Abstract
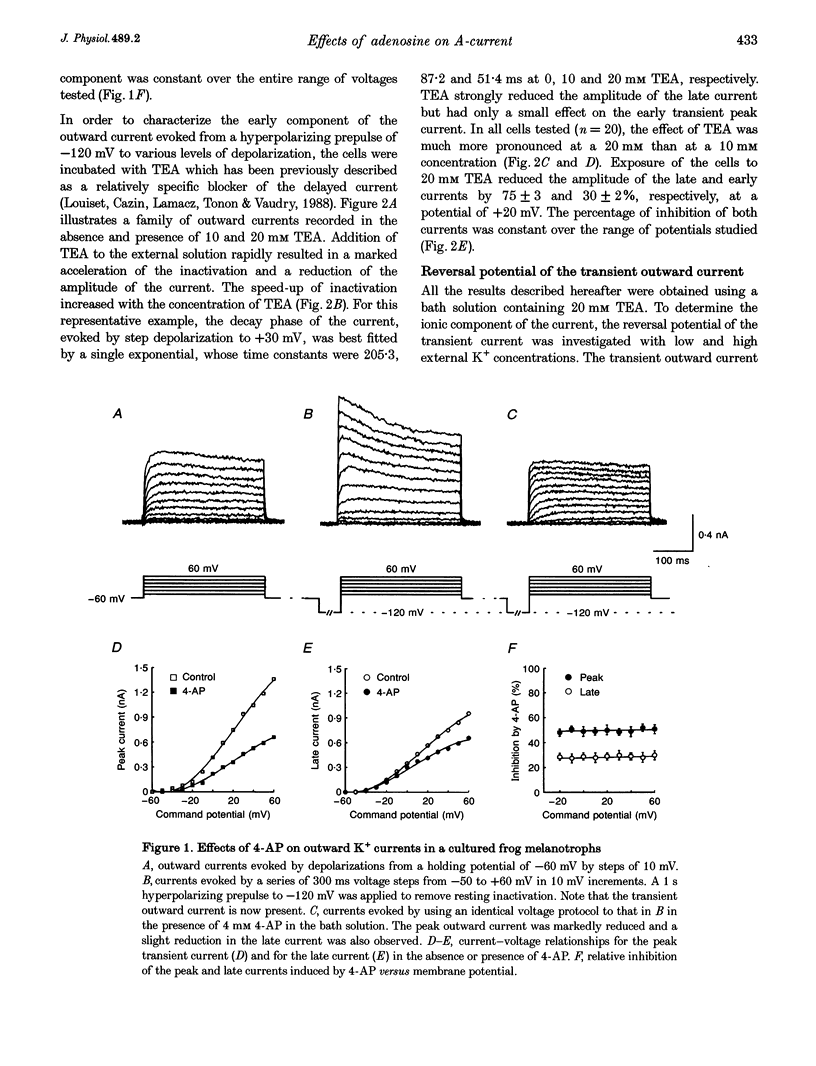
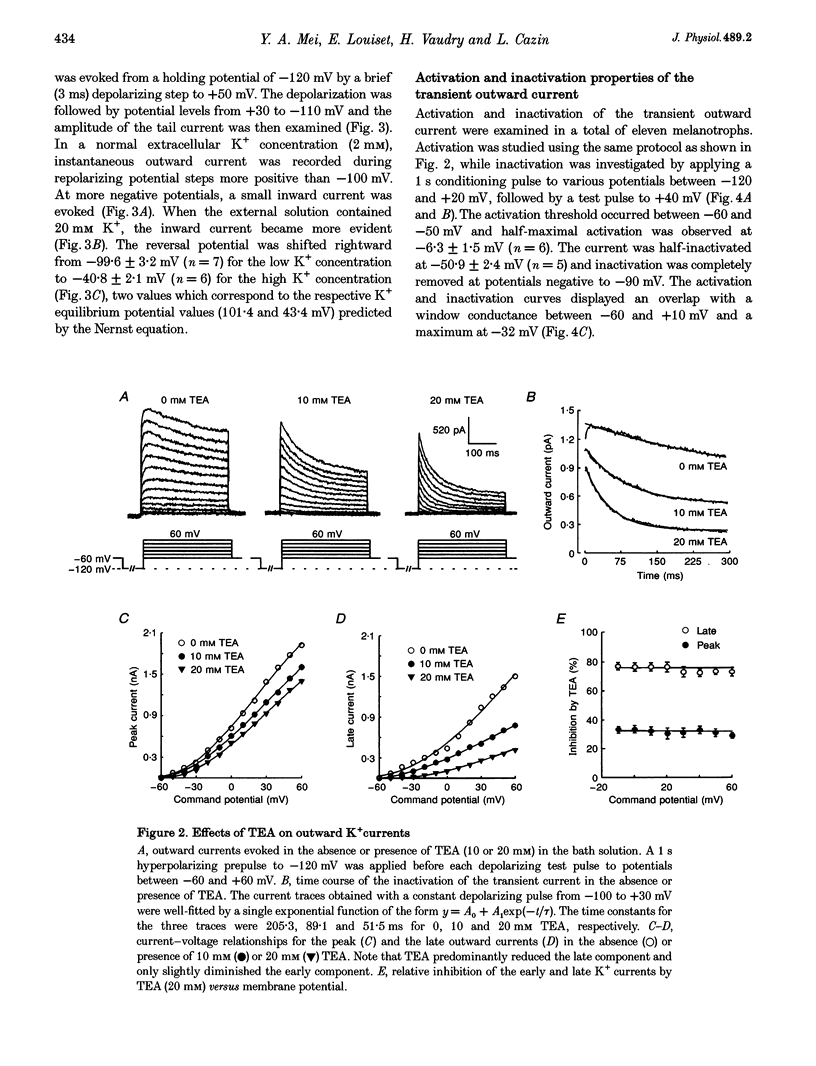
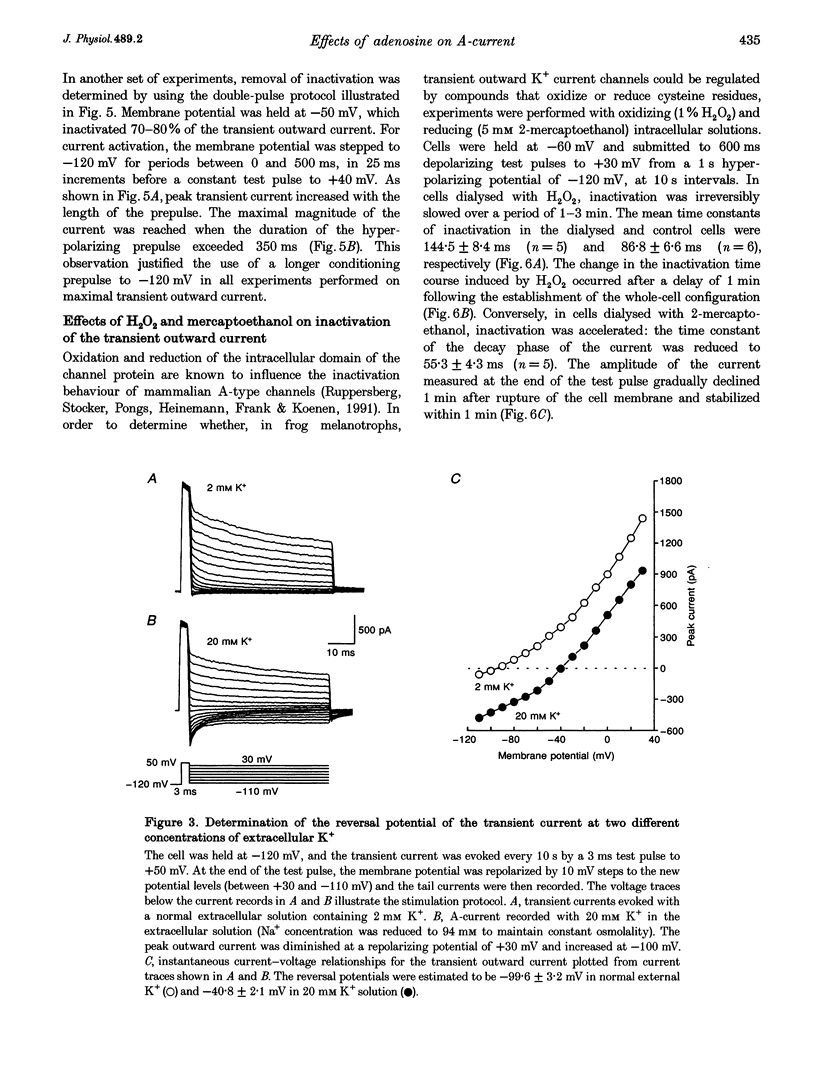
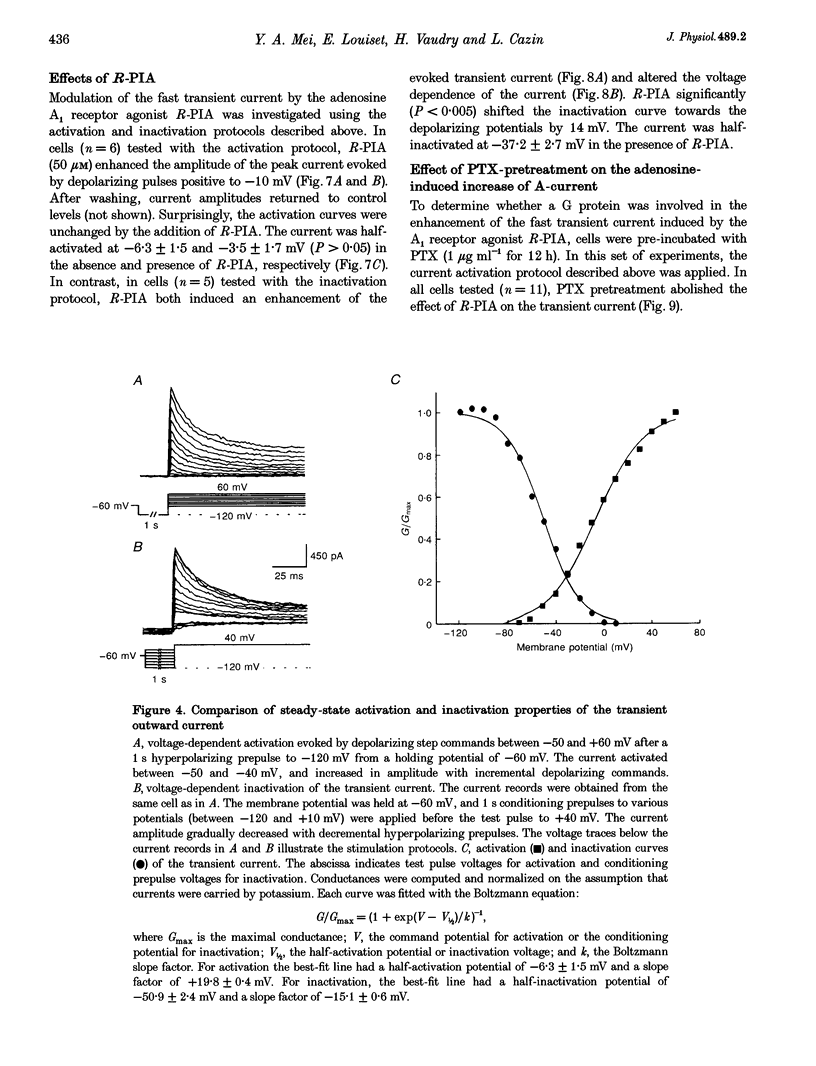
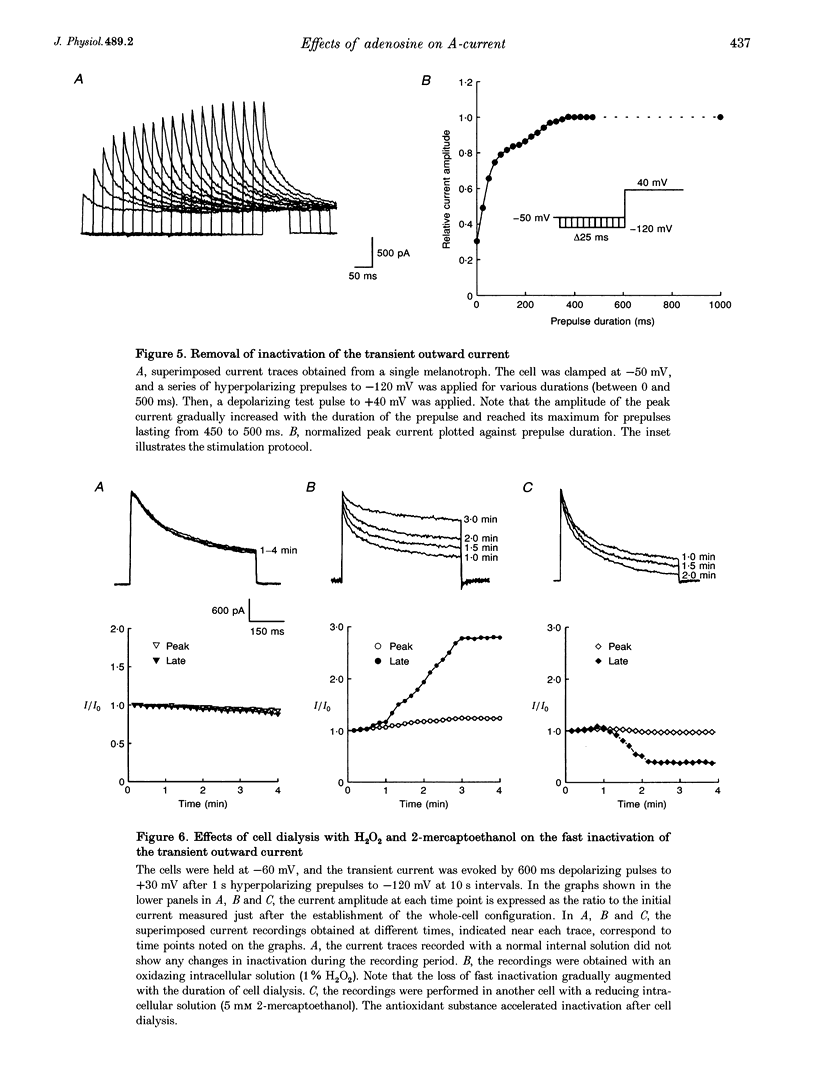
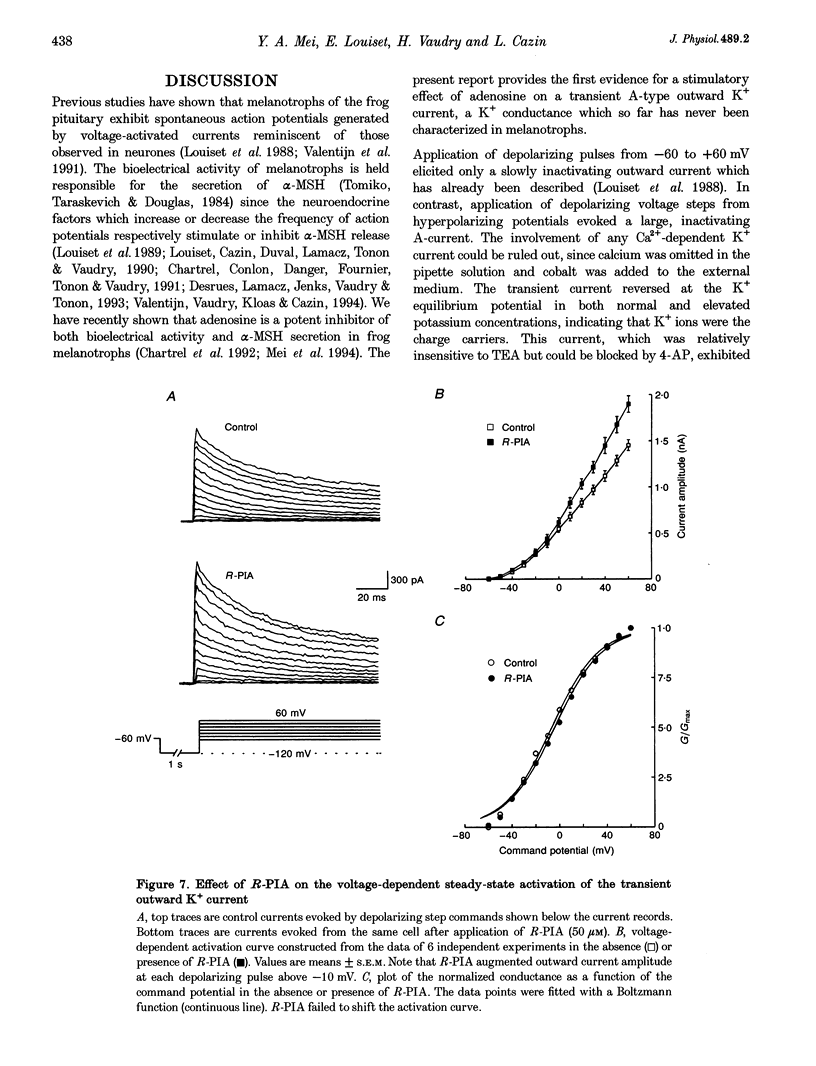
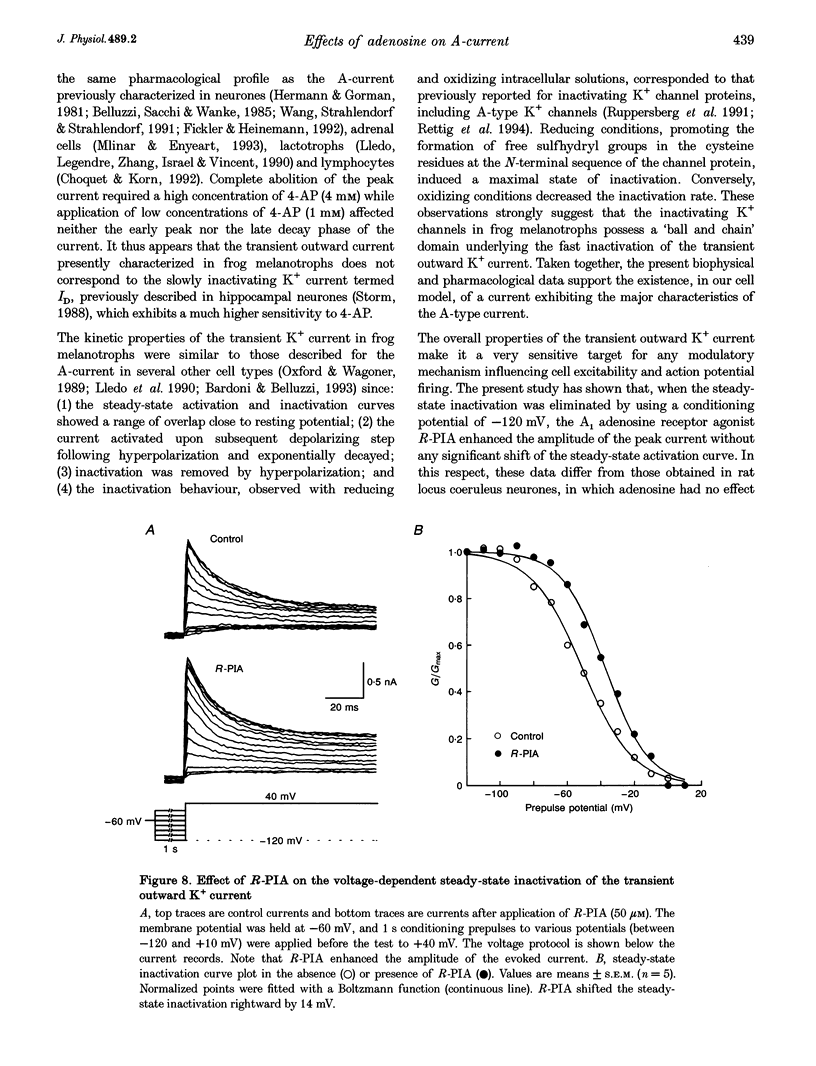
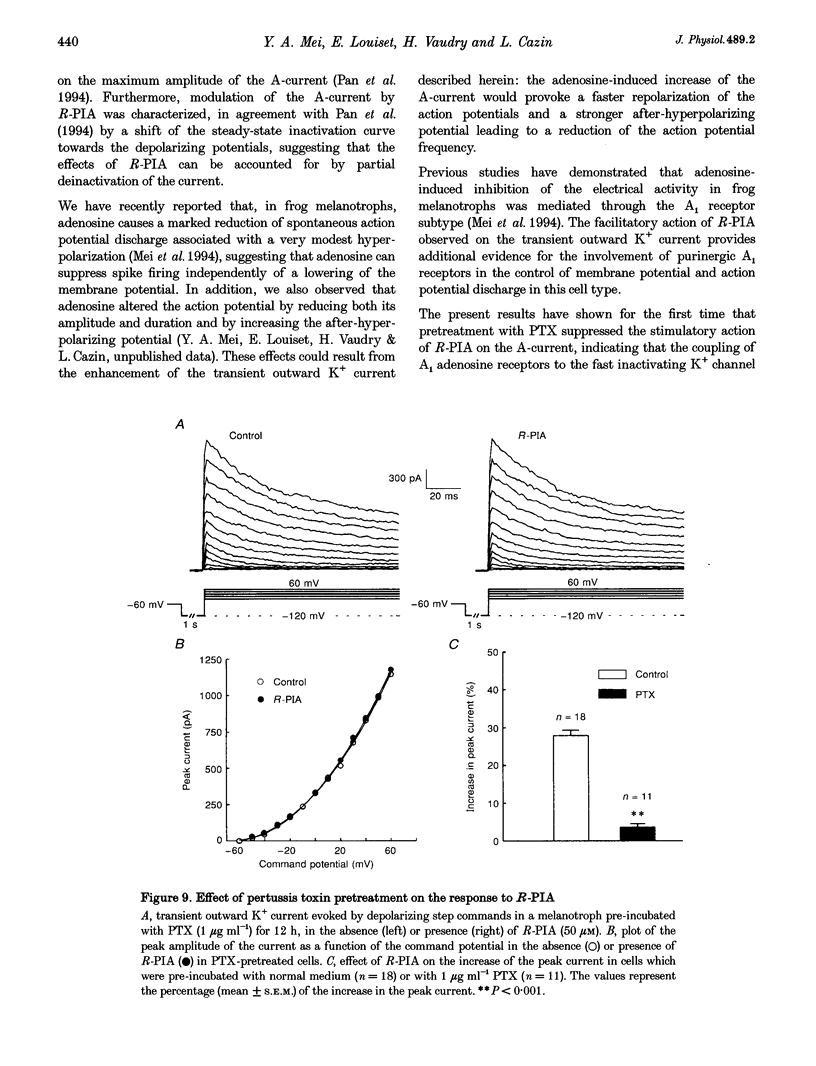
1. Transient outward current was recorded in cultured frog melanotrophs with the whole-cell configuration of the patch-clamp technique. The ionic dependence, kinetics and pharmacological properties of the current were studied. The effects of the A1 adenosine receptor agonist R-N6-phenylisopropyl-adenosine (R-PIA) on this current were also investigated. 2. In tetrodotoxin- and cobalt-containing solution, depolarization from -120 mV elicited both transient and delayed outward currents. Pulses from -60 mV activated only a sustained late current. 3. 4-Aminopyridine (4 mM) reduced the transient outward current much more than the delayed outward current. In contrast, tetraethylammonium (10-20 mM) selectively reduced the delayed current. 4. Tail current measurements showed a positive shift in the reversal potential when external K+ concentration was increased, indicating that K+ was the predominant charge carrier. 5. Steady-state inactivation was complete at potentials positive to -10 mV and removed by hyperpolarization. 6. Inactivation of the transient current was slowed and accelerated in oxidizing and reducing conditions, respectively, confirming the involvement of an inactivating 'ball and chain' peptide. 7. R-PIA increased the transient current. The steady-state inactivation curve was shifted towards more positive potentials without changing the activation kinetics. Pretreatment with pertussis toxin (1 microgram ml-1) blocked the response to R-PIA. 8. It is concluded that frog melanotrophs possess an A-type current that is likely to play an important role in excitability. This current, which is directly modulated by A1 adenosine receptors through a Gi/G(o) protein, appears to be responsible for the inhibitory effects of adenosine on electrical activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alzheimer C., ten Bruggencate G. Postsynaptic inhibition by adenosine in hippocampal CA3 neurons: Co(2+)-sensitive activation of an inwardly rectifying K+ conductance. Pflugers Arch. 1991 Oct;419(3-4):288–295. doi: 10.1007/BF00371109. [DOI] [PubMed] [Google Scholar]
- Anand-Srivastava M. B., Cantin M., Gutkowska J. Adenosine regulates the release of adrenocorticotropic hormone (ACTH) from cultured anterior pituitary cells. Mol Cell Biochem. 1989 Aug 15;89(1):21–28. doi: 10.1007/BF00228276. [DOI] [PubMed] [Google Scholar]
- Augustine G. J. Regulation of transmitter release at the squid giant synapse by presynaptic delayed rectifier potassium current. J Physiol. 1990 Dec;431:343–364. doi: 10.1113/jphysiol.1990.sp018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R., Belluzzi O. Kinetic study and numerical reconstruction of A-type current in granule cells of rat cerebellar slices. J Neurophysiol. 1993 Jun;69(6):2222–2231. doi: 10.1152/jn.1993.69.6.2222. [DOI] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Ho S. Adenosine modulation of potassium currents in preganglionic nerve terminals of avian ciliary ganglia. Neurosci Lett. 1992 Mar 16;137(1):41–44. doi: 10.1016/0304-3940(92)90293-g. [DOI] [PubMed] [Google Scholar]
- Chartrel N., Conlon J. M., Danger J. M., Fournier A., Tonon M. C., Vaudry H. Characterization of melanotropin-release-inhibiting factor (melanostatin) from frog brain: homology with human neuropeptide Y. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3862–3866. doi: 10.1073/pnas.88.9.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D., Korn H. Mechanism of 4-aminopyridine action on voltage-gated potassium channels in lymphocytes. J Gen Physiol. 1992 Feb;99(2):217–240. doi: 10.1085/jgp.99.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. M., Caldwell K. K., Boyajian C. L., Petcoff D. W., Schlegel W. Adenosine A1 receptors inhibit both adenylate cyclase activity and TRH-activated Ca2+ channels by a pertussis toxin-sensitive mechanism in GH3 cells. Cell Signal. 1989;1(1):85–97. doi: 10.1016/0898-6568(89)90023-5. [DOI] [PubMed] [Google Scholar]
- Desrues L., Lamacz M., Jenks B. G., Vaudry H., Tonon M. C. Effect of dopamine on adenylate cyclase activity, polyphosphoinositide metabolism and cytosolic calcium concentrations in frog pituitary melanotrophs. J Endocrinol. 1993 Mar;136(3):421–429. doi: 10.1677/joe.0.1360421. [DOI] [PubMed] [Google Scholar]
- Dorflinger L. J., Schonbrunn A. Adenosine inhibits prolactin and growth hormone secretion in a clonal pituitary cell line. Endocrinology. 1985 Dec;117(6):2330–2338. doi: 10.1210/endo-117-6-2330. [DOI] [PubMed] [Google Scholar]
- Ficker E., Heinemann U. Slow and fast transient potassium currents in cultured rat hippocampal cells. J Physiol. 1992 Jan;445:431–455. doi: 10.1113/jphysiol.1992.sp018932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of 4-aminopyridine on potassium currents in a molluscan neuron. J Gen Physiol. 1981 Jul;78(1):63–86. doi: 10.1085/jgp.78.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo P. M., Legendre P., Zhang J., Israel J. M., Vincent J. D. Effects of dopamine on voltage-dependent potassium currents in identified rat lactotroph cells. Neuroendocrinology. 1990 Dec;52(6):545–555. doi: 10.1159/000125650. [DOI] [PubMed] [Google Scholar]
- Louiset E., Cazin L., Duval O., Lamacz M., Tonon M. C., Vaudry H. Effect of acetylcholine on the electrical and secretory activities of frog pituitary melanotrophs. Brain Res. 1990 Nov 19;533(2):300–308. doi: 10.1016/0006-8993(90)91353-i. [DOI] [PubMed] [Google Scholar]
- Louiset E., Cazin L., Lamacz M., Tonon M. C., Vaudry H. Dual effects of thyrotrophin-releasing hormone (TRH) on K+ conductance in frog pituitary melanotrophs. TRH-induced alpha-melanocyte-stimulating hormone release is not mediated through voltage-sensitive K+ channels. J Mol Endocrinol. 1989 Nov;3(3):207–218. doi: 10.1677/jme.0.0030207. [DOI] [PubMed] [Google Scholar]
- Louiset E., Cazin L., Lamacz M., Tonon M. C., Vaudry H. Patch-clamp study of the ionic currents underlying action potentials in cultured frog pituitary melanotrophs. Neuroendocrinology. 1988 Nov;48(5):507–515. doi: 10.1159/000125057. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984 Sep;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y. A., Vaudry H., Cazin L. Inhibitory effect of adenosine on electrical activity of frog melanotrophs mediated through A1 purinergic receptors. J Physiol. 1994 Dec 1;481(Pt 2):349–355. doi: 10.1113/jphysiol.1994.sp020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlinar B., Enyeart J. J. Voltage-gated transient currents in bovine adrenal fasciculata cells. II. A-type K+ current. J Gen Physiol. 1993 Aug;102(2):239–255. doi: 10.1085/jgp.102.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S., Wagoner P. K. The inactivating K+ current in GH3 pituitary cells and its modification by chemical reagents. J Physiol. 1989 Mar;410:587–612. doi: 10.1113/jphysiol.1989.sp017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W. J., Osmanović S. S., Shefner S. A. Adenosine decreases action potential duration by modulation of A-current in rat locus coeruleus neurons. J Neurosci. 1994 Mar;14(3 Pt 1):1114–1122. doi: 10.1523/JNEUROSCI.14-03-01114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol. 1981;16(3-4):187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Rettig J., Heinemann S. H., Wunder F., Lorra C., Parcej D. N., Dolly J. O., Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994 May 26;369(6478):289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Shimahara T. Presynaptic modulation of transmitter release by the early outward potassium current in Aplysia. Brain Res. 1983 Mar 14;263(1):51–56. doi: 10.1016/0006-8993(83)91199-x. [DOI] [PubMed] [Google Scholar]
- Simpson R. E., O'Regan M. H., Perkins L. M., Phillis J. W. Excitatory transmitter amino acid release from the ischemic rat cerebral cortex: effects of adenosine receptor agonists and antagonists. J Neurochem. 1992 May;58(5):1683–1690. doi: 10.1111/j.1471-4159.1992.tb10041.x. [DOI] [PubMed] [Google Scholar]
- Spain W. J., Schwindt P. C., Crill W. E. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1987 May;57(5):1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- Storm J. F. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988 Nov 24;336(6197):379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Haas H. L., Gähwiler B. H. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiko S. A., Taraskevich P. S., Douglas W. W. Effects of veratridine, tetrodotoxin and other drugs that alter electrical behaviour on secretion of melanocyte-stimulating hormone from melanotrophs of the pituitary pars intermedia. Neuroscience. 1984 Aug;12(4):1223–1228. doi: 10.1016/0306-4522(84)90016-2. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987 Oct;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn J. A., Louiset E., Vaudry H., Cazin L. Dopamine-induced inhibition of action potentials in cultured frog pituitary melanotrophs is mediated through activation of potassium channels and inhibition of calcium and sodium channels. Neuroscience. 1991;42(1):29–39. doi: 10.1016/0306-4522(91)90147-g. [DOI] [PubMed] [Google Scholar]
- Valentijn J. A., Vaudry H., Kloas W., Cazin L. Melanostatin (NPY) inhibited electrical activity in frog melanotrophs through modulation of K+, Na+ and Ca2+ currents. J Physiol. 1994 Mar 1;475(2):185–195. doi: 10.1113/jphysiol.1994.sp020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Strahlendorf J. C., Strahlendorf H. K. A transient voltage-dependent outward potassium current in mammalian cerebellar Purkinje cells. Brain Res. 1991 Dec 13;567(1):153–158. doi: 10.1016/0006-8993(91)91449-b. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Ikeda S. R. Adenosine modulates voltage-gated Ca2+ channels in adult rat sympathetic neurons. J Neurophysiol. 1993 Aug;70(2):610–620. doi: 10.1152/jn.1993.70.2.610. [DOI] [PubMed] [Google Scholar]


