Abstract
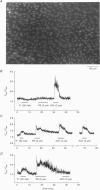
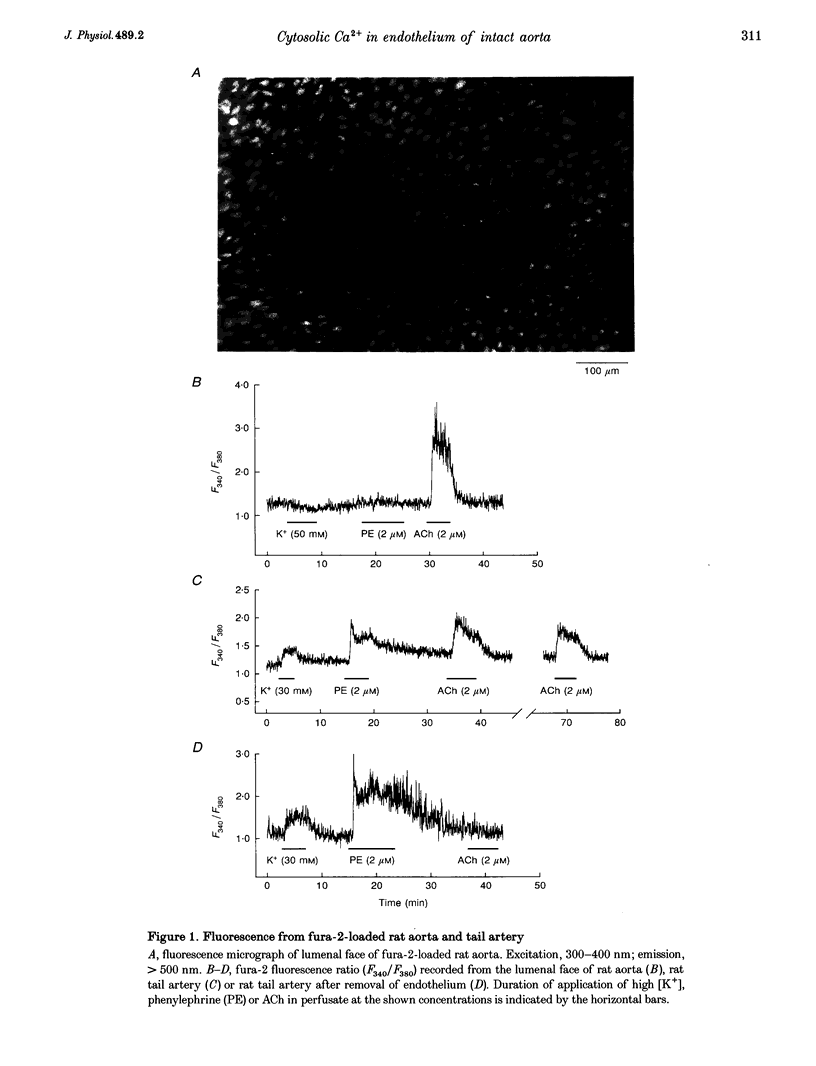
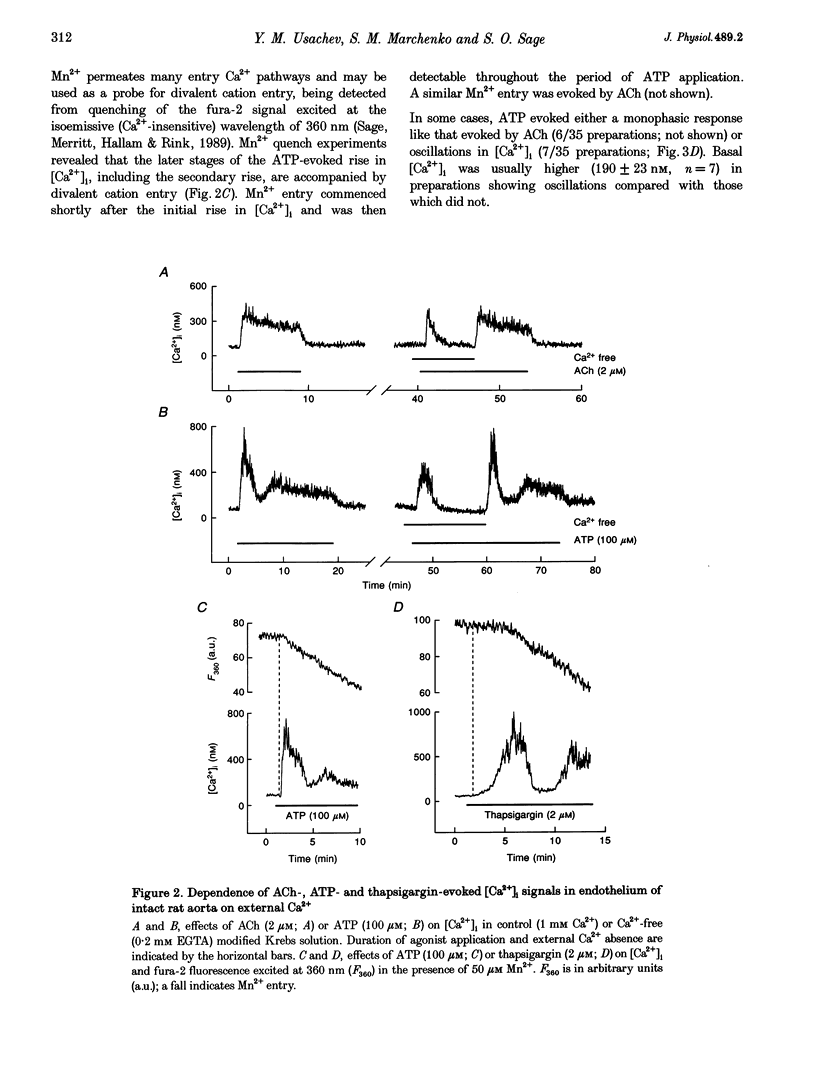
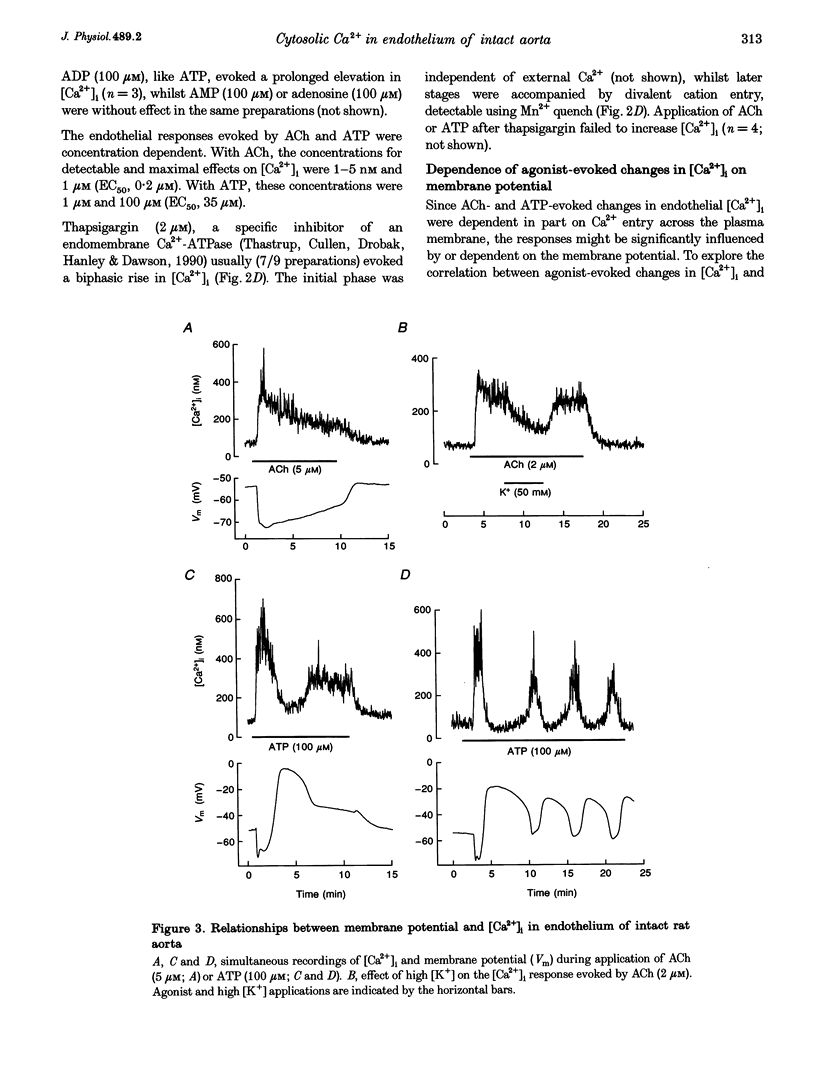
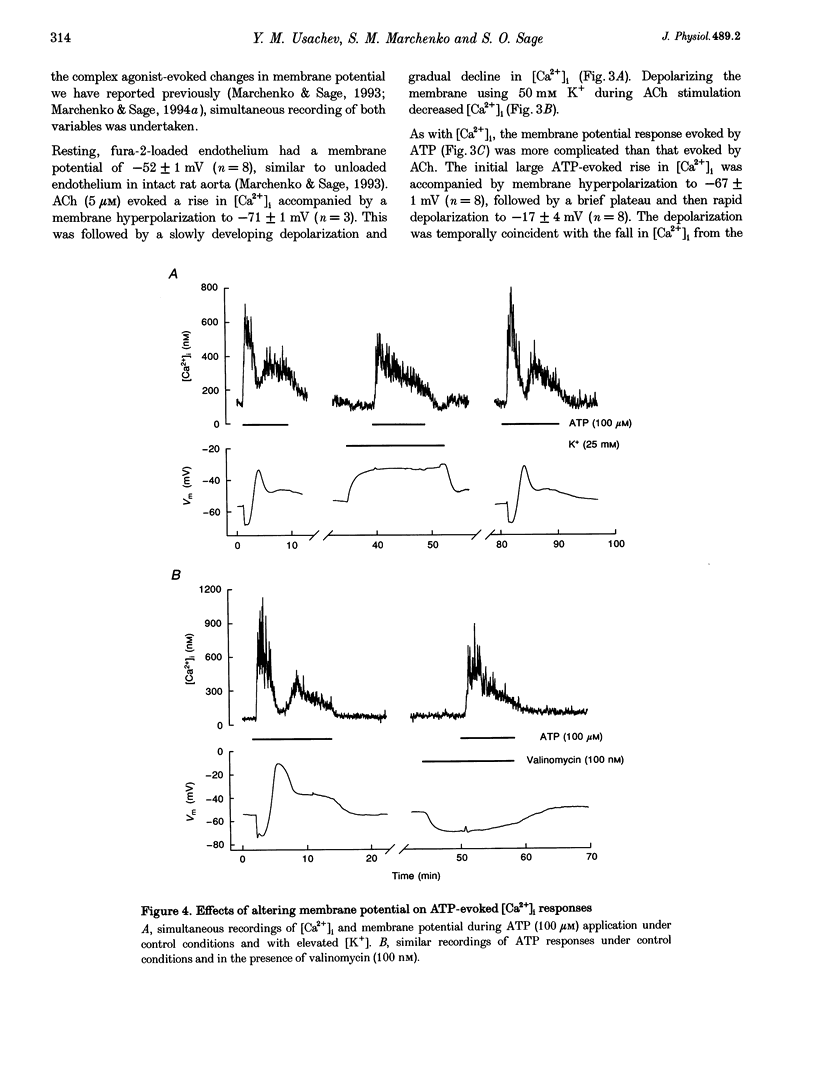
1. Optical fibres were used to excite and record fluorescence from the lumenal face of rat aorta or tail artery loaded with fura-2. 2. Acetylcholine (ACh) evoked an endothelium-dependent rise in the fura-2 340/380 nm excitation ratio in both vessels. High [K+] or phenylephrine evoked an endothelium-independent rise in ratio in tail artery but failed to increase the ratio in aorta. These observations indicate that fura-2 fluorescence and therefore cytosolic calcium concentration ([Ca2+]i) may be selectively recorded from the endothelium of intact rat aorta. 3. In aortic endothelium, resting [Ca2+]i was 95 +/- 8 nM (n = 44). ACh evoked a monophasic rise in [Ca2+]i which was temporally coincident with a membrane hyperpolarization. 4. ATP in most (22/35) preparations evoked a rise in [Ca2+]i which declined towards resting and was followed by a secondary rise. The biphasic [Ca2+]i responses were accompanied by biphasic electrical responses of initial hyperpolarization followed by depolarization above the resting potential and subsequent restoration towards rest. In the presence of high [K+] or the K+ ionophore valinomycin, ATP did not evoke changes in membrane potential and only monophasic rises in [Ca2+]i were observed. In some (7/35) preparations, ATP evoked oscillations in [Ca2+]i, with membrane potential oscillating in antiphase. 5. These data suggest interplay between [Ca2+]i and membrane potential in the generation of agonist-evoked responses in native endothelium in situ. The observed oscillations in [Ca2+]i imply spatio-temporal synchronization of Ca2+ signalling in large groups of endothelial cells in intact rat aorta.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batlle D. C., Godinich M., LaPointe M. S., Munoz E., Carone F., Mehring N. Extracellular Na+ dependency of free cytosolic Ca2+ regulation in aortic vascular smooth muscle cells. Am J Physiol. 1991 Nov;261(5 Pt 1):C845–C856. doi: 10.1152/ajpcell.1991.261.5.C845. [DOI] [PubMed] [Google Scholar]
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Carter T. D., Ogden D. Acetylcholine-stimulated changes of membrane potential and intracellular Ca2+ concentration recorded in endothelial cells in situ in the isolated rat aorta. Pflugers Arch. 1994 Oct;428(5-6):476–484. doi: 10.1007/BF00374568. [DOI] [PubMed] [Google Scholar]
- Falcone J. C., Kuo L., Meininger G. A. Endothelial cell calcium increases during flow-induced dilation in isolated arterioles. Am J Physiol. 1993 Feb;264(2 Pt 2):H653–H659. doi: 10.1152/ajpheart.1993.264.2.H653. [DOI] [PubMed] [Google Scholar]
- Gosink E. C., Forsberg E. J. Effects of ATP and bradykinin on endothelial cell Ca2+ homeostasis and formation of cGMP and prostacyclin. Am J Physiol. 1993 Dec;265(6 Pt 1):C1620–C1629. doi: 10.1152/ajpcell.1993.265.6.C1620. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D., Needham L. A. Thrombin-stimulated elevation of human endothelial-cell cytoplasmic free calcium concentration causes prostacyclin production. Biochem J. 1988 Apr 1;251(1):243–249. doi: 10.1042/bj2510243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., Cannell M., van Breemen C. Calcium entry-dependent oscillations of cytoplasmic calcium concentration in cultured endothelial cell monolayers. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1690–1694. doi: 10.1073/pnas.89.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., van Breemen C. Cytosolic [Ca2+] measurements in endothelium of rabbit cardiac valves using imaging fluorescence microscopy. Am J Physiol. 1994 May;266(5 Pt 2):H2130–H2135. doi: 10.1152/ajpheart.1994.266.5.H2130. [DOI] [PubMed] [Google Scholar]
- Marchenko S. M., Sage S. O. Electrical properties of resting and acetylcholine-stimulated endothelium in intact rat aorta. J Physiol. 1993 Mar;462:735–751. doi: 10.1113/jphysiol.1993.sp019579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko S. M., Sage S. O. Mechanism of acetylcholine action on membrane potential of endothelium of intact rat aorta. Am J Physiol. 1994 Jun;266(6 Pt 2):H2388–H2395. doi: 10.1152/ajpheart.1994.266.6.H2388. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer S., Trautwein W., Pelzer D. J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994 Apr;74(2):365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986 Dec;103(6 Pt 1):2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Adams D. J., van Breemen C. Synchronized oscillations in cytoplasmic free calcium concentration in confluent bradykinin-stimulated bovine pulmonary artery endothelial cell monolayers. J Biol Chem. 1989 Jan 5;264(1):6–9. [PubMed] [Google Scholar]
- Sage S. O., Merritt J. E., Hallam T. J., Rink T. J. Receptor-mediated calcium entry in fura-2-loaded human platelets stimulated with ADP and thrombin. Dual-wavelengths studies with Mn2+. Biochem J. 1989 Mar 15;258(3):923–926. doi: 10.1042/bj2580923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]