Abstract
Background
The intestinal epithelial barrier is the first line of defense against pathogens and noxious substances entering the body from the outside world. Through proliferation and differentiation, intestinal stem cells play vital roles in tissue regeneration, repair, and the maintenance of intestinal homeostasis. Inflammatory bowel disease (IBD) is caused by the disruption of intestinal homeostasis through the invasion of toxic compounds and pathogenic microorganisms. Hylotelephium erythrostictum (Miq.) H. Ohba (H. erythrostictum) is a plant with diverse pharmacological properties, including antioxidant, anti-inflammatory, antidiabetic, and antirheumatic properties. However, the roles of H. erythrostictum and its bioactive compounds in the treatment of intestinal injury are unknown.
Methods
We examined the protective effects of H. erythrostictum water extract (HEWE) and H. erythrostictum butanol extract (HEBE) on Drosophila intestinal injury caused by dextran sodium sulfate (DSS) or Erwinia carotovoracarotovora 15 (Ecc15).
Results
Our findings demonstrated that both HEWE and HEBE significantly prolonged the lifespan of flies fed toxic compounds, reduced cell mortality, and maintained intestinal integrity and gut acid‒base homeostasis. Furthermore, both HEWE and HEBE eliminated DSS-induced ROS accumulation, alleviated the increases in antimicrobial peptides(AMPs) and intestinal lipid droplets caused by Ecc15 infection, and prevented excessive ISC proliferation and differentiation by inhibiting the JNK, EGFR, and JAK/STAT pathways. In addition, they reversed the significant changes in the proportions of the gut microbiota induced by DSS. The bioactive compounds contained in H. erythrostictum extracts have sufficient potential for use as natural therapeutic agents for the treatment of IBD in humans.
Conclusion
Our results suggest that HEWE and HEBE are highly effective in reducing intestinal inflammation and thus have the potential to be viable therapeutic agents for the treatment of gut inflammation.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-024-04686-w.
Keywords: Hylotelephiumerythrostictum, Inflammatory bowel disease, Drosophila, Intestinal homeostasis, Intestinal stem cell, Pathway
Introduction
Inflammatory bowel disease (IBD) is a chronic disease that causes intestinal injury and disturbance and affects the gastrointestinal tract, with Crohn’s disease (CD) and ulcerative colitis (UC) being the most prevalent forms [1]. As IBD is difficult to treat completely and many medications reduce only symptoms, there is a need for more effective therapeutic medicines for IBD [2]. Drosophila melanogaster has become an outstanding model for studying disease mechanisms and drug screening in recent years [3]. The gastrointestinal tract of flies is composed of self-regenerating digestive and absorbent tissues and has similar properties to their mammalian counterparts, including the stomach, small intestine, and colon [4].Compared to those of the human small intestine, the posterior midgut possesses the highest metabolic activity and immune response [5]. When a fly consumes toxic chemicals or microorganisms, intestinal homeostasis is disrupted, leading to the development of IBD. As a result, the Drosophila intestine has become an ideal subject for IBD research.
TheDrosophila intestinal epithelium contains four distinct cell types: intestinal stem cells (ISCs), enteroblasts (EBs), enterocytes (ECs), and enteroendocrine cells (EEs). Through self-renewal and differentiation processes, ISCs play a crucial role in maintaining intestinal homeostasis [5, 6]. Drosophila intestinal epithelial homeostasis is maintained by a number of signaling pathways. The Notch signaling pathway initiates ISC differentiation, identifies EB terminal cell fates, and possibly inhibits erroneous ISC proliferation, thereby decreasing ISC turnover [5]. Moreover, EGFR signaling plays a significant role in regulating ISC proliferation [7]. The JAK/STAT and c-Jun N-terminal kinase (JNK) signaling pathways are involved in inflammation and infection and promote ISC proliferation and differentiation. The microbiota also modulates the activity of ISCs by causing cell death and replacement, as well as modifying the physiology of the gut and the body as a whole [8].
Recent research efforts have focused on the development of new and efficacious natural medications for the treatment of IBD. H. erythrostictum is a perennial herbaceous plant with fleshy leaves that belongs to the Crassulaceae family. H. erythrostictum, commonly known as the garden stonecrop, has been widely applied in urban greening and ecological gardening in China, due to its remarkable ornamental value and strong stress resistance [9, 10]. H. erythrostictum is widely recognized as a multifunctional plant due to its exceptional pharmacological and ornamental properties. H. erythrostictum has also been used as a traditional Chinese medicine, and according to the Chinese encyclopedia dictionary “Cihai”, the whole plant is employed as a therapeutic agent for the treatment of rheumatism and diabetes [11]. Extracts from the aerial portions of H. erythrostictum were found to have potent antioxidant and anti-inflammatory properties [10]. In addition, the chemical composition and antibacterial activity of the ethyl acetate extract were revealed [12]. Previous research on H. erythrostictum has focused on drought stress [13], salt stress [14], chloroplast genome completion [9], and α-glucosidase inhibition [11]. However, the protective effects of H. erythrostictum extracts and their bioactive compounds against intestinal injury have not been determined.
Using Drosophila melanogaster as a model organism, this study revealed the protective function and regulatory mechanism of H. erythrostictum water extract (HEWE) and butanol extract (HEBE) against the disruption of intestinal homeostasis caused by the ingestion of dextran sodium sulfate (DSS) or Erwinia carotovoracarotovora 15 (Ecc15) infection. In addition, the functional compounds of these extracts were analyzed. Supplementation with HEWE or HEBE significantly increased adult fly survival and restored ROS levels, acid‒base homeostasis, and midgut lipid droplet accumulation induced by DSS or Ecc15, according to our findings. Furthermore, both HEWE and HEBE inhibited ISC proliferation and differentiation by inhibiting the activation of the JNK, EGFR, and JAK/STAT signaling pathways. Additionally, both HEWE and HEBE restored the equilibrium of the disturbed intestinal microflora and preserved intestinal homeostasis. Moreover, several effective compounds for treating inflammatory bowel disease were identified in both HEWE and HEBE. In conclusion, our results demonstrated that H. erythrostictum extracts possess a distinct mechanism of protection against intestinal damage and have the potential to serve as a novel, effective treatment for gut inflammation.
Materials and methods
Hylotelephium erythrotictum water extract (HEWE) and butanol extract (HEBE)
Hylotelephiumerythrostictum (Miq.) H. Ohba (Crassulaceae) was collected in September 2013 on the campus of Yanbian University in Yanji City, identified by Professor Huizi Lv (College of Pharmacy, Yanbian University). A voucher specimen (number: YB-HE-1309) was deposited at the Pharmacognosy Laboratory, College of Pharmacy, Yanbian University. The fresh medicinal plant stems were dried in the shade until their true quality remained unchanged and were then crushed in a grinder. All filtration operations were carried out in a Buchner funnel under vacuum. A total of 185 g of desiccated H. erythrostictum stems was macerated with 70% EtOH (200 ml×5) for 3 h (each time) under reflux in a 1 L three-necked round-bottomed flask in an oil bath with a drug‒solvent ratio (DSR) = 1:1. Under vacuum, the combined extracts were concentrated to produce a crude extract. After being suspended in water (20 ml), the crude extract was successively partitioned with petroleum ether (20 ml×3, resulting in a drug-to-extract ratio (DER) = 1:10), dichloromethane (20 ml×3, resulting in a drug-to-extract ratio (DER) = 1:18), ethyl acetate (20 ml×3, resulting in a drug-to-extract ratio (DER) = 1:19), and n-butanol (20 ml×3, resulting in a drug-to-extract ratio (DER) = 1:5) in a 500 ml separating funnel at normal pressure and temperature. All the extracts were dried under vacuum drying mode. Accordingly, 1.19 g of dry water-insoluble extract, 1.76 g of dry petroleum ether extract, 1.34 g of dry dichloromethane extract, 1.25 g of dry ethyl acetate extract, 4.10 g of dry n-butanol extract, and 44.67 g of dry water-soluble extract were obtained. For experimental repeatability, the obtained water extract (10 mg) and n-butanol extract (10 mg) were dissolved in deuterated methanol, after which the nuclear magnetic resonance (NMR) hydrogen and carbon spectra were measured on a Bruker AV500 nuclear magnetic resonance spectrometer for fingerprint authentication and standardization.
Drosophila stocks
All flies used in this study were as follows: the esg-Gal4 UAS-GFP/CyO line was obtained from Rongwen Xi [15]. The Delta-lacZ line was a gift from Bruno Lemaitre [16]. Jose Carlos Pastor-Pareja donated the upd3-Gal4 UAS-GFP/CyO line [17]. The Bloomington Stock Center was the source for the w1118, Vn-lacZ, puc-lacZ, UAS-hepWT/CyO, GstD1-GFP, UAS-EGFRCA, UAS-Cat RNAi, UAS-Sod2 RNAi, and UAS-hopCA lines. The Tsinghua Fly Center was the source for the Su(H)GBE-lacZ, esgts-Gal4 UAS-GFP, 10XSTAT-GFP, and Dipt-GFP lines. The Def-GFP line was generated by Jean-Luc Imler [18]. The Drs-GFP line was provided by Zongzhao Zhai as a gift [19]. All flies were maintained on a standard diet of cornmeal and yeast at 25 °C and 60% humidity with a 12-hour light cycle.
Survival experiments
Three- to five-day-old adult w1118 flies (thirty per group, half female and half male) were used as experimental animals for survival experiments. After starving for 2 h, the flies were transferred to plastic vials containing 5 layers of filter paper soaked in 5% sucrose medium (w/v), 5% sucrose medium containing 4% DSS (w/v; MP Biomedicals, LLC, Santa Ana, CA), 5% sucrose medium containing 0.6% SDS (w/v; Sigma‒Aldrich), and DSS or SDS medium supplemented with HEWE (4, 6, and 8 mg/ml) or HEBE (1, 2, and 4 mg/ml). The filter papers were changed daily at the same time, and survival rates were recorded daily. Three independent experimental repeats were performed.
Immunostaining
The intestines of 15 to 20 female flies per group were dissected in cool PBS and then fixed for 30 min at room temperature in 4% PFA (paraformaldehyde). After fixation, the samples were flushed four times (for 10 min each) in blocking buffer (PBST containing 5% goat serum) and blocked for 30 min. Then, the samples were incubated overnight at 4 °C with primary antibodies in the same buffer. After washing with PBST four times for 10 min each, the intestines were incubated for two hours at room temperature with secondary fluorophore-conjugated antibodies in PBST. The samples were cleaned a total of 10 times for 5 min in PBST, stained with Hoechst (1:500 in PBST), and then mounted in glycerol at a concentration of 70%. A Carl Zeiss Axioscope A1 microscope was used to acquire the images. The primary antibodies used were as follows: rabbit anti-PH3 (1:200, Millipore), mouse anti-β-gal (1:200, Promega), rabbit anti-GFP (1:200, Thermo Fisher Scientific), mouse anti-Prospero (1:200, DSHB), mouse anti-armadillo (Arm) (1:30, DSHB), rabbit anti-p-JNK (1:200, Promega), and rabbit anti-p-Erk (1:50, Cell Signaling Technology). At a ratio of 1:200, the secondary antibodies used were Alexa Fluor 488- and Alexa Fluor 568-conjugated antibodies (Thermo Fisher Scientific). Three independent experimental repeats were performed.
BODIPY staining
Adult intestines were dissected, fixed in 4% paraformaldehyde for 30 min at room temperature, and washed four times for 5 min with PBST (0.1% Triton X-100 in PBS). The samples were stained with 2 µg/ml BODIPY and incubated for 1 h. The excess stain was then removed, and each sample was rinsed three times for 5 min in PBST. Next, the samples were incubated with 2 µg/ml Hoechst for 10 min before being mounted on glass transparencies with 70% glycerol. Three independent experimental repeats were performed.
Monitoring gut acidification
To determine the acidity of the Drosophila intestine, 100 ml of 2% BPB (bromophenol blue sodium; Sigma, B5525) was deposited on the surface of the food, and a pipette tip was used to ensure that the BPB solution was absorbed. After 12 h, the intestines were dissected and photographed in ice-cold PBS. Three independent experimental repeats were performed.
Quantitative real-time PCR for AMP expression
TRIzol (Thermo Fisher Scientific) was used to extract total RNA from 30 guts per group, and cDNA was synthesized using M-MLV Reverse Transcriptase RNase H Minus Point Mutant (Promega). Quantitative real-time PCR was performed on a LightCycler 480 II (Roche) using ROX FastStart Universal SYBR Green Master Mix reagents. The following primers were used in the experiments: rp49: F-AGTCGGATCGATATGCTAAGCTGT and R-TAACCGATGTTGGGCATCAGATACT; Drs (drosomycin): F-CTTGTTCGCCCTCTTCGCTGTC and R-AGCACTTCAGACTGGGGCTGCA; Dpt (diptericin): F-ATGCAGTTCACCATTGCCGTC and R-TCCAGCTCGGTTCTGAGTTG; Def (defencin): F-CGCTTTTGCTCTGCTTGCTTGC and R-TAGGTCGCATGTGGCTCGCTTC. As an internal reference, relative AMP expression was normalized to that of the rp49 gene. Five independent experimental repeats were performed.
Smurf assay
The Smurf assay, which was used to assess the intestinal barrier integrity of Drosophila, was performed using 2.5% (w/v) blue dye (FD&C Blue #1) in accordance with a previously described method [20]. After 5 days of feeding, the female flies were transferred to medium containing blue dye. The ‘Smurf’ phenotype was observed after 9 h if the fly’s entire body was determined to be blue. Three independent experimental repeats were performed.
Bacterial cultures and oral infection
Ecc15 was grown in LB medium at 29 °C for 12 h. Female flies aged 3 to 5 days were starved for 2 h at 25 °C before being transferred to receptacles containing five pieces of filter paper. As a feeding medium, filter papers were saturated with 5% sucrose, and Ecc15 bacteria (final OD600nm = 200) were cultured in 5% sucrose.
7-Aminoactinomycin D (7-AAD), DHE, and CM-H2DCFDA staining
7-AAD staining was used to identify dead cells, while DHE and CM-H2DCFDA staining were used to measure intestinal ROS levels. The intestines of female adult flies were dissected in PBS and stained for 30 min at room temperature with 5 µM 7-AAD, 5 µM DHE, or 1 µM CM-H2DCFDA. The dissected intestines were then fixed in 4% PFA and stained with Hoechst for 10 min. Finally, the digestive tract was affixed with 70% glycerol. Three independent experimental repeats were performed.
Gut microbiota analysis
Using 16 S rDNA analysis, the intestinal microbial composition of Drosophila was analyzed. Using the Fast DNA Spin Kit, intestinal microbial DNA was extracted, and the extracted genomic DNA was analyzed via 1% agarose gel electrophoresis. The sequencing results for 16 S rDNA were obtained from Shanghai Majorbio Bio-Pharm Technology Co., Ltd. A paired-end library was constructed using the NEXTFLEX Rapid DNASeq Kit in accordance with Illumina’s recommendations for genomic DNA library preparation. The amplicons were subsequently sequenced with a MiSeq Reagent Kit v3.
Widely targeted metabolomics
H. erythrostictum extracts were subjected to metabolite profiling at Shanghai Hoogen Biotechnology Co., Ltd. (Shanghai, China) using a widely targeted metabolomic method (http://www.hoogen-bio.com/). The following conditions were used for ultracentrifugation (UCPLC) analysis. The column used was an Acquity UPLC HSS T3 (1.8 μm, 2.1 × 100 mm) column; mobile phase A was 0.1% formic acid in water, and mobile phase B was acetonitrile. A gradient of water/acetonitrile was applied, starting with a ratio of 98:2 V(A)/V(B) at 0 min, maintaining this ratio until 0.5 min, then transitioning to 50:50 V(A)/V(B) at 10 min, 5:95 V(A)/V(B) at 11.0 min, 5:95 V(A)/V(B) at 13.0 min, returning to 98:2 V(A)/V(B) at 13.1 min, and finally maintaining this ratio until 15.0 min. The flow rate was 0.40 ml/min, the temperature of the column was 40 °C, the temperature of the autosampler was 4 °C, and the injection volume was 2 µl. The UHPLC instrument was integrated with tandem mass spectrometry (MS/MS) (Applied SCIEX 6500 QTRAP + triple quadrupole with an IonDrive Turbo V ESI ion source). The following were the MS conditions: IonSpray Voltage was set to + 5500/-4500 V, Curtain Gas was set to 35 psi, Temperature was set to 400 °C, Ion Source Gas 1 was set to 60 psi, Ion Source Gas 2 was also set to 60 psi, and DP was set to ± 100 V. SCIEX Analyst Workstation Software (Version 1.6.3) was used to acquire and evaluate the MRM (multiple reaction monitoring) method’s data. Using MSconverter, the original MS data (wiff) files were converted to the TXT format. Using an in-house R program and database, peak detection and annotation were performed.
Targets prediction of HEWE and HEBE
Compounds with relative percentages > 0.1% obtained as SMILES in PubChem were identified through widely targeted metabolomics. The SwissADME platform was subsequently used to predict the ADME properties of these compounds, with a focus on identifying compounds with high gastrointestinal absorption and excellent drug-like properties. Subsequently, the SwissTarget prediction platform was used to predict potential targets of the selected compounds. For prospective drug targets, only those with a probability greater than 0.1% were considered.
IBD-related and hub-related objectives
To identify targets associated with inflammatory bowel disease (IBD), several databases, including OMIM, CTD, DisGeNet, GeneCards, and TTD [21–24] were used. Direct evidence of correlation was the criterion for selecting targets from CTD. In DisGeNet, targets with scores greater than 0.02 were obtained. The GeneCards tool was used to screen for targets with correlation coefficients greater than the median. To normalize the datasets, UniProt [25] was used. The resulting targets were visualized as Venn diagrams by jvenn [26].
Statistical analysis
ImageJ was used for image analysis, GraphPad Prism 6.0 was used for graph generation and results analysis by Student’s t test. Normality is analyzed using the Kolmogorov-Smirnov test.All data were shown as means ± SEM.P values > 0.05 indicated no significance. P < 0.05 was considered to indicate statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Results
H. erythrostictumextracts enhance protection against toxic compounds
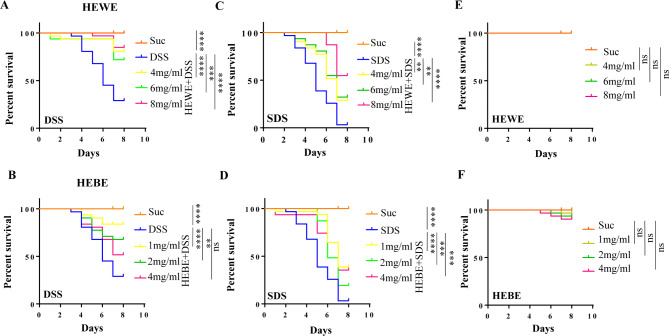
To determine whether H. erythrostictum water extract (HEWE) and butanol extract (HEBE) have protective effects against toxic substances in the intestine, adult flies were fed DSS or SDS, respectively. These chemicals are toxic compounds that disrupt normal intestinal barrier function and cause intestinal inflammation. To determine the survival rate, adult flies were fed multiple concentrations of HEWE (4, 6, and 8 mg/ml) or HEBE (1, 2, and 4 mg/ml). After 7 days, the survival rates of the flies in the 4% DSS and 0.6% SDS groups substantially decreased by 71% and 96.8%, respectively (Fig. 1A-D). However, compared to those in the control groups, the survival rates of the experimental groups administered HEWE or HEBE were significantly greater, with 8 mg/ml HEWE and 1 mg/ml HEBE having the greatest protective effects. After exposure to 4% DSS, supplementation with 8 mg/ml HEWE or 1 mg/ml HEBE increased survival by 55.8% (P < 0.0001) and 54.2% (P < 0.0001), respectively (Fig. 1A, B). The survival rates increased substantially by 51.6% (P < 0.0001) and 35.5% (P < 0.0001), respectively, when 0.6% SDS was utilized as an inflammatory agent. (Fig. 1C, D). The toxicity of H. erythrostictum extracts to Drosophila was subsequently evaluated. As shown in Fig. 1E and F, the survival rates of the group fed HEWE or HEBE did not differ from the survival rate of the group fed sucrose alone, indicating that the H. erythrostictum extracts were not toxic to Drosophila. Overall, 8 mg/ml HEWE and 1 mg/ml HEBE significantly enhanced the survival rates of flies exposed to the inflammatory factors DSS and SDS. Next, we used only DSS to induce intestinal dysfunction.
Fig. 1.
Both HEWE and HEBE enhance the survival of flies exposed to DSS or SDS. (A-D) Wild type w1118 flies were reared on various media. Suc, 5% sucrose medium; DSS, 5% sucrose containing 4% DSS; SDS, 5% sucrose containing 0.6% SDS; experimental group, DSS or SDS medium supplemented with HEWE (4, 6, and 8 mg/ml) or HEBE (1, 2, and 4 mg/ml), respectively. Supplementation with HEWE or HEBE at each concentration significantly increased the survival rate of flies exposed to toxic compounds. (E, F) Flies were reared in 5% sucrose medium supplemented with HEWE (E) or HEBE (F) without toxic compounds. Neither HEWE nor HEBE at any concentration was harmful to fruit flies. Differences in survival were analyzed using the log-rank test. nsP > 0.05,**P < 0.01,***P < 0.001,****P < 0.0001
H. erythrostictum extracts protect against the degradation and dysfunction of intestinal epithelial cells caused by DSS
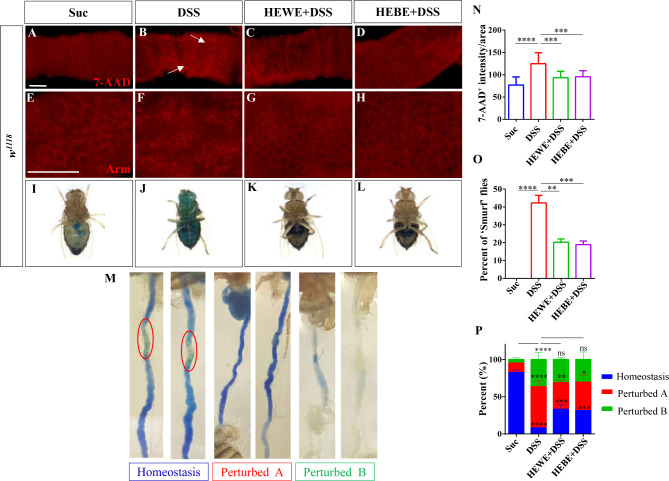
As H. erythrostictum extracts improved Drosophila survival after ingestion of toxic compounds, we hypothesized that they may protect intestinal function. 7-AAD staining was used to evaluate DSS-induced intestinal epithelial cell death. The fluorescence intensity of 7-AAD was substantially greater in DSS-treated flies than in sucrose-fed flies (P < 0.0001, Fig. 2A, B, N). However, the fluorescence signal decreased by more than 76% after supplementation with H. erythrostictum extracts (HEWE:P < 0.001, HEBE: P < 0.001, Fig. 2C, D, N). In addition, adherens junctions regulate cell adhesion and cytoskeletal organization in a variety of cell types. Armadillo (Arm) is a crucial protein that binds to other proteins to form a cell‒cell junction complex [27]. Previous research has demonstrated that DSS stimulation disrupts and disorganizes the adhesion of intestinal epithelial cells [28]. To assess cell adhesion against intestinal damage, we utilized anti-Arm antibodies. In the sucrose-fed group, clear small progenitor cells and large ECs, which make up the majority of the intestinal epithelium, were observed, whereas in the DSS-treated group, cell boundaries were disorganized and cell types could not be distinguished (Fig. 2E, F). After supplementation with HEWE or HEBE, the cell adhesions resembled those of the sucrose-fed group in terms of clarity and orderliness (Fig. 2G-H).
Fig. 2.
Both HEWE and HEBE can prevent DSS-induced intestinal epithelial cell death and dysfunction. (A-D) w1118 flies were exposed to 6% DSS for 72 h, and 7-AAD was used to detect dead cells. The addition of HEWE or HEBE significantly reduced DSS-induced cell death. (E-H) The cell membranes were stained with anti-Arm antibodies. Following the administration of DSS, the intestinal cell arrangement was disrupted; however, supplementation with HEWE or HEBE restored the aberrant cellular arrangement. (I-L) Images of ‘Smurf’ flies on different foods. ‘Smurf’ flies discharge blue dye from the intestine into other tissues. (M) Illustration of intestinal acid‒base homeostasis indicated by the bromophenol blue pH indicator. ‘Homeostasis’ indicates that the CCR is yellow, ‘Perturbed A’ indicates that the CCR is blue, and ‘Perturbed B’ indicates that the flies were not administered bromophenol blue and that the intestinal tract was not stained with a pH indicator. (N) Fluorescence intensity of 7-AAD as measured in A-D (n = 12–16). Three separate experimental repeats were performed. (O) Quantification of the percentage of Smurf flies in I-L. Sixty flies were included in each group. Three separate experimental repeats were performed. (P) The percentage of intestinal acid‒base homeostasis in M. Each group consisted of 60 flies. Three experimental repeats were performed. Quantification results represent mean ± SEM.ns P > 0.05, *P < 0.05, **P < 0.01,***P < 0.001,****P < 0.0001. Scale bars: 50 μm
Cell mortality and disordered cell arrangement may induce disruption of the epithelial barrier, thereby increasing intestinal permeability. Next, we determined the integrity of the intestinal epithelial barrier in a Smurf assay. In the Smurf experiment, a nonabsorbable blue food dye (FD&C Blue #1) was used, and flies whose entire bodies exhibited blue color after ingesting the dye were referred to as ‘Smurf’ flies [29]. In the DSS-fed group, 42% of flies exhibited the ‘Smurf’ phenotype (P < 0.0001, Fig. 2I, J, O). In contrast, supplementation with 8 mg/ml HEWE or 1 mg/ml HEBE significantly reduced the Smurf phenotype to 20% (P < 0.01) and 18% (P < 0.001), respectively (Fig. 2K, L, O).
Previous research has suggested that intestinal acid‒base homeostasis plays an essential role in metabolic regulation [30], and instability of intestinal epithelial cells results in a loss of acid‒base homeostasis [31]. The copper cell region (CCR) of fruit flies is located in the middle midgut and is composed of a group of copper cells implicated in gastric acid secretion [32]. Bromophenol blue (BPB) is a pH sensitive dye that transforms from yellow at pH 3.0 to blue at pH 4.6 or higher. Therefore, when Drosophila consumes BPB, the CCR under intestinal homeostasis appears yellow. We used BPB to determine whether DSS administration could disrupt the acid‒base homeostasis of CCR in the intestine. In the DSS-fed group, acid-base homeostasis was significantly reduced by 74.5% (P < 0.0001) compared to that in the sucrose-fed group; however, in the HEWE- and HEBE-supplemented groups, acid-base homeostasis was significantly improved by 25% (P < 0.001) and 23.4% (P < 0.001), respectively, compared to that in the DSS-fed group (Fig. 2M, P). These results suggested that H. erythrostictum extracts could protect against the disruption of intestinal morphology and function caused by DSS administration.
H. erythrostictum extracts reduce excessive AMP levels and steatosis caused by Ecc15
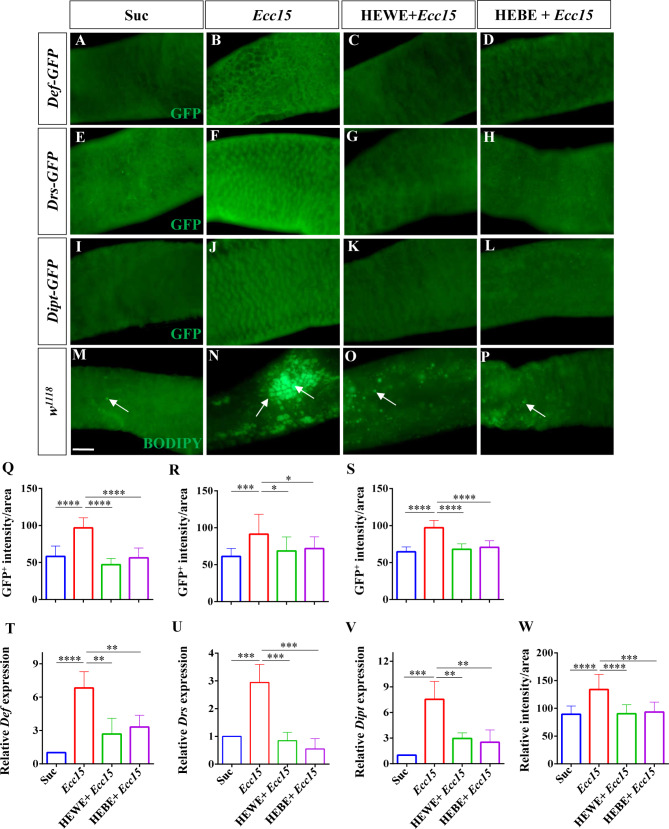
Antimicrobial peptides (AMPs) are small, functional proteins with potent antiviral, antibacterial, and antifungal activities [33]. AMPs for host defense against microbial infection in Drosophila are produced essentially in adipocytes, but also in gut epithelial cells [34]. According to previous studies, AMP can protect intestinal homeostasis in Drosophila by responding to gram-negative bacterial infection [34], but excessive expression of AMP is detrimental to epithelial cells [35–37]. In this study, we examined the expression levels of the reporter genes defencin (Def)-GFP, drosomycin (Drs)-GFP, and diptericin (Dipt)-GFP following infection with the gram-negative bacterium Ecc15. The AMP levels were substantially greater in the Ecc15-infected group than in the sucrose-fed group (Def: P < 0.0001, Drs: P < 0.001, Dipt: P < 0.0001); however, after the addition of 8 mg/ml HEWE or 1 mg/ml HEBE, the AMP levels decreased to levels comparable to those in the uninfected group (Def: HEWE P < 0.0001, HEBE P < 0.0001; Drs: HEWE P < 0.05, HEBE P < 0.05; Ditp: HEWE P < 0.0001, HEBE P < 0.0001, Fig. 3A-L, Q-S). To further confirm these results, we performed qRT-PCR analysis of AMP gene expression. As anticipated, the Def, Drs, and Dipt transcript levels were 5.8-, 1.9-, and 6.5-fold greater, respectively, in the infected group than in the uninfected group (Def: P < 0.0001, Drs: P < 0.001, Ditp: P < 0.001). In contrast, supplementation with H. erythrostictum extracts significantly decreased AMP gene transcription (Def: HEWE P < 0.01, HEBE P < 0.01; Drs: HEWE P < 0.001, HEBE P < 0.001; Ditp: HEWE P < 0.01, HEBE P < 0.01, Fig. 3T-V).
Fig. 3.
Both HEWE and HEBE can reduce excessive AMP levels and steatosis during Ecc15 infection. (A-L) Using anti-GFP antibodies, immunofluorescence images of the posterior midgut were obtained. The transgenic fly lines Def-GFP (A-D), Drs-GFP (E-H), and Dipt-GFP (I-L) were infected with Ecc15 for 16, 16, and 24 h, respectively. Infection with Ecc15 substantially increased Def, Drs, and Dipt levels. However, supplementation with HEWE or HEBE significantly reduced the increase in AMP levels caused by Ecc15 infection. (M-P) Bodipy was used to obtain representative images of midgut lipid droplets (LDs). Ecc15 infection causes LD accumulation in the midgut, but supplementation with HEWE or HEBE significantly decreases LD accumulation. (Q, R, S) Fluorescence intensities of Def-GFP (Q), Drs-GFP (R), and Dipt-GFP (S) in A-D, E-H, and I-L, respectively (n = 12–17). (T-V) After Ecc15 infection for 6 h, qRT-PCR analysis of Def (T), Drs (U), and Dipt (V) was performed. All mRNA levels were normalized to rp49 expression. (W) Quantification of the fluorescence intensity of LDs in M-P (n = 12–14). Quantification results represent mean ± SEM.*P < 0.05, **P < 0.01,***P < 0.001,****P < 0.0001. Scale bars: 50 μm
Adult fly bacterial infection induces intestinal steatosis due to lipid accumulation, and intestinal steatosis is an antibacterial immune response marker and modulator [38]. BODIPY staining revealed significant lipid accumulation in response to Ecc15 infection (P < 0.0001); however, supplementation with HEWE or HEBE reduced lipid droplet numbers in the intestine (HEWE: P < 0.0001, HEBE: P < 0.001, Fig. 3M-P, W). Taken together, these findings showed that H. erythrostictum extracts have intestinal-protective effects by regulating excessive AMP levels and lipogenesis in the Drosophila gut caused by Ecc15 infection.
H. erythrostictum extracts eliminate the excessive accumulation of ROS in the intestine
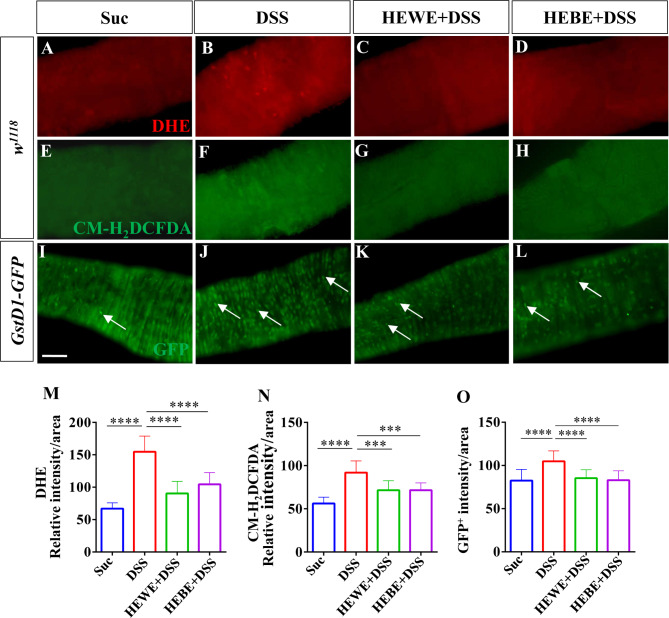
In clearing microbes from the gut, reactive oxygen species (ROS), which are highly effective immune effector molecules, exert broad-spectrum microbicidal effects [39]. However, ROS are destructive to intestinal epithelial cells; therefore, their effects must be transient and at low levels [40]. Excessive amounts of ROS cause tissue damage by oxidizing proteins, lipids, and DNA [41]. In the present study, for the first time, the ROS-scavenging activity of H. erythrostictum extracts was examined. The levels of ROS in the intestine were measured using dihydroethidium (DHE) and 5-(6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate (CM-H2DCFDA). After exposure to DSS, flies had a much stronger fluorescence intensity in the posterior midgut than did those fed sucrose (DHE: P < 0.0001, CM-H2DCFDA: P < 0.0001). However, 8 mg/ml HEWE or 1 mg/ml HEBE supplementation decreased DHE (HEWE: 41.7%, P < 0.0001; HEBE: 32.5%, P < 0.0001) or CM-H2DCFDA (HEWE: 22.2%, P < 0.001; HEBE: 22.1%, P < 0.0001) fluorescence intensity (Fig. 4A-H, M, N). Next, we evaluated intestinal ROS levels in ROS-sensitive GstD1-GFP transgenic flies [42]. In DSS-stimulated flies, the GFP intensity was substantially greater than that in sucrose-fed flies (P < 0.0001, Fig. 4I, J, O), corroborating the findings of a previous study indicating that GstD1 activity is increased in differentiated cells in response to stress [43]. As expected, supplementation with HEWE or HEBE significantly decreased GstD1-GFP levels (HEWE: P < 0.0001, HEBE: P < 0.0001, Fig. 4K, L, O).
Fig. 4.
Both HEWE and HEBE can decrease ROS levels in the gut of Drosophila fed DSS. (A-H) ROS levels were monitored with DHE and CM-H2CFDA. Wild-type w1118 flies were exposed for 48 h to 6% DSS for DHE staining and for 96 h to 3% DSS for CM-H2DCFDA staining. After ingesting DSS, ROS levels in the gut were significantly elevated (A, B, E, F). Both HEWE and HEBE ameliorated the DSS-induced increase in ROS levels (C, D, G, H). (I-L) Photographs of the midguts of gstD1-GFP transgenic flies. After treatment with 6% DSS for 48 h, the GFP levels increased significantly (I, J). Supplementation with HEWE or HEBE significantly reduced the expression of gstD1-GFP (K, L). (M) Quantification of DHE intensity in A-D (n = 10–16). (N) Quantification of CM-H2DCFDA intensity in E-H (n = 10–16). Quantification of the GFP intensity in I-L (n = 12–27). Quantification results represent mean ± SEM.***P < 0.001,****P < 0.0001. Scale bars: 50 μm
Through enzymatic and nonenzymatic antioxidant defenses, living cells have evolved a balanced system to counteract excessive ROS levels [44]. Catalase is one of the oldest known enzymes and has been extensively characterized as a critical defense system against reactive oxygen species and free radicals [45]. This enzyme decomposes H2O2 into oxygen and water and plays a crucial role in the metabolism of H2O2 [46]. In addition, superoxide dismutase (SOD) enzymes catalyze the breakdown of superoxide into hydrogen peroxide and water and are central regulators of ROS levels, the first line of defense against free radicals [47, 48]. In a previous study, when excessive ROS were induced with paraquat, ISC proliferation and an increase in progenitor cells were observed [49]. Therefore, we questioned whether Catalase and Sod2 inhibition would result in an increase in progenitor cells. The GFP signal fromesg-Gal4 UAS-GFP flies can be used to identify both ISCs and EBs in the intestinal tract of Drosophila. When Sod2 or Catalase was knocked down in ISCs/EBs using the esg > GFP driver, we showed that the numbers of progenitors and mitotic cells were significantly greater than those inesg > GFP/+ flies (Sod2 RNAi: esg+ cells P < 0.001, PH3+ cells P < 0.0001; Catalase RNAi: esg+ cells P < 0.0001, PH3+ cells P < 0.0001, Figure S1A, D, G, J, M-P). However, supplementation with H. erythrostictum extracts reduced the numbers of esg+ (HEWE: 27.6%, P < 0.0001, 41.4%, P < 0.001; HEBE: 33.4%, P < 0.001, 41.1%, P < 0.0001) and PH3+ (HEWE: 49.6%, P < 0.01, 50.4%, P < 0.0001; HEBE, 36.6%, P < 0.01, 55.9%, P < 0.01) cells compared to those in cells with low levels of antioxidant enzyme-induced increases in cell numbers (Figure S1B, C, E, F, H, I, K, L, M-P). These results indicated that both HEWE and HEBE possess ROS-scavenging activity, which, by reducing ROS accumulation, can reduce progenitor overproliferation and differentiation.
H. erythrostictum extracts inhibit the proliferation and differentiation of stem cells induced by DSS
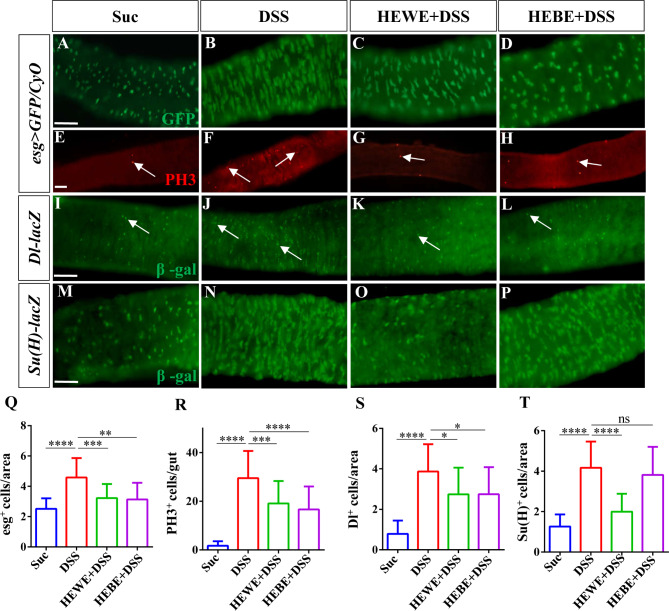
To preserve the epithelial barrier, microbial or chemical damage to epithelial surfaces activates a number of signal transduction pathways that accelerate progenitor proliferation and differentiation [40]. In a previous experiment, DSS-exposed Drosophila intestinal epithelial cells died, and intestinal integrity was compromised. Therefore, we examined whether H. erythrostictum extracts could maintain intestinal epithelial homeostasis to protect intestinal integrity. In the midgut epithelium of the sucrose-fed group, modest and dispersed subpopulations of esg+ cells were observed (Fig. 5A). Many large and concentrated esg+ cells were observed after 72 h of feeding with 3% DSS, and the number of progenitor cells increased by more than 82% (P < 0.0001, Fig. 5B, Q). Compared to those in DSS-fed group, supplementation with 8 mg/ml HEWE or 1 mg/ml HEBE significantly reduced the numbers of esg+ cells by 29.6% (P < 0.001) and 31.6% (P < 0.01), respectively (Fig. 5C, D, Q). To further confirm the presence of mitotic stem cells, we analyzed cell proliferation with an anti-phospho-histone H3 (anti-PH3) antibody. After DSS stimulation, the number of PH3+ cells in the entire midgut significantly increased (P < 0.0001); however, supplementation with HEWE or HEBE inhibited the number of PH3+ cells by 35.35% (P < 0.001) and 43.6% (P < 0.0001), respectively (Fig. 5E-H, R).
Fig. 5.
Both HEWE and HEBE prevent excessive proliferation and differentiation of ISCs induced by DSS. (A-P) Representative images of the posterior midguts of esg > GFP/Cyo(ISC/EB marker, A-H), Dl-lacZ (ISC marker, I-L) and Su(H)-lacZ (EB marker, M-P) transgenic flies. Guts were stained using anti-GFP (green), anti-PH3 (red), and anti-β-gal antibodies (green). After treatment with 3% DSS for 72 h, the numbers of esg+ cells, PH3+ cells, ISCs, and EBs significantly increased. Both HEWE and HEBE supplementation significantly reduced the increases in all types of targeted cells. However, HEBE did not reduce the increase in EBs. (Q-T) Quantification of the numbers of esg+ cells in A-D (n = 13–30), the numbers of PH3+ cells in E-H (n = 25–32), the numbers of ISCs in I-L (n = 12–14), and the numbers of EBs in M-P (n = 12–14). Quantification results represent mean ± SEM. nsP > 0.05, *P < 0.05, **P < 0.01,***P < 0.001,****P < 0.0001. Scale bars: 50 μm
Next, we utilized transgenic flies expressing the reporter genes Delta-lacZ (an ISC-specific marker) and Su(H)GBE-lacZ (an EB-specific marker) to analyze the numbers of ISCs and EBs, respectively. As expected, the numbers of Dl+ and Su(H)+ cells increased substantially after three days of treatment with 3% DSS (Dl+ cells: P < 0.0001, Su(H)+ cells: P < 0.0001). However, both the Dl+ and Su(H)+ cell numbers decreased by 29.2% (P < 0.05) and 52.0% (P < 0.0001), respectively, in the HEWE-supplemented group, while only the Dl+ cell number decreased by 28.9% (P < 0.05) in the HEBE-supplemented group (Fig. 5I-P, S, T). Our results demonstrated that H. erythrostictum extracts have a protective effect against DSS-induced abnormal proliferation and differentiation of progenitors.
H. erythrostictum extracts modulate progenitor overproliferation and differentiation by inhibiting the ROS-associated JNK signaling pathway
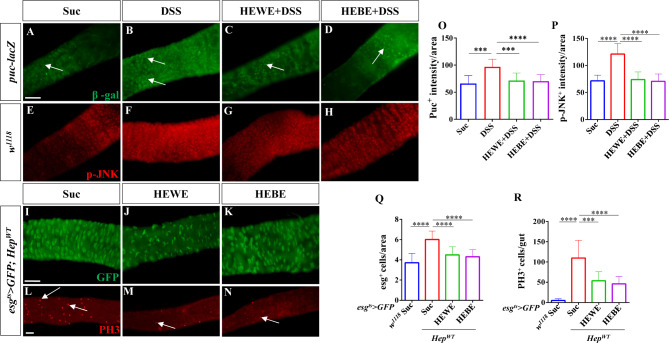
ROS-induced cellular damage and mortality, as well as bacterial or chemical ingestion, can activate JNK stress signaling pathways in ECs [50]. Activation of these pathways leads to the nuclear translocation and activation of the transcription factor AP1 (downstream of the JNK pathway), which induces cytokine and growth factor expression. Activation of JNK signaling pathways was observed by monitoring target gene expression. Using puc-lacZflies, we first measured the expression level of a reporter of JNK signaling. After 3 days of treatment with 3% DSS, the number of lacZ+ cells significantly increased compared to that in the sucrose-fed group (P < 0.0001). However, this increase was completely reversed by supplementation with 8 mg/ml HEWE or 1 mg/ml HEBE (HEWE: P < 0.0001, HEBE: P < 0.001, Fig. 6A-D, O). An anti-p-JNK antibody was subsequently used to assess JNK pathway activation. H. erythrostictum extracts significantly attenuated increase in the fluorescence intensity of p-JNK (HEWE: P < 0.0001, HEBE: P < 0.0001, Fig. 6E-H, P).To confirm the inhibitory effect of H. erythrostictum extracts on the JNK signaling pathway, we specifically overexpressed the JNK kinase hemipterous (Hep) in progenitor cells and counted the numbers of progenitor and mitotic cells. As a controls, the esgts> GFP transgene was crossed with wild-type w1118. In esgts> GFP; HepWT transgenic flies, the numbers of esg+ and PH3+ cells dramatically increased (P < 0.0001, P < 0.0001); however, after feeding with 8 mg/ml HEWE or 1 mg/ml HEBE, the numbers of progenitor and mitotic cells decreased by more than 25.4% (HEWE: P < 0.0001, HEBE: P < 0.0001) and 51.1% (HEWE: P < 0.001, HEBE: P < 0.0001), respectively (Fig. 6I-N, Q, R). These findings demonstrated that H. erythrostictum extracts can inhibit excessive progenitor proliferation and differentiation by decreasing JNK pathway activity.
Fig. 6.
Both HEWE and HEBE inhibited the activation of the JNK pathway by DSS. (A-H) Representative images of the posterior midguts of puc-lacZ (A-D) and w1118 (E-H) flies. Guts were stained with anti-β-gal antibodies (green) and anti-p-JNK antibodies (red). After treatment with 3% DSS for 72 h, the number of β-gal+ cells and the p-JNK level increased. Both HEWE and HEBE supplementation effectively decreased the increase in the number of β-gal+ cells and the level of p-JNK. (I-N) Representative images of the posterior midgut of esgts> GFP; HepWT transgenic flies stained with anti-GFP (green, I-K) and anti-PH3 (red, L-N) antibodies. In flies supplemented with HEWE or HEBE, the numbers of esg+ and PH3+ cells were significantly lower than those in the sucrose group. (O-R) Quantification of the numbers of β-gal+cells in A-D (n = 12–15), p-JNK intensities in E-H (n = 12–13), the numbers of esg+ cells in I-K, and the numbers of PH3+ cells in L-N (n = 12–16).Quantification results represent mean ± SEM. ***P < 0.001, ****P < 0.0001. Scale bars: 50 μm
H. erythrostictumextracts regulate intestinal homeostasis by inhibiting the JAK-STAT pathway
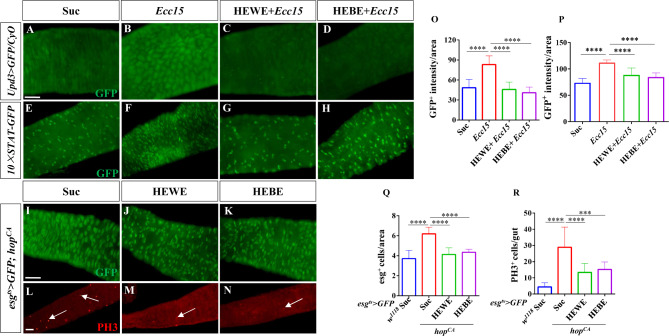
Increased JNK activity in ECs is sufficient to promote ISC proliferation by stimulating the expression of the cytokines Upd2 and Upd3. These cytokines promote regeneration by stimulating JAK/STAT signaling in ISCs to promote their proliferation and in EBs to promote their differentiation [40, 51]. Upd3 is one of the main cytokines that acts as a signal to elicit antibacterial and reparative host responses during oral infection with entomopathogenic bacteria such as Ecc15 [20, 51]. Therefore, we analyzed Upd3 expression in upd3 > GFP/CyO flies. As expected, after oral infection with Ecc15, GFP levels increased by more than 71.6% (P < 0.0001); however, after supplementation with HEWE or HEBE, GFP signals decreased almost to control levels (HEWE: P < 0.0001, HEBE: P < 0.0001, Fig. 7A-D, O). Next, the activity of the JAK/STAT pathway was evaluated using the 10XSTAT-GFP reporter, a common JAK/STAT signaling reporter containing 10 Stat92E-binding sites derived from the suppressor of cytokine signaling at 36E (Soc-s36E) gene [52]. As expected, supplementation with 8 mg/ml HEWE or 1 mg/ml HEBE reduced the number of GFP+ cells induced by Ecc15 (HEWE: P < 0.0001, HEBE: P < 0.0001, Fig. 7E-H, P).
Fig. 7.
Both HEWE and HEBE inhibited the activation of the JAK–STAT pathway byEcc15. (A-H) Representative images of the posterior midguts of upd3 > GFP/CyO (A-D) and 10×STAT-GFP (E-H) flies. Guts were stained with anti-GFP antibodies (green). After infection with Ecc15 for 16 h, the intensity of GFP and the number of GFP+ cells increased. Both HEWE and HEBE supplementation effectively reduced the increase in the intensity of GFP and the number of GFP+ cells. (I-N) Representative images of the posterior midgut of esgts> GFP; hopCA transgenic flies. Guts were stained with anti-GFP (green, I-K) and anti-PH3 (red, L-N) antibodies. In flies supplemented with HEWE or HEBE, the numbers of esg+ and PH3+ cells were significantly lower than those in the sucrose group. (O-R) Quantification of GFP intensities in A-D (n = 11–15), GFP+ cell numbers in E-H, the numbers of esg+ cells in I-K, and the numbers of PH3+ cells in L-N (12–19) (n = 13–15). Quantification results represent mean ± SEM. ***P < 0.001, ****P < 0.0001. Scale bars: 50 μm
We overexpressed hopscotch (hop) of Drosophila JAK kinase in ISCs/EBs using the esgts> GFP driver to confirm the inhibitory effect of H. erythrostictum extracts on the JAK/STAT signaling pathway. The numbers of progenitor and mitotic cells increased by approximately 1.7- and 5.6-fold, respectively (esg+ cells: P < 0.0001, PH3+ cells: P < 0.0001). However, supplementation with HEWE or HEBE significantly suppressed the JAK/STAT pathway-induced proliferation and differentiation of cells. The numbers of progenitor and mitotic cells drastically decreased, with the progenitor number resembling that in the sucrose group (esg+ cells: HEWE P < 0.0001, HEBE P < 0.0001; PH3+ cells: HEWE P < 0.001, HEBE P < 0.0001, Fig. 7I-N, Q, R). In conclusion, these findings demonstrated that H. erythrostictum extracts inhibit the JAK-STAT pathway to maintain intestinal homeostasis.
H. erythrostictum extracts inhibit intestinal injury induced by DSS via the EGFR pathway
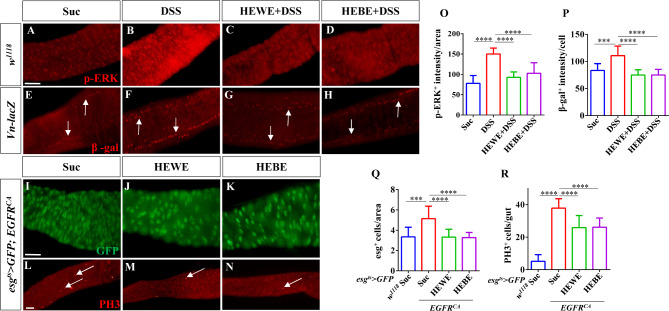
The EGFR/Ras/MAPK pathway is crucial for ISC proliferation, and JNK and JAK/STAT signaling induce ISC proliferation by activating EGFR signaling [7, 16]. Therefore, we examined whether H. erythrostictum extracts could protect against intestinal injury induced by DSS via the EGFR pathway. We initially stained the midgut with an anti-p-Erk antibody that recognizes the activated form of MAPK [53]. After 3 days of stimulation with 3% DSS, the p-Erk intensity in the midgut increased by approximately 92.5% compared to that in the sucrose-fed group (P < 0.0001); however, supplementation with 8 mg/ml HEWE or 1 mg/ml HEBE significantly decreased the p-Erk intensity (P < 0.0001, P < 0.0001, Fig. 8A-D, O). Three EGFR ligands, Vein, Spitz, and Keren, are expressed in the intestine of Drosophila. Vein is expressed in the visceral mesoderm (VM) surrounding the intestinal epithelium in a stable state and is the most essential ligand for stimulating ISC division [7, 54, 55]. The expression level of the EGFR signaling pathway ligand was subsequently detected using the Vein (Vn)-lacZ reporter. The fluorescence intensity of Vn-lacZ+ cells in the midgut was significantly greater in the DSS-fed group than in the sucrose-fed group (P < 0.001). Supplementation with 8 mg/ml HEWE or 1 mg/ml HEBE significantly decreased Vein expression in response to DSS stimulation (HEWE: P < 0.0001, HEBE:P < 0.0001, Fig. 8E-H, P).
Fig. 8.
Both HEWE and HEBE inhibited the activation of theEGFR pathway by DSS. (A-H) Representative images of the posterior midguts of w1118 (A-D) and Vn-lacZ (E-H) flies. Guts were stained with anti-p-ERK (red) and anti-β-gal antibodies (red). After treatment with 3% DSS for 72 h, the levels of p-ERK and β-gal increased. Both HEWE and HEBE supplementation effectively decreased the elevated p-ERK and β-gal levels. (I-N) Representative images of the posterior midgut of esgts> GFP; EGFRCA transgenic flies. Guts were stained with anti-GFP (green, I-K) and anti-PH3 (red, L-N) antibodies. In flies supplemented with HEWE or HEBE, the numbers of esg+ and PH3+ cells were significantly those the sucrose group.(O-R) Quantification of p-ERK intensities in A-D (n = 13–15), β-gal intensities in E-H (n = 11–13), the numbers of esg+ cells in I-K, and the numbers of PH3+ cells in L-N (12–19). Quantification results represent mean ± SEM. ***P < 0.001,****P < 0.0001. Scale bars: 50 μm
The inhibitory effect of H. erythrostictum extracts on the activation of the EGFR pathway was also evaluated. Compared to those in the control group, the numbers of progenitor and mitotic cells in the EGFR pathway-activated flies increased by approximately 1.5- and 7.3-fold, respectively (esg+ cells: P < 0.001, PH3+ cells: P < 0.0001). The numbers of esg+ and PH3+ cells decreased substantially after supplementation with 8 mg/ml HEWE or 1 mg/ml HEBE (esg+ cells: HEWE P < 0.0001, HEBE P < 0.0001; PH3+ cells: HEWE P < 0.0001, HEBE P < 0.0001, Fig. 8I-N, Q, R). In response to DSS-induced intestinal injury, these findings demonstrated that H. erythrostictum extracts can protect against intestinal epithelial homeostasis by inhibiting the EGFR signaling pathway.
Effects of H. Erythrostictum extracts on the composition and abundance of the gastrointestinal microbiota
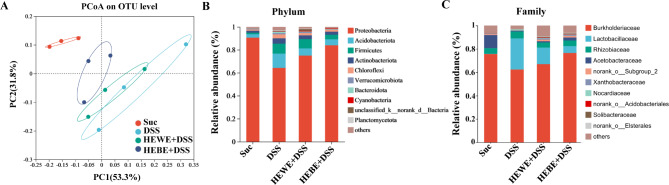
The gut microbiota may play a role in modulating ISC proliferation following tissue damage and boosting the host immune system [8]. Therefore, we used 16 S rDNA sequencing to determine whether the anti-inflammatory effects of H. erythrostictum extracts on DSS-induced inflammation are associated with alterations in the intestinal microbial structure. Alpha diversity analysis was performed to calculate the species diversity of samples using indices such as Ace, Shannon, and Simpson indices, as depicted in Figure S2A-C. There were no significant differences among the four distinct diet groups. A Venn diagram was generated to illustrate the unique and overlapping gut operational taxonomical units (OTUs) among the different groups. Following supplementation with HEWE or HEBE, the number of OTUs induced by DSS in the intestine decreased (Figure S2D). Beta diversity analysis was also performed to investigate the similarities and differences in community composition between samples from distinct groups. Principal coordinate analysis (PCoA) based on Bray curtis values was performed to visualize the similarity between microbiota compositions. As shown in Fig. 9A, the Suc, DSS, HEWE + DSS, and HEBE + DSS groups had distinct gut microbial compositions, whereas the HEWE + DSS and HEBE + DSS groups had similar gut microbial compositions, indicating that H. erythrostictum extracts modulated gut microbiota dysregulation in DSS-fed flies.
Fig. 9.
Effects of HEWE and HEBE on the gut microbiome structure in DSS-fed flies. (A) Beta diversity analysis of the gut microbiota in each group using the PCoA method based on Bray curtis values. (B) Relative abundance at the phylum level for each group (n = 3). (C) Relative abundance at the family level for each group (n = 3)
Figure 9B shows the relative abundance levels of the top 10 bacterial taxa in the intestines of the four distinct groups at the phylum level. Proteobacteria, Acidobacteriota, and Firmicutes were demonstrated to be the predominant phyla in eachgroup. Compared to those in the sucrose-fed group, the Acidobacteriota (12.4%) and Firmicutes (8.6%) in the DSS-fed group increased, and the relative abundance of Proteobacteria decreased (64.3%). Nevertheless, the HEWE and HEBE groups exhibited relative decreases in Acidobacteriota (6.3% and 5.2%) and Firmicutes (8.1% and 4.0%) and relative increases in Proteobacteria (74.9% and 83.9%).
Subsequently, we analyzed the gut microbiota composition of each group at the family level.As shown in Fig. 9C, Burkholderiaceae, Lactobacillaceae, Rhizobiaceae, and Acetobacteraceae were the dominant family found in the gut of each group, respectively.Compared to those in the sucrose-fed group, the Lactobacillaceae (26.6%) and Rhizobiaceae (5.9%) in the DSS-fed group increased, and the relative abundance of Burkholderiaceae (62.4%) and Acetobacteraceae (1.05%) decreased. Nevertheless, the HEWE and HEBE groups exhibited relative decreases in Lactobacillaceae (14.0% and 5.8%) and Rhizobiaceae (4.5% and 4.8%) and relative increases in Burkholderiaceae (67.0% and 76.6%) and Acetobacteraceae (1.08% and 2.7%).Shifts in relative abundance of various bacterial phyla and families indicated that DSS induced substantial alterations in the gut microbiota, as demonstrated by our findings. Moreover, our findings demonstrated the effect of HEWE or HEBE supplementation on the intestinal microbiota.
Changes in the proportions of Proteobacteria, Acidobacteriota, and Firmicutesat phylum level and Burkholderiaceae, Lactobacillaceae, Rhizobiaceae, and Acetobacteraceae at family level suggested that H. erythrostictum extracts may selectively stimulate the growth of particular beneficial bacteria while inhibiting the growth of harmful bacteria. These results suggested that H. erythrostictum extracts could be a viable therapeutic agent for restoring the gut microbiota balance and improving gut homeostasis.
Analysis of active compounds and potential targets of H. erythrostictumextracts
To identify the bioactive compounds present in HEWE and HEBE, we conducted metabolite profiling of the H. erythrostictum extracts. Widely targeted metabolomics analysis identified 633 (HEWE) and 512 (HEBE) compounds in H. erythrostictum extracts. Of these compounds, 42 (HEWE) and 21 (HEBE) compounds with relative contents of more than 0.1%, high gastrointestinal absorption and excellent drug likeness were selected. Based on these results, SwissADME predicted 492 and 347 targets in HEWE and HEBE, respectively. Various databases, including OMIM (253 targets), CTD (164 targets), DisGeNet (731 targets), GeneCards (948 targets), and TTD (62 targets), were consulted to obtain the IBD targets, and the database intersections are displayed in Figure S3A. The intersection of compound targets and IBD targets revealed 166 and 120 hub targets, respectively (Figure S3B, C). These results demonstrated that H. erythrostictum extracts contain potential IBD-treating compounds. To predict the anti-inflammatory bioactive compounds of HEWE and HEBE against IBD, a network of herb-compound targets was constructed using Cytoscape software. (Figure S3D, E). The results indicated that 19 compounds in HEWE and 14 components in HEBE could be candidate bioactive compounds for IBD treatment (Tables S1, S2). These results demonstrated that H. erythrostictum extracts can be used to treat IBD effectively via multiple targets and compounds.
Discussion
Fruit flies are commonly used as powerful models for drug testing. Xiu et al. emphasized that Drosophila is a suitable model for high-throughput screening of drugs derived from natural products [56]. The Drosophila intestinal epithelium is an important defense barrier that prevents infection and invasion of the microbiota or toxic substances; moreover, the fly intestine is structurally, genetically, and functionally similar to the mammalian intestine, making it an ideal model for studying the mechanisms of intestinal disorders [57, 58]. Thus, numerous researchers are currently examining natural molecules and herbal extracts with distinct protective and therapeutic effects on intestinal inflammation [56].
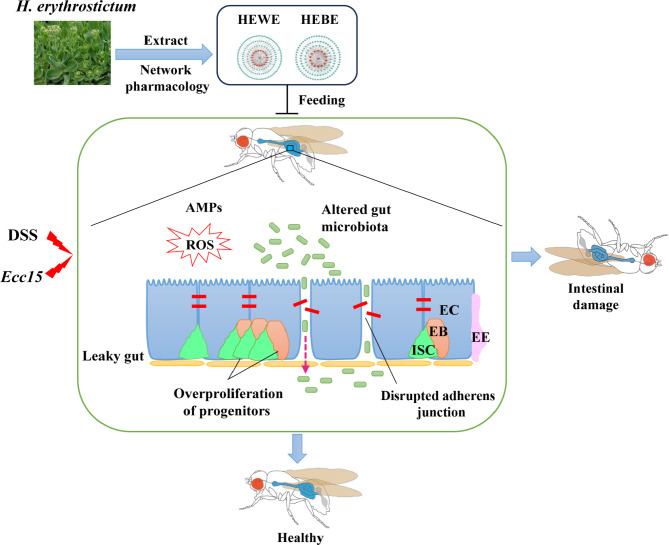
The protective effects of H. erythrostictum water extract (HEWE) and H. erythrostictum butanol extract (HEBE) on preventing intestinal injury caused by DSS stimulation or Ecc15 infection have been described in this study (Fig. 10). DSS or SDS was administered with various concentrations of HEWE or HEBE, and 8 mg/ml HEWE and 1 mg/ml HEBE resulted in greater survival rates. Our previous studies revealed that ingestion of SDS or DSS in fruit flies can lead to damage of intestinal barrier integrity and epithelial cell death, significantly reducing the survival rate of the flies [59–61]. Supplementation with HEWE and HEBE was able to reverse intestinal epithelial cell death and resulted in disorganized cell localization and disruption of intestinal barrier integrity and acid‒base homeostasis in Drosophila intestinal disorders induced by DSS. In addition, we did not observe a difference in food intake between the control group and those fed HEWE or HEBE.
Fig. 10.
Mechanism of action of HEWE and HEBE against intestinal injury induced by DSS and Ecc15
We observed that infection with the gram-negative bacterium Ecc15 significantly increased intestinal AMP levels. It has been well established that AMPs serve as effective antimicrobial agents against a variety of pathogens. However, increasing evidence suggests that excessive AMPs may have other physiological effects that may be harmful to host tissues, such as modulating the microbiome and being involved in nervous system activity and aging [62]. Our data demonstrated that H. erythrostictum extracts can reduce excessive AMP levels induced by Ecc15. In addition, Harsh et al. described the function of lipid droplets (LDs) in the context of host–pathogen interactions and immunity. Systemic infection with Photorhabdus bacteria causes gastrointestinal steatosis characterized by LD accumulation, which is related to the activation of immune signaling pathways. Moreover, intestinal steatosis is an indicator and regulator of the antibacterial immune response [38]. Our results revealed substantial accumulation of intestinal LDs upon infection with Ecc15, which was consistent with previous results of infection with gram-negative Photorhabdus bacteria [38]. Nonetheless, supplementation with HEWE or HEBE substantially decreased LDs accumulation, indicating that H. erythrostictum extracts can modulate the immune response by inhibiting Ecc15-induced excessive lipogenesis.
ROS are groups of active molecules derived from molecular oxygen and are typical examples of double-edged swords [63]. Appropriate levels of ROS serve as signaling molecules that modulate particular signaling pathways to maintain normal cell function and survival [64]. However, excessive ROS can injure tissues through the oxidation of proteins, lipids, and DNA [42]. Moreover, in Drosophila, elevated ROS levels can cause intestinal injury by increasing the proliferation of ISCs [65]. This study revealed that HEWE and HEBE inhibited DSS-induced accumulation of ROS in the midgut. In addition, HEWE and HEBE inhibited UAS-Cat RNAi- and UAS-Sod2 RNAi-induced proliferation and mitosis of ISC progenitors. These findings demonstrated that H. erythrostictum extracts possess ROS-scavenging activity and can inhibit the excessive proliferation and differentiation of progenitors caused by elevated ROS levels.
ISC differentiation and proliferation are required to maintain intestinal homeostasis in the presence of toxic compounds or bacterial infection; however, excessive proliferation and differentiation can lead to a variety of diseases, including inflammatory bowel disease and cancer. By inhibiting the JNK, JAK-STAT, and EGFR pathways, HEWE or HEBE supplementation contributed to the maintenance of ISC homeostasis in the context of DSS ingestion and Ecc15 infection. In addition, 16 S rDNA sequencing of the gut microbiota revealed that the amelioration of DSS-induced inflammation by H. erythrostictum extracts was associated with alterations in the structure of the gut microbiota. After supplementation with HEWE or HEBE, the proportions of Proteobacteria, Acidobacteriota, and Firmicutes changed significantly at the phylum level.
We performed metabolite profiling and network pharmacology analysis of H. erythrostictum extracts to investigate the essential components and mechanisms of HEWE and HEBE in the treatment of IBD. Based on our network pharmacology analysis, 19 major components of HEWE and 14 major components of HEBE were linked to IBD hub targets (Tables S1, S2). Previous studies have shown that Ecc15 infection causes intestinal injury, increases intestinal progenitor numbers, and leads to morphological changes [16]. However, there are no studies describing whether Ecc15 can induce IBD, though it may contribute to inflammatory triggers or exacerbate IBD. However, the specific functions and mechanisms of these active ingredients in treating inflammatory intestinal injury have not been fully elucidated, necessitating further clarification through the use of multiple IBD models.
Conclusion
These findings demonstrate that H. erythrostictum extracts have a potential effect in alleviating intestinal inflammation caused by DSS ingestion or Ecc15 infection in a Drosophila model. Therefore, H. erythrostictum extracts have distinct protective effects against intestinal damage and may be novel, effective treatments for gut inflammation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- H.erythrostictum
Hylotelephium erythrostictum (Miq.) H. Ohba
- HEWE
Hylotelephium erythrostictumwater extract
- HEBE
Hylotelephium erythrostictumbutanol extract
- IBD
Inflammatory bowel disease
- ROS
Reactive oxygen species
- DSS
Dextran sodium sulfate
- SDS
Sodium dodecyl sulfate
- Ecc15
Erwinia carotovoracarotovora 15
- LD
Lipid droplet
- AMP
Antimicrobial peptide
- ISC
Intestinal stem cell
- EB
Enteroblast
- EC
Absorbent enterocyte
- EE
Enteroendocrine cell
- PBS
Phosphate buffered saline
- PBST
Triton X-100 in PBS
- PFA
Paraformaldehyde
- BPB
Bromophenol blue sodium
- 7-AAD
7-Aminoactinomycin D
- Def
Defencin
- Drs
Drosomycin
- Dipt
Diptericin
- OTUs
Operational taxonomical units
- CCR
Copper cell region
Author contributions
Conceptualization, LHJ and HIK; methodology, LHJ and HIK; software, HMX, GHY and JHZ; validation, HIK, HMX and XYY; formal analysis, HMX and XYY; investigation, HIK and LHJ; resources, LHJ; data curation, TJE, GHY and JHZ; writing—original draft preparation, HIK and TJE; writing—review and editing, SHW and LHJ; visualization, LHJ and SHW; supervision, LHJ and SHW; project administration, LHJ; funding acquisition, LHJ. and SHW All authors have read and agreed to the published version of the manuscript.
Funding and acknowledgments
This research was funded by the National Natural Science Foundation of China (21964017) and the Fundamental Research Funds for the Central Universities (2572022DQ07). We thank Rongwen Xi, Bruno Lemaitre, Jose Carlos Pastor-Pareja, Jean-Luc Imler and Zongzhao Zhai, the Bloomington Stock Center and the Tsinghua Fly Center for fly strains, and the DSHB for antibodies.
Data availability
Data from this study are available from the corresponding author upon reasonable request.
Declarations
Competing interest
The authors declare that this work was conducted without any commercial or financial affiliations that could be perceived as possible conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sihong Wang, Email: shwang@ybu.edu.cn.
Li Hua Jin, Email: lhjin2000@hotmail.com.
References
- 1.Ferguson LR, Shelling AN, Browning BL, Huebner C, Petermann I. Genes, diet and inflammatory bowel disease. Mutat Res. 2007;622(1–2):70–83. [DOI] [PubMed] [Google Scholar]
- 2.Yang S, Li X, Xiu M, Dai Y, Wan S, Shi Y, Liu Y, He J. Flos Puerariae ameliorates the intestinal inflammation of Drosophila via modulating the Nrf2/Keap1, JAK-STAT and wnt signaling. Front Pharmacol. 2022;13:893758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitani-Morii F, Friedland RP, Yoshida H, Mizuno T. Drosophila as a model for Microbiota studies of Neurodegeneration. J Alzheimers Dis. 2021;84(2):479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LiuX HJJ. BuchonN: Drosophila as a model for homeostatic, antibacterial, and antiviral mechanisms in the gut. PLoS Pathog. 2017;13:e1006277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439(7075):475–9. [DOI] [PubMed] [Google Scholar]
- 6.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439(7075):470–4. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Grenley MO, Bravo M-J, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8(1):84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonfini A, Liu X, Buchon N. From pathogens to microbiota: how Drosophila intestinal stem cells react to gut microbes. Dev Comp Immunol. 2016;64:22–38. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Zhou H, Gong W. The complete chloroplast genome of Hylotelephium erythrostictum (Miq.) H. Ohba (Crassulaceae). Mitochondrial DNA B Resour. 2022;7(2):365–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan MHA, Elwekeel A, Moawad A, Afifi N, Amin E, Amir DE. Phytochemical constituents and biological activity of selected genera of family Crassulaceae: a review. South Afr J Bot. 2021;141:383–404. [Google Scholar]
- 11.Quan YS, Zhang XY, Yin XM, Wang SH, Jin LL. Potential alpha-glucosidase inhibitor from Hylotelephium erythrostictum. Bioorg Med Chem Lett. 2020;30(24):127665. [DOI] [PubMed] [Google Scholar]
- 12.Yin XM, Wang SH, Li DH. Antibacterial activity and chemical composition of Hylotelephiumerythrostictumethyl acetate extracts. Nat Prod Res Dev. 2018;30:930–5. [Google Scholar]
- 13.Zhang P, Bai J, Liu Y, Meng Y, Yang Z, Liu T. Drought resistance of ten ground cover seedling species during roof greening. PLoS ONE. 2020;15(6):e0220598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Zhao X, Hu Z, Leng P. Nitric oxide modulating ion balance in Hylotelephium erythrostictum roots subjected to NaCl stress based on the analysis of transcriptome, fluorescence, and ion fluxes. Sci Rep. 2019;9(1):18317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang F, Xi R. Silencing transposable elements in the Drosophila germline. Cell Mol Life Sci. 2017;74(3):435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. In: BMC biology. vol. 8; 2010: 152. [DOI] [PMC free article] [PubMed]
- 17.Pastor-Pareja JC, Wu M, Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Models Mech. 2008;1(2–3):144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzou P, Ohresser S, Ferrandon D, Capovilla M, Reichhart J-M, Lemaitre B, Hoffmann JA, Imler J-L. Tissue-specific Inducible expression of Antimicrobial peptide genes in DrosophilaSurface Epithelia. Immunity. 2000;13(5):737–48. [DOI] [PubMed] [Google Scholar]
- 19.Zhai Z, Boquete J-P, Lemaitre B. Cell-specific Imd-NF-κB responses enable simultaneous antibacterial immunity and intestinal epithelial cell shedding upon bacterial infection. Immunity. 2018;48(5):897–e910897. [DOI] [PubMed] [Google Scholar]
- 20.Zhou F, Rasmussen A, Lee S, Agaisse H. The UPD3 cytokine couples environmental challenge and intestinal stem cell division through modulation of JAK/STAT signaling in the stem cell microenvironment. Dev Biol. 2013;373(2):383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis AP, Wiegers TC, Johnson RJ, Sciaky D, Wiegers J, Mattingly Carolyn J. Comparative toxicogenomics database (CTD): update 2023. Nucleic Acids Res. 2023;51(D1):D1257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piñero J, Saüch J, Sanz F, Furlong LI. The DisGeNET cytoscape app: exploring and visualizing disease genomics data. Comput Struct Biotechnol J. 2021;19:2960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y et al. The GeneCards suite: from Gene Data Mining to Disease Genome sequence analyses. Curr Protocols Bioinf 2016, 54(1). [DOI] [PubMed]
- 24.Zhou Y, Zhang Y, Lian X, Li F, Wang C, Zhu F, Qiu Y, Chen Y. Therapeutic target database update 2022: facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res. 2022;50(D1):D1398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bateman A, Martin M-J, Orchard S, Magrane M, Ahmad S, Alpi E, Bowler-Barnett EH, Britto R, Bye-A-Jee H, Cukura A, et al. UniProt: the Universal protein knowledgebase in 2023. Nucleic Acids Res. 2023;51(D1):D523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. Jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15(1):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marygold SJ, Vincent J-P. Armadillo levels are reduced during mitosis in Drosophila. Mech Dev. 2003;120(2):157–65. [DOI] [PubMed] [Google Scholar]
- 28.Lei X, Zhou Z, Wang S, Jin LH. The protective effect of safranal against intestinal tissue damage in Drosophila. Toxicol Appl Pharmcol 2022, 439. [DOI] [PubMed]
- 29.Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T Jr., Jones DL, Walker DW. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14(5):623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin WS, Huang CW, Song YS, Yen JH, Kuo PC, Yeh SR, Lin HY, Fu TF, Wu MS, Wang HD, et al. Reduced gut acidity induces an obese-like phenotype in Drosophila melanogaster and in mice. PLoS ONE. 2015;10(10):e0139722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Qi Y, Jasper H. Preventing Age-Related decline of gut compartmentalization limits Microbiota Dysbiosis and extends Lifespan. Cell Host Microbe. 2016;19(2):240–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu F, Ohlstein B. Monitoring Gut Acidification in the Adult Drosophila Intestine. J Vis Exp 2021(176). [DOI] [PubMed]
- 33.Lazzaro BP, Zasloff M, Rolff J. Antimicrobial peptides: application informed by evolution. Science 2020, 368(6490). [DOI] [PMC free article] [PubMed]
- 34.Nehme NT, Liegeois S, Kele B, Giammarinaro P, Pradel E, Hoffmann JA, Ewbank JJ, Ferrandon D. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 2007;3(11):e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bischoff V, Vignal C, Duvic B, Boneca IG, Hoffmann JA, Royet J. Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog. 2006;2(2):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsichritzis T, Gaentzsch PC, Kosmidis S, Brown AE, Skoulakis EM, Ligoxygakis P, Mosialos G. A Drosophila ortholog of the human cylindromatosis tumor suppressor gene regulates triglyceride content and antibacterial defense. Development. 2007;134(14):2605–14. [DOI] [PubMed] [Google Scholar]
- 37.Lee JE, Edery I. Circadian regulation in the ability of Drosophila to combat pathogenic infections. Curr Biol. 2008;18(3):195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harsh S, Heryanto C, Eleftherianos I. Intestinal lipid droplets as novel mediators of host-pathogen interaction in Drosophila. Biol Open 2019, 8(7). [DOI] [PMC free article] [PubMed]
- 39.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310(5749):847–50. [DOI] [PubMed] [Google Scholar]
- 40.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23(19):2333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry G, Raina AK, Nunomura A, Wataya T, Sayre LM, Smith MA. How important is oxidative damage? Lessons from Alzheimer’s disease. Free Radic Biol Med. 2000;28(5):831–4. [DOI] [PubMed] [Google Scholar]
- 42.Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14(1):76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan SWS, Yip GW, Suda T, Baeg GH. Small maf functions in the maintenance of germline stem cells in the Drosophila testis. Redox Biol. 2018;15:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jena AB, Samal RR, Bhol NK, Duttaroy AK. Cellular Red-Ox system in health and disease: the latest update. Biomed Pharmacother. 2023;162:114606. [DOI] [PubMed] [Google Scholar]
- 45.Alfonso-Prieto M, Biarnés X, Vidossich P, Rovira C. The molecular mechanism of the catalase reaction. J Am Chem Soc. 2009;131(33):11751–61. [DOI] [PubMed] [Google Scholar]
- 46.Sepasi Tehrani H, Moosavi-Movahedi AA. Catalase and its mysteries. Prog Biophys Mol Biol. 2018;140:5–12. [DOI] [PubMed] [Google Scholar]
- 47.Areekul S, Boonme Y. Superoxide dismutase and catalase activities in red cells of patients with Plasmodium falciparum. 1987. [PubMed]
- 48.Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev. 2005;126(3):365–79. [DOI] [PubMed] [Google Scholar]
- 49.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3(4):442–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagai H, Kurata S, Yano T. Immunoglobulin superfamily beat-Ib mediates intestinal regeneration induced by reactive oxygen species in Drosophila. Genes Cells. 2020;25(5):343–9. [DOI] [PubMed] [Google Scholar]
- 51.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137(7):1343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7(3):323–31. [DOI] [PubMed] [Google Scholar]
- 53.Gabay L, Seger R, Shilo B-Z. In situ activation pattern of Drosophila EGF receptor pathway during development. Science. 1997;277(5329):1103–6. [DOI] [PubMed] [Google Scholar]
- 54.Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011;138(6):1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R, EGFR. Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011;354(1):31–43. [DOI] [PubMed] [Google Scholar]
- 56.Xiu M, Wang Y, Yang D, Zhang X, Dai Y, Liu Y, Lin X, Li B, He J. Using Drosophila melanogaster as a suitable platform for drug discovery from natural products in inflammatory bowel disease. Front Pharmacol. 2022;13:1072715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medina A, Bellec K, Polcownuk S, Cordero JB. Investigating local and systemic intestinal signalling in health and disease with Drosophila. Dis Model Mech 2022, 15(3). [DOI] [PMC free article] [PubMed]
- 58.Lin J, Hackam DJ. Worms, flies and four-legged friends: the applicability of biological models to the understanding of intestinal inflammatory diseases. Dis Models Mech. 2011;4(4):447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Z, Chen Y, Zhang H, Jin LH. Crocus sativus L. protects against SDSinduced intestinal damage and extends lifespan in Drosophila melanogaster. Mol Med Rep. 2016;14(6):5601–6. [DOI] [PubMed] [Google Scholar]
- 60.Zhu C, Guan F, Wang C, Jin LH. The protective effects of Rhodiola crenulata extracts on Drosophila melanogaster gut immunity induced by bacteria and SDS toxicity. Phytother Res. 2014;28(12):1861–6. [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, Wang S, Jin LH. Acanthopanax senticosus polysaccharide regulates the intestinal homeostasis disruption induced by toxic chemicals in Drosophila. Phytother Res. 2020;34(1):193–200. [DOI] [PubMed] [Google Scholar]
- 62.Staczek S, Cytrynska M, Zdybicka-Barabas A. Unraveling the role of antimicrobial peptides in insects. Int J Mol Sci 2023, 24(6). [DOI] [PMC free article] [PubMed]
- 63.Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol. 2014;9:119–45. [DOI] [PubMed] [Google Scholar]
- 64.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iatsenko I, Boquete JP, Lemaitre B. Microbiota-Derived Lactate activates production of reactive oxygen species by the Intestinal NADPH Oxidase Nox and Shortens Drosophila Lifespan. Immunity. 2018;49(5):929–e942925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study are available from the corresponding author upon reasonable request.