Abstract
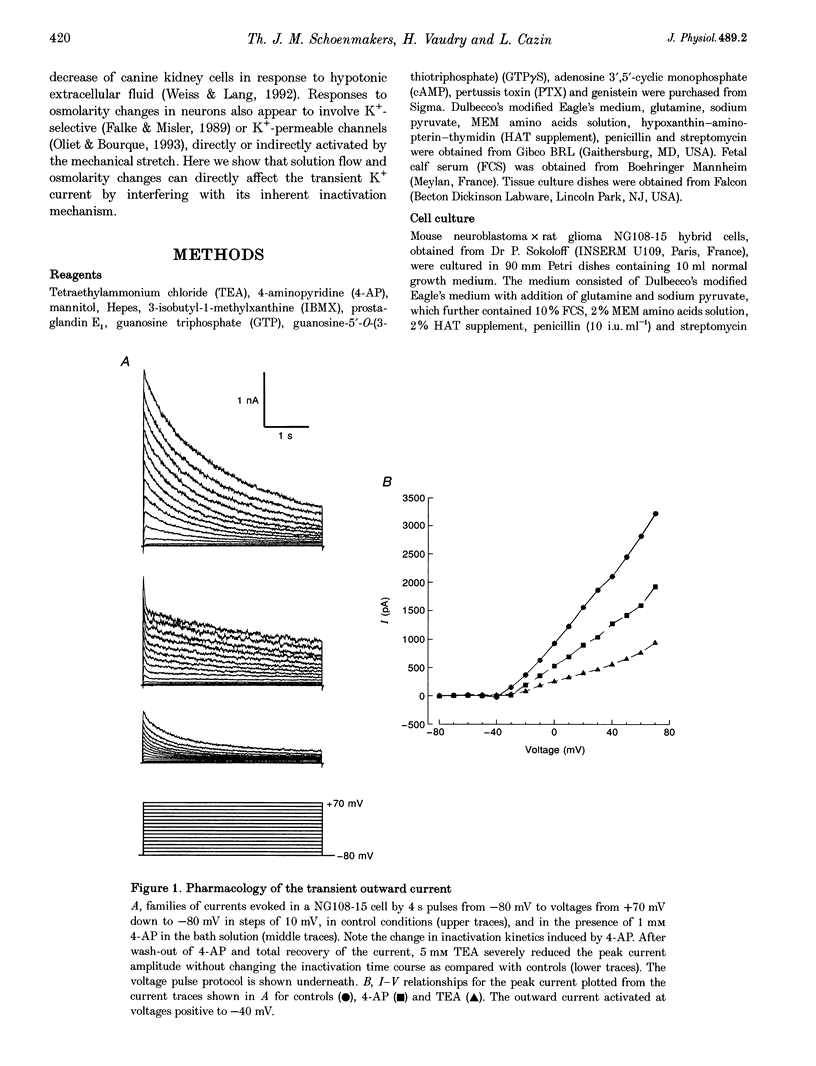
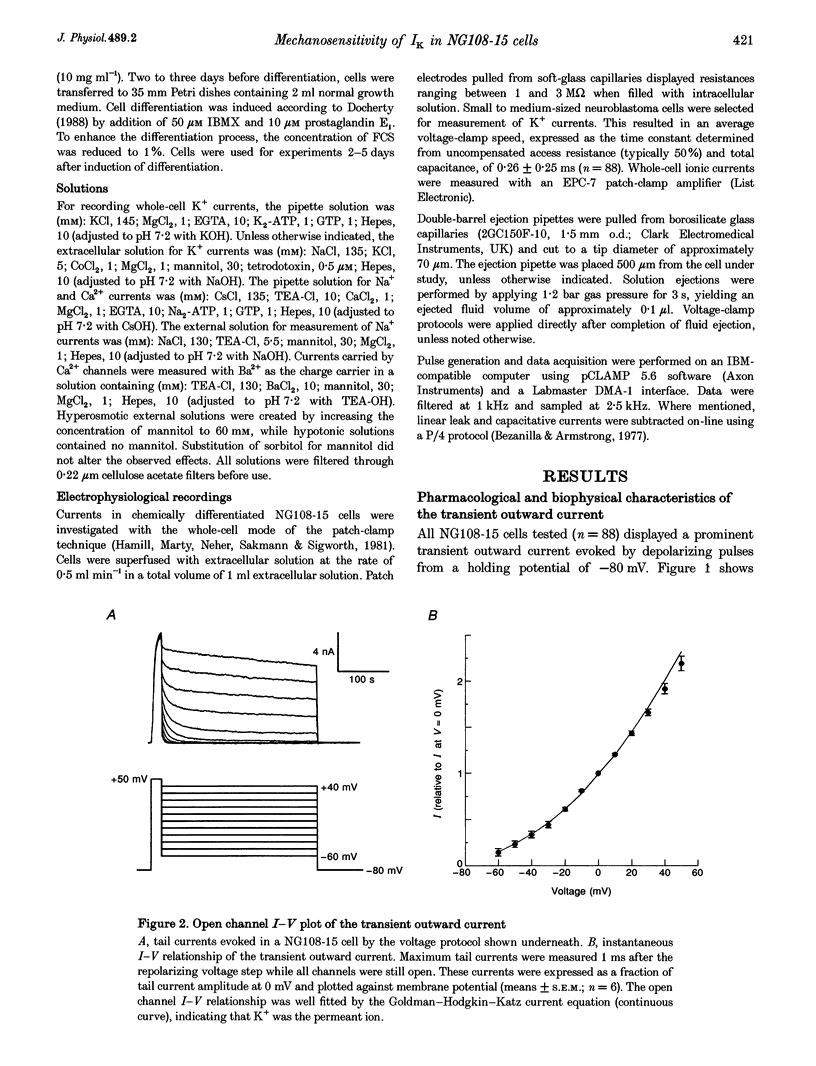
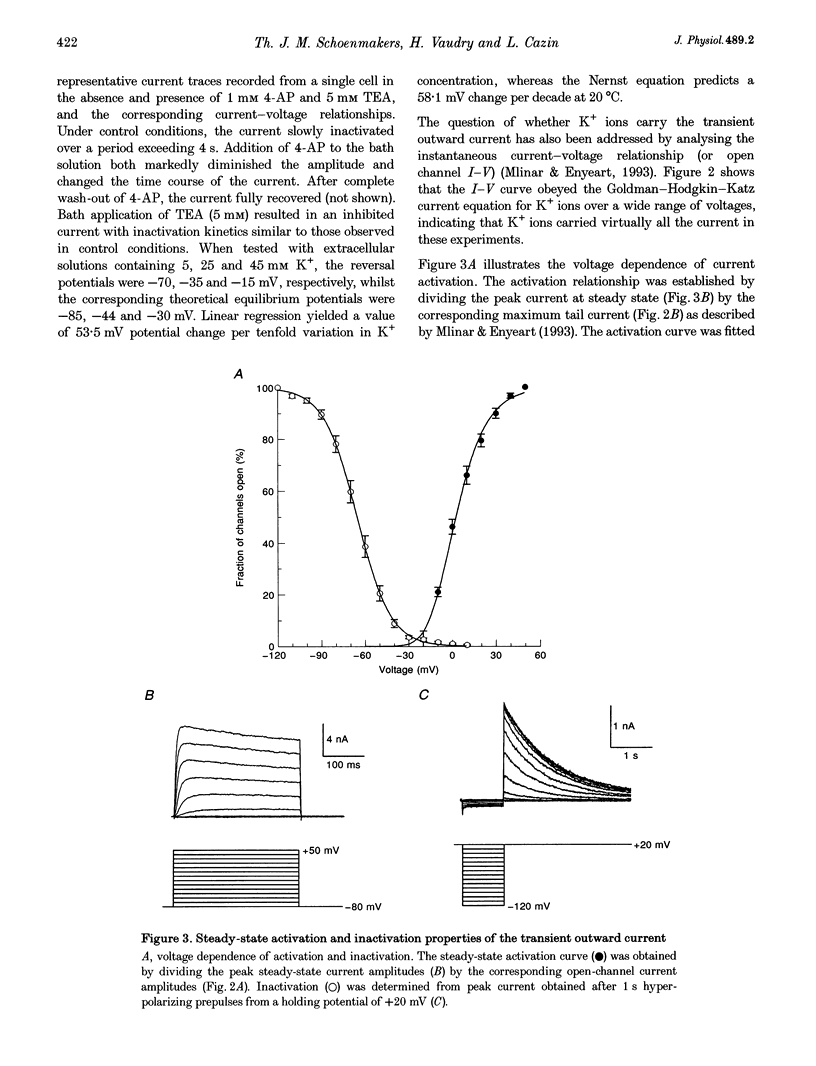
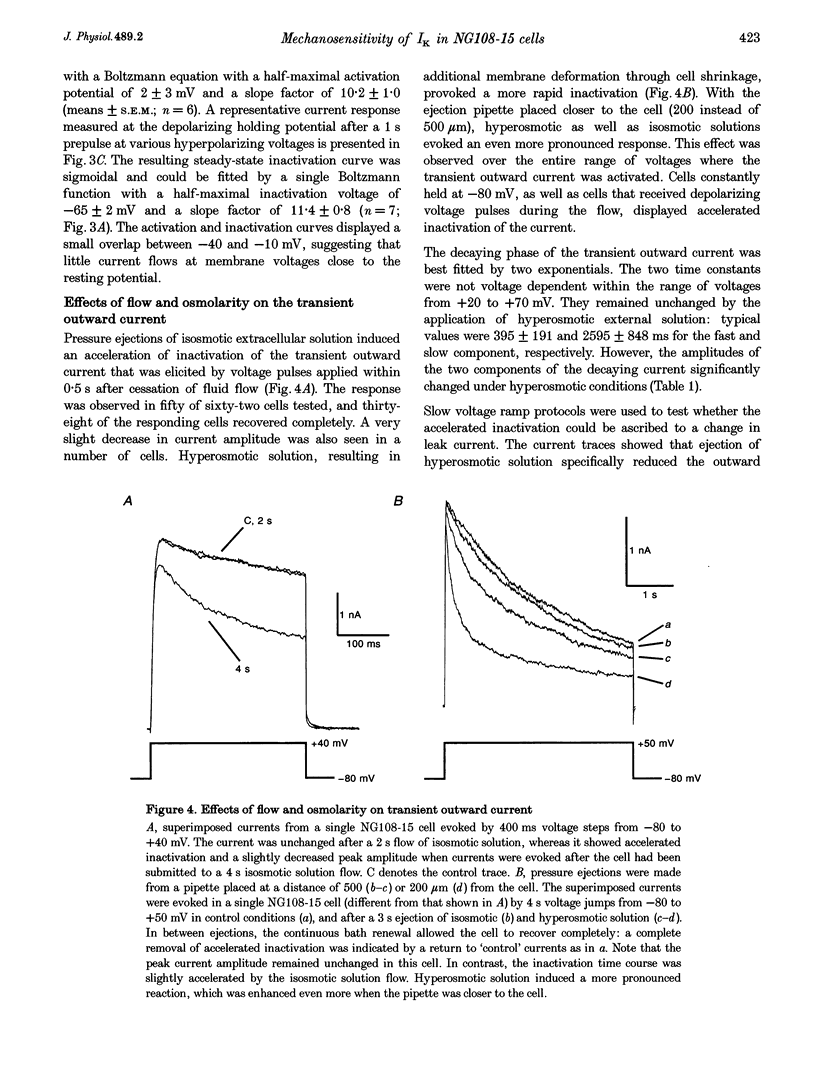
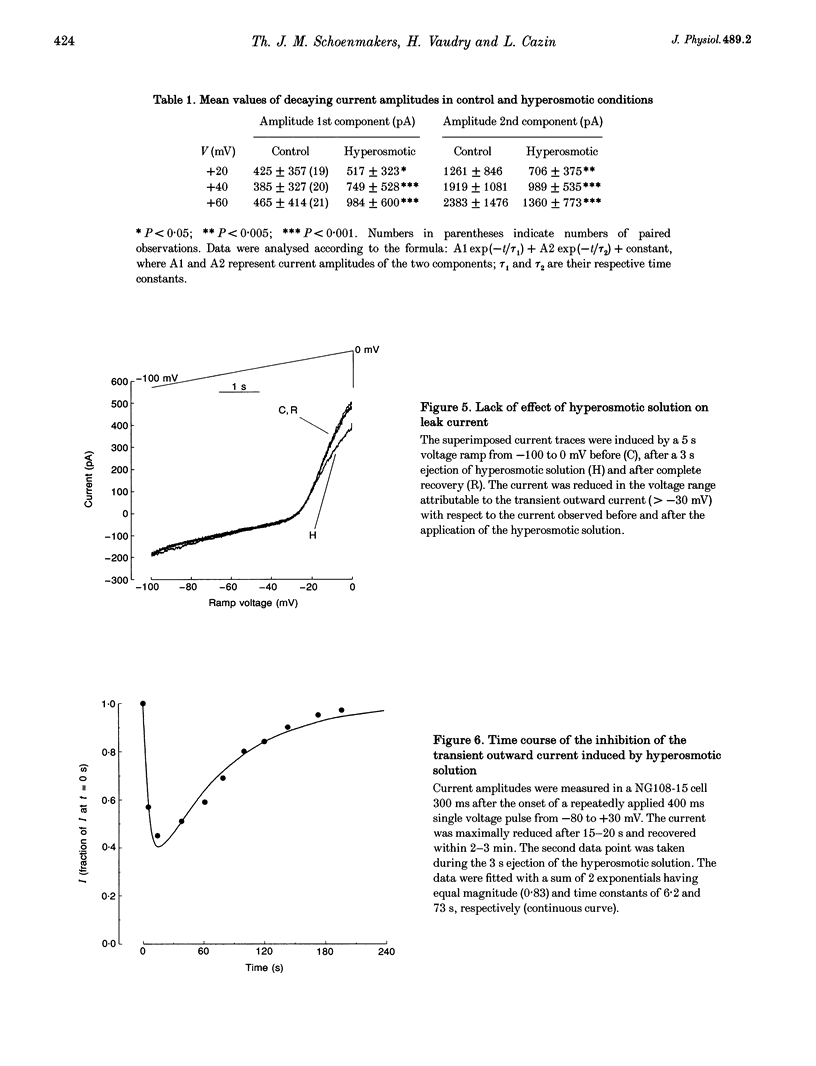
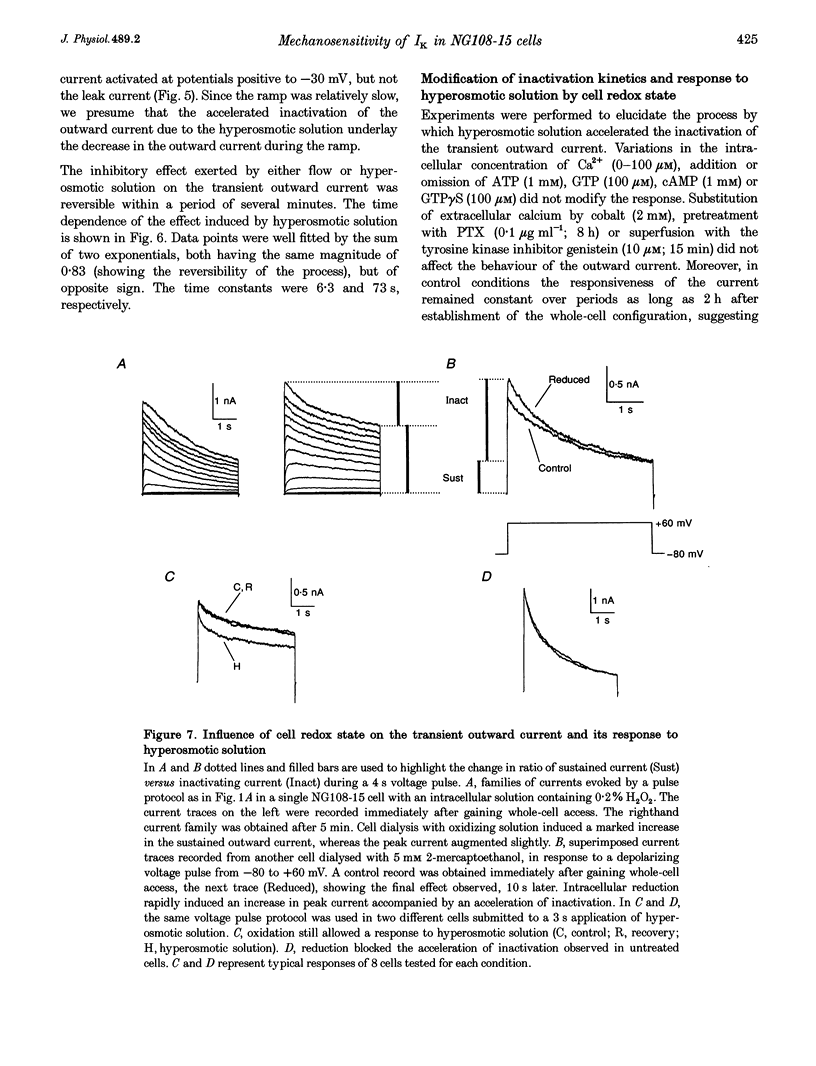
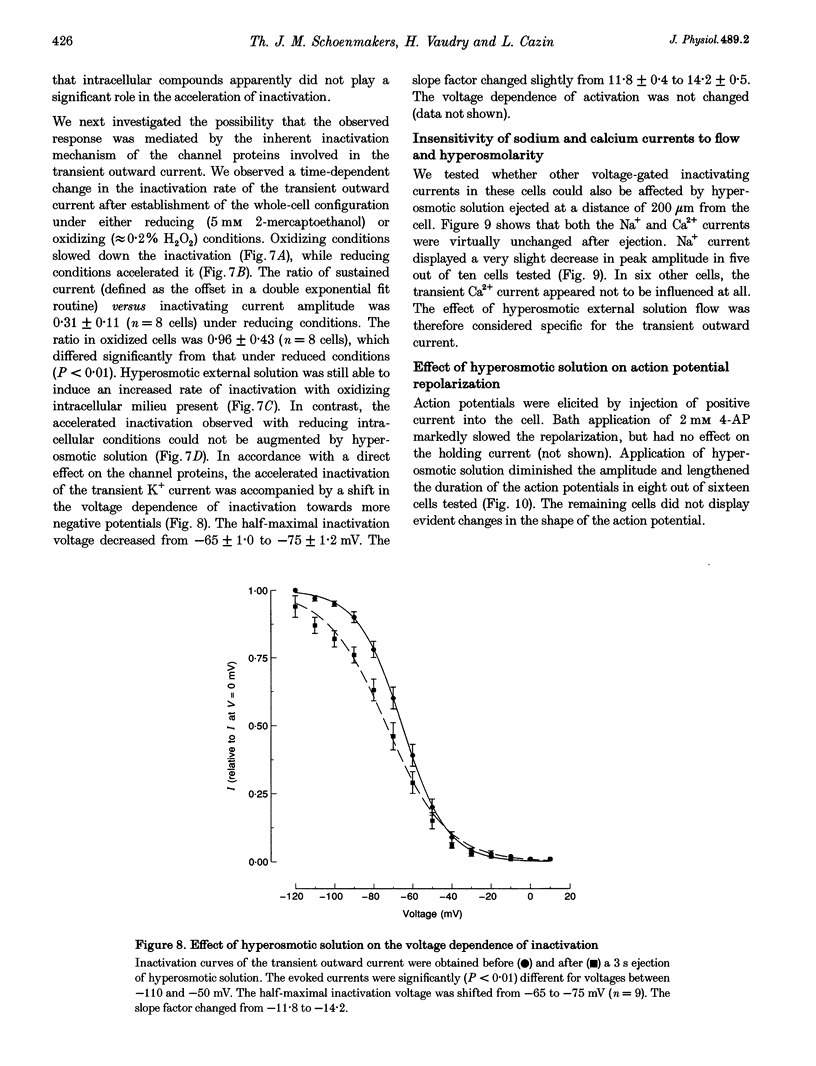
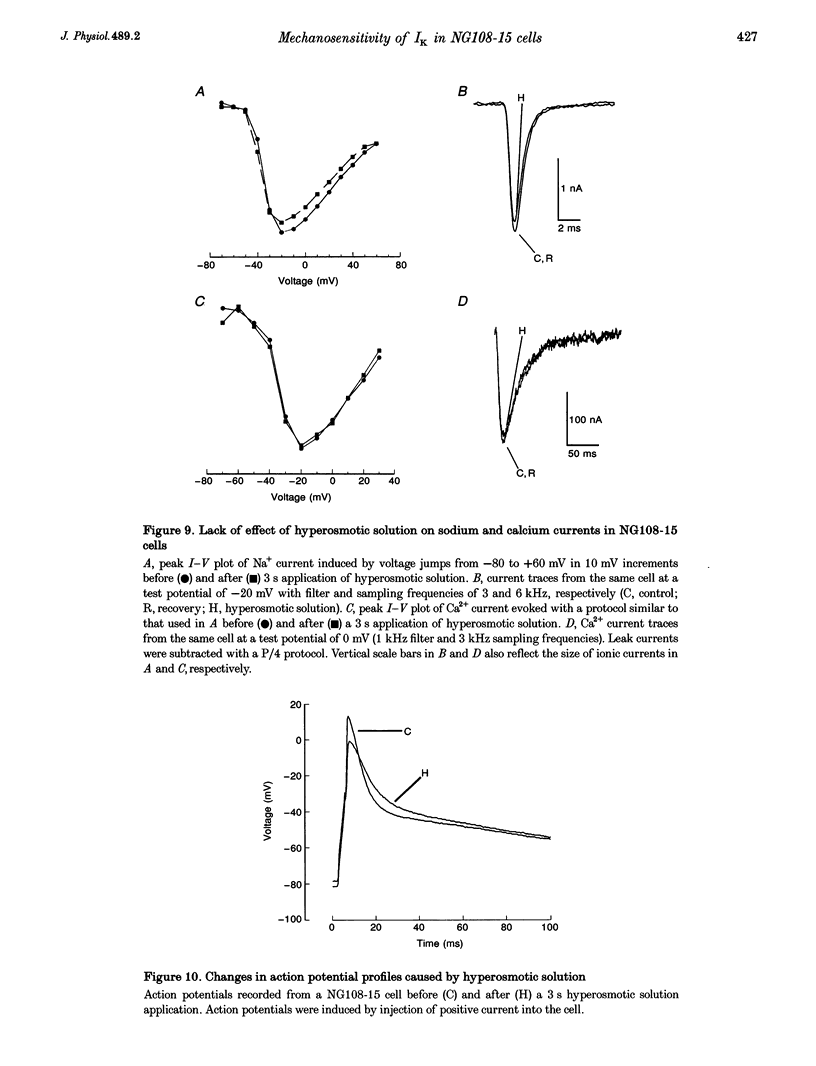
1. The transient outward current in NG108-15 cells was investigated with the whole-cell patch-clamp technique. The current was inhibited by external 4-aminopyridine or tetra-ethylammonium. The reversal potential shifted rightward with increased external K+ concentrations. 2. Current inactivation was markedly accelerated in hyperosmotic media (+30 mosmol l-1) and after nearby ejection of isosmotic solution with maximal acceleration occurring after 15-20 s and full recovery within 2-4 min, thus demonstrating an osmo- and mechanosensitivity of this current. Voltage-dependent Na+ and Ca2+ currents were unaffected. 3. Hyperosmotic solution shifted the voltage dependence of inactivation leftward. Inactivation was sensitive to reducing and oxidizing intracellular conditions. Reduction blocked the acceleration of current inactivation induced by hyperosmotic media, while oxidation did not hamper the response. 4. Action potentials had a decreased amplitude and a slower repolarization after hyperosmotic ejections. 5. It is concluded that the transient K+ current is osmo- and mechanosensitive, thus providing a mechanism for extracellular osmolarity to modulate neuronal excitability. The response appeared to be mediated through a changed sensitivity of the inactivating principle to the membrane electric field and was dependent on the redox state of the cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beirão P. S., Davies N. W., Stanfield P. R. Inactivating 'ball' peptide from Shaker B blocks Ca(2+)-activated but not ATP-dependent K+ channels of rat skeletal muscle. J Physiol. 1994 Jan 15;474(2):269–274. doi: 10.1113/jphysiol.1994.sp020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Tabou S., Keller E., Nussinovitch I. Mechanosensitivity of voltage-gated calcium currents in rat anterior pituitary cells. J Physiol. 1994 Apr 1;476(1):29–39. [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Voltage- and calcium-activated potassium currents in mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:149–165. doi: 10.1113/jphysiol.1988.sp016993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton F. L., Hutter O. F. Sensitivity to flow of intrinsic gating in inwardly rectifying potassium channel from mammalian skeletal muscle. J Physiol. 1990 May;424:253–261. doi: 10.1113/jphysiol.1990.sp018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano M. A., Liu L. X., Monsma F. J., Jr, Sibley D. R., Kapatos G., Chiodo L. A. Transfected D2 short dopamine receptors inhibit voltage-dependent potassium current in neuroblastoma x glioma hybrid (NG108-15) cells. Mol Pharmacol. 1993 Sep;44(3):649–656. [PubMed] [Google Scholar]
- Charness M. E., Hu G., Edwards R. H., Querimit L. A. Ethanol increases delta-opioid receptor gene expression in neuronal cell lines. Mol Pharmacol. 1993 Dec;44(6):1119–1127. [PubMed] [Google Scholar]
- Docherty R. J. Gadolinium selectively blocks a component of calcium current in rodent neuroblastoma x glioma hybrid (NG108-15) cells. J Physiol. 1988 Apr;398:33–47. doi: 10.1113/jphysiol.1988.sp017027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty R. J., McFadzean I. Noradrenaline-Induced Inhibition of Voltage-Sensitive Calcium Currents in NG108-15 Hybrid Cells. Eur J Neurosci. 1989 Mar;1(2):132–140. doi: 10.1111/j.1460-9568.1989.tb00780.x. [DOI] [PubMed] [Google Scholar]
- Falke L. C., Misler S. Activity of ion channels during volume regulation by clonal N1E115 neuroblastoma cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3919–3923. doi: 10.1073/pnas.86.10.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991 Oct;7(4):547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Islas L., Pasantes-Morales H., Sanchez J. A. Characterization of stretch-activated ion channels in cultured astrocytes. Glia. 1993 Jun;8(2):87–96. doi: 10.1002/glia.440080204. [DOI] [PubMed] [Google Scholar]
- Kasai H., Neher E. Dihydropyridine-sensitive and omega-conotoxin-sensitive calcium channels in a mammalian neuroblastoma-glioma cell line. J Physiol. 1992 Mar;448:161–188. doi: 10.1113/jphysiol.1992.sp019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara K., Matsuzaki K. A stretch-activated cation channel in the apical membrane of A6 cells. Jpn J Physiol. 1993;43(6):817–832. doi: 10.2170/jjphysiol.43.817. [DOI] [PubMed] [Google Scholar]
- Mackler S. A., Eberwine J. H. Cellular adaptation to opiates alters ion-channel mRNA levels. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):385–389. doi: 10.1073/pnas.91.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlinar B., Enyeart J. J. Voltage-gated transient currents in bovine adrenal fasciculata cells. II. A-type K+ current. J Gen Physiol. 1993 Aug;102(2):239–255. doi: 10.1085/jgp.102.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. E., Horn R. Failure to elicit neuronal macroscopic mechanosensitive currents anticipated by single-channel studies. Science. 1991 Mar 8;251(4998):1246–1249. doi: 10.1126/science.1706535. [DOI] [PubMed] [Google Scholar]
- Morris C. E. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Morris C. E., Sigurdson W. J. Stretch-inactivated ion channels coexist with stretch-activated ion channels. Science. 1989 Feb 10;243(4892):807–809. doi: 10.1126/science.2536958. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Clapham D. E., Davies P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988 Jan 14;331(6152):168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Oliet S. H., Bourque C. W. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993 Jul 22;364(6435):341–343. doi: 10.1038/364341a0. [DOI] [PubMed] [Google Scholar]
- Quasthoff S. A mechanosensitive K+ channel with fast-gating kinetics on human axons blocked by gadolinium ions. Neurosci Lett. 1994 Mar 14;169(1-2):39–42. doi: 10.1016/0304-3940(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Rettig J., Heinemann S. H., Wunder F., Lorra C., Parcej D. N., Dolly J. O., Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994 May 26;369(6478):289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Robbins J., Sim J. A. A transient outward current in NG108-15 neuroblastoma x glioma hybrid cells. Pflugers Arch. 1990 Apr;416(1-2):130–137. doi: 10.1007/BF00370234. [DOI] [PubMed] [Google Scholar]
- Robbins J., Trouslard J., Marsh S. J., Brown D. A. Kinetic and pharmacological properties of the M-current in rodent neuroblastoma x glioma hybrid cells. J Physiol. 1992;451:159–185. doi: 10.1113/jphysiol.1992.sp019159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppersberg J. P., Frank R., Pongs O., Stocker M. Cloned neuronal IK(A) channels reopen during recovery from inactivation. Nature. 1991 Oct 17;353(6345):657–660. doi: 10.1038/353657a0. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Seabrook G. R., Kemp J. A., Freedman S. B., Patel S., Sinclair H. A., McAllister G. Functional expression of human D3 dopamine receptors in differentiated neuroblastoma x glioma NG108-15 cells. Br J Pharmacol. 1994 Feb;111(2):391–393. doi: 10.1111/j.1476-5381.1994.tb14746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M., Rogawski M. A., Barker J. L. A transient potassium conductance regulates the excitability of cultured hippocampal and spinal neurons. J Neurosci. 1984 Feb;4(2):604–609. doi: 10.1523/JNEUROSCI.04-02-00604.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandorpe D. H., Small D. L., Dabrowski A. R., Morris C. E. FMRFamide and membrane stretch as activators of the Aplysia S-channel. Biophys J. 1994 Jan;66(1):46–58. doi: 10.1016/S0006-3495(94)80749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H., Lang F. Ion channels activated by swelling of Madin Darby canine kidney (MDCK) cells. J Membr Biol. 1992 Mar;126(2):109–114. doi: 10.1007/BF00231909. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990 Oct 26;250(4980):568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]


