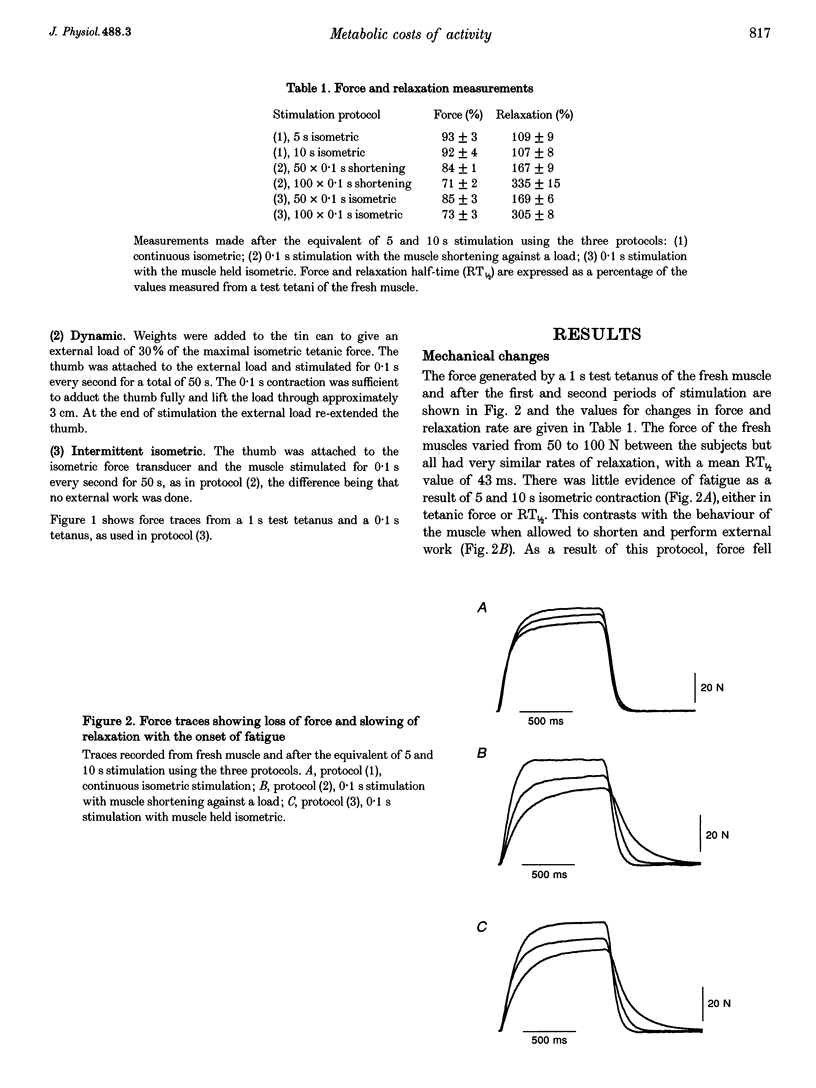
Abstract
1. The metabolic costs and physiological consequences of shortening contractions of a human muscle working in situ have been compared with those of the muscle maintaining a continuous isometric contraction and when performing repeated brief isometric contractions. 2. After a total of 10 s stimulation, the shortening and intermittent brief isometric protocols had very similar effects, causing a 30% loss of force and a threefold increase in the half-time of relaxation. This was in contrast to the continuous isometric contraction protocol where there was less than 10% loss of force or slowing of relaxation. 3. The ATP cost over the first 5 s of the continuous isometric protocol was 27 mmol (l intracellular water)-1 while for the shortening and repeated brief isometric protocols the costs were 48 and 46 mmol (l intracellular water)-1, respectively. 4. The results show that shortening and repeated brief isometric contractions are considerably more energetically demanding, and hence more fatiguing, than sustained isometric contractions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay C. J., Constable J. K., Gibbs C. L. Energetics of fast- and slow-twitch muscles of the mouse. J Physiol. 1993 Dec;472:61–80. doi: 10.1113/jphysiol.1993.sp019937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström M., Hultman E. Relaxation and force during fatigue and recovery of the human quadriceps muscle: relations to metabolite changes. Pflugers Arch. 1991 Mar;418(1-2):153–160. doi: 10.1007/BF00370464. [DOI] [PubMed] [Google Scholar]
- Bogdanis G. C., Nevill M. E., Boobis L. H., Lakomy H. K., Nevill A. M. Recovery of power output and muscle metabolites following 30 s of maximal sprint cycling in man. J Physiol. 1995 Jan 15;482(Pt 2):467–480. doi: 10.1113/jphysiol.1995.sp020533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady E. B., Jones D. A., Lynn J., Newham D. J. Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol. 1989 Nov;418:311–325. doi: 10.1113/jphysiol.1989.sp017842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A. Heat production and chemical changes during isometric contractions of the human quadriceps muscle. J Physiol. 1975 Oct;251(2):303–315. doi: 10.1113/jphysiol.1975.sp011094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H., Young A., Hosking G. P., Jones D. A. Human skeletal muscle function: description of tests and normal values. Clin Sci Mol Med. 1977 Mar;52(3):283–290. doi: 10.1042/cs0520283. [DOI] [PubMed] [Google Scholar]
- Fürst P., Jonsson A., Josephson B., Vinnars E. Distribution in muscle and liver vein protein of 15N administered as ammonium acetate to man. J Appl Physiol. 1970 Sep;29(3):307–312. doi: 10.1152/jappl.1970.29.3.307. [DOI] [PubMed] [Google Scholar]
- Greenhaff P. L., Söderlund K., Ren J. M., Hultman E. Energy metabolism in single human muscle fibres during intermittent contraction with occluded circulation. J Physiol. 1993 Jan;460:443–453. doi: 10.1113/jphysiol.1993.sp019480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman E., Sjöholm H. Energy metabolism and contraction force of human skeletal muscle in situ during electrical stimulation. J Physiol. 1983 Dec;345:525–532. doi: 10.1113/jphysiol.1983.sp014994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion A. F., Jakeman P. M., Dunnett M., Harris R. C., Willan P. L. Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans. Eur J Appl Physiol Occup Physiol. 1992;64(1):47–50. doi: 10.1007/BF00376439. [DOI] [PubMed] [Google Scholar]
- Round J. M., Jones D. A., Chapman S. J., Edwards R. H., Ward P. S., Fodden D. L. The anatomy and fibre type composition of the human adductor pollicis in relation to its contractile properties. J Neurol Sci. 1984 Nov-Dec;66(2-3):263–272. doi: 10.1016/0022-510x(84)90015-7. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Alvestrand A., Brandt R., Hultman E. Intracellular pH and bicarbonate concentration in human muscle during recovery from exercise. J Appl Physiol Respir Environ Exerc Physiol. 1978 Sep;45(3):474–480. doi: 10.1152/jappl.1978.45.3.474. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Styles P., Matthews P. M., Arnold D. A., Gadian D. G., Bore P., Radda G. K. Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med. 1986 Feb;3(1):44–54. doi: 10.1002/mrm.1910030107. [DOI] [PubMed] [Google Scholar]



