Abstract
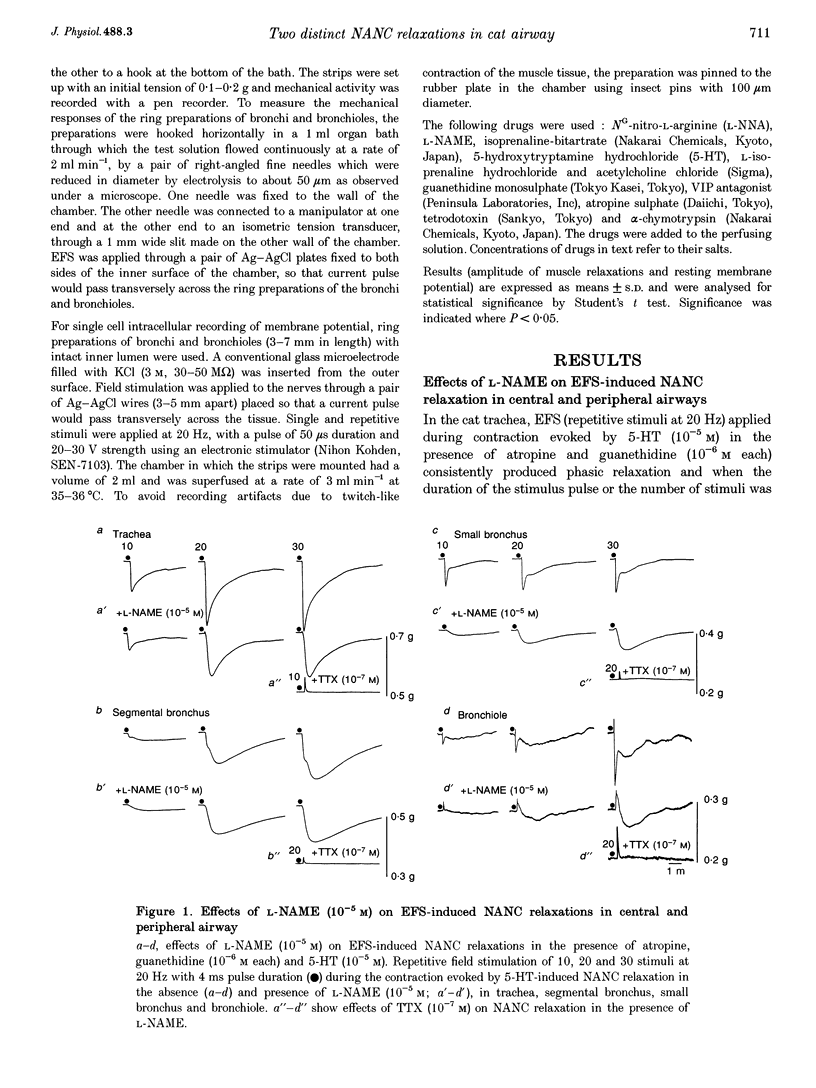
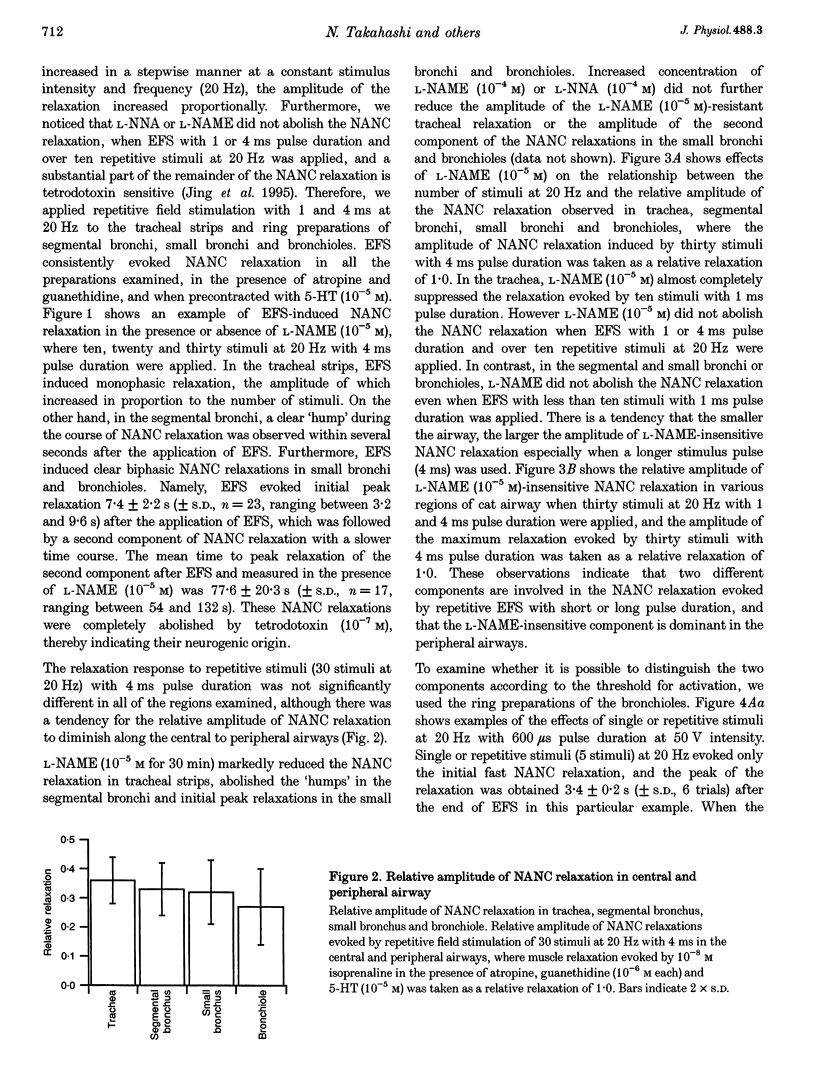
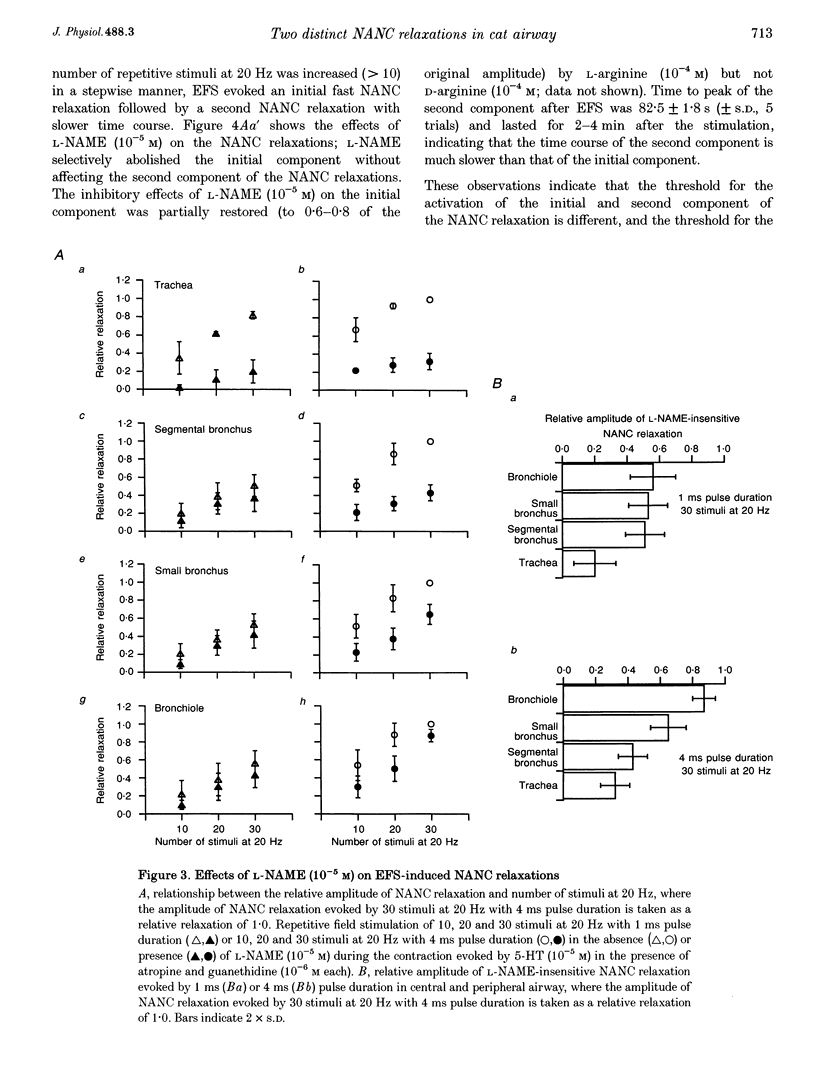
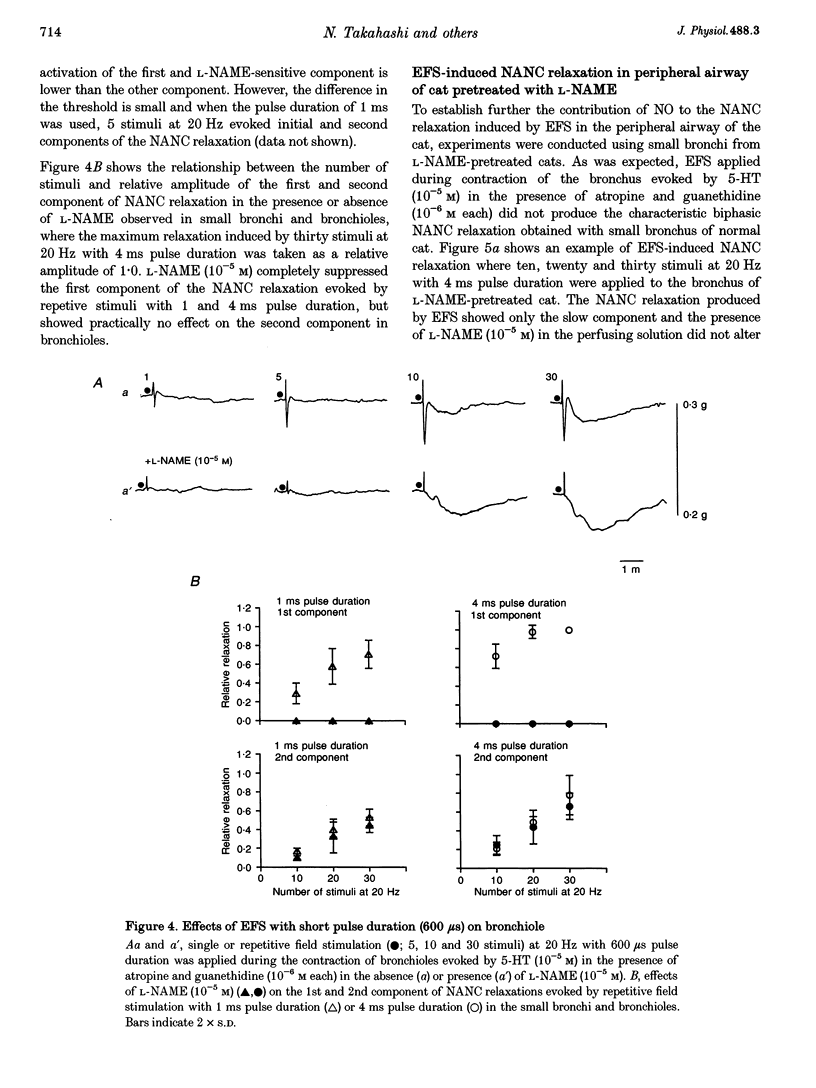
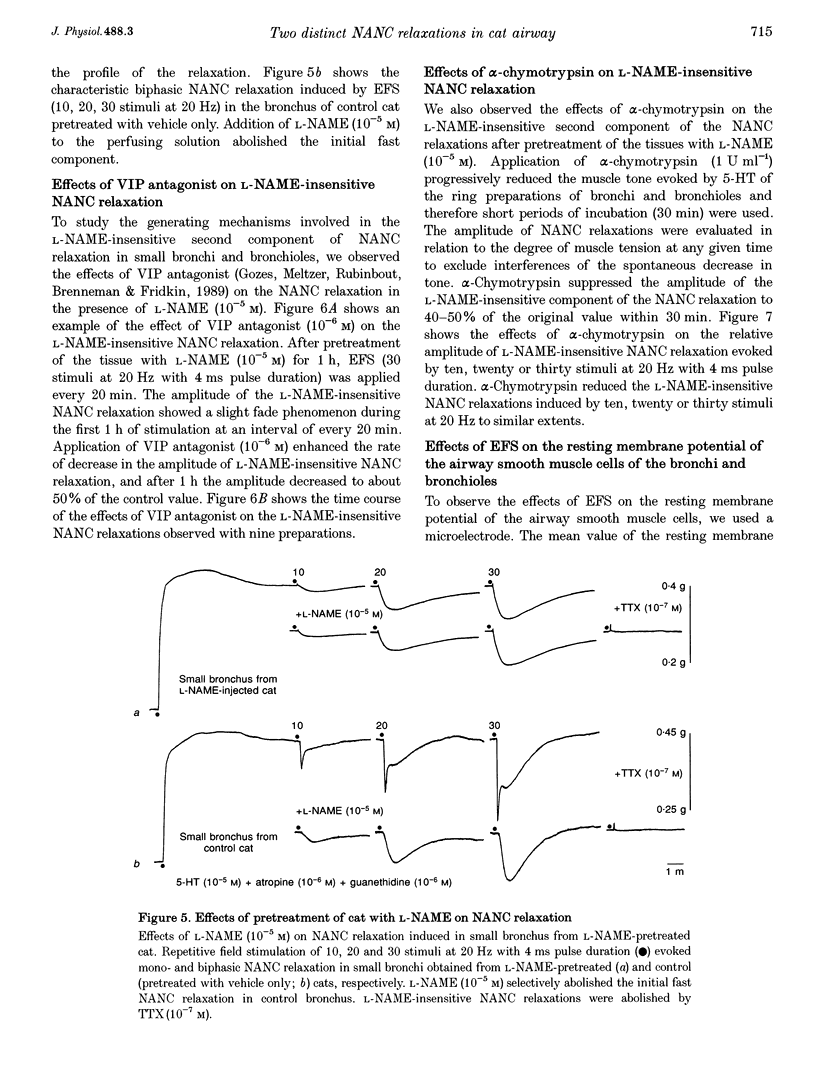
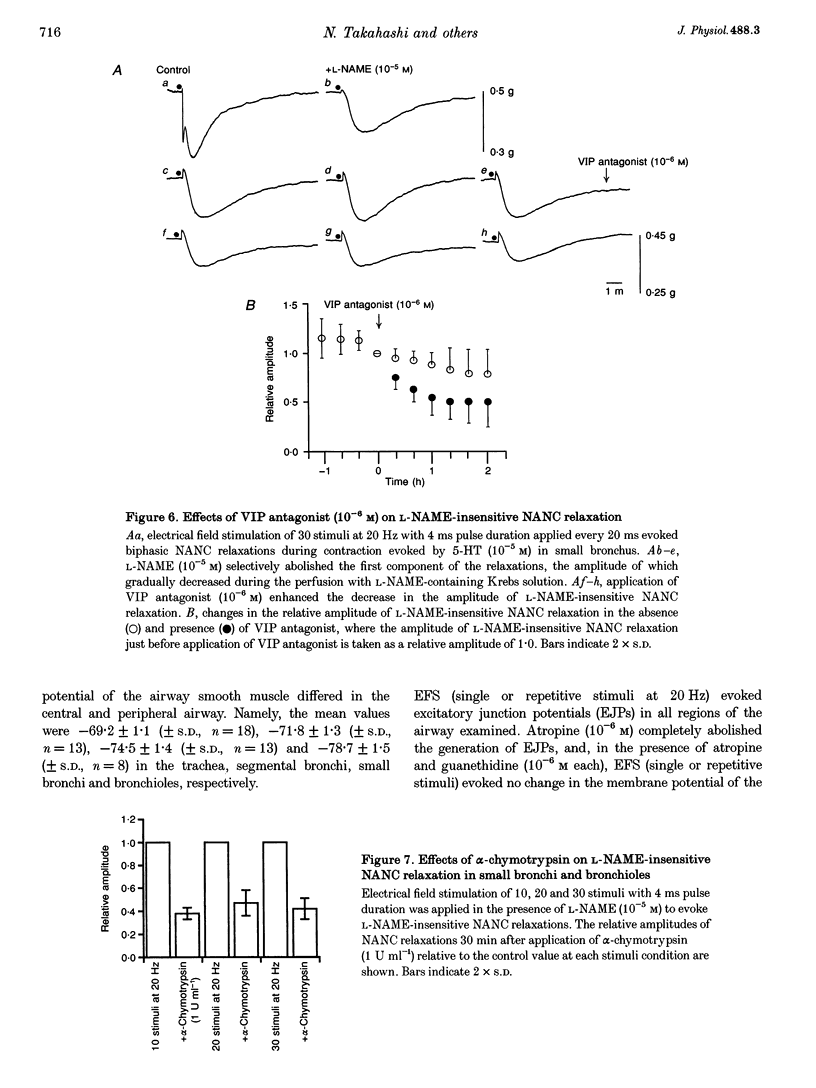
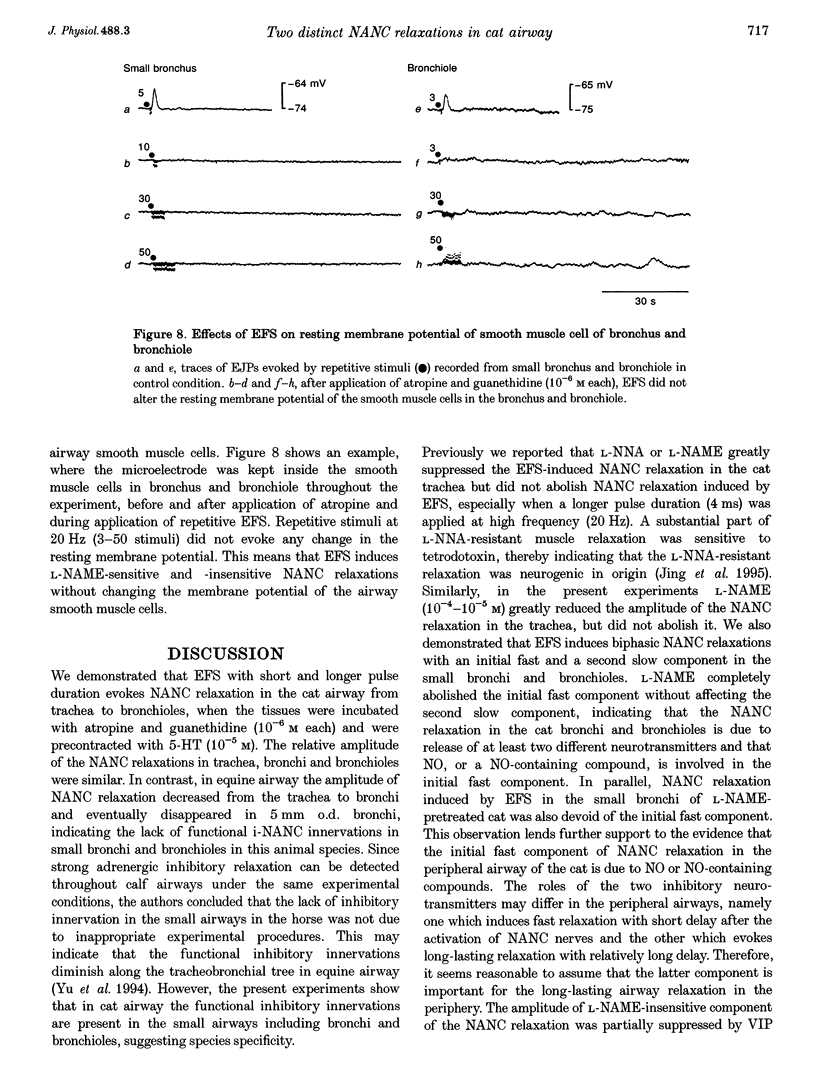
1. To investigate the distribution profile of functional inhibitory non-adrenergic non-cholinergic (i-NANC) nerves and the contribution of NO to the NANC relaxation in the cat, we studied the effects of N omega-nitro-L-arginine methyl ester (L-NAME) on NANC relaxation elicited by electrical field stimulation (EFS) in the trachea, bronchus and bronchiole. 2. EFS applied to the tracheal smooth muscle during contraction induced by 5-HT (10(-5) M) in the presence of atropine (10(-6) M) and guanethidine (10(-6) M) elicited a monophasic NANC relaxation. By contrast, NANC relaxation elicited in the peripheral airway was biphasic, comprising an initial fast followed by a second slow component and L-NAME (10(-5) M) selectively abolished the first component without affecting the second one. In the trachea, L-NAME (10(-5) M) completely suppressed the monophasic NANC relaxation when single or short repetitive stimuli (< 5) with 1 ms pulse duration were applied. However, at higher repetitive stimuli (> 10) with 1 or 4 ms pulse duration, suppression of NANC relaxation was incomplete. 3. In the small bronchi obtained from L-NAME-pretreated cats, EFS applied during contraction induced by 5-HT (10(-5) M) elicited only the slow component of NANC relaxation which is sensitive to tetrodotoxin. 4. In the peripheral airway, a newly synthesized VIP antagonist (10(-6) M) or alpha-chymotrypsin (1 U ml-1) considerably attenuated the amplitude of L-NAME-insensitive relaxation. 5. Single or repetitive EFS consistently evoked excitatory junction potentials (EJPs) in the central and peripheral airways. When tissues were exposed to atropine (10(-6) M) and guanethidine (10(-6) M), single or repetitive EFS did not alter the resting membrane potential. 6. These results indicate that at least two neurotransmitters, possibly NO or NO-containing compounds and VIP, are involved in i-NANC neurotransmission and the distribution profile of the two components differs in the central and peripheral airway of the cat.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amis T. C., McKiernan B. C. Systematic identification of endobronchial anatomy during bronchoscopy in the dog. Am J Vet Res. 1986 Dec;47(12):2649–2657. [PubMed] [Google Scholar]
- Barnes P. J. Neuropeptides and asthma. Am Rev Respir Dis. 1991 Mar;143(3 Pt 2):S28–S32. doi: 10.1164/ajrccm/143.3_Pt_2.S28. [DOI] [PubMed] [Google Scholar]
- Belvisi M. G., Stretton C. D., Miura M., Verleden G. M., Tadjkarimi S., Yacoub M. H., Barnes P. J. Inhibitory NANC nerves in human tracheal smooth muscle: a quest for the neurotransmitter. J Appl Physiol (1985) 1992 Dec;73(6):2505–2510. doi: 10.1152/jappl.1992.73.6.2505. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Tomita T. Evidence for nonadrenergic inhibitory nerves in the guinea pig trachealis muscle. Am J Physiol. 1973 May;224(5):1072–1080. doi: 10.1152/ajplegacy.1973.224.5.1072. [DOI] [PubMed] [Google Scholar]
- Cumming G. Airway morphology and its consequences. Bull Physiopathol Respir (Nancy) 1972 May-Jun;8(3):527–533. [PubMed] [Google Scholar]
- Dupuy P. M., Shore S. A., Drazen J. M., Frostell C., Hill W. A., Zapol W. M. Bronchodilator action of inhaled nitric oxide in guinea pigs. J Clin Invest. 1992 Aug;90(2):421–428. doi: 10.1172/JCI115877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. L., Farmer S. G. Effects of peptidases on non-adrenergic, non-cholinergic inhibitory responses of tracheal smooth muscle: a comparison with effects on VIP- and PHI-induced relaxation. Br J Pharmacol. 1989 Mar;96(3):521–526. doi: 10.1111/j.1476-5381.1989.tb11848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. L., Farmer S. G. The effects of vasoactive intestinal peptide (VIP) antagonists, and VIP and peptide histidine isoleucine antisera on non-adrenergic, non-cholinergic relaxations of tracheal smooth muscle. Br J Pharmacol. 1989 Mar;96(3):513–520. doi: 10.1111/j.1476-5381.1989.tb11847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. L., Undem B. J. Inhibition by L-NG-nitro-L-arginine of nonadrenergic-noncholinergic-mediated relaxations of human isolated central and peripheral airway. Am Rev Respir Dis. 1992 Dec;146(6):1543–1547. doi: 10.1164/ajrccm/146.6.1543. [DOI] [PubMed] [Google Scholar]
- Ellis J. L., Undem B. J. Non-adrenergic, non-cholinergic contractions in the electrically field stimulated guinea-pig trachea. Br J Pharmacol. 1990 Dec;101(4):875–880. doi: 10.1111/j.1476-5381.1990.tb14174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. T., Anderson J. W., Waldron M. A. Nonadrenergic noncholinergic neurotransmitter of feline trachealis: VIP or nitric oxide? J Appl Physiol (1985) 1993 Jan;74(1):31–39. doi: 10.1152/jappl.1993.74.1.31. [DOI] [PubMed] [Google Scholar]
- García-Pascual A., Triguero D. Relaxation mechanisms induced by stimulation of nerves and by nitric oxide in sheep urethral muscle. J Physiol. 1994 Apr 15;476(2):333–347. doi: 10.1113/jphysiol.1994.sp020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Meltzer E., Rubinrout S., Brenneman D. E., Fridkin M. Vasoactive intestinal peptide potentiates sexual behavior: inhibition by novel antagonist. Endocrinology. 1989 Dec;125(6):2945–2949. doi: 10.1210/endo-125-6-2945. [DOI] [PubMed] [Google Scholar]
- Hakoda H., Xie Z. Q., Aizawa H., Inoue H., Hirata M., Ito Y. Effects of immunization against VIP on neurotransmission in cat trachea. Am J Physiol. 1991 Oct;261(4 Pt 1):L341–L348. doi: 10.1152/ajplung.1991.261.4.L341. [DOI] [PubMed] [Google Scholar]
- Håkanson R., Sundler F., Moghimzadeh E., Leander S. Peptide-containing nerve fibres in the airways: distribution and functional implications. Eur J Respir Dis Suppl. 1983;131:115–140. [PubMed] [Google Scholar]
- Ito Y., Inoue T. Contracture and change in membrane potential produced by sodium removal in the dog trachea and bronchiole. J Appl Physiol (1985) 1989 Nov;67(5):2078–2086. doi: 10.1152/jappl.1989.67.5.2078. [DOI] [PubMed] [Google Scholar]
- Ito Y., Tajima K. Actions of indomethacin and prostaglandins on neuro-effector transmission in the dog trachea. J Physiol. 1981;319:379–392. doi: 10.1113/jphysiol.1981.sp013915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Takeda K. Non-adrenergic inhibitory nerves and putative transmitters in the smooth muscle of cat trachea. J Physiol. 1982 Sep;330:497–511. doi: 10.1113/jphysiol.1982.sp014355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L., Inoue R., Tashiro K., Takahashi S., Ito Y. Role of nitric oxide in non-adrenergic, non-cholinergic relaxation and modulation of excitatory neuroeffector transmission in the cat airway. J Physiol. 1995 Feb 15;483(Pt 1):225–237. doi: 10.1113/jphysiol.1995.sp020580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan M. S., Johnson D. E. Nitric oxide mediates the neural nonadrenergic, noncholinergic relaxation of pig tracheal smooth muscle. Am J Physiol. 1992 Apr;262(4 Pt 1):L511–L514. doi: 10.1152/ajplung.1992.262.4.L511. [DOI] [PubMed] [Google Scholar]
- Kowaluk E. A., Fung H. L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther. 1990 Dec;255(3):1256–1264. [PubMed] [Google Scholar]
- Laitinen A., Partanen M., Hervonen A., Pelto-Huikko M., Laitinen L. A. VIP like immunoreactive nerves in human respiratory tract. Light and electron microscopic study. Histochemistry. 1985;82(4):313–319. doi: 10.1007/BF00494059. [DOI] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Evidence that part of the NANC relaxant response of guinea-pig trachea to electrical field stimulation is mediated by nitric oxide. Br J Pharmacol. 1991 Jan;102(1):91–94. doi: 10.1111/j.1476-5381.1991.tb12137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M. Evidence for coexistence of vasoactive intestinal polypeptide (VIP) and acetylcholine in neurons of cat exocrine glands. Morphological, biochemical and functional studies. Acta Physiol Scand Suppl. 1981;496:1–57. [PubMed] [Google Scholar]
- Matsuzaki Y., Hamasaki Y., Said S. I. Vasoactive intestinal peptide: a possible transmitter of nonadrenergic relaxation of guinea pig airways. Science. 1980 Dec 12;210(4475):1252–1253. doi: 10.1126/science.6254154. [DOI] [PubMed] [Google Scholar]
- Mitchell H. W., Sparrow M. P., Tagliaferri R. P. Inhibitory and excitatory responses to field stimulation in fetal and adult pig airway. Pediatr Res. 1990 Jul;28(1):69–74. doi: 10.1203/00006450-199007000-00015. [DOI] [PubMed] [Google Scholar]
- Mortensen J. D., Young J. D., Stout L., Stout A., Bagley B., Schaap R. N. A numerical identification system for airways in the lung. Anat Rec. 1983 May;206(1):103–114. doi: 10.1002/ar.1092060112. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Palmer J. B., Cuss F. M., Barnes P. J. VIP and PHM and their role in nonadrenergic inhibitory responses in isolated human airways. J Appl Physiol (1985) 1986 Oct;61(4):1322–1328. doi: 10.1152/jappl.1986.61.4.1322. [DOI] [PubMed] [Google Scholar]
- Richardson J., Béland J. Nonadrenergic inhibitory nervous system in human airways. J Appl Physiol. 1976 Nov;41(5 Pt 1):764–771. doi: 10.1152/jappl.1976.41.5.764. [DOI] [PubMed] [Google Scholar]
- Russell J. A. Noradrenergic inhibitory innervation of canine airways. J Appl Physiol Respir Environ Exerc Physiol. 1980 Jan;48(1):16–22. doi: 10.1152/jappl.1980.48.1.16. [DOI] [PubMed] [Google Scholar]
- Smith R. B., Taylor I. M. Observations on the intrinsic innervation of trachea, bronchi and pulmonary vessels in the sheep. Acta Anat (Basel) 1971;80(1):1–13. doi: 10.1159/000143669. [DOI] [PubMed] [Google Scholar]
- Tucker J. F., Brave S. R., Charalambous L., Hobbs A. J., Gibson A. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxations of guinea-pig isolated tracheal smooth muscle. Br J Pharmacol. 1990 Aug;100(4):663–664. doi: 10.1111/j.1476-5381.1990.tb14072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Hakoda H., Ito Y. Airway epithelial cells regulate membrane potential, neurotransmission and muscle tone of the dog airway smooth muscle. J Physiol. 1992 Apr;449:619–639. doi: 10.1113/jphysiol.1992.sp019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Wang Z., Robinson N. E., Leblanc P. H. Inhibitory nerve distribution and mediation of NANC relaxation by nitric oxide in horse airways. J Appl Physiol (1985) 1994 Jan;76(1):339–344. doi: 10.1152/jappl.1994.76.1.339. [DOI] [PubMed] [Google Scholar]


