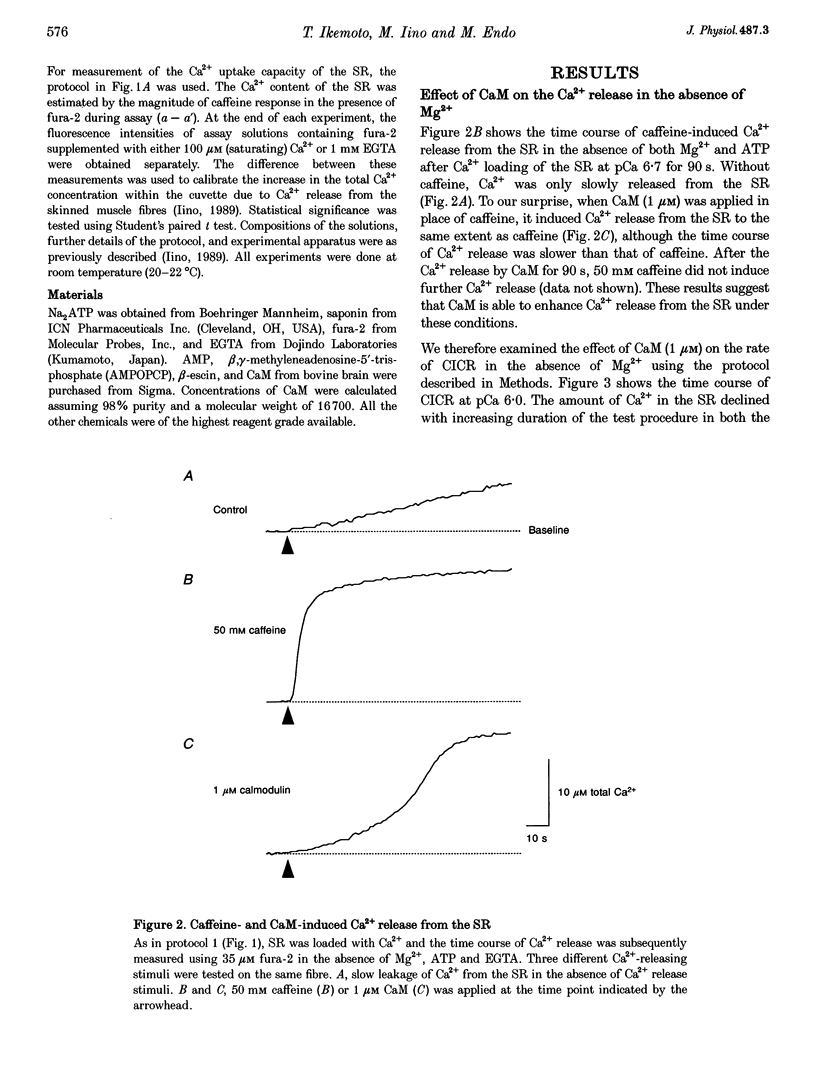
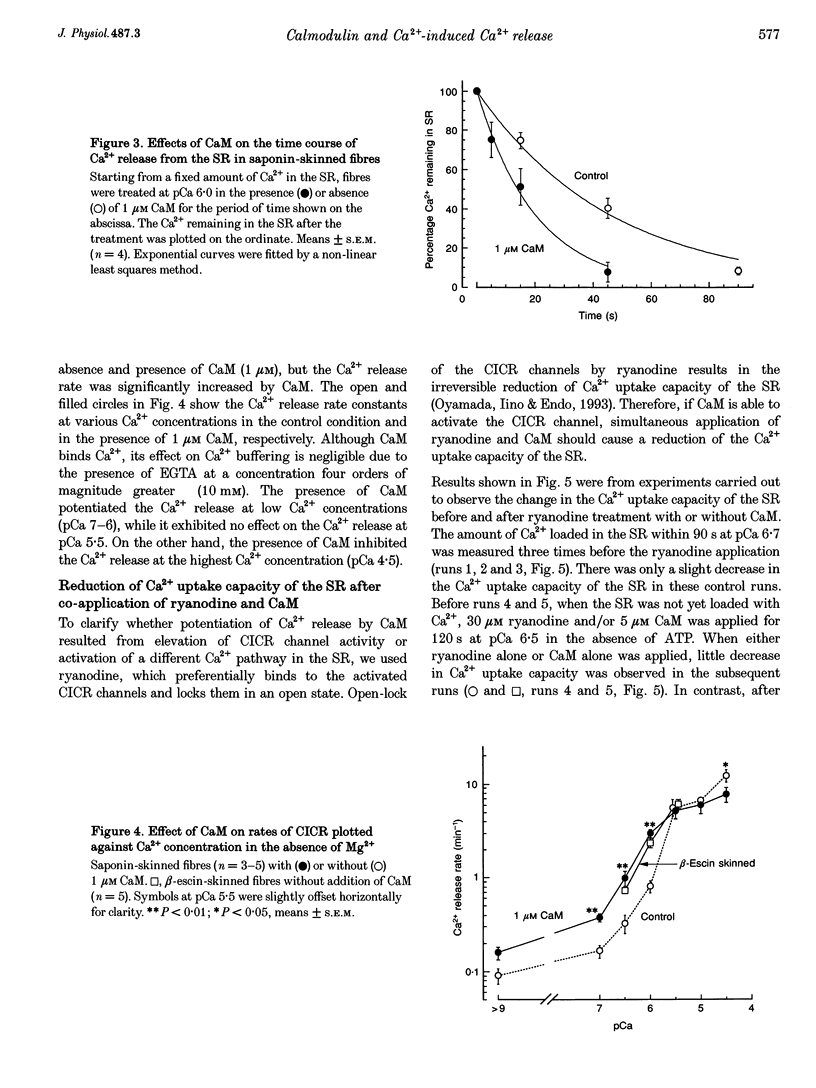
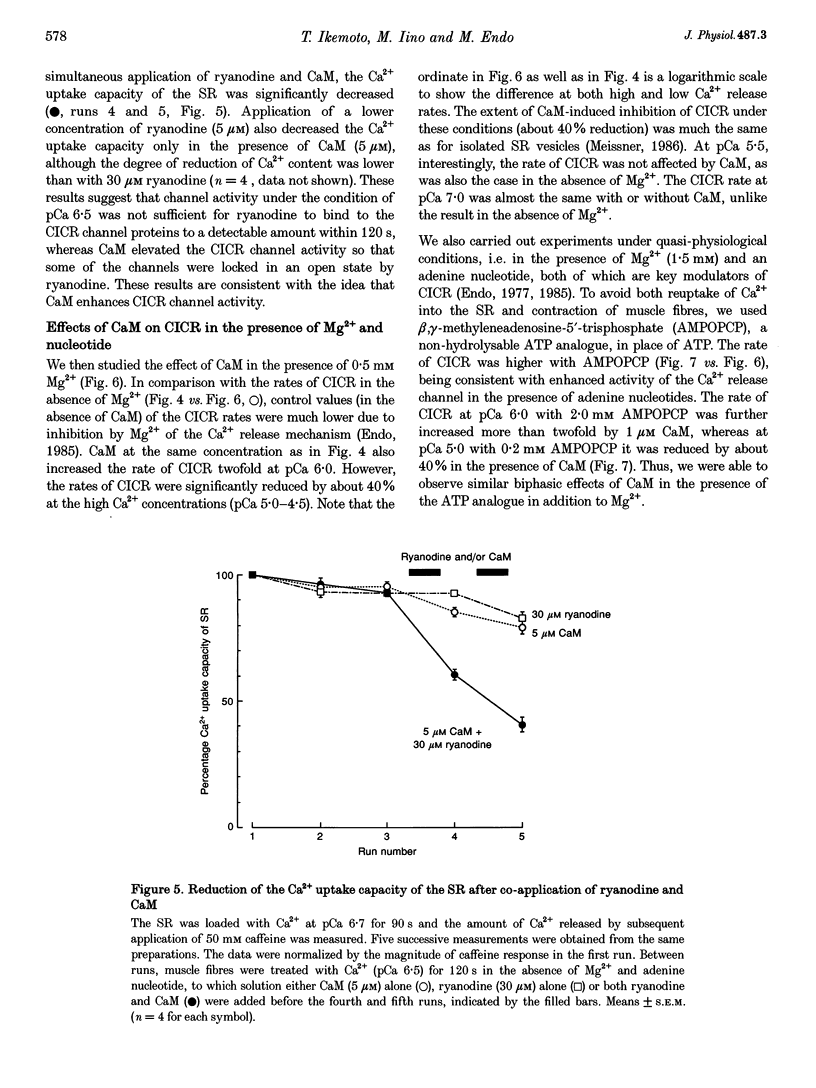


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Ebashi S. Excitation-contraction coupling and the mechanism of muscle contraction. Annu Rev Physiol. 1991;53:1–16. doi: 10.1146/annurev.ph.53.030191.000245. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Iino M. Measurement of Ca2+ release in skinned fibers from skeletal muscle. Methods Enzymol. 1988;157:12–26. doi: 10.1016/0076-6879(88)57064-7. [DOI] [PubMed] [Google Scholar]
- Endo M., Iino M. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J Muscle Res Cell Motil. 1980 Mar;1(1):89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hakamata Y., Nakai J., Takeshima H., Imoto K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett. 1992 Nov 9;312(2-3):229–235. doi: 10.1016/0014-5793(92)80941-9. [DOI] [PubMed] [Google Scholar]
- Hymel L., Inui M., Fleischer S., Schindler H. Purified ryanodine receptor of skeletal muscle sarcoplasmic reticulum forms Ca2+-activated oligomeric Ca2+ channels in planar bilayers. Proc Natl Acad Sci U S A. 1988 Jan;85(2):441–445. doi: 10.1073/pnas.85.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989 Aug;94(2):363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa T., Smith J. S., Coronado R., Campbell K. P. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987 Dec 5;262(34):16636–16643. [PubMed] [Google Scholar]
- Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989 Oct 25;264(30):17997–18004. [PubMed] [Google Scholar]
- Konishi M., Watanabe M. Molecular size-dependent leakage of intracellular molecules from frog skeletal muscle fibers permeabilized with beta-escin. Pflugers Arch. 1995 Feb;429(4):598–600. doi: 10.1007/BF00704168. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Control of calcium release and the effect of ryanodine in skinned muscle fibres of the toad. J Physiol. 1990 Apr;423:519–542. doi: 10.1113/jphysiol.1990.sp018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson P. S., Campbell K. P. Characterization of the major brain form of the ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993 Sep 15;268(26):19785–19790. [PubMed] [Google Scholar]
- Meissner G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. J Biol Chem. 1984 Feb 25;259(4):2365–2374. [PubMed] [Google Scholar]
- Meissner G., Darling E., Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986 Jan 14;25(1):236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Meissner G. Evidence of a role for calmodulin in the regulation of calcium release from skeletal muscle sarcoplasmic reticulum. Biochemistry. 1986 Jan 14;25(1):244–251. doi: 10.1021/bi00349a034. [DOI] [PubMed] [Google Scholar]
- Meissner G., Henderson J. S. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987 Mar 5;262(7):3065–3073. [PubMed] [Google Scholar]
- Nakai J., Imagawa T., Hakamat Y., Shigekawa M., Takeshima H., Numa S. Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett. 1990 Oct 1;271(1-2):169–177. doi: 10.1016/0014-5793(90)80399-4. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Callewaert G., Cleemann L., Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Otsu K., Willard H. F., Khanna V. K., Zorzato F., Green N. M., MacLennan D. H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem. 1990 Aug 15;265(23):13472–13483. [PubMed] [Google Scholar]
- Oyamada H., Iino M., Endo M. Effects of ryanodine on the properties of Ca2+ release from the sarcoplasmic reticulum in skinned skeletal muscle fibres of the frog. J Physiol. 1993 Oct;470:335–348. doi: 10.1113/jphysiol.1993.sp019861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev. 1991 Jul;71(3):849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- Saito A., Inui M., Radermacher M., Frank J., Fleischer S. Ultrastructure of the calcium release channel of sarcoplasmic reticulum. J Cell Biol. 1988 Jul;107(1):211–219. doi: 10.1083/jcb.107.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Schneider M. F. Control of calcium release in functioning skeletal muscle fibers. Annu Rev Physiol. 1994;56:463–484. doi: 10.1146/annurev.ph.56.030194.002335. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+. J Gen Physiol. 1986 Nov;88(5):573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Rousseau E., Meissner G. Calmodulin modulation of single sarcoplasmic reticulum Ca2+-release channels from cardiac and skeletal muscle. Circ Res. 1989 Feb;64(2):352–359. doi: 10.1161/01.res.64.2.352. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Iino M., Takekura H., Nishi M., Kuno J., Minowa O., Takano H., Noda T. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994 Jun 16;369(6481):556–559. doi: 10.1038/369556a0. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Yagi K., Yazawa M., Kakiuchi S., Ohshima M., Uenishi K. Identification of an activator protein for myosin light chain kinase as the Ca2+-dependent modulator protein. J Biol Chem. 1978 Mar 10;253(5):1338–1340. [PubMed] [Google Scholar]
- Yang H. C., Reedy M. M., Burke C. L., Strasburg G. M. Calmodulin interaction with the skeletal muscle sarcoplasmic reticulum calcium channel protein. Biochemistry. 1994 Jan 18;33(2):518–525. doi: 10.1021/bi00168a017. [DOI] [PubMed] [Google Scholar]
- Zorzato F., Fujii J., Otsu K., Phillips M., Green N. M., Lai F. A., Meissner G., MacLennan D. H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990 Feb 5;265(4):2244–2256. [PubMed] [Google Scholar]


