Abstract
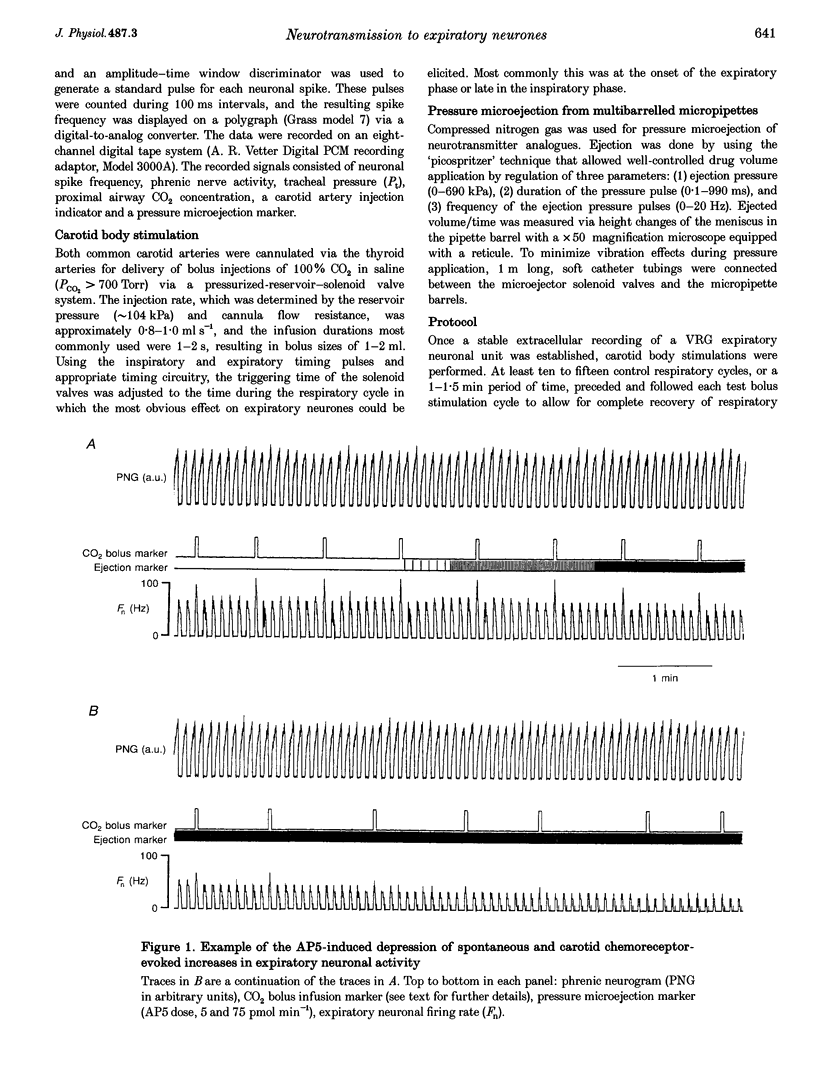
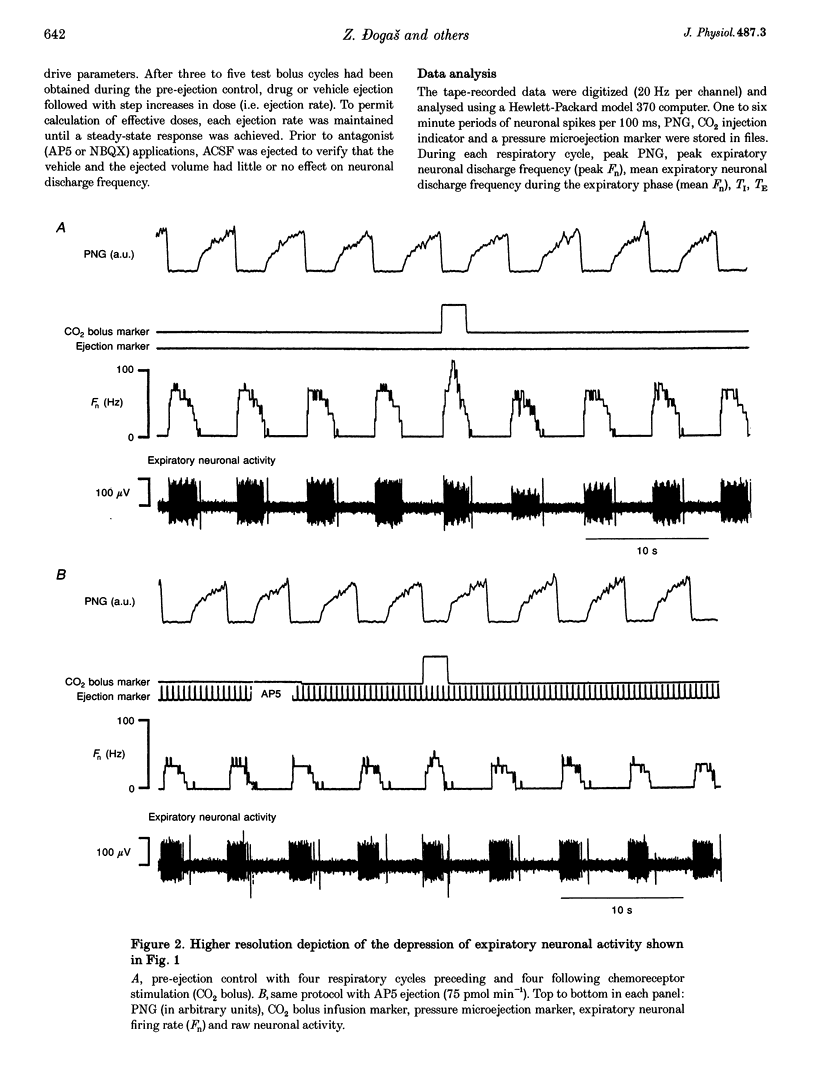
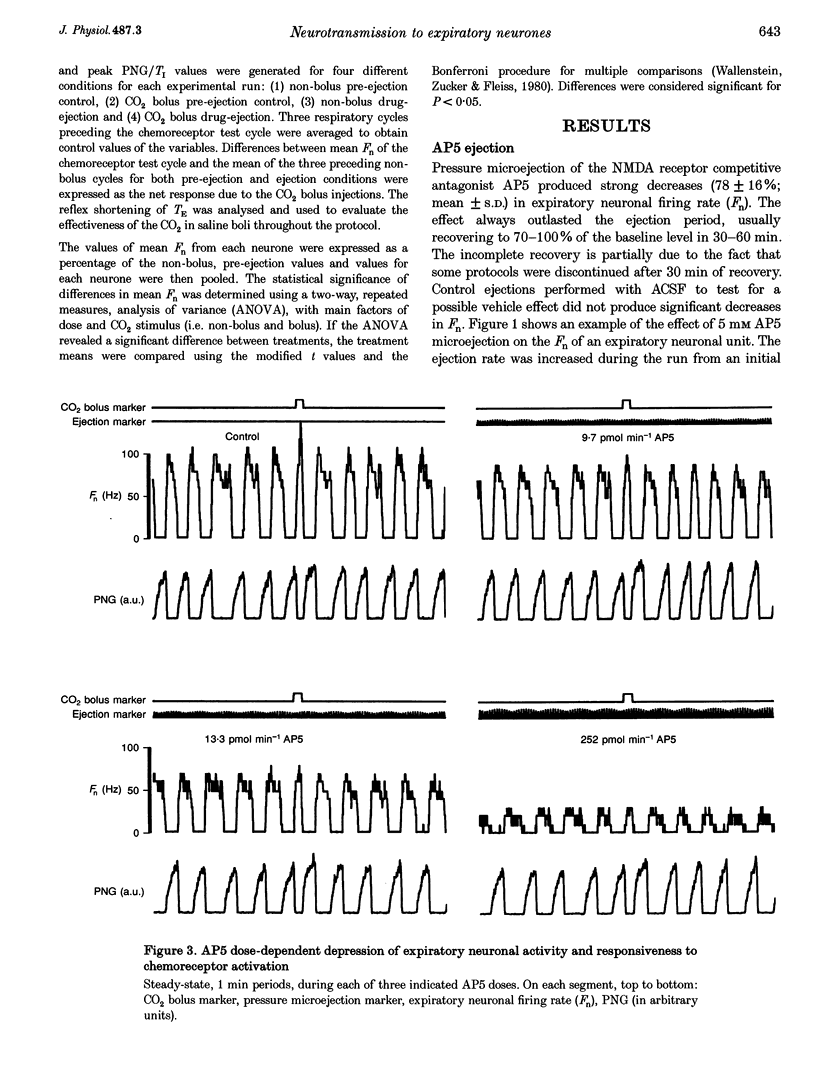
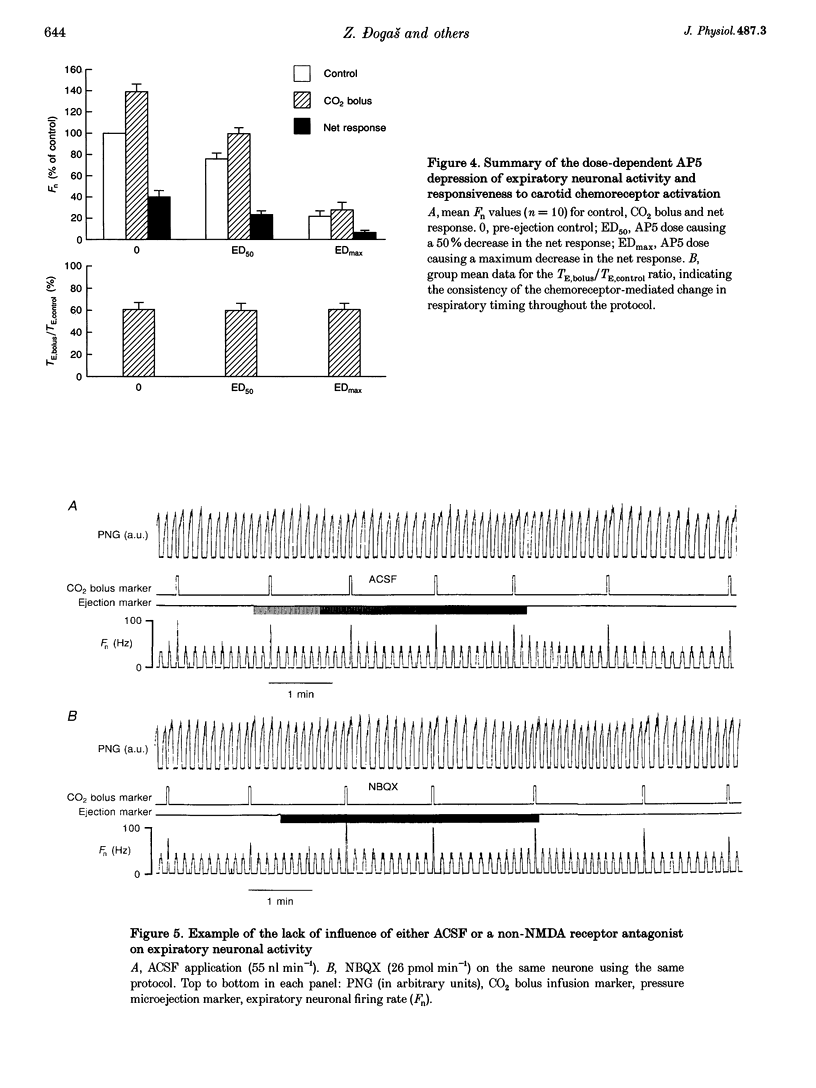
1. This study tested the hypothesis that excitatory amino acid receptors mediate the excitatory response of expiratory bulbospinal neurones to carotid body chemoreceptor inputs. 2. Studies were carried out in thiopental sodium anaesthetized, paralysed, ventilated, vagotomized dogs. 3. Brisk, short-duration chemoreceptor activation was produced by bilateral bolus injections of CO2-saturated saline (PCO2 > 700 mmHg) into the autoperfused carotid arteries. A pressurized-reservoir-solenoid valve system was used to deliver the CO2 bolus injections just prior to the onset of the neural expiratory phase, as determined from the phrenic neurogram, about once per minute. 4. Multibarrelled micropipettes were used to record neuronal unit activity and deliver neurotransmitter agents. Net responses of expiratory bulbospinal neurones to peripheral chemoreceptor activation were determined by subtracting the mean discharge frequencies (Fn) during three control expiratory cycles from the Fn during administration of a CO2 test bolus. The role of excitatory amino acid receptors in mediating this response was determined by comparing the baseline and bolus expiratory neuronal Fn before, during and after the pressure microejection of the NMDA receptor antagonist 2-amino-5-phosphonovalerate (AP5) or the non-NMDA receptor antagonist 2,3-dihydroxy-6-nitro-7-sulphamoyl- benzo(f)quinoxaline (NBQX). Ejection rates of AP5 and NBQX were measured by monitoring the movement of the pipette meniscus. 5. AP5 reduced Fn during both the control and bolus cycles, as well as reducing the change in Fn between control and bolus cycles. NBQX had no effect on either baseline or bolus responses. 6. AP5 did not prevent excitation of expiratory bulbospinal neurones by AMPA. Coadministration of AMPA with AP5 prevented the AP5-mediated decrease in Fn but not the dose-dependent reduction in the CO2 bolus response. 7. Taken together, these data strongly suggest that the carotid chemoreceptor-mediated excitation of expiratory bulbospinal neurones is dependent on NMDA but not non-NMDA glutamate receptors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton C. R., Kirkwood P. A. The effect of carbon dioxide on the tonic and the rhythmic discharges of expiratory bulbospinal neurones. J Physiol. 1979 Nov;296:291–314. doi: 10.1113/jphysiol.1979.sp013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajić J., Zuperku E. J., Tonković-Capin M., Hopp F. A. Expiratory bulbospinal neurons of dogs. I. Control of discharge patterns by pulmonary stretch receptors. Am J Physiol. 1992 Jun;262(6 Pt 2):R1075–R1086. doi: 10.1152/ajpregu.1992.262.6.R1075. [DOI] [PubMed] [Google Scholar]
- Bajić J., Zuperku E. J., Tonković-Capin M., Hopp F. A. Interaction between chemoreceptor and stretch receptor inputs at medullary respiratory neurons. Am J Physiol. 1994 Jun;266(6 Pt 2):R1951–R1961. doi: 10.1152/ajpregu.1994.266.6.R1951. [DOI] [PubMed] [Google Scholar]
- Davies R. O., Edwards M. W., Jr Medullary relay neurons in the carotid-body chemoreceptor pathway of cats. Respir Physiol. 1975 Jun;24(1):69–79. doi: 10.1016/0034-5687(75)90122-x. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. Expiratory effects of brief carotid sinus nerve and carotid body stimulations. Respir Physiol. 1976 May;26(3):395–410. doi: 10.1016/0034-5687(76)90009-8. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. The importance of timing on the respiratory effects of intermittent carotid sinus nerve stimulation. J Physiol. 1972 Apr;222(2):297–318. doi: 10.1113/jphysiol.1972.sp009798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley J. C., Katz D. M. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992 Feb 14;572(1-2):108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Harris K. M., Miller R. J. CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) antagonizes NMDA-evoked [3H]GABA release from cultured cortical neurons via an inhibitory action at the strychnine-insensitive glycine site. Brain Res. 1989 Jun 5;489(1):185–189. doi: 10.1016/0006-8993(89)90023-1. [DOI] [PubMed] [Google Scholar]
- Hopp F. A., Seagard J. L., Bajić J., Zuperku E. J. Respiratory responses to aortic and carotid chemoreceptor activation in the dog. J Appl Physiol (1985) 1991 Jun;70(6):2539–2550. doi: 10.1152/jappl.1991.70.6.2539. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepchen H. P., Klüssendorf D., Philipp U. Mechanisms of central transmission of respiratory reflexes. Acta Neurobiol Exp (Wars) 1973;33(1):287–299. [PubMed] [Google Scholar]
- Lipski J., Bellingham M. C., West M. J., Pilowsky P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Methods. 1988 Dec;26(2):169–179. doi: 10.1016/0165-0270(88)90166-5. [DOI] [PubMed] [Google Scholar]
- Lipski J., McAllen R. M., Spyer K. M. The carotid chemoreceptor input to the respiratory neurones of the nucleus of tractus solitarus. J Physiol. 1977 Aug;269(3):797–810. doi: 10.1113/jphysiol.1977.sp011930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek W., Prabhakar N. R., Loeschcke H. H. Electrical stimulation of arterial and central chemosensory afferents at different times in the respiratory cycle of the cat: I. Ventilatory responses. Pflugers Arch. 1985 Apr;403(4):415–421. doi: 10.1007/BF00589255. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Benveniste M., Patneau D. K., Vyklicky L., Jr Pharmacologic properties of NMDA receptors. Ann N Y Acad Sci. 1992 May 11;648:194–204. doi: 10.1111/j.1749-6632.1992.tb24538.x. [DOI] [PubMed] [Google Scholar]
- Mitchell G. S., Selby B. D. Ventilatory responses to lung inflation and arterial CO2 in halothane-anesthetized dogs. J Appl Physiol (1985) 1988 Apr;64(4):1433–1438. doi: 10.1152/jappl.1988.64.4.1433. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Guet T., Cordi A., Lepagnol J. M. Competitive inhibition by NBQX of kainate/AMPA receptor currents and excitatory synaptic potentials: importance of 6-nitro substitution. Eur J Pharmacol. 1992 May 14;215(2-3):237–244. doi: 10.1016/0014-2999(92)90033-z. [DOI] [PubMed] [Google Scholar]
- Sheardown M. J., Nielsen E. O., Hansen A. J., Jacobsen P., Honoré T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990 Feb 2;247(4942):571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Subtypes of NMDA receptors. Gen Pharmacol. 1993 Jul;24(4):825–832. doi: 10.1016/0306-3623(93)90155-q. [DOI] [PubMed] [Google Scholar]
- Suzdak P. D., Sheardown M. J., Honoré T. Characterization of the metabotropic glutamate receptor in mouse cerebellar granule cells: lack of effect of 2,3-dihydroxy-6-nitro-7-sulphamoylbenzo(F)-quinoxaline (NBQX). Eur J Pharmacol. 1993 May 15;245(3):215–220. doi: 10.1016/0922-4106(93)90099-u. [DOI] [PubMed] [Google Scholar]
- Tonković-Capin M., Zuperku E. J., Bajić J., Hopp F. A. Expiratory bulbospinal neurons of dogs. II. Laterality of responses to spatial and temporal pulmonary vagal inputs. Am J Physiol. 1992 Jun;262(6 Pt 2):R1087–R1095. doi: 10.1152/ajpregu.1992.262.6.R1087. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]


